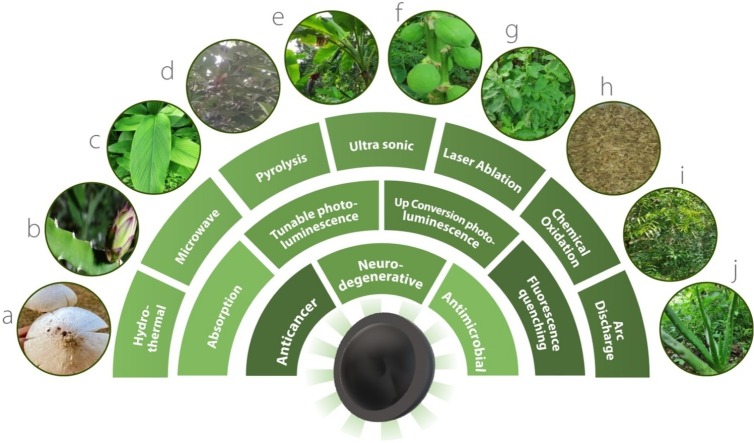
Graphical abstract
Various NCDs with antimicrobial, anticancer and neurodegenerative drug delivery applications. a) Pleurotus species, b) Hylocereus undatus, c) Curcuma longa L., d) Manilkara zapota (L.) P. Royen, e) Banana leaf, f) Carica papaya, g) Ocimum Sanctum, h) Foeniculum vulgare Mill seeds, i) Azadirachta indica, j) Aloe vera (L.) Burm.f.
Keywords: Natural carbon quantum dots, Synthesis, Optical mechanism, Drug delivery applications, Clinical status
Highlights
-
•
The source and rationale behind natural carbon quantum dots (NCDs) are carefully discussed.
-
•
The various synthesis method and their merits have been summarized.
-
•
The optical properties and toxicological profile of NCDs are highlighted.
-
•
The drug delivery applications of NCDs are detailed.
-
•
The clinical status of NCDs are presented.
Abstract
Natural carbon based quantum dots (NCDs) are an emerging class of nanomaterials in the carbon family. NCDs have gained immense acclamation among researchers because of their abundance, eco-friendly nature, aqueous solubility, the diverse functionality and biocompatibility when compared to other conventional carbon quantum dots (CDs).The presence of different functional groups on the surface of NCDs such as thiol, carboxyl, hydroxyl, etc., provides improved quantum yield, physicochemical and optical properties which promote bioimaging, sensing, and drug delivery. This review provides comprehensive knowledge about NCDs for drug delivery applications by outlining the source and rationale behind NCDs, different routes of synthesis of NCDs and the merits of adopting each method. Detailed information regarding the mechanism behind the optical properties, toxicological profile including biosafety and biodistribution of NCDs that are favourable for drug delivery are discussed. The drug delivery applications of NCDs particularly as sensing and real-time tracing probe, antimicrobial, anticancer, neurodegenerative agents are reviewed. The clinical aspects of NCDs are also reviewed as an initiative to strengthen the case of NCDs as potent drug delivery agents.
1. Introduction
Drug delivery is an approach to deliver drugs to the site of action in order to achieve therapeutic outcomes in an organism [1]. Nanoparticles with the potential of improved bioavailability, increased circulation times, reduced toxicity, and controlled drug delivery have emerged as a promising approach to develop new therapies and improve the efficacy of drugs. However, many nano-based drug delivery systems succumb to biological systems. Therefore, a nanoparticle-based drug delivery, which could not only support the systemic and intracellular evaluation of drug but also retain the original properties of the vehicle or carrier with high resolution, high sensitivity is required [2]. Quantum dots (QDs) have invited the attention of researchers over the past decades due to its versatile surface chemistry, small size, incredible electromagnetic, and luminescent properties which facilitate real-time monitoring of QDs vehicle transport and drug release at both systemic as well as cellular levels. These QDs are inorganic nanomaterials that are indicative of broad luminescence excitation spectra with narrow symmetrical excitation spectra encountering large stokes shifts [3].The optical society of America accredited Brus, Efros and Ekimov for its discovery in 1980 [4]. QDs consist of atoms in which the elements range from groups II-VI or III-V in the periodic table with physical dimensions of the particles smaller than the excited Bohr radius (radius of the first orbit of the atom) [5]. Typically, QDs consist of a metallic core material (capable of transmitting fluorescence) which is surrounded by a coating material to avoid photobleaching or leaking. The core material is selected with the aim to produce maximum quantum yield which is indicative of the brightness of fluorescent material and calculated by the ratio of the light emitted to the light absorbed by a fluorophore. It is believed that every excited electron leaves a hole in the ground state and when this excited electron reaches the ground state it combines to its hole to generate fluorescence. The electron absorbs energy to travel from ground to excited state which comes from U.V light as well as emits the same when traveling back. The distance travelled by an electron from the ground to excited state is termed as bandgap and the amount of energy emitted by the electron depends upon this bandgap. On the one hand, the band gaps of smaller QDs are larger, hence emit blue light due to more energy or higher frequency wavelength and on the other hand, bigger QDs have a smaller bandgap, therefore, emit red light due to lower frequency [5]. Modern QDs are fabricated to serve various purposes such as modified by functionization with acquiescent molecules, adopt hydrophilic nature by undergoing suitable modification, flexibly designed in the size ranging from 2−4 nm and possess innate properties such as optical, fluorescent as well as electronic properties. The size of the self-assembled QDs depends upon the manufacturing process. Long reaction periods consequently increase the particle size which could be stopped if the temperature is reduced or the solution is withdrawn at every interval and kept at room temperature [5]. These QDs are widely accepted because of the dominant striking characteristics including biocompatibility, photoluminescence (PL), morphology, size, and stability, despite high toxicity even at low levels due to the presence of heavy metals. Among commonly used graphene quantum dots, cadmium quantum dots and CDs, the latter have anchored firmly as nanostructures because CDs are highly cost-effective, synthesized easily on a large scale, agreeable chemical composition, uncomplicated functionalization, harmonising fluorescence emission, high solubility, and photochemical stability [6,7]. However, these semi conducting quantum dots witness toxicity problems because of the presence of heavy metals, and therefore many of the clinical studies are interrupted. Even at low doses, they tend to exert a harmful effect on human beings and environment due to the involvement of heavy metals in their preparation [8]. These limitations triggered the development of CDs and eventually, Xu et al. discovered CDs possessing fluorescent properties, during the separation and purification of carbon nanotubes. In 2006, Sun et al. proposed the name ‘Carbon quantum dots’ and proposed the synthetic route to develop CDs along with surface passivation to enhance fluorescence. These CDs are quasi-spherical nanoparticles which can be synthesized by two different methods namely top-down and bottom-up route. The extraordinary ability of CDs to either possess appropriate chemically reactive groups for functionalization or to link with numerous inorganic, organic, polymeric, biological or natural materials for surface passivation is because of the numerous carboxyl components affixed to the surface of the CDs. Surface functionalization ameliorates the solubility in both aqueous solutions as well as non-aqueous solutions and surface passivation improves fluorescence [6]. The synthesized fluorescent CDs are reported to emit in a range from deep blue (430 nm) to near infrared (730 nm) region [9]. These attributes of CDs make them in a favourable position for achieving unprecedented performance for a broad range of applications such as photodynamic therapy, bioimaging, biosensing, electrocatalysis and drug delivery [6].
Moreover, these CDs are further catalogued under man-made CDs (graphite, candle soot, ammonium citrate) and natural CDs (NCDs) manufactured from natural products (milk, pepper, coriander, honey, garlic, curcumin, rice husks). NCDs are gaining preference because of high abundance, low price, safe and possess the ability to convert low-value biomass into useful and valuable materials [10].
The aim of this review is to focus on source and rationale behind NCDs as well as their route of synthesis and modifications. The mechanisms behind the luminescence properties that promote the success of naturally fabricated CDs for drug delivery are explored. Besides, mode of action of NCDs which support drug delivery namely quantum yield, and toxicological profile including the biosafety and biodistribution are briefed in this review, based on recent works. The review specifically focuses on the beneficial outcomes of NCDs based drug delivery especially as sensing and real-time tracing probe, antimicrobial, anticancer, neurodegenerative agents, which are probably due to their optical properties, versatility towards functionalization and flexibility towards surface modification. The clinical studies in vitro and in vivo are also discussed to understand the limitation and needs in the foreseeable future.
2. Source and rationale behind NCDs
Natural products have always intrigued researchers because their limitless source and eco-friendly nature [11]. NCDs are CDs which are synthesised from natural raw materials, which are economical, non-toxic, mostly renewable and easy to synthesize. These NCDs do not require organic solvents, but instead can be prepared in aqueous solutions resulting in increased water solubility. NCDs do require external energy for their synthesis. The continuous availability of raw materials and low production costs have made the synthesis of NCDs a suitable protocol for industries. Kong et al. used ascorbic acid to fabricate NCDs with good water solubility, biocompatibility and fluorescent properties [12]. Kumar et al. prepared orange juice-NCDs, which were high in quality and produced good yield [13]. Recently, lemon juice-NCDs were prepared and investigated by Hoan et al. for potential applications in bioimaging and optoelectronics [14]. There are numerous other naturally occurring raw materials which have been employed for the preparation of NCDs such as carrot roots [15], egg yolk oil [16], chitosan [17] sucrose [18], raw cashew gum [19], lotus root [20], konjac flour [21],curcumin [22], mangosteen peel [23],N-acetyl-l-cysteine [24]. In addition, researchers are also focusing on recycling biomass wastes because of the alarming effects of the environment. These recycled sources are low cost, eco-friendly, sustainable and help reduce the environmental burdens of the society. In this context, Dehvari et al. investigated the effects of converting crab shells into NCDs with the help of nitrogen dopants. These crab shell NCDs possessed high quantum yield, biocompatibility and proved promising for theranostic applications [25].
For any potential nanomaterial, the constituents involved in the preparation play a vital role. NCDs have improved optical properties and compound idleness [26]. Similarly, utilization of plant extracts consisting of various bioactive molecules such as basic constituents (alkaloids), acidic components (tanins, flavonoids, ascorbic acid, tartaric acid, polyphenols, citric acid) and neutral ingredients (carbohydrates) built potent CDs because the synthetic kinetics depend upon these phytoconstituents. The bioactive components present in the extract deliver several benefits such as determination of surface functional groups on the CDs, material reactivity, particle size, and fluorescence quantum yield. To illustrate, neem leaf (Azadirachta indica) have better supercapacitor properties than Ashoka (Saraca asoca) due to the presence of the bioactive components present in Azadirachta indica [27]. The phytoconstituents such as certain enzymes, proteins, polysaccharides, vitamins, and other biomolecules possess natural behaviour of reduction and capping of non-biocompatible substances [28]. The phytoconstituents present in NCDs provide adaptability to solvent and dopants, hence making them an attractive choice for developing successful CDs. Unlike man-made CDs, which require the addition of external nitrogen (N) or sulphur (S) containing compounds, these natural products proved more lucrative as they contain heteroatoms such as nitrogen and sulphur, therefore, can be conveniently prepared as heteroatom doped NCDs [29]. Since, doping with nitrogen ameliorates the fluorescence intensity, nitrogen doped NCDs are incredibly suitable for biomedicines [30]. Besides, to avoid any kind of toxicity, surface passivation via citric acid or amino acids are utilized as post synthesis treatment. To avoid any expensive precursors NCDs are prepared from fruit peels or juices (lemon, grapes, lychees, avocado, kiwi etc.), vegetables, milk, and willow bark, which have abundance of polyphenolic and phenolic compounds that exhibit antioxidant properties which further enrich their synthesis [31,32]. This green synthesis approach is highly sustainable, minimizes waste production, highly biocompatible, environmental friendly, easy handling and non-toxic. They are well suited for the production of CDs because they have high content of carbon source [28]. In addition, their synthesis method requires lower temperature and less time consuming, hence prove potent candidates for various applications [33].
3. NCDs: synthesis method and modifications
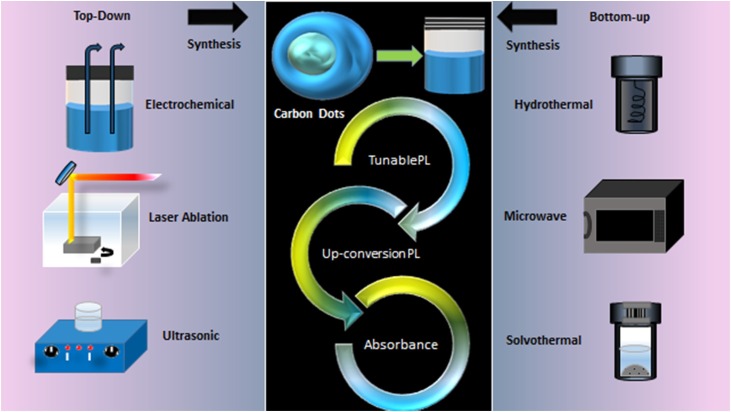
On the bases of particular application as well as for controlled and desirable form of NCDs, the choice of synthesis method is critical. Basically two main approaches are adopted for the synthesis of NCDs which are broadly categorized into ‘Bottom - up’ and ‘Top - down’ method [34]. The ‘Bottom-up’ method utilizes small organic molecules through partial dehydration and dehydrogenation accompanied by microwave, thermal pyrolysis, hydrothermal or solvothermal decomposition whereas the ‘Top-down’ method proceeds via the breakdown of relatively large particles into smaller molecules or nanoparticles via laser ablation, arc discharge, ultrasonic or chemical oxidation [35]. Despite the difference in methods both require external energy to conduct the synthesis [36].This segment intends to brief about the preparation of NCDs including different synthesis approaches, laying more emphasis on the merits and demerits of each method and modifications involved.
