Abstract
The supply of N95 filtering facepiece respirators (FFRs) may not be adequate to match demand during a pandemic outbreak. One possible strategy to maintain supplies in healthcare settings is to extend FFR use for multiple patient encounters; however, contaminated FFRs may serve as a source for the airborne transmission of virus particles. In this study, reaerosolization of virus particles from contaminated FFRs was examined using bacteriophage MS2 as a surrogate for airborne pathogenic viruses. MS2 was applied to FFRs as droplets or droplet nuclei. A simulated cough (370 l min−1 peak flow) provided reverse airflow through the contaminated FFR. The number and size of the reaerosolized particles were measured using gelatin filters and an Andersen Cascade Impactor (ACI). Two droplet nuclei challenges produced higher percentages of reaerosolized particles (0.21 and 0.08%) than a droplet challenge (<0.0001%). Overall, the ACI-determined size distribution of the reaerosolized particles was larger than the characterized loading virus aerosol. This study demonstrates that only a small percentage of viable MS2 viruses was reaerosolized from FFRs by reverse airflow under the conditions evaluated, suggesting that the risks of exposure due to reaerosolization associated with extended use can be considered negligible for most respiratory viruses. However, risk assessments should be updated as new viruses emerge and better workplace exposure data becomes available.
Keywords: infection control, influenza transmission, respiratory protection
INTRODUCTION
National Institute for Occupational Safety and Health (NIOSH)-certified N95 filtering facepiece respirators (FFRs) are part of a hierarchy of control strategies recommended by the Centers for Disease Control and Prevention (CDC) to reduce exposure to airborne respiratory pathogens such as Mycobacterium tuberculosis (TB), Severe Acute Respiratory Syndrome (SARS)-associated coronavirus (SARS-CoV), and most recently 2009 H1N1 influenza (CDC, 1999, 2005, 2009, 2010a). The ever-present concerns of a pandemic influenza, elevated by the confirmed transmission of avian influenza (H5N1) to humans, and the recent 2009 H1N1 influenza pandemic have focused efforts on pandemic preparedness including FFR availability and supply (Claas et al., 1998; Buxton Bridges et al., 2000; Bailar et al., 2006; CDC, 2009). These concerns, supported with estimates of FFR supply requirements for pandemic influenza and corroborated with occurrence of FFR shortages during the SARS outbreak, have opened discussions and initiated research into extending the useful life and maintaining supplies of FFRs as part of pandemic influenza preparedness planning (Bailar et al., 2006).
Many strategies have been identified to maintain FFR supplies during a pandemic including minimizing the number of individuals who need respiratory protection, the use of alternative types of respiratory protective devices, prioritization (e.g. for those with the highest risk of exposure), stockpiling, FFR reuse, and FFR extended use (CDC, 2010b). Since the SARS outbreak, several studies have been completed or initiated to assess some of these strategies. For example, stockpiling of FFRs occur on many organizational levels from individual hospitals to hospital networks and among local, state, and federal government bodies. Viscusi et al. (2009) examined the effects of extended storage of FFRs on filtration performance, while other studies have examined the potential to decontaminate or inactivate viruses on the surfaces of FFRs so that they may be used again (Viscusi et al., 2007; Fisher et al., 2009; Viscusi et al., 2009; Vo et al., 2009; Bergman et al., 2010; Fisher et al., 2010; Fisher and Shaffer, 2010; Fisher and Shaffer, 2011; Heimbuch et al., 2011). These studies have highlighted the complexities of effective FFR decontamination. A successful decontamination method must inactivate the virus, not harm the filtration performance of the filtering medium, not affect the fit of the FFR, present no health or irritation concerns to the wearer due to residual chemicals, and be easily performed in a timely manner. The issue of toxic residues remaining on FFRs has been previously evaluated (Salter et al., 2010). In that study, very few toxic residues were detected following decontamination with a variety of chemical decontamination agents. Although research has proved promising, there are currently no guidance or recommendations for FFR decontamination and reuse. Among the strategies proposed for maintaining supplies during a pandemic, the simplest approach may be to extend the use of FFRs, which are routinely disposed of after each patient encounter (Siegel, 2007). Extended use of FFRs (e.g. wearing the same FFR over multiple patient encounters as it is commonly practiced by healthcare workers treating patients with TB) would significantly cut down on the number of FFRs used, but the potential health risks of this process have not been extensively examined for many respiratory pathogens.
Concerns have been raised that the extended use of FFRs could result in additional opportunities for influenza transmission to co-workers and patients. For example, extended use may result in a risk of contact transmission by touching the contaminated surface of a respirator and subsequently touching the mucous membranes of the face. Recommendations to minimize the threat of contact transmission including limiting the handling of the FFR while in use or practicing hand hygiene after touching the respirator have been provided (CDC, 2008). Another concern of extended use is that a contaminated FFR may serve as a potential source for the airborne transmission of virus containing particles (VCPs). A healthcare worker's FFR may become contaminated with VCPs when in close contact with an infected patient. If the healthcare worker continues using the contaminated FFR and enters the room of a subsequent patient, it is possible that VCPs may reaerosolize due to the airflow (breathing, coughing, and sneezing) generated by the healthcare worker. The reaerosolized VCPs then become a hazard to current and subsequent occupants of the area. The number and size distribution of the reaerosolized VCPs has risk assessment implications for extended use of FFRs. The objective of this study was to assess the reaerosolization characteristics of VCPs from highly contaminated NIOSH-certified FFRs during simulated user-generated airflow (e.g. cough).
