Abstract
Oxidative stress (OS) refers to the physiological imbalance between oxidative and antioxidative processes leading to increased oxidation, which then results in the inflammatory infiltration of neutrophils, increased protease secretion, and the production of a large number of oxidative intermediates. Oxidative stress is considered an important factor in the pathogenesis of cardiovascular disease (CVD). At present, active components of Chinese herbal medicines (CHMs) have been widely used for the treatment of CVD, including coronary heart disease and hypertension. Since the discovery of artemisinin for the treatment of malaria by Nobel laureate Youyou Tu, the therapeutic effects of active components of CHM on various diseases have been widely investigated by the medical community. It has been found that various active CHM components can regulate oxidative stress and the circulatory system, including ginsenoside, astragaloside, and resveratrol. This paper reviews advances in the use of active CHM components that modulate oxidative stress, suggesting potential drugs for the treatment of various CVDs.
1. Introduction
According to the World Health Organization (WHO) statistics, cardiovascular diseases (CVDs) were responsible for the highest number of deaths in 2019 [1]. Increasing and aging populations further complicate the situation, and 22.2 million CVD-related deaths are expected to occur in 2030 [2]. There is an important link between complex oxidation reactions and the development of atherosclerosis [3, 4]. Increasing levels of oxidative stress contribute to the subsequent formation and progression of atherosclerotic plaques [5]. A lack of endogenous antioxidants is another important cause of coronary heart disease [6]. The use of traditional medicinal plants has rapidly expanded in recent years. Medical plant research is no longer limited to chemical composition and pharmacology and now encompasses the study of metabolomics and underlying mechanisms of action [7]. Studies employ approaches ranging from simple chemical component separation and drug efficacy tests to transcriptomics and pathway research. The underlying mechanisms of the remarkable curative effects of medicinal plants are being revealed. Technological advances have greatly contributed to the increased use of medicinal plants. 25% of drugs in the global medical market are phytomedicines [8]. Further, 60% of anticancer drugs and 75% of drugs for the treatment of infectious diseases originate from medicinal plants [9]. Between 1984 and 2014, 9.1% of FDA-approved drugs were medicinal plants, while 21% were natural product derivatives [10].
Finding safe and effective drugs derived from natural products is a hot topic in the CVD field [11]. Medicinal plants have great advantages for the treatment of cardiovascular disease owing to their safety profiles [12]. Favorable effects of medicinal plants have been described for diseases such as hypertension, hyperlipidemia, atherosclerosis, and chronic heart failure, as well as for the overall reduction of cardiovascular risk [13]. Cardiovascular disease patients being informed about the benefits of medicinal plants could improve their health. Medicinal plant-containing diets may be used to effectively control hypertension, for example [14]. The sufficient intake of fruits, vegetables, nuts, red wine, coffee, and others can also effectively prevent the occurrence of CVD [15]. This is due to such foods often having antioxidant biological activities. Similarly, medicinal plants possess antioxidant pharmacological effects, making them likely drug candidates. Within medicinal plant research for CVD, oxidative stress inhibition is a very advanced research line. Accumulating evidence suggests that flavonoids, phenolics, and saponin from medical plants could reduce oxidative stress [16]. Our studies have evaluated the active ingredients of 20 different natural compounds, including ginsenoside, resveratrol, astragaloside A, and quercetin. The therapeutic mechanisms of these different active ingredients were addressed in the context of CVD. Herein, we provide an overview of the oxidative stress reduction mechanisms of medicinal plants for the treatment of CVD. The questions addressed include the following: (1) which medicinal plant components have antioxidative pharmacological effects? (2) What is the regulatory role of antioxidant medicinal plant components in CVD? (3) What is the mechanism of action of antioxidant medicinal plant components in CVD?
2. Methodology and Strategy
In this review, we performed a literature search for information on the bioactive components of medicinal plants and their effect on oxidative stress. The Institute for Scientific Information (ISI) Web of Knowledge, MEDLINE, PubMed, Scopus, Google Scholar, and the China National Knowledge Infrastructure (CNKI) databases were searched using relevant keywords and phrases, including “natural drugs and oxidative stress”, “natural active ingredients and oxidative stress”, “traditional Chinese medicine and oxidative stress”, “phytochemistry/medicinal plant extracts and oxidative stress”, “medicinal plants and cardiovascular diseases”, and “natural active ingredients and cardiovascular diseases”. From the search results, we manually selected original articles discussing natural medicines or their active ingredients and their effect(s) on oxidative stress or CVDs. Our search selection criteria were mainly based on the following:
(1) Different Mechanisms Used by Natural Medicines and Active Ingredients to Treat Cardiovascular Diseases. For example, total flavonoids of matsuba can dilate the coronary artery, increase blood flow, and reduce abnormal electron transfer of the myocardial cell membrane and the production of oxygen free radicals, thereby improving myocardial ischemia and treating coronary heart disease [17]. Orientin can protect red blood cells from oxidative damage by reducing oxidative stress, increasing the activity of antioxidant enzymes, and maintaining the structural integrity of red blood cells [18].
(2) Different Categories of Natural Medicines and Active Ingredients with Therapeutic Efficacy in Cardiovascular Diseases. The active ingredients of natural medicines including flavonoids, saponins, polyphenols, polysaccharides, and anthraquinones can exert different therapeutic effects. For example, saponin-based active ingredients have multiple functions such as antiviral activity, scavenging oxygen free radicals, expanding blood vessels, strengthening the heart, and reducing the synthesis of reactive oxygen species (ROS) and malondialdehyde (MDA) [19]. Polyphenols inhibit the formation of hydroxyl free radicals, increase endogenous superoxide dismutase (SOD) activity, inhibit lipid peroxidation, and increase cellular energy metabolism [20].
(3) Different Therapeutic Targets of Natural Medicines and Active Ingredients. For example, berberine can regulate adenosine 5′-monophosphate- (AMP-) activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathways [21]. Curcumin can inhibit phosphatidylinositol 3-kinase- (PI3K-) serine/threonine protein kinase- (AKT-) mTOR signal transduction [22]. Further, some active ingredients can regulate multiple signal pathways and can also regulate the growth, proliferation, differentiation, migration, and apoptosis of a variety of cells.
3. Oxidative Stress and Cardiovascular Disease
Oxidative stress refers to the pathological state of reactive oxygen species (ROS) accumulation caused by the excessive production of oxygen radicals or a compromised intracellular antioxidant defense system [23]. Oxidative stress plays an important role in the regulation of the cardiovascular system and has become a new target for CVD prevention and treatment. Oxidative stress can cause severe functional damage to endothelial cells and cardiomyocytes [24, 25]. In addition, oxidative stress is involved in the pathogenesis of hypertension, myocardial ischemia-reperfusion injury, atherosclerosis, and other associated diseases by regulating inflammation and stimulating vascular smooth muscle proliferation [26–28].
As shown in Figure 1, there are various biological markers of oxidative stress, among which ROS are the most closely linked to oxidative stress. In the normal physiological state of the human body, relatively small levels of endogenous ROS are produced, and, at a certain level, ROS play an important role in the protection of myocardial cells [29, 30]. However, in a pathological state, as ROS are scavenged at a rate that is much lower than the rate of their production, ROS will accumulate, directly leading to oxidative stress. Increased oxidative stress causes lipid peroxidation, protein and enzyme denaturation, DNA damage, and other events damaging the myocardial cell membrane or cardiovascular epithelial cells [31]. Further, oxidative stress and inflammation will cause myocardial injury and remodeling, leading to the occurrence and aggravation of CVD.