3.1. Preparation of NCDs
The preparation of NCDs involves the selection of the appropriate methods of synthesis that is either Bottom-up or Top-down approach as per the required application, followed by the removal of large-sized particles.
In the Bottom-up approach, the hydrothermal method is the most popular which proceeds by directly heating aqueous solutions containing the intended natural source [36]. This single method is capable of covering four crucial stages namely dehydration, polymerization, passivation and carbonization. This method is widely accepted because it can be easily controlled, simple, economic, energy-efficient and results in non-toxic NCDs [37]. However, the requirement of high temperature, lengthy process time, low quantum yield and necessarily require high temperature furnace, which is not practically available in laboratories are some major drawbacks [36,38]. Ramar et al. developed NCDs with Pomelo juice (Citrus maxima or Citrus grandis) by one-step hydrothermal method at 200 °C for 7 h which proved efficient as photocatalytic agent [26]. There are various other natural products which are successfully fabricated as NCDs by hydrothermal method which exhibit high photostability and prove promising as sensing probe. The one-step green hydrothermal synthesis (250 °C for 6 h) of glycerol and succinic acid without any addition of acid or base catalysts. By varying the reaction time, blue and green fluorescence with quantum yield of 11 and 7 %, respectively, were obtained. The blue fluorescent CDs acted as dual mode sensors for the detection of hydrogen peroxide (H2O2) and ferrous ion (Fe2+).The cell viability was 90 % at concentration of 1 g L−1, indicating high biocompatibility in cell imaging test. Hence, these hydrothermally synthesized succinic acid and glycerol NCDs proved promising as chemical and intracellular probes. NCDs prepared from rice husk (mesoporous silica) by hydrothermal method at 200 °C for 6 h and calcination methods, were 4–5 meters in diameters with quantum yield of 3 %. These NCDs exhibited high photostability and were capable of detecting alcohol vapors at room temperature [39]. Besides, ethanol, methanol and many volatile organic compounds were easily distinguished by optical electronic nose system [40]. Another economical and readily available natural resource, radish, was used as precursor to prepare CDs by hydrothermal method at 200 °C for 7 h. The quantum yield for these NCDs was found to be 15 % and demonstrated blue emission under U.V. illumination. The radish NCDs were capable of sensing acetic acid vapour and Cu2+ions [41]. Another method is Microwave-assisted synthesis which involves electromagnetic radiation for transforming the precursors into desired NCDs. This method is highly efficient, selective, high yield, short reaction times, easy, rapid, energy-saving, controlled temperature, controlled size, low impurity, improved safety, better reproducibility, and cost-effective [37]. This is because this method provides contactless heat transfer to the reactant sample because the energy is produced directly within the material by molecular interaction with the electromagnetic field. However, this method lack in in-depth penetration [38]. l-asparagine CDs synthesized by Wang et al. through one–pot microwave assisted method at 180 °C and held for 15 min proved to pose high stability and negligible cytotoxicity even at 800 μg/mL (incubation concentration) [42]. Ultra-small CDs with Acacia concinna seeds (Shikakai) were prepared by microwave method at 800 W for 2 min., which were helpful in multicolour imaging of fungal cell (Penicillium sp.) and other bioanalytical applications [43]. Pyrolysis is yet another method which utilises irrevocable thermal exposure to the precursors to undergo physical and chemical modification. This approach is not much in demand because of the requirement of high temperature and immense energy consumption. The one-step pyrolysis method for the preparation of NCDs derived from Aloe vera extract was demonstrated in a study by Devi et al. The extract was subjected to high temperature (160−250 °C) for 10−30 min for carbonization and diluted with water. This method produced NCDs with a quantum yield of 12.3 % and proved beneficial in exhibiting bright luminescence under U.V. light with excitation dependent emission behavior as studied by PL, ultraviolet (U.V) spectroscopy, fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. Aloe vera NCDs exhibited light-activated antibacterial activity and metal ion sensing quality [44].
In the ‘Top-down’ approach, the ultrasonic method is most popular which involves high intensity wavelength to produce NCDs via chemical modifications. The credit to this method is consumption of low external energy and ameliorated precursor reactivity [36]. However, these modifications results in low yield [37]. Recently, Ring et al. synthesized curcumin quantum dots (Cur-NCDs) by employing unique combination of mechanical and ultrasonic techniques. In this technique, zirconia beads (10 g) were mixed with curcumin (600 mg) in ethanol (15 mL), oscillated for 15 min and concentrated by rotary evaporator at 60 °C for 15 min. This concentrated solution was subjected to ultrasonication on an ice bath for 20 min with power of 750 W, frequency 20 kHz, the intensity of 30 W/cm2, pulse ratio 50/10 s on/off and 40 mL hot water was added drop-wise. This ultrasonication procedure was repeated again and dried at 70 °C to form efficient Cur-NCDs with 13.7 nm average size, and zeta potential of +13.3 mV. Cur- NCDs formed promising antibacterial and antimicrobial agent [45]. Besides, laser ablation utilises direct UV-pulsed laser irradiation to target moieties that are submerged in aqueous medium to produce NCDs. Another method under this category is electrochemical synthesis using precursors of electrodes in electrolyte of water or ionic liquid which operates by voltage differences. This method is simple, requires mild experimental conditions including no exhausted toxic gas and with slight alterations in electrolyte pH, pure water or phosphate buffer solution successful CDs could be prepared in ionic liquid free electrolyte which prove both economic and convenient [46]. Chemical oxidation serves as another possible method that utilizes oxidative agents namely H2SO4 and HNO3 to oxidize the substrate. This method is also convenient, non-requirement of elaborate methods, repeatability in production and numerous oxygen function groups can be utilized such as −COOH and −OH to provide hydrophilicity and emission control [47]. Moreover, arc discharge method functions under inert atmosphere and in the presence of catalyst to vaporize carbon. It is considered to be a tedious method because of the formation of complex compound that needs further separation and purification [48]. The NCDs prepared from natural source using different method are given in Table 1 .
Table 1.
Natural carbon quantum dots (NCDs) synthesized by different method.
| S. No. | NCDs | Methods | Quantum Yield (%) | Size (nm) | Reference |
|---|---|---|---|---|---|
| ‘Bottom-up’ approach | |||||
| 1 | White flowering plant | Hydrothermal, 250 °C, 4 h | 28.2 | 5 | [11] |
| 2 | Ascorbic acid | Hydrothermal, 180 °C, 12 h | 47 | 4.5 | [12] |
| 3 | Orange juice | Hydrothermal,120 °C, 150 min | 26 | 1.5−4.5 | [13] |
| 4 | Lemon juice | Hydrothermal, 120–280 °C, 12 h | 14.86 to 24.89 | 12−15 (200 °C), 3−5 (280 °C) | [14] |
| 5 | Carrot roots | Hydrothermal, 170 °C, 12 h | 7.6 | 2.3 | [15] |
| 6 | Pomelo peels | Hydrothermal, 220 °C,2 h | 6.9 | 3 | [26] |
| 7 | Papaya | Hydrothermal, 200 °C, 5 h | 18.39−18.98 | 20 | [64] |
| 8 | Tulsi leaves | Hydrothermal, 200 °C, 4 h | 3.06 | 5 | [79] |
| 9 | Curcumin | Hydrothermal, 180, 1h | 3.6 | 1.6 | [86] |
| 10 | Egg yolk oil | Hydrothermal, 200 °C,60 min | 5.01 | 10 | [16] |
| 11 | Chitosan | Microwave,450 W, 5 min | 2−10 | 20 | [17] |
| 12 | Sucrose | Microwave, 100 W, 3 min 40 s | – | 3−10 | [18] |
| 13 | Raw cashew gum | Microwave, 800 W, 30 - 40 min | – | 9 | [19] |
| 14 | Lotus root | Microwave, 800 W, 6 min | – | – | [20] |
| 15 | L-Asparagine | Microwave, 180 W, 15 min | – | 2.17 ± 0.70 | [42] |
| 16 | Konjac flour | Pyrolysis, 470 °C, 1.5 h | – | – | [21] |
| 17 | Curcumin | Pyrolysis,180 °C | 17 | 2−8 | [22] |
| 18 | Aloe vera | Pyrolysis, 160–250 °C | 12.37 | [44] | |
| ‘Top-down’ approach | |||||
| 19 | Curcumin | Ultrasonic | – | 13.7+4.9 | [45] |
| 20 | Fruit juice (Avacado, kiwi, pear) | Ultrasonic,30 °C, hydrothermal treatment 200 °C for 12 h |
35, 23, 20 | 4.42 + 0.05, 4.35 + 0.04, 4.12 +0.03 | [32] |
| 21 | N-acetyl-l-cysteine | Laser ablation | – | 34.1−40.8 | [24] |
| 22 | Neem Leaves | Chemical oxidation | 1−2 | 5−6 | [27] |
After synthesis, the prepared uneven sized NCDs are subjected to filtration, centrifugation and/or dialysis to remove the larger particles and to obtain the desired particle size.
3.2. Modification through Hetro-atom doping and surface functionalization
NCDs can be modified either by hetro-atom doping or surface functionalization, where the former involves the addition of Nitrogen (N), Sulphur (S) or Boron (B) to improve the fluorescence properties of NCDs which further broadens their applications whereas later bring alterations in the functional group of NCDs by the process of addition, removal or conversion [3]. Surface modification or passivation is an approach to improve the properties especially, the quantum yield of NCDs that consists of abundant functional groups which readily bind to the functional ligands (DNA, proteins, polymers, organic molecules) via electrostatic, amidation and coordination interactions. NCDs prepared from sugarcane bagasse through chemical oxidation and exfoliation were surface passivated by organic solvent (toluene) to improve biocompatibility, stable fluorescence, high quantum yield, high crystalinity, with size 4.1+0.17 nm (longitudinal dimensions) and roughness of 5 nm as analysed by UV–vis absorption, fluorescence microscope, X-ray photoelectron microscopy (XPS), X-ray diffraction (XRD), high resolution transmission electron microscopy (HR-TEM), atomic force microscopy (AFM), respectively [49]. However, as surface passivation is a tedious and complex approach, therefore, hetro-atom doping is favoured because this strategy is direct and facile [50]. Hetro atom doping can be done either with metallic or non-metallic elements that help to change the electron distribution and surface structure of CDs as well as NCDs to improve their fluorescent properties by adjusting the gap between conduction band and valence band. Non-metallic dopants (nitrogen or silicon) improve quantum yield whereas metallic dopants (manganese or copper) control the reactions of CDs with carboxyl and amino groups of the precursors during carbonization or dehydration via chelation between chemical group and metal ions as well as modulate the band structure of CDs [51]. Various studies have illustrated the amelioration of the quantum yield of NCDs by doping especially, nitrogen doping, which synergistically act to improve the fluorescence effect through chelation of amino group to numerous functional group of NCDs. Nitrogen-doped CDs of Hylocereus undatus (H. undatus) via hydrothermal route show strong blue fluorescence at 400 nm as well as excitation dependent emission properties. The doping with nitrogen (aqueous ammonia) was confirmed by energy dispersive spectroscopy (EDS) as well as FT-IR and size was confirmed by HR-TEM. These fluorescent N-CDs show less cytotoxicity which is confirmed by 3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay, excellent biocompatibility on human breast adenocarcinoma cells (Michigan cancer foundation [MCF]-7)and Lymphoblastoid (L)-929 cells and good catalytic activity towards reduction of methylene blue by sodium borohydride [8]. Similarly, aqueous ammonia as nitrogen dopant proved beneficial for the preparation of CDs with Phyllantus emblica (P.emblica). Various characteristics were analysed: nitrogen doping of NCDs were confirmed by energy dispersive x-ray spectroscopy (EDX) and FTIR ; size was found to be 4.08 nm as analysed from HR-TEM ; excitation at 320 nm, an intense blue fluorescence was emitted around 400 nm, which was confirmed by EDS, Raman spectroscopy and the catalytic property by using NaBH4. These observations proved favourable in the reduction of textile effluents [52]. Besides, the nitrogen doped NCDs were efficiently prepared using the extract of unripe Prunus mume (P. mume), by hydrothermal carbonization at different pH via adjustments in aqueous ammonia solution (25 %).These NCDs emitted high fluorescence at pH 9 as analysed by UV–vis and fluorescence spectroscopy with size about 9 nm (analysed by HR-TEM), interlayer distance of 0.21 nm (determined by XRD).The doping was confirmed by XPS and FT-IR. These NCDs proved potent as a staining probe for fluorescence cell imaging with very low cytotoxicity [52].