METHODS
Experimental design
Reverse airflow reaerosolization of VCPs from contaminated FFRs was examined using bacteriophage MS2 as a surrogate for airborne pathogenic viruses. MS2 is commonly used as a surrogate virus in aerosol and filtration studies (Balazy et al., 2006; Eninger et al., 2008; Fisher et al., 2009; Vo et al., 2009; Woo et al., 2010). MS2 was applied to FFRs as droplets or droplet nuclei. Droplets are large wet particles and represent the aerosol threat that would be associated with an immediate deposition onto an FFR from a direct sneeze or cough. Droplet nuclei are small desiccated particles, remain airborne for an extended time period and present an inhalation hazard. Separate loading systems, designed in-house, were used to apply MS2 containing droplets and droplet nuclei to FFRs. The concentration of MS2 applied to the FFRs was 104 or 105 plaque forming units (p.f.u.) cm−2. There is a paucity of research on the amount of pathogens in aerosols generated by an infected patient; therefore, the rationale for selecting these MS2 loading levels was, in part, based on the sensitivity of the test method. A breathing machine was used to provide reverse airflow (simulated cough) through the contaminated FFR housed within a reaerosolization chamber. The number and size of the reaerosolized particles were measured using gelatin filters and an Andersen Cascade Impactor (ACI). The results were analyzed to determine the effects of loading particle size and type (droplet nuclei or droplet) on reaerosolization of VCP from contaminated FFRs. Particle sizers such as the Scanning Mobility Particle Sizer and the Laser Aerosol Spectrometer were used in the initial characterization of the test systems but were not used in conjunction with the reaerosolization tests as viable particle detection was the focus of this research.
Filtering facepiece respirator
All experiments were performed using a NIOSH approved N95 FFR (Gerson 1730; Louis M. Gerson Co., Inc, Middleboro, MA, USA). This FFR model is consists of several layers of material including hydrophilic strata on the exterior surfaces. The approximate surface area of this model FFR is 170 cm2.
Viruses, bacteria, and media
MS2 was prepared using standard protocols (EPA, 2001). The MS2 suspension was centrifuged to remove cell debris and the supernatant was filtered through a 0.22-μm cellulose acetate filter. The stock MS2 was diluted into filtered deionized water to obtain the target suspension concentration for aerosolization. The suspension concentration was adjusted depending on the target loading level, which ranged from 106 to 108 p.f.u. ml−1.
Droplet nuclei loading system
The majority of experiments were performed by loading the FFR using a submicron aerosol containing MS2. The test system used for loading the FFRs with an aerosol of MS2 is illustrated schematically in Fig. 1 and consisted of a nebulizer, dryer, charge neutralizer, exposure chamber, reference sampler, and breathing machine. The MS2 aerosol was generated with a 6-jet Collison nebulizer (BGI, Inc., Waltham, MA, USA) operated at 138 kPa (20 psig). The aerosol exiting the nebulizer was diluted with filtered house air and then passed through a Kr-85 charge neutralizer (Model 3012; TSI, Shoreview, MN, USA) prior to delivery to the test chamber. The test chamber measured ∼90 × 90 × 90 cm and contained two small mixing fans to minimize concentration gradients. Excess challenge was vented through a HEPA filter and exhausted into the hood. The FFR sample holder had a conical shape with an inlet of ∼7.5 cm inner diameter and an outlet of ∼2.5 cm inner diameter. It was connected to the breathing machine by tubing with a 2.5 cm inner diameter. The breathing machine inhaled and exhaled through the FFR at a tidal volume of 1.6 l breath−1 and a rate of 25 breaths min−1, which corresponds to a mean occupational volumetric breathing rate reported previously (Caretti et al., 2004). VCPs within the range of 0.65–1.1 μm were the most common aerodynamic sized particle produced by the aerosol system as measured by the ACI.
Fig. 1.
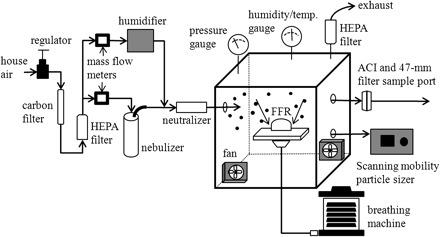
Schematic of aerosol generating system.
Droplet loading system
A limited subset of experiments was performed by spraying the FFR surface with large droplets to better simulate direct contamination such as that from a sneeze. The pneumatic droplet generating system consisted of a compressed air source to aspirate and disseminate a controlled liquid volume (0.125 ml) of MS2 suspension and a vacuum pump to pull 40 l min−1 of air through the FFR. A solenoid valve was used to pulse air from the pressurized cylinder through a 1-cm inner diameter tube and entrain 0.125 ml of suspension that subsequently impacted the FFR positioned 40 cm from the outlet of the droplet generator. The number median diameter and mass median diameter as measured 8 cm from the outlet using a Phase Doppler Anemometry instrument were ∼10 and 60 μm, respectively.