Figure 1.
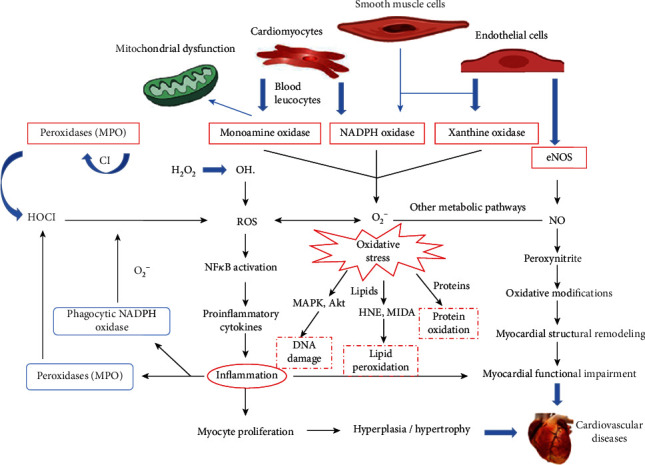
The factors that cause cardiovascular disease are complex, including NADPH, NOX, eNOS, NO, and ROS. ROS is the most obviously biological markers of oxidative stress. The increase in oxidative stress directly causes lipid peroxidation, protein and enzyme denaturation, nucleic acid DNA damage, and other mechanisms of the myocardial cell membrane or cardiovascular epithelial cells. These are also important pathways for the action of antioxidant drugs.
4. Application of Antioxidant Natural Drugs for Cardiovascular Disease
In order to reduce oxidative damage in cardiovascular tissue, an increasing number of medicinal plants are used as natural antioxidants in the clinic [32]. Active components with antioxidant activity can be isolated from various medicinal plants [33, 34]. Examples include phenols, flavonoids, and polysaccharides from traditional Chinese herbal medicine. As shown in Table 1, natural antioxidant drugs can inhibit the production of free radicals by enhancing specific and nonspecific immune function or by directly preventing free radical-induced cellular and tissue damage. The modern clinical treatment of CVD includes the use of natural drugs. Free radicals contain unpaired electrons, which have a tendency to pair. In the process of pairing, free radicals will generate more radicals, forming a chain reaction. Active components of traditional Chinese medicine can terminate this chain reaction both directly and indirectly. Further, these components can also regulate oxidative stress by reducing lipid peroxidation and free radical production, enhancing the scavenging of radicals, improving the activity of antioxidant enzymes, and upregulating the anti-inflammatory response [35]. Compared to synthetic antioxidants, natural medicinal plants with antioxidant properties have lower toxicity and thus a more favorable safety profile. Through these advantages, natural antioxidant medicinal plants may be used for targeting oxidative stress in the clinical treatment of CVD in the future.
Table 1.
Detailed information on bioactive ingredients targeting oxidative stress.
| No. | Active ingredients | Natural drug | Mechanism of action | Treatment disease |
|---|---|---|---|---|
| 1. | Ginsenoside Rb1 | Panax ginseng | (1) Inhibits the expression of proapoptotic genes Bax, Bad, and Fas (2) Increases the activity of antioxidant enzyme (3) Reduces the oxygen free radicals |
(1) Coronary heart disease (2) Ischemia-reperfusion injury |
| 2. | Ginsenoside Rg1 | Panax ginseng | (1) Inhibition of caspase-3, Bax, and ap-JNK expression (2) Increases the expression of p-ERK (3) Reduces ROS |
(1) Coronary heart disease (2) Ischemia-reperfusion injury |
| 3. | Ginsenoside Rg2 | Panax ginseng | (1) Inhibition of CK and LDH (2) Reduced LPO (3) Increases the activity of SOD, CAT, and GSH-Px |
(1) Coronary heart disease (2) Ischemia-reperfusion injury |
| 4. | Delphinidin-3-glucoside | Anthocyanidin | (1) Inhibits the expression of NOX2/NOX4 and caspase-3 (2) Reduces ROS (3) Induces autophagy through AMPK/SIRT1 |
(1) Coronary heart disease (2) Ischemia-reperfusion injury |
| 4. | Total flavonoids of matsuba | Matsuba | (1) Reduce O2 and H2O2 (2) Inhibit the formation of LPO and MDA (3) Increase the activity of SOD, GSH-Px, and CAT |
(1) Coronary heart disease (2) Atherosclerosis (3) Hyperlipidemia |
| 5. | Orientin | Passiflora leaves | (1) Reduces ROS (2) Increases the activity of antioxidant enzyme (3) Regulating AMPK, Akt, mTOR, and Bcl-2 |
(1) Coronary heart disease (2) Atherosclerosis |
| 6. | Hawthorn leaf flavonoids | Genus | (1) Inhibit the formation of LPO (2) Increase the activity of antioxidant enzyme (3) Inhibit free radical reaction |
(1) Coronary heart disease (2) Atherosclerosis (3) Hyperlipidemia |
| 7. | Anemarrhenoside | Anemarrhena asphodeloides | (1) Promoting the production of SOD (2) Inhibit the expression of prooxidative stress protein (3) Reduce ROS |
Ischemia-reperfusion |
| 8. | Hesperidin | Citrus fruits | (1) Regulating Nrf2/ARE/HO-1 and TGF-beta1/Smad3 signal transduction (2) Regulating PI3K/Akt/mTOR signaling pathway |
Ischemia-reperfusion |
| 9. | Resveratrol | Peanuts, red wine, mulberries, etc. | (1) Inhibits the formation of oxygen free radicals (2) Reduces ROS (3) Increases the expression of eNOS by activating SIRT1 |
(1) Hypertension (2) Ischemia-reperfusion |
| 10. | Tea polyphenols | Tea | (1) Increase the activity of SOD (2) Inhibit the formation of LPO (3) Downregulating Hcy metabolic enzymes |
(1) Hypertension (2) Ischemia-reperfusion |
| 11. | Saponins of Panax notoginseng | Panax notoginseng | (1) Inhibit the formation of LPO and MDA (2) Increase the activity of SOD (3) Upregulate the expression of GSH and CAT |
Hypertension |
| 12. | Berberine | Rhizoma coptidis | Regulates the AMPK/mTOR signaling pathway | Hypertension |
| 13. | Allicin | Allium in Liliaceae | (1) Scavenging free radicals (2) Inhibits the formation of ROS (3) Increases the activity of antioxidant enzyme |
Hypertension |
| 14. | Curcumin | Rhizome of a turmeric plant | (1) Reducing the formation of peroxides (2) Inhibiting the expression of Bax, beclin-1, BNIP3, and SIRT1 (3) Inhibiting PI3K-AKT-mTOR signal transduction |
Hypertension |
| 15. | Astragaloside IV | Astragalus propinquus | (1) Increases the activity of T-SOD, GSH-PX, and CAT (2) Inhibits the formation of MPO, NADPH, MDA, and ROS (3) Inhibits the activity of CPK and LDH |
(1) Heart failure (2) Heart remodeling (3) Ventricular dysfunction |
| 16. | Tetramethylpyrazine | Ligusticum chuanxiong | (1) Increased the expression level of microRNA-499a (2) Upregulation of sirtuin1 |
(1) Heart failure (2) Coronary heart disease |
| 17. | Gastrodin | Gastrodia elata | (1) Inhibits the formation of LPO and oxygen free radicals (2) Increases the activity of SOD |
Heart failure |
| 18. | Safflower | Crocus sativus L. | (1) Inducing autophagy (2) Increasing the expression of Nrf2/HO-1/NADPH/NQO1 |
Heart failure |
| 19. | Ferulic acid | Angelica sinensis | (1) Inhibition of CK and LDH (2) Activating the PI3K/Akt/mTOR signaling pathway (3) Reduces ROS |
Heart failure |
| 20. | Paeonol | Paeonia suffruticosa | (1) Increases the activity of SOD (2) Inhibits the formation of LPO and oxygen free radical |
(1) Arrhythmia (2) Coronary heart disease |
| 21. | Matrine | Sophora flavescens | Increases the activity of antioxidant enzyme | Arrhythmia |
| 22. | Astragalus polysaccharide | Astragalus propinquus | (1) Increases the activity of SOD (2) Inhibits the formation of LPO, ROS, and oxygen free radical (3) Increased the expression of 8-OH-AD |