4. Mechanism behind optical properties useful for drug delivery of NCDs
CDs are well suited for monitoring the drug release and real-time tracking of individual nanocarriers, because of the fact that CDs absorb light over a wide UV spectrum to emission wavelength of the particle and also emit light with high intensity in a narrow spectral range, which depends upon the core size of the dots. Besides, common organic fluorophores are too dim to detect with high sensitivity and get quenched rapidly under continuous illumination. This feature is compromised in CDs which possess high brightness, long term monitoring and can also be improved by surface functionalization and passivation of CDs. Moreover, the most biological molecules emit light in the blue-green spectral range and CDs shift emission toward red and near infrared region (NIR). Therefore, clear contrast could be easily achieved between CDs and the tissue displaying auto-fluorescence allowing excitation by the blue green light. CDs which emit light in the NIR region are useful for in vivo fluorescence imaging because of the absorption and scattering of visible light by biological tissues. The small dimension CDs with optical properties as well as extremely large Red (Stokes) shift prove promising model as nanocarrier for real-time intravital tracking and biodistribution study [2].
4.1. Photoluminescence (PL)
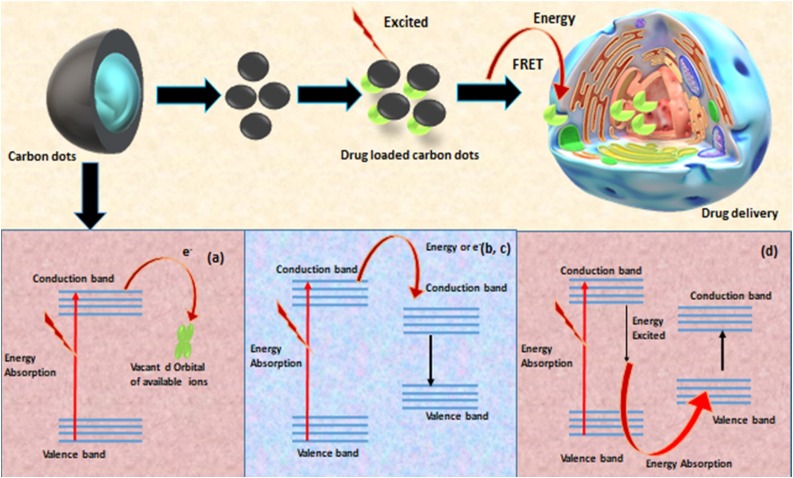
Understanding the fundamental of PL is necessary. The mechanism proposed for CDs can be also applied for NCDs [29]. Although, the mechanism of PL is not clear, there are many postulated theories evolved regarding the source for PL. It is considered that PL occurs due to the isolation of sp2 domains, which are ingrained in sp3 domain. The isolation of sp2 domain are responsible for the band gaps that forms broad emission wavelength in the range from UV to near infrared region due to excitonic state formed by high density p electrons in sp2 hybridised islands and week emission, due to quenching through radiation devoid relaxation to the ground state that occurs during migration of exciton to energy traps [9]. Zhi et al. studied the optical properties of NCDs especially, the band gap energies by utilising green and inexpensive malic acid to form NCDs via the utilization of ethylenediamine, as a self-passivating agent and cross-linker through microwave assisted synthesis. Malic acid NCDs were separated into fractions having different particle size by C-18 reversed-phase silica gel column chromatography which facilitated the study of optical properties of these CDs especially, band gap energies. An extinction shoulder at 470 nm was observed reflecting a red shift that denoted the narrowing of band gap, which was calculated by optical band gap energy Eg = h*c λ cut-off, where h is the planks constant (6.626 × 10−34 Joules sec), c is the speed of light (3.0 × 108 meter/sec and λ cut-off (in nm x10-9 m).The Eg values with respect to particle size 6.2 + 2.0, 9.2 + 1.7,15.6 + 6.0 nm were found to be 2.97, 2.91, 2.21 eV, respectively. Other parameters, influenced by the particle size range 6.2 + 2.0, 9.2 + 1.7,15.6 + 6.0 nm were stokes shift 16822, 4959, 2644.3 cm-1; quantum yield 30, 24, 27.6 % and average fluorescence lifetime 6.48 ± 0.10, 7.14 ± 0.63, 4.25 ± 0.09 ns, respectively. Hence, size of the NCDs influences the band gap energy and PL properties [53]. The adoption of suitable synthesis method and particle size of NCDs tune the band gap energies and channel the PL properties [54].The various synthesis method and mechanism involved are given in Fig. 1 . Presently, the most accepted fluorescence mechanism are the bandgap transition related to conjugated π-domains, surface defects, quantum size effect and quantum confinement effect that give rise to tunable fluorescence, up-conversion fluorescence and absorption which are helpful in imaging and sensing in drug delivery applications. Therefore, the understanding the fluorescence mechanism of CDs is important and can be understood by employing various detection techniques such as single molecule fluorescence microscopy, lifetime microscopy and super-resolution technique [55].
Fig. 1.
Different synthesis method and optical properties of Natural carbon quantum dots.
Apart from the sp2 or sp3 hybridized carbons around NCDs or CDs, other functionalized surface defects (carbonyl related localised electronic states) of NCDs or CDs possess non-perfect sites that produce surface energy traps that engulf solid hosts responsible for exhibiting multicolour emission in the blue green regions of visible spectrum. The p state in the sp2 and sp3 sites determine the optical properties of CDs due to the recombination of electron-hole pairs in the strongly localised p* and p electronic levels of the sp2 sites. These sites lie between the bandage of the s and s* of the sp3 matrix, which lead to strong visible emission. These electronic transitions exhibit weak absorption in the UV–vis region however, strong emission in the visible region and functionalization or surface passivation help brighter fluorescence properties [56].The fabrication of NCDs with dehydrated shiitake mushroom by hydrothermal synthesis via nitrogen doping which resulted in oxygen rich NCDs having carboxyl, hydroxyl and amine group with diameter 4.2 nm. The PL as well as the excitation dependent fluorescence was observed with these NCDs, which might be due to the varied emissive trap at their surface and size [57]. Besides, ethylenediamine is a strong nitrogen dopant that increases the surface defects of NCDs prepared from microwave synthesis of potato starch resulting in non-radiative transmission of electrons [58]. The various PL mechanisms are given in Table 2 [59].
Table 2.
Different Photoluminescence mechanism.
| S. No. | Photoluminescence Mechanism | Features | Reference |
|---|---|---|---|
| 1 | Bandgap transition related to conjugated π-domains |
|
[58] |
| 2 | Surface defects |
|
[61] |
| 3 | Quantum size effect |
|
[59] |
| 4 | Quantum confinement effect |
|
[59] [61] |
| 5 | Fluorescence Quenching |
|
[63] |
| |||
| |||
| |||
| |||
| |||
| |||
|
It is because of these varied emissive surface traps that enables optical selection. Quantum size effect is another factor governing fluorescence properties of NCDs which help control the light emission wavelength as reviewed by Zhu et al. To illustrate, varied sized CDs prepared by electrochemical method demonstrated that CDs with size of about 1.2, 1.5–3, 3.8 nm can be categorised as small (UV light emission), medium (visible light emission) and large (near to infrared region), respectively, possibly because of the conduction band- valence band gap dependence on graphene fragment (size of the fragment is inversely proportional to gap).Therefore, large CDs with small ratios of sp2 clusters possess larger band gaps [60]. Quantum confinement effect is dependent on crystal boundary and exhibit band size and band gap dependent energy relation process [61].The size of the sp2 domain controls the quantum confinement effect via particle size of CDs /NCDs [55]. The PL mechanism is well explained by Ramezani, et al. with quince fruit (Cydonia oblonga) powder as carbon precursors. The quince NCDs prepared by microwave and hydrothermal methods exhibited excitation dependent PL, which were because of the different energy levels exerted by different surface groups. In addition, this emission dependent excitation increased the emission intensity at 450 nm while excited between 310–350 nm, same between excitation range of 350−360 nm and decreased when excited to 370−390 nm, which explained the quantum confinement effect. Moreover, when excited to 400–500 nm, a decrease in emission intensity and red shift in emission wavelength were observed. The red shift occurs due to the surface functional groups which engulf different surface states. This emission dependent excitation is also dependent upon the adopted synthesis method, because this influence the properties of the nanoparticles formed [62].
4.2. Fluorescence quenching
NCDs or CDs exhibit excitation dependent emission, which is helpful in the detection of analytes, bioimaging and drug delivery. This phenomenon arises due to the interaction between CDs and analytes either by increasing the fluorescence via suppressing the quenching effect or decreasing the fluorescence by quenching. This quenching mechanism can be subcategorised into dynamic, static, energy transfer, inner filter effect (IFE) and photoinduced electron transfer (PET).The energy transfer is further divided into dexter energy transfer (DET), surface energy transfer (SET) and foster resonance energy (FRET). FRET was discovered in 1948 by a German scientist, which are basically the photonic energy of donor (first fluorophore) required by Bacceptor (second fluorophore) and emitted by second fluorophore [63]. Chitosan based NCDs were investigated for fluorescence quenching by using nitroaromatics with different ring substitutes, which indicated that the primary quenching mechanism behind NCDs was FRET quenching [29,63].
4.3. Tunable PL and up-conversion PL
Tunable PL and upconversion PL are features of NCDs/CDs, which are affected by surface defects, quantum size or confinement effect. In addition, the well-defined crystalline core, the structural diversity and the method of synthesis of CDs/NCDs are responsible for different as well as tunable PL, up-conversion PL, and absorption. Tunable PL phenomenon of NCDs demonstrate excitation dependent PL along with strong emission, which decline rapidly from blue-wavelength region to red-wavelength region. Kasababu et al. studied the green chemistry of NCDs formed from Carica papaya juice as precursors. All NCDs formed exhibited absorbance at 343 nm and excitation to 383 nm, yielded maximum emission peak at 461 nm. It was also observed that after prolonged heating for 12 h and at temperature 170 °C, maximum absorbance and strong blue emission were detected under U.V. illumination at 365. Besides, excitation wavelength higher than 400 nm, the emission intensities decreased rapidly and exhibited red shift with excitation to longer wavelength [64].The manipulation in the size of CDs also affects PL due to the quantum size effect. These modifications in the chemistry and size of CDs could also ameliorate the red-shift emission, consequently narrowing the band gap between conduction band and valence band. Therefore, there are various factors can be employed to control the size and conjugation of CDs such as synthesis, fractionation or surface modification. In certain cases the interference due to surface defects including either heteroatoms or functional groups restricts quantum size effects. These radiative or non-radiative surface defects form recombination centres’ for excited electrons or holes, which drastically affects PL.
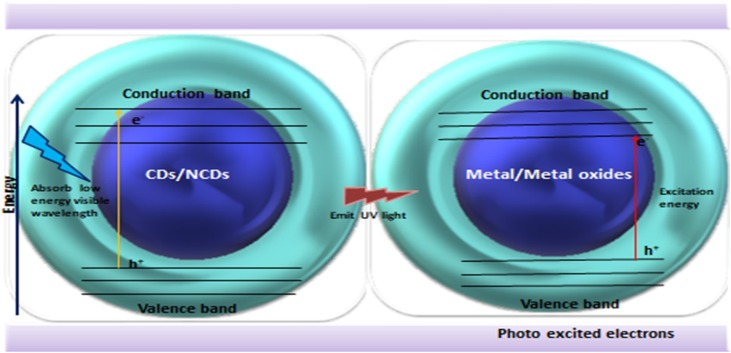
Up-conversion fluorescence is a type of tunable PL, where sequential absorption of two or more photons leads to the emission of light shorter than the involved excitation wavelength, helpful in in vivo bioimaging as bioimaging in the NIR region (longer wavelength) due to the diminished background auto-fluorescence and ameliorated photon tissue penetration (Fig. 2 ). Up-conversion arises due to the electron relaxation from p-orbital of high energy conduction band to the s-orbital as low energy photons excite electrons that transit to the conduction band. Even electrons in s-orbital can get excited but exhibit conventional down-conversion. Another reason postulated for up-conversion phenomenon is that certain upconversion emission is artefact from the conventional PL because it can be excited by leaking the component from the second diffraction in the monocromator of the spectrofluorometer [65]. Generally, in fluorescence spectrophotometer, continuous spectrum from the xenon lamp passes through a diffraction grating. The grating splits the single wavelength light in different directions as a monochromatic excitation light. The diffracted light will demonstrate maxima at angles θm= arc sin (m λ/d- sin θi), where d is the grating spacing, θi is the angle of the incident light on the grating, λ is the wavelength of light and integer m is 0, 1, 2 (order of the diffraction maxima).The zero order (m = 0) is the direct transmission for a transmission grating or specular reflection grating, the first order maximum is the excitation beam of largest intensity and second order maximum of the light is half wavelength of the excitation wavelength. The second order maxima can also simultaneously pass through the slit but with a lower intensity and can be easily removed by inserting a long pass filter into the excitation pathway. As up-conversion phenomenon is an emission photon at shorter wavelength than the excitation wavelength which is attained by absorbing two or more photons. Hence, it is difficult or extremely less observed in commercial fluorescence spectrophotometer because the excitation source is low intensity and incoherent xenon lamp. The long pass filter help confirm that there is no observable upconversion fluorescence for CDs/NCDs [66].
Fig. 2.
Up conversion photoluminescence of CDs/NCDs: absorb light of visible wavelength and emit ultraviolet light, which is absorbed by metal/metal oxides to form photo-excited electrons.