Reaerosolization system
The reaerosolization chamber, illustrated in Fig. 2 was constructed of Lucite with an internal volume of ∼27 l (30 × 30 × 30 cm). The chamber was maintained at a slightly positive pressure [less than ∼25 Pa (0.1 in. H2O)] to ensure particles did not leak into the chamber. The chamber was equipped with a HEPA filter for removal of air displaced by the cough and a mixing fan to enhance mixing within the chamber. The front panel of the chamber contained an opening for attaching the filter holder with mounted FFR. A breathing machine was used to simulate the cough through the FFR. It was equipped with a check valve such that HEPA filtered air was inhaled and directed through the FFR. The chamber was equipped with a 1.3-cm outer diameter sample port for collecting aerosol samples subsequent to the simulated cough. Prior to experimentation, the reaerosolization chamber was tested for particle distribution uniformity using polystyrene latex spheres. Duplicate sampling in five locations demonstrated a coefficient of variation of 10%.
Fig. 2.
Schematic of reaerosolization system.
Aerosol samplers
Samples of the loading aerosol were collected with 47-mm gelatin filters (Sartorius Stedim Biotech S.A., Aubagne Cedex, France) to determine the time-averaged MS2 aerosol concentration. The filters were housed in an in-line filter holder and a vacuum pump was used to draw 10 l min−1 through the filter. The collected viruses were extracted from the filter and a bioassay was performed to determine the number of viable organisms collected. The viable airborne concentration was then estimated based on the sample volume. The 47-mm gelatin filters were also used to collect the MS2 reaerosolized from the FFR. The filters were housed in in-line holders and the sample flow rate was 28 l min−1.
An ACI (BGI Inc., Waltham, MA, USA) was used to measure the viable particle size distribution of both the loading and reaerosolized MS2 aerosols. The ACI consists of six stages, each of which contains a glass Petri dish filled with a solid nutrient medium seeded with Escherichia coli. The bin sizes for each stage are provided in Fig. 3. A 47-mm gelatin filter was placed in-line downstream of the ACI to collect particles with aerodynamic diameters <0.65 μm. The ACI sampled at a flow rate of 28 l min−1. Each particle that impacts the agar surface and contains at least one viable virus is assumed to result in a p.f.u. It is possible that more than one particle could impact the same location on the plate leading to understated values; however, this phenomenon is more likely with higher aerosol concentrations. As such, the ACI provides the size distribution based on number of VCPs.
Fig. 3.
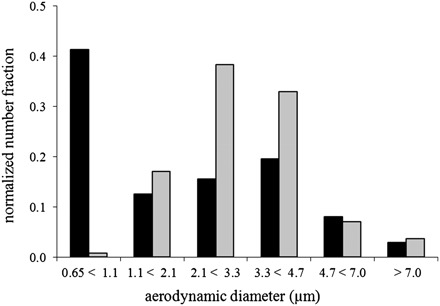
Size distribution of loading (104 p.f.u. cm−2) and reaerosolized droplet nuclei. ACI measured particle distribution over the range 0.65–7.0 μm. The black bars (n = 1) represent the loading aerosol size distribution and the gray bars (n = 5) represent the reaerosolized particle size distribution.
Virus loading of FFRs
Virus contamination of FFRs was conducted under ambient temperature (22 ± 3°C) and relative humidity (30 ± 10%). Virus was applied as droplet nuclei, using two loading levels (104 and 105 p.f.u. cm−2) or as droplets (105 p.f.u. cm−2) as described below. Virus loading of the FFR models was performed under a cyclic flow of 40 l min−1 (1.6 l breath−1 and 25 breaths min−1), simulating the mean occupational volumetric breathing rate (Caretti et al., 2004). Three swatches (area = 4 cm2 swatch−1) were recovered from the FFR for extraction and bioassay to confirm the loading level.
Droplet nuclei.
The FFR was sealed to the holder using hot melt glue (Part No. BAP5-4; Arrow Fastener Company, Inc., Saddlebrook, NJ, USA) and mounted to the loading chamber. The chamber was sealed and the breathing machine was started and operated at 40 l min−1. The aerosol generation system was then started. A stop watch was used to measure the loading duration, typically 30 min. A total of four reference filters were collected during loading to quantify the viable aerosol concentration during loading. All reference filters were collected after the chamber had reached steady state concentrations; two sets of filter pairs were collected for 10 min each starting at 5 min into the test and at 16 min into the test. The loading level (PL, p.f.u. cm−2) was estimated based on the loading duration (tL, minutes), time-averaged challenge concentration (CChal, p.f.u. cm−3), flow rate (Q, cm3 min−1), filter surface area (A, cm2), and fractional filtration efficiency (f) using the following relationship:
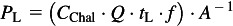 |
The fractional filtration efficiency was assigned to be 0.95. This was based on previous measurements of filtration efficiency made on the same brand of FFR in the size range of the loading aerosol (Richardson et al., 2006). At the desired loading level, the aerosol generation system and breathing machine were stopped and the chamber flushed with HEPA filtered air. The filter holder and FFR were then removed from the test system for reaerosolization testing as described below.
Droplet.