(1) Coronary heart disease (2) Acute myocardial infarction |
| 23. | Quercetin | Dendrobium nobile | (1) Inhibits the expression of aldose reductase (2) Inhibits the formation of MPO and NADPH (3) Inhibits the oxidation of low-density lipoprotein (LDL) |
(1) Acute myocardial infarction (2) Ischemia-reperfusion |
| 24. | Tanshinone II-A | Salvia miltiorrhiza | (1) Upregulation of Nrf-2 (2) Regulates the autophagy genes ATG and LC3 |
(1) Coronary heart disease (2) Acute myocardial infarction |
| 25. | Gypenoside | Gynostemma pentaphyllum | (1) Increases the activity of SOD (2) Inhibits the formation of oxygen free radical |
Acute myocardial infarction |
| 26. | Soybean isoflavone | Glycine max | (1) Increases the activity of SOD and GSH-Px (2) Inhibits the activity of NADPH and NOX |
(1) Acute myocardial infarction (2) Hyperlipidemia |
| 27. | Hydroxy safflower yellow | Carthamus tinctorius L. | (1) Activating the PI3K/Akt signaling pathway (2) Increases the expression of NADPH and NQO1 |
Acute myocardial infarction |
ROS: reactive oxygen species; ap-JNK: C-Jun N-terminal kinase- (JNK-) c-Jun/activated protein (AP); p-ERK: protein kinase R- (PKR-) like endoplasmic reticulum kinase; CK: creatine kinase; LDH: lactate dehydrogenase; LPO: lipid peroxidation; SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde; GSH-Px: glutathione peroxidase; eNOS: endothelial nitric oxide synthase; NADPH: nicotinamide adenine dinucleotide phosphate; NOX2: NADPH oxidase 2; NOX4: NADPH oxidase 4; AMPK: adenosine 5′-monophosphate- (AMP-) activated protein kinase; SIRT1: sirtuin1; PI3K: phosphatidylinositol 3-kinase; Akt: serine/threonine kinase Akt; mTOR: mammalian target of rapamycin; Nrf2: nuclear factor erythroid 2-related factor 2; ARE: antioxidant response element; HO-1: heme oxygenase 1; TGF-beta1: transforming growth factor beta 1; NQO1: NAD(P)H quinone dehydrogenase 1.
4.1. Coronary Atherosclerotic Heart Disease
Coronary atherosclerotic heart disease (CHD) is a CVD caused by stenosis or obstruction of the coronary artery [36, 37]. It is characterized by the formation of lipid-filled atherosclerotic plaques under the tunica intima of the great and middle arteries, leading to stenosis or occlusion of the arterial lumen, which then causes myocardial ischemia and hypoxia [38, 39]. Various factors that lead to CHD may cause lipid peroxidation damage, promoting the occurrence and development of atherosclerosis [40, 41].
During early atherosclerosis, nutrients cannot pass freely due to the reduced blood flow, which in turn leads to a decrease in endogenous ATP levels, activates AMPK, inhibits mTOR, and causes increased ROS, leading to oxidative stress. Excessive ROS can cause endothelial and smooth muscle cell dysfunction. It can also lead to the activation of inflammatory signaling and cardiomyocyte mitochondria-mediated apoptosis, accelerating the occurrence and development of atherosclerosis and CHD [42]. ROS can also act as second messengers, responding to the binding of extracellular signals to cell surface receptors through changes in their concentration. Through Ca2+ signal transduction, the mitogen-activated protein kinase signaling pathway, and the protein kinase B signaling pathway, ROS play a regulatory role in the process of cardiomyocyte signal transmission [43, 44]. Modern pharmacological studies have shown that various Chinese herbal extracts can treat CHD through their antioxidant effects.
4.1.1. Ginsenosides
Ginsenosides are a group of nontoxic bioactive components with antioxidant effects produced by plants of the genus Panax [45, 46]. Ginsenosides Rb1, Rg1, and Rg2 have antioxidant effects, scavenge free radicals, improve myocardial ischemia and hypoxia, reduce intracellular calcium overload, and protect the myocardium [19, 47]. A large body of evidence suggests that ginsenosides Rb1, Rg1, and Rg2 possess protective abilities against CHD.
Ginsenoside Rb1 can reduce the occurrence of cardiomyocyte apoptosis, inhibit oxidative stress, inhibit the expression of apoptosis-promoting genes Bax and Fas, and upregulate mTOR signaling [48]. A recent study indicated that Rb1 could enhance the activity of antioxidant enzymes and reduce free radical-induced damage to the myocardium via activation of the PI3K/Akt/Nrf2 signaling pathway [49, 50].
Ginsenoside Rg2 could reduce oxidative stress injury and improve myocardial ischemia and hypoxia by regulating the activities of serum creatine kinase (CK), lactate dehydrogenase (LDH), lipid peroxides (LPOs), superoxide dismutase (SOD), and glutathione peroxidase (GPX) in rats [51]. Ginsenoside Rg2 was also shown to reduce oxidative stress in human epidermal keratinocytes [52].
Ginsenoside Rg1 may play a role in antioxidant defense by upregulating the AMPK/Nrf2/HO-1 signaling pathway. In addition, it exhibited protective effects against STZ-induced cardiac dysfunction [53]. Further studies indicated that Rg1 increased cell survival, promoted the expression of antioxidant proteins, and reduced ROS and apoptosis through the Nrf2/ARE signaling pathway [54]. Thus, it is suggested that Rg1 may be beneficial for the survival of cardiomyocytes via the inhibition of oxidative stress.
4.1.2. Delphinidin-3-glucoside
Delphinidin-3-glucoside (DPg) is a bioflavonoid with strong antioxidant effects. DPg could decrease the expression of NOX2/NOX4 and caspase-3 induced by oxidized LDL (oxLDL), while reducing ROS production, p38 MAPK phosphorylation, NF-κB p65 activity, and, importantly, the damage induced by oxidative stress [55].
Further, studies reported that DPg could induce autophagy through the AMPK/SIRT1 signaling pathway, thus protecting human umbilical vein endothelial cells (HUVECs) from oxLDL-induced oxidative stress [56].
4.1.3. Total Flavonoids of Matsuba
Total flavonoids of matsuba are a natural extract from pine needles. It possesses antioxidant, anti-inflammatory, and antibacterial activities [57, 58]. The total flavonoids of matsuba can inhibit oxidative stress by upregulating the activity of SOD, GSH-PX, and CAT, while reducing malondialdehyde (MDA) content. In addition, the total flavonoids of matsuba can reduce the oxidative modification of LDL, directly capture and remove O2 and H2O2 radicals, and block the free radical-induced oxidative stress chain reaction, while also inhibiting the formation of toxic substances such as LPO and copolyludiene [17]. Therefore, the total flavonoids of matsuba may be a promising agent for treating CHD.