4.4. Absorption
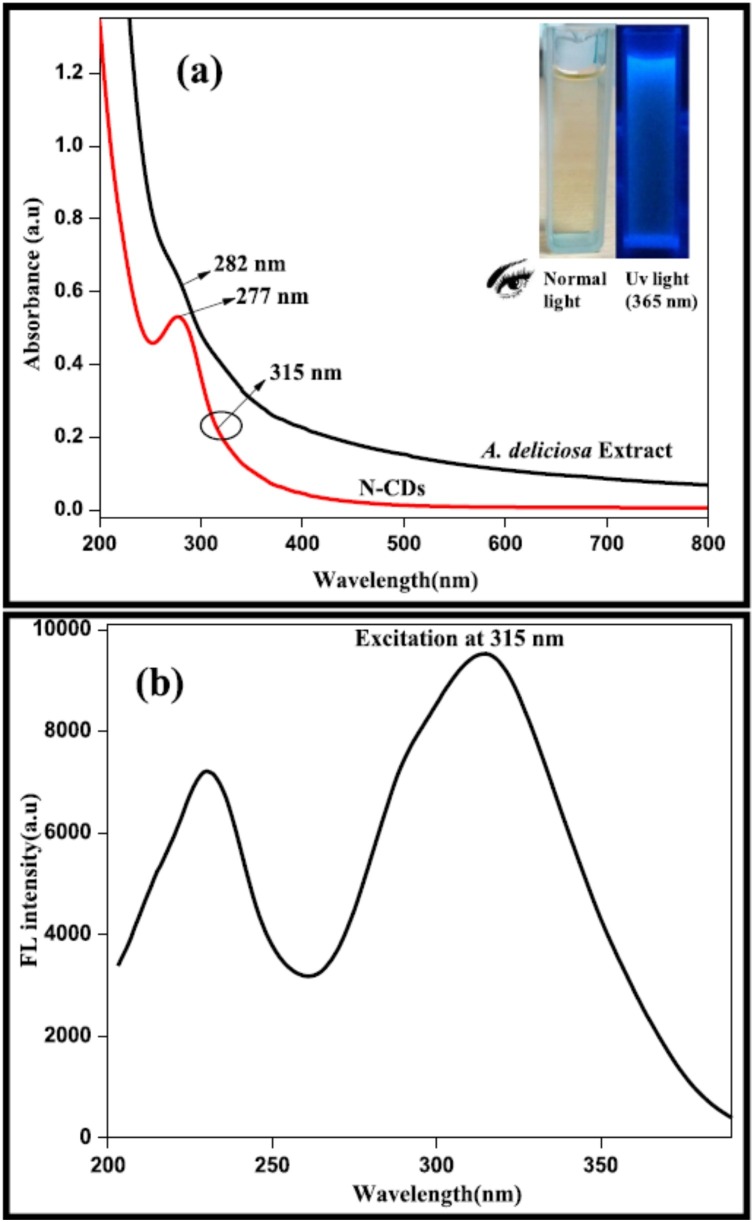
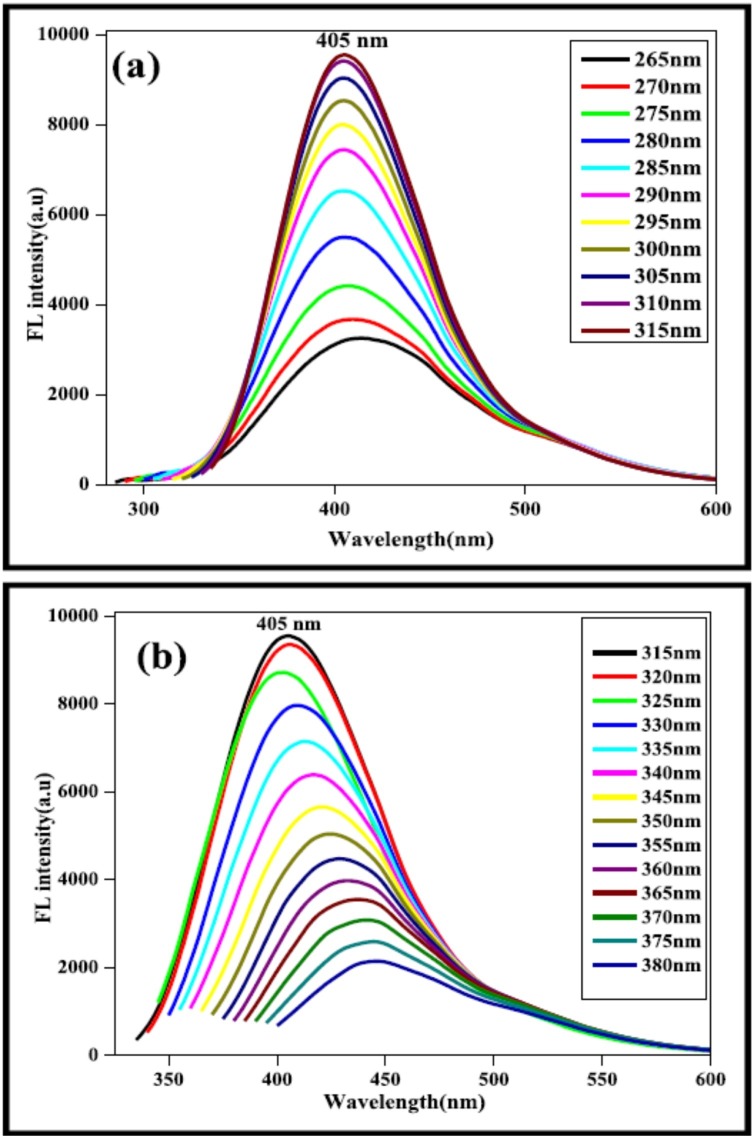
Most NCDs or CDs demonstrate a strong absorption band that shifts from UV to visible region [29]. This shift is also speculated to be due to surface modification or heteroatom doping. The obtained absorption peaks are similar for most of the NCDs or CDs and are ascribed to π-π* transitions of conjugated electron as well as n-π* transitions of hybridization with heteroatom such as N, S, P, etc. or oxygen atom [3,67]. A peculiar absorption spectrum of CDs consists of an absorption peaks that are usually in the range of 230−320 nm which extends into the visible region as absorption tail. The method adopted for synthesis also significantly affects the spectral range of absorption of CDs as the UV absorption peaks are slightly different for different methods. It is estimated that the properties observed in CDs are similar to that of NCDs [67]. The detailed absorption properties of NCDs were studied by Arul et al. In this study, Actinidia Deliciosa NCDs, which were prepared by hydrothermal method showed absorption maxima (λmax) at 282 nm. Actinidia Deliciosa NCDs revealed two distinct peaks at 277 and 315 nm, which is postulated to arise due to the n-π* as well as π-π* transition linked to carbonyl/hydroxyl and C C groups, respectively. The changes in the absorption values of NCDs were attributed to the addition of aqueous ammonia during the hydrothermal reaction, which caused the phenolic −OH to convert into ammonium phenolate ions. The excitation spectrum had two different major peaks at 289 and 315 nm that correlated with their absorption spectrum (Fig. 3 ). Besides, the detailed fluorescence study demonstrated no notable shift during the increase in the excitation wavelength in the range of 265–315 nm because of the π-π* transition of graphitic carbon cores. The emission intensity also decreased as the emission wavelength increased from 315 to 380 nm and the emission peaks were red shifted (Fig. 4 ) [68]
Fig. 3.
(a) UV–vis spectra of A. deliciosa extract and synthesized NCDs; (b) Fluorescence excitation spectrum of synthesized NCDs. Image adapted from [68].
Fig. 4.
(a) Fluorescence spectra of synthesized NCDs at different excitation wavelength from 265 nm to 315 nm; (b) Fluorescence spectra of synthesized NCDs at different excitation wavelength from 315 nm to 380 nm. Image adapted from [68].
5. Toxicological profile (biosafety and biodistribution) of NCDs
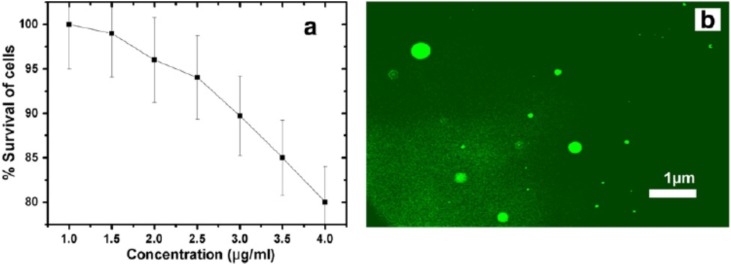
Cytotoxicity is a serious issue because it can cause severe side effects on both diseased and normal tissues. Therefore, drugs and remedial measures are developed in concern with the cytotoxicity. Quantum dots are considered to exert serious issues in concern to cytotoxicity whereas CDs exhibit more biocompatibility and less toxicity. CDs at different concentration with or without surface passivation or doping on different cell lines are biocompatible [69]. Similar is the case with NCDs which are biocompatible against most cells and to learn more about NCDs cytotoxicity, Mewada et al. attempted to reveal the biocompatibility of NCDs against MDCK cells, and prepared NCDs with Trapa Bispinosa peel extract. They found that the survival of cells were more than 80 % at all concentrations of these NCDs (1−4 μg/mL) which ought to be because of the involvement of receptor mediated endocytosis, direct binding to cell membranes and killing, if any, may be due to the obstruction of blocking transporters or channel proteins that mediate entry of important metabolites (Fig. 5 ) [70].
Fig. 5.
(a) Cytotoxicity of CDs using MDCK cells and (b) fluorescence microscope image. Image adapted from [70].
Luo et al. studied the cytotoxicity of aqueous extracts of Radix Puerariae Carbonisata (RPC), which were cultured in Dulbecco’s modified Eagle’s medium, having 20 % fetal bovine serum with 5 % CO 2 at 37 °C. The cells were seeded in 96 well plates (density 1 × 105/well, medium/well 100 μL). After the incubation period, the original medium was discarded to replace with RPC of different concentration and controls were treated with the medium alone. CCK-8(10 μL) was added to each plate and incubated for 3 h at 37 °C and observed at absorbance 450 nm with a microplate reader. The cytotoxicity test with CCK-8 assay on RAW 264.7 cells showcased low cytotoxicity even when the concentration was increased to 1000 mg/mL, the cell viability was found to be 80 % which indicated the biosafety of NCDs with RPC [71].
The main objective of a biosafety assessment is to establish the safe doses or concentration ranges for bio-applications such as diagonistics, bio-imaging, therapeutics, and drug delivery across whole animal system, organs or integrated organs, molecular, cellular levels [72]. For the global acceptance of NCDs/CDs, they should perform well in biological systems and should be devoid of any undesired as well as non-specific functions [73]. Therefore, it is essential to establish their biosafety profiles. There are many evaluations in vivo and in vitro, however, there are not much systemic comparison (in vitro and in vivo) which are important for prospective biological applications, also certain response difference including sensitivity in species (rats) towards these NCDs/CDs have been witnessed [74].The NCDs prepared from tender ginger juice by Li and his co-workers discussed the biosafety profile of these NCDs. The ginger juice NCDs exhibited greater biosafety and biocompatibility when compared with other CDs of green tea, EDTA or glycine. The ginger juice NCDs (440 μg) inhibit the growth of tumors in mice within 14 days. The study also revealed that the 50 % inhibiting concentration (IC50) value of the NCDs on Hepatocellular carcinoma cells (HepG2) to be 0.35 mg/mL. The viability percentage on different cell lines including human cervical cancer cell lines (HeLa), human lung cancer cell line (A549), human breast cancer cell line (MDA-MB-231) and HepG2 cells were more than 60 % at concentration as high as 12.5 wt % for ginger juice NCDs which demonstrate their super selectivity and higher inhibition efficiency. Hence, naturally fabricated NCDs revealed promising anticancer potential both in vitro and in vivo [75].
In addition, an in-depth in vivo toxicology assessment both qualitative and quantitative requires the knowledge of biodistribution. Biodistribution studies help to (a) determine the targeting efficiencies of all types CDs for diagnostics,(b) understand the nonspecificity towards tissues,(c)evaluation of distribution and clearance parameters for toxicity determination, (d) assess the interaction of CDs with biological systems through serum and blood analysis [76] Kang et al. reviewed the biodistribution of CDs by radiolabelling method. After intravenous administration, the CDs were found to highly accumulate in the kidney as well as the reticuloendothelial system and excreted through fecal and renal pathways. Besides, the CDs appeared to be safe at a dose of 20 mg/kg when treated in animals over a period of 3 months as analysed by histological, complete blood panel and time-course blood chemical analysis [77]. The in vivo cell imaging and histological toxicity analysis of sugarcane molasses derived NCDs were well studied by Huang et al. This study demonstrated that the major distribution of sugarcane molasses NCDs were majorly in the cytoplasm and the cell membrane, when measured by a confocal laser scanning microscopy in MCF-7 cells. The histological toxicity analysis assessed the safety of these NCDs to the major organs such as heart, lung, spleen, and kidney. No observable damages including pulmonary fibrosis, inflammatory responses morphological damage and necrosis were monitored for these NCDs when compared to the control [78]. Hence, biodistribution studies are important parameters which ensure that CDs/NCDs favour the intended site rather than any other site for the delivery of drug and toxicity assessment.
6. Applications of NCDs in drug delivery
Recently, NCDs have gained interest among researchers due to their excellent drug delivery applications. The fluorescent properties of NCDs encourage real-time tracking and sensing abilities to support drug delivery. NCDs are safe and biocompatible contrast agents, that efficiently channel the progress of drug release especially, the water insoluble drugs. The unique applications of NCDs in drug delivery are sensing and tracing probe, photo-activated antimicrobial agent, antioxidant, neurodegenerative agent.