The FFR was positioned 40 cm from the outlet of the sneeze apparatus. The vacuum pump was started to pull air through the FFR. The solenoid valve was activated to pulse air from the pressurized cylinder to create aerosolized droplets, which impacted the FFR. The vacuum pump was shut-off immediately following the simulated sneeze. The filters were allowed to dry for at least 30 min at room temperature and then were evaluated for reaerosolization as described below. A fast-response mass flow meter (Series 4000; TSI, Inc., Shoreview, MN, USA) was used to determine the duration of the pressure pulse upon activation of the solenoid valve. The flow was released from the cylinder in approximately 0.25 s. The cylinder was charged to 207 kPa (30 psig) and thus, the expelled volume was 520 cm3. The air velocity at the exit of the pneumatic system was estimated to be 26 m s−1. This velocity is consistent with sneeze source velocities measured by (Ishima et al., 2005).
Virus reaerosolization from FFRs
The reaerosolization tests were conducted in a separate chamber to minimize any background or contamination that might be incurred inside the loading chamber. The holder with FFR mounted was removed from the loading chamber and sealed to the reaerosolization chamber within 60 min of loading. The FFR was not removed from the holder or handled during transfer between the loading and reaerosolization chambers. The reaerosolization chamber was flushed with HEPA filtered air. Background samples were collected from the reaerosolization chamber prior to placement of the loaded FFR and after placement of the loaded FFR using either the 47-mm filter or ACI, depending on the sampler to be used for that trial. Both samplers operated at 28 l min−1 and the sample duration was 10 min. HEPA filtered air was supplied to the chamber at ∼29 l min−1 to replenish the sampled air and to maintain the reaerosolization chamber under slight positive pressure. The sampling media was then replaced in preparation for the reaerosolization test. Reaerosolization sampling was initiated by starting the sampler, either the 47-mm filter or the ACI, 1 min prior to initiating the cough. The breathing machine was then used to simulate the cough through the filter. All sampling was then performed for 10 min. At the conclusion of the test, the sample media was recovered for subsequent bioassay and the FFR was removed from the test system. The surfaces of the reaerosolization chamber were wiped with bleach following each trial to decontaminate the system to ensure no cross-contamination between trials. System backgrounds were collected prior to each reaerosolization test to verify the chamber had been properly decontaminated since the previous trial.
A cough profile, with a tidal volume of 1.6 l and a peak flow of about 370 l min−1 was obtained from a human subject during testing performed at the U.S. Army Edgewood Chemical Biological Center (Richardson et al., 2006). This measured waveform was programmed into a pulmonary waveform generator for the simulated cough. A single cough was performed during each reaerosolization test.
Virus enumeration
FFR swatch samples were cut from the FFR using scissors and forceps that had been decontaminated. Swatch samples were placed in 50-ml conical tubes containing 10 ml phosphate-buffered saline and extracted using a vortexer. The same method was used for FFRs loaded with the aerosol or large droplets. Sampled gelatin filters were removed from their respective filter holders using decontaminated forceps and placed in conical tubes containing 10 ml of phosphate buffered saline. All samples were serially diluted in phosphate-buffered saline and plated per standard microbiological protocols. Each sample was plated in triplicate and the plates were incubated overnight. Plaques were counted and used to determine p.f.u. ml−1 of sample. Upon completion of aerosol sampling, the ACI plates were incubated at the appropriate conditions and the p.f.u. on each stage were counted.
Scanning electron microscopy
Microphotographs were collected to visualize the distribution of aerosol or droplets on the FFR. FFR swatch samples were cut from the FFR using scissors and forceps that had been decontaminated. The coarser outer layer and the filter media were separated and imaged separately. Secondary electron images were collected on a JEOL 840A scanning electron microscope using an accelerating voltage of 20 kV. Samples were fixed to pure carbon adhesive tabs placed on aluminum stubs and lightly coated with gold prior to imaging. Samples were analyzed from an as-received FFR (i.e. no contamination), an FFR loaded with the MS2 aerosol, and an FFR loaded with liquid droplets containing MS2.
Data analysis
The percent of viable viruses reaerosolized was defined as the ratio of the number of viable viruses re-entrained to the number of viable viruses loaded onto the filter. The total number of viable viruses re-entrained was based on the number of p.f.u. collected on the 47-mm gelatin filters following the simulated cough. The viable loading on the FFR was determined through analysis of swatches removed from the FFR and/or the time-averaged challenge concentration and known minute volume and duration. The area of the FFR was estimated to be 170 cm2 and the loading was assumed to be constant over the surface. The cascade impactors were used to estimate the median aerodynamic diameters of both the loading and reaerosolization aerosols. Statistical comparison of the reaerosolization of particles deposited as droplet nuclei for both loading concentrations was performed using a two-tailed T-test (Excel 2007).
RESULTS
Table 1 summarizes the results of the reaerosolization testing to quantify the total number of viable viruses resuspended from the FFR during the simulated cough. The table contains the average measured viable loading level, the average number of airborne viruses collected following resuspension due to the cough, and the average percent of viable viruses loaded onto the FFR that were reaerosolized. The bioassay of all background filters, collected to monitor decontamination of the chamber between experiments, resulted in non-detections (<100 p.f.u.). In comparison, the number of viable viruses sampled following the cough was generally >1000 p.f.u., 10 times higher than the minimum detection limit. Thus, the organisms collected during reaerosolization can be attributed to those VCPs shed from the FFR. The percentage of reaerosolization measured 0.21 and 0.08% for the 104 and 105 p.f.u. cm−2 challenge levels of the droplet nuclei experiments, respectively. The difference in the percentage of particle reaerosolization between the two loading levels was not statistically significant (P = 0.33). The droplet challenge experiment only demonstrated reaerosolization of <0.0001% (below detection limits).