4.2. Ischemia-Reperfusion Injury
Ischemia-reperfusion injury refers to the increase in ROS and oxidative stress and the further damage of the mitochondrial ultrastructure function and metabolism due to the reoxygenation of tissue that has had its oxygen supply temporarily disrupted [59]. At present, it has become one of the decisive factors affecting the prognosis and survival of patients with CVD. The main contributors to the ROS increase are xanthine oxidase formation, neutrophil respiratory burst, mitochondrial single-electron reduction, catecholamine autoxidation, and intracellular Ca2+ overload [60]. The excessive production of ROS in the myocardial ischemic area can directly trigger myocardial cell apoptosis, inflammatory reactions, and energy metabolism disorders [61]. Several studies have shown that the active components of various CHMs can protect myocardial cells by regulating oxidative stress and have a good therapeutic effect on ischemia-reperfusion injury.
4.2.1. Orientin
Orientin is a flavonoid component present in natural plant extracts. Orientin has been demonstrated to have antioxidant and anticancer properties, while also contributing to cardiac remodeling and the prevention of myocardial ischemia-reperfusion injury through enhanced antioxidant defense [62]. Studies have shown that orientin can inhibit the oxLDL-induced increase in TNF-α, IL-6, and IL-1β, as well as reducing levels of ROS [63]. Orientin also protects red blood cells from oxidative damage by reducing oxidative stress, increasing the activity of antioxidant enzymes, and maintaining the structural integrity of red blood cells [18]. Studies have also reported that orientin can regulate apoptosis via AMPK, Akt, mTOR, and Bcl-2 signaling and the maintenance of autophagic balance. In addition, orientin protects myocardial cells against hypoxia-reoxygenation injury [64]. Overall, orientin's protective effects are related to the inhibition of oxidative stress.
4.2.2. Hawthorn Leaf Flavonoids
Hawthorn leaf flavonoids are the extract of the hawthorn dry leaves of Rosaceae. It possesses antioxidant, anti-inflammatory, and hypolipidemic activities [65]. Hawthorn leaf flavonoids were found to enhance the activity of antioxidant enzymes and inhibit the oxidative modification of LDL-C, while also improving oxidative stress-induced damage to the rat myocardium through the PKC-alpha signaling pathway. Further, the extract was shown to activate PPAR-α signaling to reduce blood triglycerides and regulate the vascular pathological response [66]. It was also found that hawthorn leaf flavonoids could protect vascular endothelial cells from free oxygen radicals by reducing lipid peroxidation and enhancing the activity of antioxidant enzymes and radical scavenging [67]. Therefore, hawthorn leaf flavonoids may be used for the prevention of myocardial injury induced by oxidative stress.
4.2.3. Anemarrhenoside
Anemarrhenoside is a steroidal saponin monomer extracted from the dried rhizome of Anemarrhena asphodeloides, while also being its most abundant component [68–70]. Saponins E-I, E-II, B-II, B-III, and A-III of Anemarrhena asphodeloides can promote the production of SOD. Anemarrhenosides E-I and E-II can also inhibit the expression of prooxidative stress proteins and the abnormal aggregation of platelets [71]. It is reported that 35 different metabolites related to oxidative stress can be found in the H2O2-induced oxidative stress injury model of PC12 cells, and Anemarrhenoside B-II may play a protective role against oxidative stress by decreasing the formation of free radicals via regulation of oxidative stress-related metabolites [72]. In addition, Anemarrhenoside A-III has been reported to regulate the formation of ROS in cells and increase SOD and catalase (CAT) in a concentration-dependent manner, thus regulating intracellular oxidative homeostasis [73].
4.2.4. Hesperidin
Hesperidin is a flavonoid widely found in lemon or citrus fruits and has strong antioxidant activity [74]. Hesperidin has been reported to have a wide range of pharmacological effects, such as regulation of lipid metabolism abnormalities, protection of cardiovascular endothelial cells, antioxidant activity, and regulation of autophagy [75, 76]. Research shows that hesperidin can inhibit oxidative stress by regulating Nrf2/ARE/HO-1 and TGF-beta1/Smad3 signal transduction [77]. The Nrf2/ARE-HO-1 axis mediated by ERS-PERK signaling is a new target for the treatment of myocardial ischemia-reperfusion injury [78]. Moreover, hesperidin can inhibit autophagy by activating the PI3K/Akt/mTOR signaling pathway, contributing to its myocardial protective effect on ischemia-reperfusion injury. Hesperidin's mechanism of action is related to the inhibition of oxidative stress [79].
4.3. Hypertension
Hypertension is a clinical syndrome characterized by high blood, which may be accompanied by functional damage of the heart, brain, and kidney [80, 81]. It has been proven that ROS play an important role in the pathophysiological process of hypertension. Increased oxidative stress is an important mediator of endothelial damage in hypertension, which is related to the increased synthesis of oxidants, such as hydrogen peroxide (H2O2) and nitric oxide (NO), and decreased antioxidant bioavailability [82, 83]. During hypertension, plasma myeloperoxidase levels and oxidative stress are significantly increased. Treatment with antioxidants inhibiting NADPH oxidase and ROS can effectively prevent the abnormal increase in blood pressure [84, 85].
ROS, as regulatory signaling molecules, can regulate the endothelial function of blood vessels as well as the relaxation and growth of vascular smooth muscle cells. In addition, ROS can stimulate cells to produce growth factor-like cell responses [86, 87]. MAPK signal transduction is a major mechanism that mediates vascular damage in hypertension. ROS-induced oxidative stress can inhibit the activity of tyrosine phosphatase and enhance the activity of MAPKs. Overexpression of MAPKs causes the abnormal activation of NF-κB and HIF-1α, induces vascular damage caused by lipid peroxidation, and thus aggravates the vascular remodeling, which occurs during hypertension [88–90]. Recent studies have found that polyphenols in medicinal plants can slow down the progress of lipid peroxidation-induced vascular damage by regulating oxidative stress. These observations provide new options for the treatment of hypertension.
4.3.1. Resveratrol
Resveratrol is a natural polyphenol present in peanuts, wine, mulberries, and other plants. It is an antioxidant compound that may be used to prevent and treat CVD [91–93]. Resveratrol can scavenge free oxygen radicals in the body [94], and studies have reported that it can prevent and treat hypertension by inhibiting oxidative stress [95, 96].
Resveratrol inhibits the formation of oxygen free radicals and reduces oxidative stress and blood pressure by enhancing the ability of redox proteins to alter the redox environment of cells [97]. Moreover, resveratrol can increase the expression of endothelial nitric oxide synthase (eNOS) by activating SIRT1 [98] and can effectively inhibit the uncoupling of eNOS and the generation of superoxide radicals through the inhibition of oxidative stress and ROS formation, thus maintaining normal vascular function and reducing blood pressure. The interaction between resveratrol and SIRT1 can also inhibit the expression of the angiotensin-II receptor, hindering vasoconstriction caused by angiotensin-II, resulting in blood vessel relaxation and a lower blood pressure [99].
4.3.2. Tea Polyphenols
Tea is a traditional drink in China and one of the most popular drinks in the world. Tea polyphenols, the most important bioactive components of tea, also have beneficial effects for the prevention and treatment of hypertension [20]. Tea polyphenols can enhance endogenous SOD activity, inhibit lipid peroxidation, increase ATP levels, and inhibit the formation of free radicals [100].