6.1. NCDs as sensing and tracing probe in drug delivery
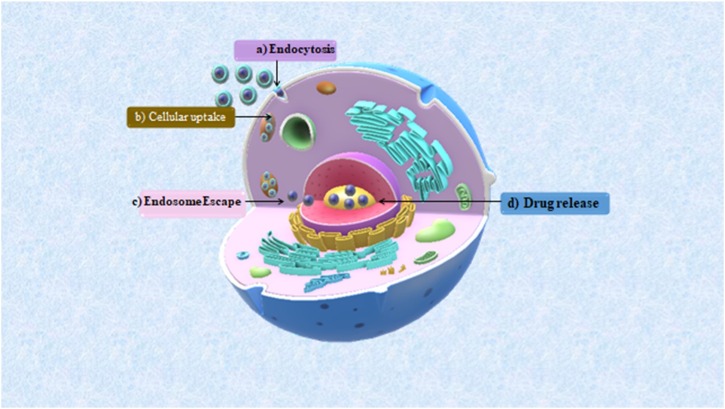
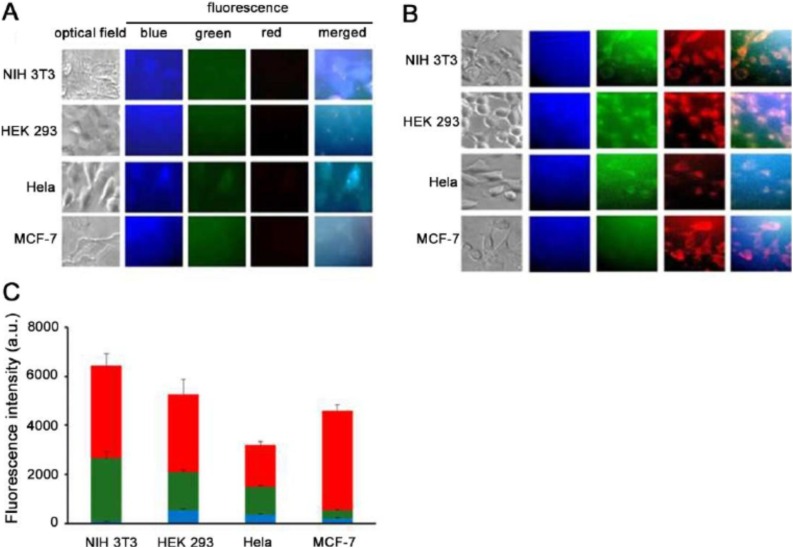
NCDs have similar fluorescence mechanism as CDs and therefore, both have similar the real-time tracking and sensing properties which facilitate drug delivery. Certain characteristics of real-time tracking that help understand the in vitro and in vivo interactions of these nanocarrier with target cells: visualization of drug translocation through the microtubules, membrane bound receptor diffusion, receptor mediated signal transduction as well as endocytic uptake, monitoring the exchange of CDs/NCDs between cells, and visualization of the virus behavior within target cells. Besides, the cell binding, uptake and intracellular drug release are unveiled by the sensing property of CDs/NCDs (Fig. 6 ). The internalization of NCDs prepared from Tulsi (Ocimum Sanctum) by hydrothermal synthesis showed efficient internalization of the NCDs in human breast cancer cell line (MDA-MB 468) cells by intense florescence images. These images obtained from fluorescence microscope revealed that the NCDs were spread in cell membrane, cell nucleus and cytoplasmic area [79]. The N-doped NCDs of cocoon silk prepared by hydrothermal method successfully worked as sensors MCF-7 at a depth of 60−120 nm [80]. Fu et al. reported that the strong luminescence property of arginine-modified CDs facilitated high cellular uptake efficiency as monitored by fluorescence microscope. The cellular luminescence exhibited red shift as arginine CDs entered the cells. This red luminescence was dependent upon the cell lines (embroyonic fibroblast cells [NIH 3T3], human embroyonic kidney cells [HEK 293], cervical cancer cell [Hela] and MCF-7 cells) promoting cell imaging. These different cell lines showed different fluorescence characteristics, as read by microplate reader and fluorescence intensities (red, green, blue), which were statistically analysed by one-way ANOVA (Fig.7 ) [81]. Similarly, nitrogen doped NCDs prepared from date kernel by hydrothermal method with Fe3+ ions proved excellent sensors by detecting trace level sample of zoledronic acid in biological samples by label-free, selective and sensitive off and on signal fluorescence as well as demonstrated good cellular uptake capability. With exceptional water solubility, photo and ionic stability and quantum yield of 12.5 %, the fluorescence intensity of these NCDs were tremendously quenched due to interaction between the functional group of NCDs and ferric ions (fluorescence sensor switch off mode) and after the addition of zoledronic acid, the interaction of its phosphate group with the functional group of NCDs removed the ferric ion from NCDs (fluorescence sensor switch on mode).The developed NCDs-Fe3+ were potent sensors for zoledronic assay had linearity of 0.1 mM–10.0 mM, precision of 2.70 % detection limit of 0.4 mM with low cytotoxicity and efficient cellular uptake (membrane permeability) as investigated in human osteosarcoma (MG-63) cell line by MTT assay [82]. Besides, it is because of their efficient fluorescent mechanism CDs/NCDs are observed in endosomal and lysosomal vesicles and the trafficking pathway of the drug loaded CDs/NCDs by transmission electron microscopy (TEM) and confocal microscopy [83]. The fluorescence property also helps evaluate the drug loading efficiency and monitoring the drug release. The sensing property of CDs/NCDs can be explained by fluorescence resonance energy transfer (FRET) chemistry in which the energy transfer from higher energy donor to lower energy acceptor fluorochrome are able to detect the physical interaction between two fluorescently labeled components, which are approximately 10 nm apart, via fluorophore FRET pairs, thereby quenching the donor and enhancing the acceptor fluorochrome. In addition, for any sample, the nanocrystalline core of CDs/NCDs provide a platform to accurately quantify the nanocarrier biodistribution as well as high imaging resolution because of the red (Stokes) shift upto 300−400 nm [2,29]. Fig. 8 denotes the various quenching mechanisms and FRET chemistry facilitating drug delivery.
Fig. 6.
Intracellular trafficking of CDs/NCDs: a) CDs/NCDs carried inside via endocytosis (cellular process) b) Cellular uptake c) Endosomes rupture to release the drug, d) Drug release.
Fig. 7.
Fluorescence images of bare CDs and arginine-modified CDs in NIH3T3, HEK293, Hela and MCF-7 cells. The cells are observed under a fluorescence microscope following treated with bare CDs (A) or arginine-modified CDs (B) for 2 h. Scale bar 20 μm (C).The total and separate fluorescence intensities in different cell lines as recorded with a microplate reader. The Data were expressed as + SEM (n = 6). Image adapted from [81].
Fig. 8.
Fluorescence quenching mechanism facilitating Drug delivery: a. PET (Photoinduced electron transfer) b. FRET (Forster resonance energy transfer) Quenching, c. SET (Surface energy transfer) Quenching, d. IFE (Inner filter effect) Quenching.
6.2. Antimicrobial drug delivery
The abundance, economical and light emitting characteristics of NCDs are drawing huge attention among researchers. These NCDs could be easily synthesized, easy surface medication with different antimicrobial agents and handle the increasing antibiotic resistance of bacterial pathogens in comparison to others nanostructures, which have limited light responsiveness, high cost and toxicity. The transmission of these infections can be controlled by various approaches, including photodynamic inactivation. NCDs with photosensitizer property, illuminate visible light by using molecular oxygen to produce microbial reactive oxygen species (ROS).These ROS react non-specifically with viral or cell component generating substantial damage and inactivate wide range of microbes such as parasites, fungi, viral and bacteria. Hence, microbes which exhibit resistance to antibiotics are eventually inactivated similarly as their drug susceptible counterparts and cause nonspecific damage to ROS, which means resistance towards NCDs are unlikely to happen [84]. Devi et al. found that NCDs of Aloe vera extract are good antibacterial agent against Staphylococcus aureus and Escherichia coli (E.coli) [85].The Aloe vera extract NCDs demonstrated bright blue luminescence under UV light and a quantum yield of 12.3 %.The antimicrobial actions can be further subcategorized into antiviral, antibacterial and antifungal activities. Majorly, the antiviral and antibacterial activities of NCDs have been discussed further.
6.2.1. Antiviral drug delivery
NCDs, as antiviral drug, are promising candidate for treating contagious viral infections by contributing towards the pandemic chaos created by certain virus especially, coronavirus. Cur- NCDs were successfully investigated for the antiviral property on porcine epidemic diarrhea virus (PEDV), which was supposed as a model for coronavirus model. Uniform, cationic CDs were prepared by hydrothermal method, which were studied for its inhibitory effect on viral replication by provoking the production of proinflammatory cytokines and interferon stimulating genes (ISGs). The virology tests revealed that the treatment with Cur-NCDs could alter the surface protein structure of virus, enabling inhibition of viral entry, reduction in the synthesis of negative strand RNA of virus, suppression of accumulation of ROS and inhibition of the budding of virus [86]. The antiviral potential of Cur-NCDs against Enterovirus 71 (EV71) demonstrated high biocompatibility, decreased mortality as well as delivered high protection against lethal dose of EV71.These CUR-NCDs were prepared in single step by heating CUR at 180 °C, and evaluated for antiviral potential. The study suggested that the inhibitory activity against EV1 infection in RD cells was insignificant and the half maximal effective concentration (EC50) was > 200 μg ml−1, however, the half- maximal cytotoxic concentration (CC50) was < 13 μg ml−1, which indicated high cytotoxicity towards RD cells. The EC50 for 0.2 μg ml-1 was >1000-fold lower whereas CC50 for 452.2 μgml−1 was >34- fold higher. These results demonstrated that the antiviral potential depends highly upon the synthesis of the bioactive, which was considered to undergo a series of structural changes via dehydration, polymerization as well as carbonization to form core shell NCDs with pyrolytic-curcumin like polymer structure and also preserved most polymeric moieties of curcumin that promoted ameliorated antiviral characteristics [87].
Currently, the world is under the threat of coronavirus, which is biologically diverse and has the capability of rapid mutation. Therefore, therapeutic options for highly pathogenic human coronavirus infection are urgently required. Basically, the viral infection cycle produces crucial structural and biological changes in the host cell which consequently causes cell damage. The most convenient approach is the interference of the drug moiety with the infected cells or viral replication to alleviate the viral infection as well as the propagation of the infection [88]. Hence, these studies confirm the antiviral potential of NCDs specially CUR-NCDs and can be employed effectively under this approach which could prove highly promising to contain the severity of this contagious and life threatening disease.
6.2.2. Antibacterial drug delivery
Certain pressing issues including rapid transmission of pathogens from infected surfaces to host and development of antimicrobial resistance have been an increasing threat to human race. Therefore, to overcome such situation inactivation of microbial growth via photodynamic therapy is promising. CDs utilizing green approach for synthesis are reported to be beneficial because they are found abundantly, no chromatographic purifications, economical, small size, biocompatible, scalable photosentizing property and multicolour emissive properties [84]. In this context, Bhamore et al. found that the multicolor emissive NCDs prepared from Manilkara zapota fruit were promising imaging agents for bacterial cells of E.coli, Fomitopsis sp and Aspergillus aculeatus because of their ultra-small size as well as biocompatibility. The nano size facilitated distribution in the cytoplasm and non-toxic nature revealed their biocompatibility which widen their scope in imaging and antibacterial drug delivery [89].The NCDs prepared by oyster mushroom (Pleurotus species) have also demonstrated promising antibacterial activity against Klebsiella pneumonia, Staphylococcus aureus and Pseudomonas aeruginosa as observed by MIC assay (MIC value – 30 μg/mL). The NCDs inhibited the growth of bacteria in a dose dependent manner [90].The antibacterial activity of NCDs prepared from sea weeds derived from κ- carrageenan and lemon juice by hydrothermal synthesis and then quaternised by benzalkonium chloride (improve the fluorescence property) were promising. These NCDs efficiently inhibited the E. coli bacteria (gram negative) [91].
In addition, NCDs coupled with various agents to enhance their fluorescence have proved promising as potent antibacterial agent. Karfa et al. studied the antibacterial efficiency of cysteine derived NCDs bound gold (Au) nanocomposite. It was observed that this nanocomposite inhibited the growth of E.coli as the MIC value was 20.0 ngml−1 [92].The antibacterial activity of quaternized NCDs with glucose and polyethyleneimine (PEI) and Cur NCDs showed excellent inhibition results, on both gram-positive and gram negative bacteria as observed by broth minimum inhibitory concentration assay (MIC) [93,45]. In addition, MIC assay demonstrated varied susceptibility on the wide spectrum of bacteria to Cur-NCDs ranging from 7.8 to 62.5 mg/mL, recording lowest for Klebsiella pneumonia and Stretococcus sp. The antibacterial activity of Cur-NCDs was bacteriostatic as evaluated by high minimal bactericidal concentration (MBC).The bacterial growth were lower when compared to control and 99 % bacterial cells were killed at MBC concentration. Cur-NCDs displayed a concentration dependent killing efficiency on K. pneumonia and Streptococcus sp. These promising results suggest further clinical studies to be conducted on these NCDs for their antibacterial efficiency [45].