Table 1.
Viable MS2 reaerosolized from FFRs during a simulated cough when contaminated with droplets or droplet nuclei. [The load and reaerosolized values represent the average MS2 (p.f.u. cm−2) and MS2 (p.f.u.), respectively recovered from 3–5 replicate experiments. The percentage of MS2 reaerosolized was calculated for each of the 3–5 replicate experiments and then reported here as the average.]
| Particle type | Load (p.f.u. cm−2) | Reaerosolized (p.f.u.) | Reaerosolized (%) |
| Droplet nuclei | 1.3E + 04 | 2.4E + 03 | 0.21 ± 0.22 |
| Droplet nuclei | 1.3E + 05 | 1.7E + 04 | 0.08 ± 0.08 |
| Droplet | 3.6E + 05 | <1.0E + 02 | <0.0001 |
The ACI results shown in Fig. 3 represent the loading and simulated cough resuspended aerosol size distributions of VCPs >0.65 μm for the droplet nuclei sample set with a determined load of ∼1.3 × 104 p.f.u. cm−2. Roughly 41% of the loaded aerosol contained VCPs <1.1 μm with the remaining 60% distributed among the five larger bin sizes at 13, 16, 20, 8, and 3% for each increasing category, respectively. The measured VCPs in the reaerosolized particles demonstrated an overall shift to the right in particle size distribution with the majority of the VCPs residing in the two bins for particle sizes between 2.1 and 4.7 μm. The higher loading level (1.3 × 105 p.f.u. cm−2) experiment demonstrated similar trends; however, the loading aerosol sample was overloaded with MS2 p.f.u. for the two stages measuring particles in the range of 0.65–2.1 μm and was not quantified.
DISCUSSION
The results of this study are congruent with the limited published investigations of reaerosolization of particles from FFRs. Qian et al. (1997a) and Willeke and Qian (1998) evaluated the reaerosolization of biological aerosols from three different models of N95 FFRs at velocities up to 300 cm s−1, intended to represent violent sneezing or coughing. The microorganisms Bacillus subtilis and Bacillus megatherium were loaded onto FFRs (105 particles cm−2) with a constant airflow of 85 l min−1. Reaerosolization was found to be <0.2% under all conditions tested similar to the results demonstrated in this study (Table 1). Kennedy and Hinds (2004) found that <0.3% of polystyrene latex microspheres (1.0 μm in diameter) reaerosolized from FFRs when dropped from a height of 3 ft. In similar studies, Birkner et al. (2011) found that particle release tended to increase with drop height and particle size. Qian et al. (1997a) also characterized the reaerosolization of inert particles to assess the effect of particle size, particle type, and filter type. For particles <1.0 μm, <0.025% of the particles became reaerosolized. For the condition of violent sneezing or coughing, ∼1% of 3 μm and 6% of 5 μm particles became reaerosolized. A similar trend is evident in this study; in general, larger particles were detected in the aerosol generated from contaminated FFRs when compared to the loading aerosol (Fig. 3). The percentage of particles detected in the largest size categories, ≥4.2 μm, were no different between the loading and reaerosolized samples, which may be due to low particle counts in this size range. It is possible that some of the reaerosolized particles detected in this study were agglomerates formed after shedding from the FFR. Virus viability may be higher in large agglomerates than in smaller particles, which would overstate the measured reaerosolization of larger particles; however, previous characterization of reaerosolized monodispersed inert particles suggests that larger particles may be more susceptible to reaerosolization due to the larger aerodynamic drag forces seen with larger particles (Qian et al., 1997a). Qian et al. (1997b) found that multiple pulses (i.e. coughs) did not result in any measureable reaerosolization because drag forces from the airflow over the particle were not enough to exceed the particle's force of adhesion to the filter fiber.
Reaerosolization of particles from FFRs contaminated using the droplet method was below the detection limit. The microphotographs of Fig. 4 demonstrate a difference in the presentation of the contaminants on the FFR fibers for droplet and droplet nuclei exposed FFRs. There is no visual evidence of contamination on FFR fibers exposed to VCPs as droplets. This may be due to a capillary or wicking mechanism that has been previously speculated and studied (Belkin, 1996; Li et al., 2006; Li, 2008; Roberge, 2008). Upon deposition, the wet droplet particle may be distributed over a wide area, including multiple layers, of the FFR. It appears that wicking of droplets into the media of an FFR may reduce the potential of reaerosolization; however, the capillary movement of infectious particles to the inside of the FFR from the exterior may present another potential danger to the wearer and is the focus of a future study at NIOSH.
Fig. 4.