Tea polyphenols have no regulatory effect on normal blood pressure but can significantly reduce the abnormal increases in blood pressure. The underlying mechanism is related to the increase in antioxidant enzyme activity and the decrease in oxidative stress [101]. It has been reported that intravenous injection of tea polyphenols can reduce blood pressure in rats with acute hypertension while also effectively inhibiting ROS formation, downregulating homocysteine-metabolizing enzymes and related metabolites in the rat aorta, and effectively reducing hypertension [102].
4.3.3. Saponins of Panax notoginseng
Saponins of Panax notoginseng (SPN) have significant antioxidant effects on hypertension [103]. SPN can inhibit the formation of free oxygen radicals and regulated erythrocyte rheology in patients with hypertension. SPN can also significantly reduce MDA, increase the expression of SOD, increase the deformability, and reduce the aggregation of red blood cells [104]. Panax notoginseng, a medicinal plant, also contains ginsenoside Rb3, which can increase the endothelium-dependent relaxation of spontaneously hypertensive rats in vitro. The regulatory pathways behind this mechanism are related to antioxidant signaling [105]. SPN can also reduce the content of LPO in the brain and blood, allowing for enhanced resistance against aging and oxidative stress. Further, it increases the levels of GSH and CAT in serum, enhancing antioxidant defenses and lowering blood pressure [106]. Thus, SPN represents a novel option for the treatment of hypertension.
4.3.4. Berberine
Berberine is a natural extract from Rhizoma coptidis. It is reported that berberine has therapeutic effects on a variety of CVDs. Further, berberine has antioxidant, anti-inflammatory, antiatherosclerosis, and antihypertensive effects [107–109].
The antihypertensive activity of berberine is due to inhibiting the activity of cholinesterase, thus activating the M-receptor on vascular endothelial cells and promoting the release of the vasodilator NO from endothelial cells, which results in peripheral vascular smooth muscle relaxation. This mechanism may also contribute to the antioxidant effect of berberine [110, 111]. In addition, it has been reported that berberine can regulate AMPK signaling and inhibit the overexpression of p-mTOR. Further, berberine can reduce the levels of CRP, TNF-α, and IL-6 in plasma. This extract is also able to reduce myocardial autophagy and apoptosis through the AMPK/mTOR pathway, thus alleviating myocardial injury [21]. In conclusion, berberine may be used to regulate blood pressure and prevent myocardial injury.
4.3.5. Allicin
Allicin is a sulfur-containing compound extracted from the bulb of Allium (Liliaceae). Allicin is modified by alliinase and has strong hydrophobicity. It can quickly reach the intracellular space through the cell membrane [112, 113]. Allicin can exert an antioxidant effect by scavenging free radicals, reducing reactive oxygen species, inducing glutathione production, and regulating NOS. It has also been described as a potential drug for the prevention and treatment of hypertension [114, 115].
Studies have reported that allicin can strongly inhibit the formation of ROS, reduce H2O2-induced apoptosis, and increase SOD and NO levels, as well as the expression of eNOS. It is suggested that allicin protects vascular endothelial function through its antioxidant activity, thus reducing vascular endothelial damage caused by oxidative stress [116]. Studies have revealed that allicin can reduce the vascular response to angiotensin-II, downregulate the expression of AT1R/KEAP1, increase the expression of Nrf2, upregulate antioxidant enzymes, reduce oxidative stress, and relieve the high tension of blood vessels [114]. Therefore, allicin may be another promising agent for treating hypertension.
4.3.6. Curcumin
Curcumin is a polyphenol compound extracted from the rhizome of a turmeric plant. Curcumin has anti-inflammatory, antioxidant, antifibrosis, and antitumor pharmacological activities [117–119]. Experimental studies have demonstrated curcumin's strong antioxidant effect. Curcumin inhibits oxidative stress by reducing the formation of peroxides in blood vessels, reduces vascular resistance, restores vascular reactivity, and inhibits the occurrence and development of hypertension [120]. Curcumin can also inhibit H/R-induced apoptosis and autophagy in H9c2 cardiomyocytes by upregulating Bcl-2 and inhibiting the expression of Bax, BECN1, BNIP3, and SIRT1 [22]. Curcumin regulates autophagy by inhibiting PI3K-AKT-mTOR signal transduction, promoting the dissociation of BECN1 and Bcl-2, preventing FOXO1 acetylation, and reducing oxidative stress, thus protecting vascular endothelial cell function and controlling blood pressure [121].
4.4. Heart Failure
Heart failure is considered the end-stage of various heart diseases, and cardiomyocyte apoptosis caused by oxidative stress has been described as the most important factor in heart failure [122]. Since the discovery of SOD in 1969, animal experiments and clinical trials have strongly supported the close relationship between oxidative stress and heart failure. Antioxidant drugs can prevent some of the pathological processes leading to heart failure, including cardiac hypertrophy, cardiomyocyte apoptosis, and ischemia-reperfusion injury [123]. Research by Liu et al. revealed that during the stage of compensatory cardiac hypertrophy, SOD and GSH-PX levels increased, LPO decreased, animal blood flow was stable, and the enhanced activity of the endogenous antioxidant system could effectively resist damage induced by exogenous ROS and reperfusion [124, 125]. It has been reported that in animal models of compensatory cardiac hypertrophy, the production of ROS by NADPH oxidases will gradually increase to a peak, which is at the level of heart failure decompensation [126].
Research has also reported that the increase in oxidative stress is related to the increase in autophagy during heart failure following pressure overload. In H9c2 cardiomyocytes, high concentrations of H2O2 increased autophagy. Therefore, the autophagy and oxidative stress may contribute to heart failure after chronic pressure overload [122]. Active components of CHM can regulate oxidative stress and autophagy and, thus, may be helpful in the treatment of heart failure [127, 128].
4.4.1. Astragaloside IV
Astragaloside IV (As-IV) is a natural saponin purified from Astragalus membranaceus. As an exogenous antioxidant, As-IV can significantly protect myocardial cells and mitochondria during the process of heart failure [129–131]. This protective effect is mainly achieved by an increase in the reserve respiratory capacity of cardiomyocytes and mitochondrial ATP after oxidative stress injury, as well as through the increased activity of T-SOD, GSH-Px, and CAT in cardiomyocytes [132, 133]. As-IV can also improve the metabolic rate of cardiomyocytes, reduce the release of MDA and NOS, and inhibit the generation of free oxygen radicals, alleviating damage to the membrane of cardiomyocytes and thus improving their viability [134].
As-IV has also been reported to inhibit ROS and NADPH production by upregulating PGC-1α and TFAM, as well as to promote mitochondrial autophagy and mitochondrial biogenesis, contributing to the protection of damaged mitochondria through its antioxidant activity [135]. As-IV can also reduce the activities of CPK and LDH, reduce the loss of mitochondrial membrane potential, and ultimately slow down cardiomyocyte apoptosis [136–138].
4.4.2. Tetramethylpyrazine
Tetramethylpyrazine (TMP) is the main active alkaloid ingredient of Ligusticum [139, 140]. TMP can reverse the PI3K/Akt signal pathway inactivation caused by hypoxia and reduce oxidative stress-induced cardiomyocyte apoptosis by downregulating miR-499a and upregulating SIRT1 signaling [141]. Oxidative stress induced by oxygen deficiency after heart failure causes great damage to cardiomyocytes, and TMP can directly enhance myocardial protection by reducing oxidative damage. Further, it can also inhibit cardiomyocyte apoptosis by regulating the expression of apoptosis-related proteins such as Bcl-2, Bax, and caspase-3 and the NF-κB pathway [142].