6.3. Anticancer drug delivery
Generally, any nano vehicle has the ability to accumulate on disease tissues via conjugation should possess certain characteristics such as abundance, high affinity, flexible to chemical modification and binding specificity to cell surface receptors. The ligand-receptor, aptamer targeting or antigen-antibody interactions promote the molecular identification of the diseased cells at diseased site. This ability of conjugation along with varied submicron size range promotes CDs as an excellent candidate with the ability to cross the physiological barriers as well as capability to reach varied tissues facilitating intracellular internalization and cellular uptake [94]. Hence, CDs facilitate maximum uptake that are capable of targeting the required quantity of the drug to the desired locations through conjugation [69]. Similar is the case with green CDs. The eco-friendly fluorescent CDs prepared by Daucus carota subsp. Sativus (carrot) for the delivery of mitomycin drug through hydrogen bonding that break in the acidic tumor extracellular microenvironment of pH 6.8 to release the drug. This formulation possessed ultra-small size and biocompatibility, which enabled high affinity towards cancer cell membrane that facilitate high degree internalization of mytocin CDs by Bacillus subtilis cells. The in vitro experimental model suggested that the drug was well loaded into the CDs nano sized vesicles, effectively entered the tumor cell, the release pattern was pH dependent and biocompatible with MCF-7 cancer cells [15]. The hydrothermal method to conjugate folic acid with the help of citric acid to form CDs (FA-CDs) was successful in targeted imaging of MCF-7 and HeLa. The quantum yield of FA-CDs was 50 % and their physico-chemical properties were evaluated by TEM, XRD, dynamic light scattering (DLS) and fluorescence spectroscopy. The MTT assay revealed that the cell viability of CDs and FA-CDs were 95 and 97 %, respectively, towards MCF-7 cells after 24 h incubation. [95].These studies highlight that the CDs/NCDs are promising drug carriers by successfully accomplishing the purpose of slow and sustained release of drug by maintaining the effective concentration at the target site and ameliorate cellular uptake by improved drug utilization with high degree of drug administration. The NCDs derived from walnut oil by hydrothermal method were analyzed for cytotoxic as well as apoptogenic properties and mechanism on human cancer cell lines PC3, HT-29 and MCF-7 by MTT assay, mitochondrial membrane potential (MMP) and caspase-3 assay kit. These walnut oil NCDs proved excellent cytotoxic agent with attractive features: an average size of 12 nm; emission and excitation wavelength of 420 and 350 nm, respectively; fluorescent quantum yield (QY) of 14.5 % against quinine sulphate (QY 54 %) as reference standard; super pH and photo stabilities. NCDs were observed to induce apoptosis with no significant involvement of mitochondrial pathway and increase the caspase-3 activation, however, witnessed no change in caspase-9 activity [96].
NCDs have been proved their potential for delivering anticancer drug to the target cancer cells. Fahmi et al. utilized this nontoxic green approach for synthesizing bamboo leaf cellulose into NCDs, intended for the delivery of doxorubicin to Hela tumor cells by modifying it with 4-carboxybenzylboronic acid to enrich target specificity. The in vitro confocal microscopy studies demonstrated blue fluorescence as well as cellular pathways, highlighting the folate receptor mediated endocytosis mechanism for reaching Hela cells. The selective uptake of NCDs was confirmed by flow cytometry and cell viability data [[97]97].
Another case, denoted the anticancer potential of NCDs from different spices namely red chilli, turmeric, black pepper, cinnamon prepared by hydrothermal synthesis. These NCDs were characterized by UV–vis fluorescence, Raman spectroscopy, FTIR, TEM and DLS. The quantum yield was found to be 43.6 % with good optical performance. The cytotoxicity was compared for bioimaging and cell viability between human glioblastoma cancer cell line LN-229 and human kidney non-cancerous cell line HK-2 at different concentrations 0.1–2 mg.ml−1. It was observed that NCDs exhibited higher toxicity on LN-229 cells than HK-2 cells as the cell viability was inhibited, dose-dependently after a period of 24 h. The uptake of NCDs in cancer cells than non-cancer cells was higher. Besides, the presence of functional group on NCDs and the starting material used played a crucial role on the cytotoxicity as determined by ESI-QTOF-MS. Selective cytotoxicity was monitored in black pepper NCDs because of the presence of piperine structure and NCDs of citric acid neither show toxicity in cancer cells nor non-cancerous cells [98].
A comparison between free Eudragit RS 100 nanoparticles, quantum dots curcumin loaded Eudragit RS 100 nanoparticles and unencapsulated curcumin nanoparticles by Khan et al. demonstrated that the quantum dots of curcumin loaded eudragit rs 100 nanoparticles had better inhibitory action on bacterial cells then the other two formulation. It also suggested that the anticancer properties of quantum dots of curcumin loaded Eudragit RS 100 nanoparticles on colon cancer cells (HCT-116) and MCF-7 cells had cell viability of 10.64 and 10.32 %, respectively, when compared to the action of free Eudragit RS 100 nanoparticles and unencapsulated curcumin nanoparticles [99]. The biological applications of Cur loaded CDs when coprecipitated with rutile phase of titanium oxide (TiO2) nanoparticles were studied by Sawant et al. These nanostructures were characterized for their structure and optic properties by TEM, XRD, FTIR, UV–vis, PL. The surface engineering of these NCDs reduced the hydrophobicity of curcumin and increased their biocompatibility. Besides, the anticancer potential on MCF-7 breast cancer cells and the antipsoriatic effect on keratinocyte skin cells (HaCaT) were observed to be higher for Cur loaded CDs coated TiO2 nanoparticles then unloaded nanoparticles [100].
6.4. Neurodegenerative disease
The basic strategy for treating different disorders by any nanomaterials depends upon the efficacy, cellular uptake and transport of drugs to the desired organ, cell or tissue. In case of neurodegenerative diseases the scope for drug delivery poses problems due to the blood-brain barrier, which is a tightly packed endothelial cell layer [101]. A nontoxic vehicle especially, CDs of nano size range synthesized using chitosan through carbonization facilitated consistent release of dopamine monitored through in vitro release study at different pH denoting efficient drug delivery through the blood-brain barrier. The cytotoxicity on IC-21 and SH-SY5Y cell lines were found to be about 97 % cell viability proving promising against neurodegenerative diseases. The HR-TEM images, Raman spectra (D, G, 2D bands) confirmed the synthesis of CDs, the particle size was confirmed to be 3 nm through DLS study and the PL property denoted excitation at 510 nm with an emission peak at 550 nm that proved efficient for bioimaging for tracing the drug delivery [102]. Further, there are many CDs and NCDs (with or without surface modifications), which have successfully encapsulated synthetic as well as natural drug for various drug delivery applications, which are tabulated in Table 3 [[103], [104], [105], [106], [107], [108]].
Table 3.
Types of drug delivered by NCDs.
| S. No. | Types of NCDs | Types of drug | Applications | Reference |
|---|---|---|---|---|
| NCDs for synthetic drugs | ||||
| 1 | Daucus carota subsp. Sativus (carrot)NCDs | Mitomycin | Anticancer | [15] |
| 2 | Bamboo leaf cellulose NCDs | Doxorubicin | Anticancer | [97]97] |
| 3 | Nitrogen doped-persimmon fruit NCDs | Doxorubicin and Gemcitabine | Anticancer | [103] |
| 4 | Saffron NCDs | Prilocane | Local anesthetic | [104] |
| 5 | Pasteurized milk NCDs | Lisinopril | Hypertension and renal diseases | [105] |
| 6 | Mulberry leaves (Morus alba L.) | Lycorine | Anticancer | [106] |
| 7 | Radix Puerariae Carbonisata NCDs | Baicalin | Anticancer, Antiviral | [71] |
| 8 | Nitrogen doped chrysanthemum buds NCDs | Curcumin | [107] | |
| 9 | O-carboxymethyl chitosan nanoparticles (OCMC-MMA NPs)CDs | Telmisartan | Anticancer | [108] |
| Man-made Carbon quantum dots (Cds) for natural drugs | ||||
| 10 | CDs coated Titanium dioxide (TiO2) nanoparticles | Curcumin | Anticancer, Antipsoriasis | [100] |
| 11 | Eudragit RS 100 CDs | Curcumin | Anticancer, Antibacterial | [99] |
7. Clinical status
There are numerous studies which highlight the merits of QDs as effective probes of cancer cells and diagnostic tools both in vitro and in vivo, which create an excellent platform for CDs/NCDs. In a 90 day study on rheus macques, non-human primates, who were subjected to chloroform dispersed phospholipid micelle QDs/CDs through intravenous injection, demonstrated no acute toxicity with chronic exposure of QD. Besides, after 3 months, high levels of CDs were observed in spleen, liver and kidneys as analysed by inductively coupled mass spectroscopy. However, a slow breakdown of QDs were observed, which resulted in accumulation of heavy metals in most of the organs of the primates that may possibly affect health and life span. Apart from this, there are vast research reports available on the positive outcomes of CDs/NCDs also and it is suggested that the therapeutic and diagnostic possibilities of these CDs/NCDs should be given priority and more clinical trials should be conducted in a collective and organised manner. There are only very few studies reported regarding the in vitro or in vivo clinical trials of NCDs. In a novel study, NCDs proved efficient in haemorrhage control especially with aqueous extract of Pollen Typhae carbonisata (PTC).These NCDs were characterised by TEM, HR-TEM, FTIR, U.V and fluorescence spectroscopy to demonstrate monodisperse, spherical and narrow distribution size range between 2–8 nm.80 male Kunming mice were divided into four groups according to their weight (mg/kg): 8 (high), 4 (medium), 2 (low) and control. They were analysed for antihaemorrhage effects and the coagulation effect, by using 40 Sprague-Dawley rats. The tests revealed that after the treatment with PTC-CDs, the mice exhibited decrease in bleeding time (Hemostasis effect) whereas in rats, the activated partial thromboplastin time decreased and fibrinogen as well as platelet count (coagulation effect) increased. The in vivo animal study revealed that NCDs fabricated by PTC by pyrolysis method are promising antihaemorrhage agents and widen the scope for NCDs as potent drug delivery agents [109] Another study reflected the wound healing property of green chilli NCDs, synthesized by microwave irradiation through in vitro and in vivo MSCs cell (wharton’s jelly derived mesenchymal stem cells isolated from umblical cord) study. The cells were observed for 21 days for wound healing and analyzed the ROS scavenging activity by MTT assay. This study proved that these NCDs were beneficial for wound healing and responded by forming micro-vessels, altering granulation of tissue distribution and down regulated ROS gene scavenging enzyme gene expression [110]. Mostly, the actual entities that CDs/NCDs possess is unknown, therefore, more research which showcase the actual entities of CDs/NCDs should be focused to overcome the hurdle faced for ethical approval so that more in vivo data could be gathered. Moreover, quantum dots have already entered clinical trials for breast cancer which open new possibilities for NCDs. The ability to use of natural materials to synthesize NCDs efficiently are drawing immense attention among researchers as these nanomaterials take advantages of having not only found in abundance, cheap and nontoxic but also possess similar light emitting characteristics as of CDs (narrow emission spectra and increased photostability), surface medication both in vivo and in vitro, which ameliorate their applicability. This versatile functional behaviour specially the light emitting property to emit light within the infrared spectrum facilitate penetration of light into tumor or diseased tissues or cells to visualize the structures, providing non-invasive diagnostic imaging and therapeutic remedies especially drug delivery applications intriguing the need for more clinical trials including large scale trials on humans [111].
8. Outlook and future perspectives
NCDs are naturally synthesized carbonaceous nanomaterial which are upcoming agents because they are abundantly found, non-toxic, photo stable, water solubility and possess numerous functional group. There are different methods categorised under ‘bottom up’ and ‘top down’ method which can be chosen according to the advantages and intended applications for the synthesis of NCDs such hydrothermal, microwave, pyrolysis, ultrasonic, laser ablation. It is observed that even though natural products carry numerous functional groups on their surface, modification by doping with hetro atom or surface passivation ameliorate their fluorescence properties. The PL mechanism including surface state, quantum confinement, bandgap theories, fluorescence quenching help provide a better understanding of optical properties of NCDs that act as carriers for drug delivery. These contrast agents help in sensing and tracing at cellular levels, which facilitate drug delivery. Various drug delivery applications of NCDs as antimicrobial agent, anticancer, neurodegenerative agent is highly valuable and could significantly contribute to the knowledge matrix for the broad implications and applications potential of NCDs. There are only few studies related to the clinical trials of these eco-friendly NCDs and more studies need to be conducted. However, before heading towards more serious clinical studies on larger scale, a need to evaluate the concentration of NCDs and optimising the passivating agent used in animal model and cell cultures to understand their long term effect is essential to widen the horizon of NCDs for their successful establishment as potent nanomedicines.