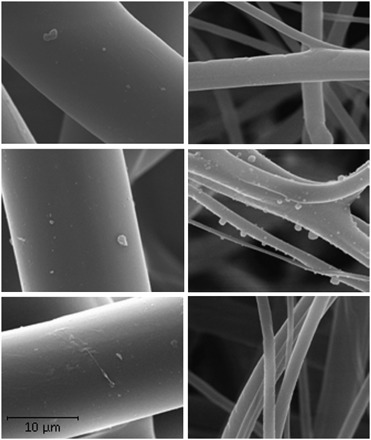
Microphotographs of the particle deposition on FFR layers. Top row, as-received FFR; middle row, droplet nuclei exposed FFR; and bottom row, droplet exposed FFR (left: outer layer and right: filter media layer). The scale bar is applicable to each image.
For risk assessment purposes, it is paramount that the data and experimental approach be put into the proper context of possible in-use scenarios. At first consideration, the total average number of reaerosolized VCPs in the droplet nuclei experiment (2400 and 17 000 p.f.u. in 100 l of sampled air) may seem alarming in regards to potential respiratory hazards. However, it is possible that the reverse airflow generated by the simulated cough was higher for the tested FFRs compared to what would be expected in-use. The tested FFRs were sealed to the holders with hot glue; therefore, the airflow was forced through the medium of the contaminated FFR and not around the FFR edges. Tang et al. (2009) used Schlieren imaging of human subjects to demonstrate that a portion of the airflow generated by coughs leaks around the edges of the FFRs, which would lessen the threat of reaerosolization due to decreased airflow passing through the filter medium. In addition, the level of contamination used in this study is unlikely as infectious aerosols are typically extremely dilute (Roy and Milton, 2004). Data on typical particle concentrations of infectious pathogens in the healthcare setting are limited due to low concentration of particles, insensitivity of virus detection methods, and virus viability (Tellier, 2009), but some recent studies have measured airborne levels of respiratory viruses in several workplace settings, presumably generated via a combination of sources (e.g. talking, breathing, coughing, and sneezing). Lindlsey et al. (2010) measured influenza RNA concentration exceeding 3 pg m−3 of air in an urgent care clinic, while Blachere et al. (2009) measured ∼17 000 and 21 000 TCID50-equivalent RNA particles in the lower and upper airspace of an emergency waiting room and children's waiting room, respectively, after 4–5 h of sampling. Yang et al. (2011) measured influenza concentrations in healthcare centers, a daycare, and on aeroplanes and determined the average concentration to be 1.6 ± 0.9 × 104 genome copies m−3 in the samples containing detectable virus. The average concentration was used to estimate inhalation doses of 30 ± 18, 236 ± 140, and 708 ± 419 TCID50 for 1, 8, and 24-h exposures periods, respectively. These studies did not measure viable influenza and the quantification methods make estimating a particle count difficult. Among three studies that sampled from within hospital rooms occupied by SARS patients, only a total of two viruses were detected (Wan et al., 2004; Booth et al., 2005; Tsai et al., 2006). Likewise, the difficulty in detecting TB in air samples of isolation rooms of infected patients supports the likelihood of dilute infectious aerosols in healthcare settings (Fennelly et al. 2004).
To assess the risk posed by the reaerosolization of respiratory viruses such as influenza, various techniques based upon modeling data (Nicas et al., 2005; Nicas and Gang, 2006; To and Chao, 2010) or actual exposure assessment data can be used. For the case of influenza, the Yang et al. (2011) data discussed above is most convenient because estimated inhalation doses are calculated. To apply this data to reaerosolization from FFRs, the inhalable dose is used to estimate FFR contamination levels. As discussed above, the inhalation dose estimated for an 8 h exposure (work shift) with an adult breathing rate of 20 m3 day−1 was 236 ± 140 TCID50. Even if 100% contamination of the FFR is assumed (worst case situation), this level of contamination would be far less than the levels used experimentally in this study. Airflow equivalent to the type of simulated coughs used in our study could reaerosolize up to 0.21% or 0.496 TCID50 from the contaminated FFR. Assuming equal mixing and no air changes, the concentration of the reaerosolized virus would be 0.018 TCID50 m−3 air for a patient room measuring 27 m3 (3.0 m × 3.0 m × 3.0 m), which is much less than the human infectious dose (ID50) of 0.6–3 that have been reported to be adequate to induce infection (Alford et al., 1966; Yang et al., 2011). This risk assessment assumes only one cough but considers a constant 8 h exposure, which are both unlikely events. Likewise, the estimation was based on measurements of genome copies converted to TCID50 equivalents, which may grossly overstate the viable influenza in the inhalation estimation.
Overall, these results suggest that a small amount of micron-sized particles, which have the potential to remain airborne, can be reaerosolized. Because of the paucity of exposure data and myriad number of scenarios in which FFRs are used, it is difficult to completely characterize all the risks associated with extended use to healthcare workers and patients from reaerosolization of virus, if it were a virulent species. However, based on the percentage of viable reaerosolized particles measured from FFR contaminated via droplet nuclei and droplet loading methods and the estimated loading levels found in workplace settings, the potential threat from the reaerosolization of most respiratory viruses appears to be insignificant and unlikely to pose a significant risk to healthcare workers and patients. Similar conclusions were made for bacteria (Willeke and Qian, 1998). As new respiratory pathogens emerge (with increased and/or unknown levels of virulence) and more exposure assessment data become available, this risk assessment should be reevaluated.