Recent studies show that TMP could relieve vascular tension and counteract oxidative stress by scavenging ROS, downregulating ERK1/MAPK signaling, and inhibiting NF-κB. TMP can also protect vascular endothelial cells from H2O2-induced injury by increasing the content of phosphatidylcholine, reducing the release of arachidonic acid, and inhibiting the phosphorylation of cytosolic phospholipase A [143, 144]. TMP, as an NADPH oxidase inhibitor and ROS scavenger, may be a potential antioxidant drug for the treatment of heart failure [145].
4.4.3. Gastrodin
Gastrodin is a glucoside extracted from the rhizome of Gastrodia elata Blume [146]. It was found that gastrodin could inhibit the formation of and scavenge oxygen radicals as well as inhibiting LPO in the myocardium during the decompensated stage of heart failure [147, 148]. Further, gastrodin was found to inhibit oxidative stress by activating ERK1/2 signaling and reducing GSK-3β overexpression [149].
Gastrodin can also mitigate myocardial injury caused by myocardial ischemia-reperfusion and improve the morphology of damaged myocardial tissue. These effects were related to the enhancement of SOD-mediated inhibition of oxidative stress [150]. In addition, during myocardial ischemia-reperfusion, free oxygen radical production occurs along with the outflow of potassium ions, resulting in the overexpression of inflammatory cytokines and subsequent injury of myocardial cells. Reduced numbers of inflammatory and red blood cells in the interstitial space were observed in myocardial ischemia-reperfusion injury after gastrodin pretreatment. Further, the degree of myocardial cell damage was also lower, which may be related to the antioxidant mechanism of gastrodin [151].
4.4.4. Safflower
Safflower is an extract of Crocus sativus L. and is commonly used in the treatment of CVD. Clinical studies have shown that safflower has antioxidant and antiarrhythmic effects, as well as protective effects on damaged myocardium [152–154]. Thus, safflower is an antioxidant with potential for the prevention and treatment of heart failure. It has been reported that safflower can significantly inhibit the overexpression of proapoptotic genes Bad and Bax by inducing autophagy. Further, safflower treatment reversed apoptosis induced by angiotensin-II (Ang-II) in H9c2 cells [155]. Safflower also inhibited H2O2-induced oxidative stress injury by upregulating Nrf2/HO-1/NADPH/NQO1 signaling and Akt phosphorylation [156]. Therefore, safflower may have potential for the treatment and prevention of heart failure through its antioxidant and antiapoptotic activities.
4.4.5. Ferulic Acid
Ferulic acid (FA) is a phenolic acid found in Angelica sinensis, chuanxiong, and other medicinal plants. FA has strong antioxidant capacity and not only inhibits free radical production but also downregulates free radical activity [157]. It has been found that FA can reduce the myocardial infarction area and LDH, CK, and cardiac troponin levels in a dose-dependent manner. FA protects myocardial tissue by activating PI3K/Akt/mTOR signaling and restoring autophagic flux, as well as through its antioxidant activity [158]. It can also increase the expression of Beclin-1/LC3-II and ATG5, while protecting cardiomyocytes from caspase-dependent and caspase-independent apoptosis by activating HSP70 and Bcl-2 [159]. At the same time, FA can counteract excessive ROS production and induce autophagy, thus inhibiting cell apoptosis [160]. Therefore, the antiapoptotic effect of FA may be mediated by its antioxidant and autophagy-inducing activities.
4.5. Arrhythmia
Arrhythmia is caused by abnormal sinoatrial node activity. Studies have shown that the mechanism of arrhythmia is closely related to oxidative stress [161]. Slow activation of the sinus node caused by oxidative stress or abnormal conduction can lead to arrhythmia [162]. Studies have shown that ROS, MDA, and other oxidative stress markers in the serum of patients with tachyarrhythmia were significantly increased, while the expression of SOD, TAC, GSH, and other antioxidant markers was decreased. In addition, oxidative stress can promote the occurrence of atrial fibrillation, a vicious circle that will eventually lead to the aggravation of arrhythmia symptoms [163]. These observations suggest that oxidative stress plays an important role in the pathogenesis of arrhythmia. At present, traditional antioxidants cannot achieve the desired therapeutic effect. However, active components derived from medicinal plants can regulate the heart rate by inhibiting oxidative stress, suggestive of their potential as an antiarrhythmic drug in the future.
4.5.1. Paeonol
Paeonol is an extract of the rhizome of Paeonia suffruticosa, which has antibacterial, anti-inflammatory, and antioxidant effects [164, 165]. Paeonol can prevent the occurrence of arrhythmia, shorten the duration of atrial fibrillation or the conduction block, and protect against ischemia-reperfusion myocardial injury. It has been reported that ischemia-reperfusion causes an increase in free radicals, a decrease in SOD activity, and an increase in MDA content, in parallel to accelerated myocardial injury and increased instability of cardiac bioelectrical conduction, leading to arrhythmia. However, paeonol can enhance SOD scavenging of endogenous radicals and reduce LPO levels, potentially exerting antiarrhythmic effects and improving myocardial injury. Paeonol can also block calcium channels of cardiomyocytes, inhibit the transient outward potassium current, selectively block the fast sodium channel, reduce the range of phase 0 depolarization, and shorten the time course of action potential in ventricular muscle [166]. These outcomes may be related to paeonol's ability to inhibit oxidative stress.
4.5.2. Matrine
Matrine is a natural extract from Sophora flavescens. It possesses antioxidant, antiviral, and antiarrhythmic activities [167–169]. Matrine can directly inhibit the flow of sodium ions outside the myocardial cell membrane and maintain the normal heart rhythm [170]. It can also reduce cardiomyocyte stress and improve ectopic heartbeats by affecting the potassium and sodium ion transfer system at the cardiomyocyte membrane [171]. Research has shown that matrine can prolong the refractory period of the atrium and ventricle by inhibiting oxidative stress, can reduce the excitability of the atrium and the ventricular muscle, and can pace the conduction system. Further, matrine stabilizes the heart rhythm by inhibiting oxidative stress and increasing endogenous antioxidant activity, thus protecting the structure and function of mitochondria in cardiomyocytes [172, 173].
4.6. Acute Myocardial Infarction
Acute myocardial infarction (AMI) is a disease with high mortality and is caused by persistent ischemia and hypoxia of the coronary artery. Early reperfusion following myocardial infarction is the most essential form of treatment. However, when blood supply is restored, excessive free oxygen radicals will damage tissues leading to ischemia-reperfusion injury. Studies have shown that myocardial ischemia-reperfusion injury is closely related to oxidative stress and myocardial autophagy. Autophagy can protect cardiomyocytes from ischemia and accelerate cell death during reperfusion [174, 175]. Moreover, trimetazidine treatment can reduce the oxidative stress and autophagic flux induced by acute myocardial infarction, thus reducing infarction size. Studies have indicated that cardiomyocytes, oxidative stress, and autophagy are involved in the pathological process of AMI and ischemia-reperfusion injury. In AMI, the increase in ROS/autophagy and the decrease in SOD can enhance oxidative stress and aggravate myocardial injury. The induction of autophagy may be related to the activation of the ROS-ATM-LKB1-AMPK signal axis [176]. This axis could represent a new therapeutic target in the treatment of AMI through CHM active components.