Funding source
The authors profess no funding.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Bandopadhyay S., Manchanda S., Chandra A., Ali J., Deb P.K. In: Pharmaceutical Product Development and Research. Tekade R.K., editor. Academic Press; U.S.A: 2020. Chapter 5 – overview of different carrier systems for advanced drug delivery, drug delivery systems, advances; pp. 179–233. [Google Scholar]
- 2.Probst C.E., Zrazhevskiy P., Bagalkot V., Gao X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013;65:703–718. doi: 10.1016/j.addr.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao J., Li P., Li L., Yang M. Biochemistry and biomedicine of quantum dots: from biodetection to bioimaging, drug discovery, diagnosis, and therapy. Acta Biomater. 2018;74:36–55. doi: 10.1016/j.actbio.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Wagner A.M., Knipe J.M., Orive G., Peppas N.A. Quantum dots in biomedical applications. Acta Biomater. 2019;94:44–63. doi: 10.1016/j.actbio.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilla J.C., Bozkurt F., Ansari S., Sozer N., Kokini J.L. Applications of quantum dots in food science and biology. Trends Food Sci. Technol. 2016;53:75–89. [Google Scholar]
- 6.Lim S.Y., Shen W., Gao Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015;44:362–381. doi: 10.1039/c4cs00269e. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni N.S., Guererro Y., Gupta N., Mutha A., Gupta V. Exploring potential of quantum dots as dual modality for cancer therapy and diagnosis. J. Drug Deliv. Sci. Technol. 2019;49:352–364. [Google Scholar]
- 8.Arul V., Edison T.N.J.I., Lee Y.R., Sethuraman M.G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol. B Biol. 2017;168:142–148. doi: 10.1016/j.jphotobiol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Yuan T., Meng T., He P., Shi Y.X., Li Y., Li X., Yang L.Fan S. Carbon quantum dots: an emerging material for optoelectronic applications. J. Mater. Chem. C. 2019;7:6820. [Google Scholar]
- 10.Zhang X., Wei C., Li Y., Yu D. Shining luminescent graphene quantum dots: synthesis, physicochemical properties, and biomedical applications. Trends Anal. Chem. 2019;116:109–121. [Google Scholar]
- 11.Arumugham T., Alagumuthu M., Amimodu R.G., Munusamy S., Iyer S.K. A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain. Mater. Technol. 2020;23 [Google Scholar]
- 12.Kong W., Wu D., Li G., Chen X., Gong P., Sun Z., Chen G., Xia L., You J., Wu Y. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta. 2017;165:677–684. doi: 10.1016/j.talanta.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D., Singh K., Verma V., Bhatti H.S. Synthesis and characterization of carbon quantum dots from orange juice. J. Bionanosci. 2014;8:274–279. [Google Scholar]
- 14.Hoan B.T., Tam P.D., Pham V.H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotechnol. 2019 [Google Scholar]
- 15.D’souza S.L., Chettiar S.S., Koduru J.R., Kailasa S.K. Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik. 2018;158:893–900. [Google Scholar]
- 16.Zhao Y., Zhang Y., Liu X., Kong H., Wang Y., Qin G., Cao P., Song X., Yan X., Wang Q., Qu H. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci. Rep. 2017;7:4452. doi: 10.1038/s41598-017-04073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao D., Yuan D., Hea H., Lu J. Microwave-assisted one-step green synthesis of amino-functionalized fluorescent carbon nitride dots from chitosan. Luminescence. 2013;28:612–615. doi: 10.1002/bio.2486. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Xiao N., Gong N., Wang H., Shi X., Gu W., Ye L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon. 2014;68:258–264. doi: 10.1021/la502705g. [DOI] [PubMed] [Google Scholar]
- 19.Pires N.R., Santos C.M.W., Sousa R.R., Paula R.C.M., Cunha P.L.R., Feitosa J.P.A. Novel and fast microwave-assisted synthesis of carbon quantum dots from raw cashew gum. J. Braz. Chem. Soc. 2015;26:1274–1282. [Google Scholar]
- 20.Gu D., Shang S., Yu Q., Shen J. Green synthesis of nitrogen-doped carbon dots from lotus root for Hg(II) ions detection and cell imaging. Appl. Surf. Sci. 2016;390:38–42. [Google Scholar]
- 21.Teng X., Ma C., Ge C., Yan M., Yang J., Zhang Y., Moraiscd P.C., Bi H. Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and l-lysine for bioimaging. J. Mater. Chem. B. 2014;2:4631–4639. doi: 10.1039/c4tb00368c. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Li C., Liu S., Liu Z., Zhu J., Yang J., Hu X. Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Adv. 2015;5:64790–64796. [Google Scholar]
- 23.Aji M.P., Susanto P.A., Wiguna, Sulhadi Facile synthesis of luminescent carbon dots from mangosteen peel by pyrolysis method. J. Theor. Appl. Phys. 2017;11:119–126. [Google Scholar]
- 24.Gonçalves H.M.R., Duarte A.J., Silva J.C.G.E. Optical fiber sensor for Hg(II) based on carbon dots. Biosens. Bioelectron. 2010;26:1302–1306. doi: 10.1016/j.bios.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Dehvari K., Liu K.Y., Tseng P.J., Gedda G., Girma W.M., Chang J.Y. Sonochemical-assisted green synthesis of nitrogen-doped carbon dots from crab shell as targeted nanoprobes for cell imaging. J. Taiwan Inst. Chem. Eng. 2018:1–9. 0 0 0. [Google Scholar]
- 26.Ramar V., Moothattu S., Balasubramanian K. Metal free, sunlight and white light based photocatalysis using carbon quantum dots from Citrus grandis: a green way to remove pollution. Sol. Energy. 2018;169:120–127. [Google Scholar]
- 27.Biswal M., Banerjee A., Deoab M., Ogale S. From dead leaves to high energy density supercapacitors. Energy Environ. Sci. 2013;6:1249–1259. [Google Scholar]
- 28.Kumawat M.K., Thakur M., Gurung R.B., Srivastava R. Graphene quantum dots from Mangifera indica: application in near- infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017;5:1382–1391. [Google Scholar]
- 29.Zhang X., Jiang M., Na N., Chen Z., Li S., Liu S., Li J. Review of natural product derived carbon dots: from natural products to functional materials. ChemSusChem. 2018;11:11–24. doi: 10.1002/cssc.201701847. [DOI] [PubMed] [Google Scholar]
- 30.Niu W.J., Li Y., Zhu R.H., Shan D., Fan Y.R., Zhang X.J. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuators B Chem. 2015;218:229–236. [Google Scholar]
- 31.Godavarthia S., Kumara K.M., Véleza E.V., Hernandez-Eligioc A., Mahendhirand M., Hernandez-Comoe N., Alemane M., M.Gomeza L. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. 2017;172:36–41. doi: 10.1016/j.jphotobiol.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Dias C., Vasimalai N., Sárria M.P., Pinheiro I., Vilas-Boas V., Peixoto J., Espiña B. Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials. 2019;9:199. doi: 10.3390/nano9020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himaja A.L., Karthik P.S., Sreedhar B., Singh S.P. Synthesis of carbon dots from kitchen waste: conversion of waste to value added product. J. Fluoresc. 2014;24:1767–1773. doi: 10.1007/s10895-014-1465-1. [DOI] [PubMed] [Google Scholar]
- 34.Sagbas S., Sahiner N. In: Nanocarbon and its Composites. Khan A., Jawaid M., Asiri A.M., editors. Woodhead Publishing Series; USA: 2019. Carbon dots: preparation, properties, and application; pp. 651–676. ch 22. [Google Scholar]
- 35.Shi X., Wei W., Fu Z., Gao W., Zhang C., Zhao Q., Deng F., Lu X. Review on carbon dots in food safety applications. Talanta. 2019;194:809–821. doi: 10.1016/j.talanta.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Radnia F., Mohajeri N., Zarghami N. New insight into the engineering of green carbon dots: possible applications in emerging cancer theranostics. Talanta. 2020;209 doi: 10.1016/j.talanta.2019.120547. [DOI] [PubMed] [Google Scholar]
- 37.Jayanthi M., Megarajan S., Subramaniyan S.B., Kamlekar R.K., Anbazhagan V. A convenient green method to synthesize luminescent carbon dots from edible carrot and its application in bioimaging and preparation of nanocatalyst. J. Mol. Liq. 2019;278:175–182. [Google Scholar]
- 38.Singh R.K., Kumar R., Singh D.P., Savu R., Moshkalev S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: a review. Mater. Today Chem. 2019;12:282–314. [Google Scholar]
- 39.Prathumsuwan T., Jamnongsong S., Sampattavanich S., Paoprasert P. Preparation of carbon dots from succinic acid and glycerol as ferrous ion and hydrogen peroxide dual-mode sensors and for cell imaging. Opt. Mater. 2018;86:517–529. [Google Scholar]
- 40.Thongsai N., Tanawannapong N., Praneerad J., Kladsomboon S., Jaiyong P., Paoprasert P. Real-time detection of alcohol vapors and volatile organic compounds via optical electronic nose using carbon dots prepared from rice husk and density functional theory calculation. Colloids Surf. A Physicochem. 2019;560:278–287. [Google Scholar]
- 41.Praneerad J., Thongsai N., Supchocksoonthorn P., Kladsomboon S., Paoprasert P. Multipurpose sensing applications of biocompatible radish-derived carbon dots as Cu2+ and acetic acid vapor sensors. Spectrochim. Acta A Mol. Biomol. Acta A Mol. Biomol. Spectrosc. 2019;211:59–70. doi: 10.1016/j.saa.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Gao T., Yang M., Zhao J., Jiang F., Liu Y. Microwave-assisted synthesis, characterization, cell imaging of fluorescent carbon dots using l-asparagine as precursor. New J. Chem. 2019;43:3323–3331. [Google Scholar]
- 43.Bhamore J.R., Jha S., Park T.J., Kailasa S.K. Fluorescence sensing of Cu2+ ion and imaging of fungal cell by ultra-small fluorescent carbon dots derived from Acacia concinna seeds. Sens. Actuators B Chem. 2018;277:47–54. [Google Scholar]
- 44.Devi P., Saini S., Kim K.H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019;141 doi: 10.1016/j.bios.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 45.Ring L.C., Yenn T.W., Wen-Nee T., Tumin N.D., Yusof F.A.M., Yacob L.S., Rosli M.I.H.B., Taher M.A. Synthesis of curcumin quantum dots and their antimicrobial activity on necrotizing fasciitis causing bacteria. Mater. Today Proc. 2020;31:31–35. [Google Scholar]
- 46.Yao A., Huang H., Liu Y., Kang Z. Carbon dots: a small conundrum. Trends Chem. 2019;1:235–246. [Google Scholar]
- 47.Wu Z.Li., Liu Z.X., Yuan Y.H. Carbon dots: materials, synthesis, properties and approaches to long-wavelength and multicolour emission. J. Mater. Chem. B. 2017;5:3794. doi: 10.1039/c7tb00363c. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S., Umar A., Sood S., Mehta S.K., Kansal S.K. Photoluminescent Cdots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. J. Alloys Compd. 2018;748:818–853. [Google Scholar]
- 49.Thambiraj S., Ravi Shankaran D. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016;390:435–443. [Google Scholar]
- 50.Chen B.B., Liu M.L., Li C.M., Huang C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019;270:165–190. doi: 10.1016/j.cis.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Arul V., Sethuraman M.G. Hydrothermally green synthesized nitrogen-doped carbon dots from Phyllanthus emblica and their catalytic ability in the detoxification of textile effluents. ACS Omega. 2019;4:3449–3457. doi: 10.1021/acsomega.8b03674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atchudan R., Edison T.N.J.I., Sethuraman M.G., Lee Y.R. Efficient synthesis of highly fluorescent nitrogen-doped carbon dots for cell imaging using unripe fruit extract of Prunus mume. Appl. Surf. Sci. 2016;384:432–441. [Google Scholar]
- 53.Zhi B., Cui Y., Wang S., Frank B., Williams D.N., Brown R.P., Melby E.S., Hamers R.J., Rosenzweig Z., Fairbrother D.H., Orr G., Haynes C.L. Malic acid carbon dots: from super-resolution live-cell imaging to highly efficient separation. ACS Nano. 2018;12:5741–5752. doi: 10.1021/acsnano.8b01619. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Dong T. Photoluminescence Tuning in Carbon Dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C. 2018;6:7944–7970. [Google Scholar]
- 55.Tejwan N., Saha S.K., Das J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020;275 doi: 10.1016/j.cis.2019.102046. [DOI] [PubMed] [Google Scholar]
- 56.Tuerhong M., Yang X., Xue-Bo Y. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017;45:139–150. [Google Scholar]
- 57.Wang W., Xia J., Feng J., He M., Chen M., Wang J. Green preparation of carbon dots for intracellular pH sensing and multicolor live cell imaging. J. Mater. Chem. B. 2016;4:7130–7137. doi: 10.1039/c6tb02071b. [DOI] [PubMed] [Google Scholar]
- 58.Zheng J., Liu X., Yang Y., liu X., Xu B. Rapid and green synthesis of fluorescent carbon dots from starch for white light emitting diodes. New Carbon Mater. 2018;33:276–288. [Google Scholar]
- 59.Kumar A., Chowdhuri A.R., Laha D., Mahto T.K., Karmakar P., Sahu S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017;242:679–686. [Google Scholar]