Finally, some limitations must be acknowledged. Only one FFR model, the NIOSH approved N95 Gerson 1730, was used in this study; therefore, the results generated in this study may not be applicable to other FFR models. Similarly, the use of MS2 as a surrogate for pathogenic viruses only generally defines the potential for virus reaerosolization from FFRs and does not define the potential threat of any specific virus. Viable assays were used to measure the size and concentrations of MS2 particles, thus the results may differ for other viruses, which may have distinct viability characteristics. The viability of lipid enveloped viruses could presumably be less than that of the non-enveloped MS2 bacteriophage used in this study; therefore, the reaerosolization values reported in this study may overstate the potential threat for viruses such as influenza. Future studies assessing the risks of extended FFR use should consider other factors, such as the infectious dose of the organism, stability of the organism in the environment, performance of existing engineering controls, and duration of the exposure. This study examined the reaerosolization of virus from FFRs from the perspective of healthcare workers in patient contaminated environments. A single simulated cough was used to provide high velocity airflow to determine particle reaerosolization. It is possible that the wearer of the FFR may also release particles with normal breathing, a topic worthy of further investigation; however, in Qian et al., 1997a, particle reaerosolization from FFRs were not registered for velocities under 200 cm s−1 because the air drag forces were not sufficient to overcome the adhesion forces between the particles and the filter fibers.
CONCLUSIONS
Only a small percentage (≤0.21%) of viable virus was reaerosolized from the tested FFRs by reverse airflow generated by a simulated cough. The extent of reaerosolization observed was dependent on the method used to load the FFRs. Virus applied as droplet nuclei were much more susceptible to reaerosolization than viruses loaded as droplets. The size distribution of the reaerosolized particles was larger than the loading aerosol as measured by the ACI. These data suggest that for most respiratory viruses the risks due to reaerosolization associated with extended use can be considered negligible, although risk assessments should be updated as new respiratory viruses emerge and better workplace exposure assessment data become available.
FUNDING
This project was funded intramurally by the US Centers for Disease Control and Prevention's National Institute for Occupational Safety Health.
Acknowledgments
The authors wish to express our sincere gratitude to Dennis Viscusi, Mike Bergman, Brian Heimbuch, Lisa Delaney, and Kenneth Williams for their suggestions and contributions. The findings and conclusions in this manuscript have not been formally disseminated by the NIOSH and should not be construed to represent any agency determination or policy. Mention of any company, product, policy, or the inclusion of any reference does not constitute endorsement by NIOSH.
References
- Alford RH, Kasel JA, Gerone PJ, et al. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800–4. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- Bailar JC, Brosseau LM, Cohen HJ et al. (2006) Reusability of facemasks during an influenza pandemic. Washington, DC: Institute of Medicine, National Academies Press.
- Balazy A, Toivola M, Adhikari A, et al. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–7. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Belkin NL. A century after their introduction, are surgical masks necessary? AORN J. 1996;64:602. doi: 10.1016/s0001-2092(06)63628-4. [DOI] [PubMed] [Google Scholar]
- Bergman MS, Viscusi DJ, Heimbuch B, et al. Evaluation of multiple (3-Cycle) decontamination processing for filtering facepiece respirators. J Eng Fiber Fabr. 2010;5:32–41. [Google Scholar]
- Birkner JS, Fung D, Hinds WC, et al. Particle release from respirators, part I: determination of the effect of particle size, drop height, and load. J Occup Environ Hyg. 2011;8:1–9. doi: 10.1080/15459624.2011.534975. [DOI] [PubMed] [Google Scholar]
- Blachere FM, Lindsley WG, Pearce TA, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48:438–40. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–7. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton Bridges C, Katz JM, Seto WH, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181:344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- Caretti DM, Gardner PD, Coyne KM. Report ECBC-TR-316, Edgewood Chemical Biological Center, US Army Research. Aberdeen Proving Ground, MD: Development and Engineering Command; 2004. Workplace breathing rates: defining anticipated values and ranges for respirator certification testing. [Google Scholar]
- CDC. TB respiratory protection program in health care facilities: administrator's guide. (1999) Available from http://www.cdc.gov/niosh/docs/99-143/. Accessed 8 March 2011. [Google Scholar]
- CDC. Interim domestic guidance on the use of respirator to prevent the transmission of SARS. (2005) Available from http://www.cdc.gov/ncidod/sars/respirators.htm. Accessed 8 March 2011. [Google Scholar]
- CDC. What you should know about using facemasks and respirators during a flu pandemic. (2008) Available from http://www.cdc.gov/Features/MasksRespirators/. Accessed 6 January 2010. [Google Scholar]
- CDC. Novel influenza A (H1N1) virus infections among health-care personnel—United States, April-May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:641–5. [PubMed] [Google Scholar]
- CDC. Interim guidance on infection control measures for 2009 H1N1 Influenza in healthcare settings, including protection of healthcare personnel. (2010a) Available from http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed 8 March 2011. [PubMed] [Google Scholar]