4.6.1. Astragalus Polysaccharides
Astragalus polysaccharides (APS) are water-soluble polysaccharides with biological activity, extracted from Astragalus. APS are increasingly considered potential exogenous antioxidants. It is reported that APS have antioxidant, antiviral, and anti-inflammatory pharmacological effects [177, 178]. APS can remove superoxide anions and hydrogen free radicals, improve the activity of SOD, GPX, and CAT, and reduce the levels of LPO. APS can also reduce cell apoptosis, the production of DHE, cytosolic nitrotyrosine products, and nuclear oxidative stress (8-OH-AD), while reducing ROS generation [179]. APS can also reduce the troponin and creatine phosphokinase, as well as the mRNA expression of Bcl-2, Bax, caspase-3, p53, Apaf-1, and AIF, leading to an improved antioxidant capacity and enhanced protection against cardiac injury caused by myocardial cell apoptosis [180].
4.6.2. Quercetin
Quercetin is one of the most well-known flavonoids. It can form complexes with superoxide anions (O2−) to reduce the production of oxygen radicals and couples with Fe2+ to prevent the formation of Fenton radicals. Quercetin also reduces the consumption of NADPH by inhibiting aldose reductase, improving the body's antioxidant capacity [181]. Quercetin can also scavenge free radicals produced in macrophages, inhibit the oxidation of LDL, protect tocopherol, and regenerate oxidized α-tocopherol [182].
According to a previous report, quercetin can maintain proper ST segment elevation in myocardial infarction model rats, reduce the level of LPO products in the rat serum and heart, and protect the damaged myocardium [183]. Quercetin could reverse the increase in NO, MDA, MPO, and caspase-3 activity, while decreasing GSH and SOD activity in the ischemia-reperfusion group. It has also been suggested that quercetin can alleviate tissue injury induced by AMI through its antioxidant and antiapoptotic effects [184]. Moreover, quercetin protects vascular endothelial cells and reduces blood pressure through antioxidant activity [185]. Thus, quercetin is a potential drug for the treatment of AMI and hypertension in the future.
4.6.3. Tanshinone II-A
Tanshinone II-A is a lipid-soluble phenanthraquinone extracted from the rhizome of Salvia miltiorrhiza. It possesses antioxidant properties, regulates autophagy, and has certain advantages in the protection of myocardial cells [186, 187]. Tanshinone II-A can alleviate oxidative stress injury of H9c2 cells induced by DOX, enhance autophagy in H9c2 cells, and mitigate myocardial injury [188]. Tanshinone II-A exerts its antioxidant effects through NRF-2, reducing DOX cardiotoxicity. These antioxidant properties of tanshinone II-A play an important role in protecting myocardial cells after AMI [189]. Autophagy is a protective mechanism allowing cells within plaques to fight against and resist oxidative stress. An excessive reduction or increase in autophagic activity will affect the extent of oxidative stress-induced damage and atherosclerotic plaque development. Tanshinone II-A can also affect autophagic activity by regulating autophagy genes ATG and LC3, contributing to its antioxidant effects [190].
4.6.4. Gypenoside
Gypenoside (GPS) is a commonly used drug for the prevention and treatment of CVDs [191]. Its benefits include antioxidant and antiatherosclerosis properties, as well as protection of the damaged myocardium. GPS can enhance the antioxidant capacity of aging rats by increasing SOD activity and can promote c-sis gene expression in endothelial cells, as well as the synthesis and release of NO, while also improving blood circulation of the coronary artery. GPS has also been reported to restore the normal redox state of ox-LDL in HUVECs through antioxidant regulation via PI3K/Akt. Further, GPS upregulated the ratio of Bcl-2 to Bax and inhibited the expression of caspase-3, leading to apoptosis [192]. GPS has protective effects on myocardial ischemia and systolic function in rats and exerts its cardiotonic and central inhibitory effects by inhibiting the activities of Na+/K+-ATPase in the heart [193]. GPS contributes to the resistance of oxygen radical damage to the heart, protects the integrity of the myocardial cell membrane, and supports normal diastolic function of the heart during acute myocardial ischemia [194].
4.6.5. Soybean Isoflavones
Soybean isoflavone (SI) is a bioactive secondary metabolite formed during soybean growth. Studies have shown that SI can protect the cardiovascular system through its antioxidant effects [195–197]. SI enhanced the activity of SOD, decreased the level of thiobarbituric acid reactant in plasma, and enhanced the antioxidant capacity of plasma, as well as the activity of GSH-PX in erythrocytes [198]. Moreover, SI inhibited the production of peroxides and the activity of NADPH oxidase in ischemia injury [199]. Studies have reported that different concentrations of SI can inhibit the apoptosis of vascular endothelial cells in a concentration-dependent manner, thus reducing vascular endothelial damage [200].
4.6.6. Hydroxy Safflower Yellow
Hydroxy safflower yellow (HSYA) is a natural active component of Carthamus tinctorius L. of Compositae, which has significant anticoagulant and antioxidant activity. HSYA reduces the level of LDH and caspase-3 in the heart of ischemia-reperfusion injury models, indicative of lower apoptosis rates [201]. HSYA can also inhibit the apoptosis of myocardial cells after AMI by increasing the level of Bcl-2/Bax [202], improve the dysfunction of mitochondrial energy metabolism by activating the PI3K/Akt signaling pathway, and play a protective role for the myocardium [203]. HSYA is a water-soluble antioxidant active component, capable of clearing oxygen radicals and inhibiting LPO generation, thus protecting the myocardial cell membrane. It has also been found that HSYA has a chalcone structure and various phenolic hydroxyl groups. HSYA's antioxidant effect may be related to the action of these phenolic hydroxyl groups [204]. HSYA can also increase the expression of NADPH and NQO1, increase the phosphorylation of Akt, and inhibit H2O2-induced oxidative stress injury by activating Nrf2/HO-1 [156]. Overall, there is a growing body of evidence for the antioxidant properties of HSYA.
5. Discussion
In the current review, the effects and mechanisms of action of the active components of 27 natural extracts in various CVDs were discussed with regard to oxidative stress. Previous studies found that oxidative stress regulation by these active components is achieved through a variety of pathways. Evidence shows that these active ingredients could be used for therapy in the future. However, further experimental studies are needed to elucidate the exact molecular mechanisms of components before their implementation in clinical use. Overall, compounds derived from medicinal plants work through various mechanisms to counteract oxidative stress.
The therapeutic efficacy of natural drugs and their active ingredients has been reported not only in cell and animal models but also in the clinic. Recent clinical studies have found that SI has therapeutic effects on heart failure caused by ischemic cardiomyopathy (IC). SI was shown to increase the expression levels of Nrf2 and SOD and to reduce the expression of C-reactive protein, 8-isopropanol, MDA, IL-6, and TNF-α in patients with heart failure, improving the antioxidant capacity of patients with IC by upregulating Nrf2 and treating heart failure [205].
Chekalina et al., through a clinical study of 85 patients with coronary heart disease, found that quercetin can adjust the central hemodynamic parameters of patients with stable coronary heart disease and can improve myocardial ischemia [206]. The results of previous clinical studies have shown an improvement in left ventricular systolic function and left ventricular ejection fraction (EF) of patients, after two months of quercetin treatment. These clinical studies suggest that quercetin had cardioprotective effects in patients with coronary heart disease.
Clinical studies have also found that resveratrol had therapeutic effects on central hemodynamic parameters and myocardial ischemia in patients with stable coronary heart disease. The studies showed that the left ventricular systolic and diastolic function and the left ventricular ejection fraction (EF) were improved in patients with coronary heart disease, after resveratrol treatment. The phase ratio of conduction blood flow E/A also improved, and it was dominant in the research group. After resveratrol treatment, the DT value decreased significantly along with the number of PACs and PVCs. This research suggested that resveratrol has a significant protective effect on the heart in clinical settings [207].