- 60.Zhu S., Song Y., Zhao X., Shao J., Zhang J., Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8:355–381. [Google Scholar]
- 61.Liu M.L., Chen B.B., Li C.M., Huang C.Z. Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019;21:449–471. [Google Scholar]
- 62.Ramezani Z., Qorbanpour M., Rahbar N. Green synthesis of carbon quantum dots using quince fruit (Cydonia oblonga) powder as carbon precursor: application in cell imaging and As3+ determination. Colloids Surf. A Physicochem. 2018;549:58–66. [Google Scholar]
- 63.Zu F., Yan F., Bai Z., Xu J., Wang Y., Huang Y., Zhou X. The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim. Acta. 2017;184:1899–1914. [Google Scholar]
- 64.Kasibabu B.S.B., D’souza S.L., Jha S., Kailasa S.K. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from Carica papaya juice. J. Fluoresc. 2015;25:803–810. doi: 10.1007/s10895-015-1595-0. [DOI] [PubMed] [Google Scholar]
- 65.Gan Z.X., Wu X.L., Zhou G.X., Shen J.C., Chu P.K. Is there real upconversion photoluminescence from grapheme quantum dots? Adv. Opt. Mater. 2013;1:554–558. [Google Scholar]
- 66.Wen X.M., Yu P., Toh Y.-R., Ma X.Q., Tang J. On the upconversion fluorescence in carbon nanodots and graphene quantum dots. Chem. Commun. 2014;50:4703–4706. doi: 10.1039/c4cc01213e. [DOI] [PubMed] [Google Scholar]
- 67.Wang X., Feng Y., Dong P., Huang J. A mini review on carbon quantum dots: preparation, properties, and electrocatalytic application. Front. Chem. 2019;7:PMC6787169. doi: 10.3389/fchem.2019.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arul V., Sethuraman M.G. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt. Mater. 2018;78:181–190. [Google Scholar]
- 69.Zuo P., Lu X., Sun Z., Guo Y., He H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta. 2016;183:519–542. [Google Scholar]
- 70.Mewada A., Pandey S., Shinde S., Mishra N., Oza G., Thakur M., Sharon M., Sharon M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C. 2013;33:2914–2917. doi: 10.1016/j.msec.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Luo J., Kong H., Zhang M., Cheng J., Sun Z., Xiong W., Zhu Y., Zhao Y., Qu H. Novel carbon dots-derived from Radix Puerariae Carbonisata significantly improve the solubility and bioavailability of baicalin. J. Biomed. Nanotechnol. 2019;15:151–161. doi: 10.1166/jbn.2019.2675. [DOI] [PubMed] [Google Scholar]
- 72.Dunpall R., Revaprasadu N. An in vitro and in vivo bio-interaction responses and biosafety evaluation of novel Au–ZnTe core–shell nanoparticles. Toxicol. Res. 2016;5:1078–1089. doi: 10.1039/c6tx00054a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farjadian F., Roointan A., Mohammadi-Samani S., Hosseini M. Mesoporous silica nanoparticles: synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019;359:684–705. [Google Scholar]
- 74.Zhang S., Pei X., Xue Y., Xiong J., Wang J. Bio-safety assessment of carbon quantum dots, N-doped and folic acid modified carbon quantum dots: a systemic comparison. Chin. Chem. Lett. 2020;31:1654–1659. [Google Scholar]
- 75.Li C.L., Ou C.M., Huang C.C., Wu W.C., Chen Y.P., Lin T.E., Ho L.C., Wang C.W., Shih C.C., Cheng Zhou H., Lee Y.C., Tzeng W.F., Chiou T.J., Chu S.T., Cangm J., Chang H.T. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J. Mater. Chem. B. 2014;2:4564. doi: 10.1039/c4tb00216d. [DOI] [PubMed] [Google Scholar]
- 76.Nurunnabi M., Khatun Z., Huh K.M., Park S.Y., Lee D.Y., Cho K.J., Lee Y.K. In Vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano. 2013;7:6858–6867. doi: 10.1021/nn402043c. [DOI] [PubMed] [Google Scholar]
- 77.Kang Z., Liu Y., Gao J., Zhu M. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017:1–3. [Google Scholar]
- 78.Huang G., Chen X., Wang C., Zheng H., Huang Z., Chen D., Xie H. Photoluminescent carbon dots derived from sugarcane molasses: synthesis, properties, and applications. RSC Adv. 2017;7:47840. [Google Scholar]
- 79.Bhatt S., Bhatt M., Kumar A., Vyas G., Gajaria T., Paul P. Green route for synthesis of multifunctional fluorescent carbon dots from Tulsi leaves and its application as Cr(VI) sensors, bio-imaging and patterning agents. Colloids Surf. B. 2018;167:126–133. doi: 10.1016/j.colsurfb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Li W., Zhang Z., Kong B., Feng S., Wang J., Wang L., Yang J., Zhang F., Wu P., Zhao D. Simple and green synthesis of nitrogen‐doped photoluminescent carbonaceous nanospheres for bioimaging. Angew. Chem. Int. Ed. 2013;52:8151–8155. doi: 10.1002/anie.201303927. [DOI] [PubMed] [Google Scholar]
- 81.Fu C., Qian K., Fu A. Arginine-modified carbon dots probe for live cell imaging and sensing by increasing cellular uptake efficiency. Mater. Sci. Eng. C. 2017;76:350–355. doi: 10.1016/j.msec.2017.03.084. [DOI] [PubMed] [Google Scholar]
- 82.Amin N., Afkhami A., Hosseinzadeh L., Madrakian T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of Zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta. 2018;1030:183–193. doi: 10.1016/j.aca.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Ray A., Mitra A.K. In: Nanotechnology in Intracellular Trafficking, Imaging, and Delivery of Therapeutic Agents, Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. Mitra A.K., Cholkar K., Mandal A., editors. Elsevier; Amsterdam: 2017. pp. 169–188. [Google Scholar]
- 84.Nie X., Jiang C., Wu S., Chen W., Lv P., Wang Q., Liu J., Narh C., Cao X., Ghiladi R.A., Weia Q. Carbon quantum dots: a bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J. Photochem. Photobiol. 2020;206 doi: 10.1016/j.jphotobiol.2020.111864. [DOI] [PubMed] [Google Scholar]
- 85.Devi P., Thakur A., Bhardwaj S.K., Saini S., Rajput P., Kumar P. Metal ion sensing and light activated antimicrobial activity of Aloe vera derived carbon dots. J. Mater. Sci. Mater. Electron. 2018;29:17254–17261. [Google Scholar]
- 86.Du T., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multi-site inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 87.Lin L., Luo Y., Tsai P., Wang J., Chen X. Metal ions doped carbon quantum dots: synthesis, physicochemical properties, and their applications. Trends Analyt. Chem. 2018;103:87–101. [Google Scholar]
- 88.Loczechin A., Seron K., Barras A., Giovanelli E., Belouzard S., Chen Y., Nolte N.M., Boukherroub R., Dubuisson J., Szunerits S. Functional carbon quantum dots as medical countermeasures to human coronavirus (HCoV) ACS Appl. Mater. Interfaces. 2019;11:42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhamore J.R., Jha S., Park T.J., Kailasaa S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. 2019;191:150–155. doi: 10.1016/j.jphotobiol.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 90.Boobalan T., Sethupathi M., Sengottuvelan N., Kumar P., Balaji P., Gulyás B.Z., Padmanabhan P., Selvan S.T., Arun A. Mushroom-derived carbon dots for toxic metal ion detection and as antibacterial and anticancer agents. ACS Appl. Nano Mater. 2020;3:5910–5919. [Google Scholar]
- 91.Das P., Ganguly S., Bose M., Ray D., Ghosh S., Mondal S., Aswal V.K., Das A., Banerjee S., Das N.C. Surface quaternized nanosensor as a one-arrow-two-hawks approach for fluorescence turn “on–off–on” bifunctional sensing and antibacterial activity. New J. Chem. 2019;43:6205–6219. [Google Scholar]
- 92.Karfa P., Roy E., Patra S., Kumar S., Tarafdar A., Madhuri R., Sharma P.K. Amino acid derived highly luminescent, heteroatom-doped carbon dots for label-free detection of Cd2+/Fe3+, cell imaging and enhanced antibacterial activity. RSC Adv. 2015;5:58141. doi: 10.1039/c8ra90093k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dou Q.Q., Fang X., Jiang S., Chee P.L., Lee T., Loh X.J. Multi-functional fluorescent carbon dots with antibacterial and gene delivery properties. RSC Adv. 2015;5:46817–46822. [Google Scholar]
- 94.Parveen S., Misra R., Sahoo S.K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Saljoghi H., Khakbaz F., Mahani M. Synthesis of folic acid conjugated photoluminescent carbon quantum dots with ultrahigh quantum yield for targeted cancer cell fluorescence imaging. Photodiagnosis Photodyn. Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101687. [DOI] [PubMed] [Google Scholar]
- 96.Arkan E., Barati A., Rahmanpanah M., Hosseinzadeh L., Moradi S., Hajialyani M. Green synthesis of carbon dots derived from walnut oil and an investigation of their cytotoxic and apoptogenic activities toward cancer cells. Adv. Pharm. Bull. 2018;8:149–155. doi: 10.15171/apb.2018.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fahmi M.Z., Haris A., Permana A.J., Wibowo D.L.N., Purwanto B., Nikmah Y.L., Adi I. Bamboo leaf-based carbon dots for efficient tumor imaging and therapy. RSC Adv. 2018;8 doi: 10.1039/c8ra07944g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vasimalai N., Vilas-Boas V., Gallo J., Cerqueira M.F., Menéndez-Miranda M., Costa-Fernández J.M., Diéguez L., Espiña B., Fernández-Argüelles M.T. Green synthesis of fluorescent carbon dots from spices for in vitro imaging and tumour cell growth inhibition. Beilstein J. Nanotechnol. 2018;9:530–544. doi: 10.3762/bjnano.9.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan F.A., Lammari N., Siar A.S.M., Alkhater K.M., Asiri S., Akhtar S., Almansour I., Alamoudi W., Haroun W., Louaer W., Meniai A.H., Elaissari A. Quantum dots encapsulated with curcumin inhibit the growth of colon cancer, breast cancer and bacterial cells. Nanomedicine (London) 2020;15:969–980. doi: 10.2217/nnm-2019-0429. [DOI] [PubMed] [Google Scholar]
- 100.Sawant V.J., Bamane S.R., Kanase G., Patilc S.B., Ghosh Jai. Encapsulation of curcumin over carbon dot coated TiO2 nanoparticles for pH sensitive enhancement of anticancer and antipsoriatic potential. RSC Adv. 2016;6:66745–66755. [Google Scholar]
- 101.Re F., Gregori M., Masserini M. Nanotechnology for neurodegenerative disorders. Nanomedicine. 2012;8:S51–S58. doi: 10.1016/j.nano.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Mathew S.A., Praveena P., Dhanavel S., Manikandan R., Senthilkumar S., Stephen A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020;10 doi: 10.1039/d0ra04599c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy A.S., Vo V.G., An S.S.A., Kim J. Solvent-free synthesis of fluorescent carbon dots: an eco-friendly approach for the bio-imaging and screening of anticancer activity via caspase-induced apoptosis. ACS Appl. Bio Mater. 2020;3:4873–4882. doi: 10.1021/acsabm.0c00377. [DOI] [PubMed] [Google Scholar]
- 104.Ensafi A.A., Sefat S.H., Kazemifard N., Rezaei B., Moradi F. A novel one-step and green synthesis of highly fluorescent carbon dots from saffron for cell imaging and sensing of prilocaine. Sens. Actuators B Chem. 2017;253:451–460. [Google Scholar]
- 105.Mehta V.N., Chettiar S.S., Bhamore J.R., Kailasa S.K., Patel R.M. Green synthetic approach for synthesis of fluorescent carbon dots for Lisinopril drug delivery system and their confirmations in the cells. J. Fluoresc. 2017;27:111–124. doi: 10.1007/s10895-016-1939-4. [DOI] [PubMed] [Google Scholar]
- 106.Shao Y., Zhu C., Fu Z., Lin K., Wang Y., Chang Y., Han L., Yu H., Tian F. Green synthesis of multifunctional fluorescent carbon dots from mulberry leaves (Morus alba L.) residues for simultaneous intracellular imaging and drug delivery. J. Nanopart. Res. 2020;22:229. [Google Scholar]
- 107.Bu L., Luo T., Peng H., Li L., Long D., Peng J., Huang J. One-step synthesis of N-doped carbon dots, and their applications in curcumin sensing, fluorescent inks, and super-resolution nanoscopy. Microchim. Acta. 2019;186:675. doi: 10.1007/s00604-019-3762-5. [DOI] [PubMed] [Google Scholar]
- 108.Chowdhuri A.R., Tripathy S., Haldar C., Roy S., Sahu S.K. Single step synthesis of carbon dots embedded chitosan nanoparticles for cell imaging and hydrophobic drug delivery. J. Mater. Chem. B. 2015;3(2015):9122–9131. doi: 10.1039/c5tb01831e. [DOI] [PubMed] [Google Scholar]
- 109.Yan X., Zhao Y., Luo J., Xiong W., Liu X., Cheng J., Wang Y., Zhang M., Qu H. Hemostatic bioactivity of novel Pollen Typhae Carbonisata‑derived carbon quantum dots. Int. J. Nanobiotechnology Pharm. 2017;15:PMC5584017. doi: 10.1186/s12951-017-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Das B., Pal P., Dadhich P., Dutta J., Dhara S. In vivo cell tracking, reactive oxygen species scavenging, and antioxidative gene down regulation by long-term exposure of biomass-derived carbon dots. ACS Biomater. Sci. Eng. 2019;5:346–356. doi: 10.1021/acsbiomaterials.8b01101. [DOI] [PubMed] [Google Scholar]
- 111.Clift M.J.D., Stone V. Quantum Dots: An insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics. 2012;2:668–680. doi: 10.7150/thno.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]