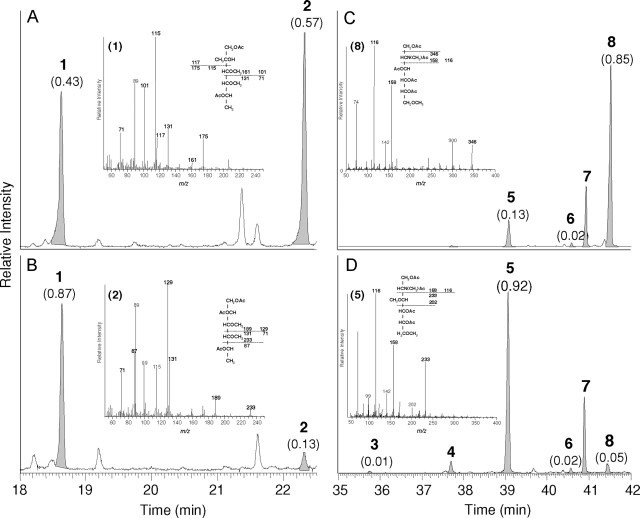
Fig. 6.
Linkage analyses of glycans released by endoglycoceramidase from total egg glycosphingolipids and egg glycosphingolipid fraction 6. (A–D), linkage analyses of oligosaccharides released from both total and fraction 6 glycosphingolipids before and after HF-treatment. Partially methylated alditol acetates obtained were separated by gas chromatography and registered in the positive ion mode after electron impact ionization. To facilitate understanding, only parts of the entire chromatograms are shown. (A and B), detection of fucose derivatives obtained from total (A) and fraction 6 (B) glycans. Peak areas normalized to the sum of terminal fucose (1) and 2-substituted fucose (2) set to 1.0 are shown in parentheses. Inset in (A): Electron impact mass spectrum of 1,5-di-O-acetyl-2,3,4-tri- O-methyl-fucitol (terminal fucose); Inset in (B): 1,2,5-tri-O-acetyl-3,4-di-O-methyl-fucitol (2-substituted fucose). Characteristic primary and some secondary fragment ions are assigned. (C and D), detection of partially methylated HexNAc-derivatives obtained from fraction 6 glycans before (C) and after HF-treatment (D). The ratios of terminal GlcNAc (3), 4-substituted GlcNAc (5), 3-substituted GlcNAc (6) and 3,4-disubstituted GlcNAc (8) set to 1.0 are shown in parentheses to underline the clear loss of 3,4-disubstituted GlcNAc after incubation of fraction 6 glycans with HF. Inset in (C): Mass spectrum of 2-deoxy-2-(N-methyl) acetamido-1,3,4,5,-tetra-O-acetyl-6-O-methylglucitol. Inset in (D): 2-deoxy-2-(N-methyl)acetamido-1,4,5,-tri-O-acetyl-3,6-di-O-methylglucitol. (1) terminal fucose; (2) 2-substituted fucose; (3) terminal GlcNAc; (4) terminal GalNAc; (5) 4-substituted GlcNAc; (6) 3-substituted GlcNAc; (7) 3-substituted GalNAc; (8) 3,4-disubstituted GlcNAc.