- CDC. Questions and answers regarding respiratory protection for preventing 2009 H1N1 Influenza among healthcare personnel. (2010b) Available from: http://www.cdc.gov/h1n1flu/guidelines_infection_control_qa.htm. Accessed 8 March 2010. [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–7. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Eninger RM, Honda T, Adhikari A, et al. Filter performance of n99 and n95 facepiece respirators against viruses and ultrafine particles. Ann Occup Hyg. 2008;52:385–96. doi: 10.1093/annhyg/men019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Method 1602 male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. Washington, DC: United States Environmental Protection Agency, Office of Water; 2001. [Google Scholar]
- Fennelly KP, Martyny JW, Fulton KE, et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169:604–9. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- Fisher E, Rengasamy S, Viscusi D, et al. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Appl Environ Microbiol. 2009;75:1500–7. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E, Shaffer R. Survival of bacteriophage MS2 on filtering facepiece respirator coupons. Appl Biosaf. 2010;15:71–76. [Google Scholar]
- Fisher EM, Shaffer RE. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J Appl Microbiol. 2011;110:287–95. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EM, Williams JL, Shaffer RE. The effect of soil accumulation on multiple decontamination processing of N95 filtering facepiece respirator coupons using physical methods. J Int Soc Respir Prot. 2010;27:16–26. [Google Scholar]
- Heimbuch BK, Wallace WH, Kinney KR, et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Ishima T, Masaaki A, Motohiko A, et al. Visualization of flow induced by human sneeze. J Vis Soc Jpn. 2005;25:107–10. [Google Scholar]
- Kennedy NJ, Hinds WC. Release of simulated anthrax particles from disposable respirators. J Occup Environ Hyg. 2004;1:7–10. doi: 10.1080/15459620490250017. [DOI] [PubMed] [Google Scholar]
- Li Y. Transmission of communicable respiratory infections and facemasks. J Multidiscip Healthc. 2008;1:17–27. doi: 10.2147/jmdh.s3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wong T, Chung J, et al. In vivo protective performance of N95 respirator and surgical facemask. Am J Ind Med. 2006;49:1056–65. doi: 10.1002/ajim.20395. [DOI] [PubMed] [Google Scholar]
- Lindsley WG, Blachere FM, Davis KA, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50:693–8. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- Nicas M, Gang S. An integrated model of infection risk in a health-care environment. Risk Anal. 2006;26:1085–96. doi: 10.1111/j.1539-6924.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–54. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Willeke K, Grinshpun SA, et al. Performance of N95 respirators: reaerosolization of bacteria and solid particles. Am Ind Hyg Assoc J. 1997;58:876–80. doi: 10.1080/15428119791012216. [DOI] [PubMed] [Google Scholar]
- Qian YG, Willeke K, Ulevicius V, et al. Particle reentrainment from fibrous filters. Aerosol Sci Technol. 1997;27:394–404. [Google Scholar]
- Richardson AW, Eshbaugh JP, Hofacre KC, et al. Respirator filter efficiency testing against particulate and biological aerosols under moderate to high flow rates. 2006. Available from http://www.cdc.gov/niosh/npptl/researchprojects/pdfs/CR-085Gardner.pdf. Accessed 8 March 2011. [Google Scholar]
- Roberge RJ. Effect of surgical masks worn concurrently over N95 filtering facepiece respirators: extended service life versus increased user burden. J Public Health Manag Pract. 2008;14:E19–E26. doi: 10.1097/01.PHH.0000311904.41691.fd. [DOI] [PubMed] [Google Scholar]
- Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med. 2004;350:1710–2. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- Salter WB, Kinney K, Wallace WH, et al. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–45. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Jackson M, et al. (2007) 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control; 35: S65–164. [DOI] [PMC free article] [PubMed]
- Tang JW, Liebner TJ, Craven BA et al. (2009) A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface; 6: S727–36. [DOI] [PMC free article] [PubMed]
- Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6:S783–90. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To GNS, Chao CYH. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YH, Wan GH, Wu YK, et al. Airborne severe acute respiratory syndrome coronavirus concentrations in a negative-pressure isolation room. Infect Control Hosp Epidemiol. 2006;27:523–5. doi: 10.1086/504357. [DOI] [PubMed] [Google Scholar]
- Viscusi DJ, Bergman MS, Eimer BC, et al. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–27. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi DJ, Bergman M, Sinkule E, et al. Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. Am J Infect Control. 2009;37:381–6. doi: 10.1016/j.ajic.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007;24:93–107. [Google Scholar]
- Vo E, Rengasamy S, Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl Environ Microbiol. 2009;75:7303–9. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan GH, Tsai YH, Wu YK, et al. A large-volume nebulizer would not be an infectious source for severe acute respiratory syndrome. Infect Control Hosp Epidemiol. 2004;25:1113–5. doi: 10.1086/502353. [DOI] [PubMed] [Google Scholar]
- Willeke K, Qian Y. Tuberculosis control through respirator wear: performance of National Institute for Occupational Safety and Health-regulated respirators. Am J Infect Control. 1998;26:139–42. doi: 10.1016/s0196-6553(98)80033-3. [DOI] [PubMed] [Google Scholar]
- Woo M-H, Hsu Y-M, Wu C-Y, et al. Method for contamination of filtering facepiece respirators by deposition of MS2 viral aerosols. J Aerosol Sci. 2010;41:944–52. doi: 10.1016/j.jaerosci.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Elankumaran S, Marr LC. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J R Soc Interface. 2011;8:1176–84. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]