Recent studies have also shown that the active ingredients of natural drugs combined with other drugs can result in synergistic therapeutic effects. A randomized controlled trial (RCT) found that tanshinone II-A sodium sulfonate injection (STS) can be used to treat CHD. The results of the study showed that the adjuvant treatment with STS significantly reduced the incidence of cardiac shock, heart failure, and arrhythmia, with no serious adverse events related to STS. STS combined with conventional medication is more effective than conventional medication alone, with fewer side effects [208].
Zhang et al. found that trimetazidine combined with berberine can have a therapeutic effect on endothelial function in patients with CHD and essential hypertension (CCP) [209]. eNOS mRNA expression (P < 0.05), NO level (50.75 ± 2.75 mol/l) (P < 0.05), and FMD value (14.02 ± 2.39) were significantly higher after the combination treatment than before the treatment (P < 0.05). The results suggested that the combination of trimetazidine and berberine increased blood NO content, promoted the endothelium-dependent relaxation function of the brachial artery, and helped treat CCP.
In summary, since the multilevel, multichannel, and multitarget action of natural medicines can effectively reduce the side effects of single-chain action, there is great potential for clinical efficacy. However, most of the research on the targeted treatment of cardiovascular diseases using natural medicines and active ingredients has been conducted in cell and animal experiments, and only a few clinical studies exist. A better understanding of the pathology of cardiovascular diseases and the pharmacological effects of natural drugs and active ingredients could aid future large-scale clinical research studies on these drugs and their targets.
Recently, the clinical application of Chinese medicine has attracted considerable attention. Traditional Chinese medicine contains a variety of active ingredients with antioxidant effects. Moreover, the active ingredients show network synergy or antagonistic effects. The clinical compatibility and application of traditional Chinese medicine follow the traditional Chinese medicine theories, and various active ingredients contained in different traditional Chinese medicines have different degrees of synergy and antagonism [210]. The interaction among several active ingredients will affect the absorption, metabolism, efficacy, and toxicity of other active ingredients. For example, the compound Huangdai is used to treat granulocytic leukemia. The main ingredients of the prescription are tetraarsenic tetrasulfide, indirubin, and tanshinone II-A. Studies have found that the combination of these three drugs can synergistically enhance anticancer activity compared with each drug alone or in combination of two drugs [211]. Danshen dripping pills and Shensongyangxin capsules commonly used in the clinical treatment of cardiovascular diseases contain Chinese herbal medicines such as ginseng, Danshen, Panax notoginseng, and Ophiopogon japonicus. The active ingredients of these drugs are ginsenosides, tanshinones, and total saponins of notoginseng, each of which can regulate oxidative stress. When combined, these active ingredients work together to produce a synergistic effect [212, 213].
Chinese medicine is complex, with diverse chemical components affecting organisms through various biological reactions. This diversity determines whether the effects of the different active ingredients in Chinese medicines will be synergistic, additive, and antagonistic. However, it is yet unclear which combinations of active ingredients have synergistic effects and antagonistic effects or which combinations may increase toxicity. At the effect level, avoid invalid and negative action modes, and which active ingredients will strengthen the primary pharmacological effect and which will negate it, as well as effective/optimal doses of each active ingredient in the combinations, need to be determined. The similarities and differences between the antioxidant mechanisms of medicinal plants and the mechanisms by which they exert therapeutic effects on the human cardiovascular system are also worth discussing. One of the advantages of medicinal plants is the synergistic action of multiple plant chemical components. Williamson first proposed the synergistic effect of natural extracts in 2001 [214]. Synergistic effect refers to multiple pathways being simultaneously targeted and engaged. There are various pathways and targets involved in oxidative stress signaling, and natural drugs or active ingredients often act on multiple pathways and targets for the effective treatment of the disease. “Synergy multitarget” and “antagonistic multitarget” may explain the effects observed from the combination of the active ingredients in traditional Chinese medicine.
In order to elucidate the mechanism of action of traditional Chinese medicine, the pathways and targets acted upon by each active ingredient alone and in different combinations need to be studied. It is also necessary to find a suitable entry point and establish a reasonable pharmacological model based on multiple omics researches such as genomics, proteomics, and metabolomics. Determining the mechanisms of action of the active ingredients can not only explain how the various ingredients in Chinese medicines function alone or in combination but more importantly may lead to the discovery of new mechanisms of action and synergistic effects of the active ingredients and lay a foundation for the innovation of Chinese medicine and the development of Chinese medicine theory.
6. Conclusions
Medicinal plants are used to treat cardiovascular disease through their antioxidant properties, and remarkable effects have been reported. The current review summarized the mechanisms of active plant components in the treatment of CHD, hypertension, heart failure, ischemia-reperfusion, and arrhythmia. The dosing and timing of active component administration require further study. Future research should also investigate the synergistic effects of multiple bioactive plant components. In addition, large-scale clinical studies should be conducted to confirm the clinical effectiveness and safety of natural medicines and their effective active ingredients.
Acknowledgments
This work was funded by the Key Project at Central Government Level: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (2060302).
Contributor Information
Zhenyu Zhao, Email: zhaozhenyu9607@163.com.
Jiahui Sun, Email: sunjh_2010@sina.com.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Mamani-Ortiz Y., San Sebastián M., Armaza A. X., et al. Prevalence and determinants of cardiovascular disease risk factors using the WHO STEPS approach in Cochabamba, Bolivia. Bmc Public Health. 2019;19(1):p. 786. doi: 10.1186/s12889-019-7064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth G. A., Forouzanfar M. H., Moran A. E., et al. Demographic and epidemiologic drivers of global cardiovascular mortality. New England Journal of Medicine. 2015;372(14):1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I., Williams K. J., Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang C., Wang X., Hu J., et al. PTPRO promotes oxidized low-density lipoprotein induced oxidative stress and cell apoptosis through toll-like receptor 4/nuclear factor κB pathway. Cellular Physiology and Biochemistry. 2017;42(2):495–505. doi: 10.1159/000477596. [DOI] [PubMed] [Google Scholar]
- 6.Wu G. S., Li H. K., Zhang W. D. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine. Chinese Journal of Natural Medicines. 2019;17(5):321–330. doi: 10.1016/S1875-5364(19)30037-8. [DOI] [PubMed] [Google Scholar]
- 7.Thomford N., Senthebane D., Rowe A., et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. International Journal of Molecular Sciences. 2018;19(6):p. 1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calixto J. B. The role of natural products in modern drug discovery. Anais da Academia Brasileira de Ciencias. 2019;91(supplement 3) doi: 10.1590/0001-3765201920190105. [DOI] [PubMed] [Google Scholar]
- 9.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 10.Newman D. J., Cragg G. M. Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products. 2015;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 11.Slovinski A. P., Hajjar L. A., Ince C. Microcirculation in cardiovascular diseases. Journal of Cardiothoracic and Vascular Anesthesia. 2019;33(12):3458–3468. doi: 10.1053/j.jvca.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Hao D. C., Xiao P. G. Impact of drug metabolism/pharmacokinetics and their relevance upon traditional medicine-based cardiovascular drug research. Current Drug Metabolism. 2019;20(7):556–574. doi: 10.2174/1389200220666190618101526. [DOI] [PubMed] [Google Scholar]
- 13.Hao P. P., Zhang Y. Clinical trials of integrated Chinese and Western medicine in treating cardiovascular disease: the past and the future. Zhonghua Xin Xue Guan Bing Za Zhi. 2019;47(9):697–702. doi: 10.3760/cma.j.issn.0253-3758.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y. Q., Jiang A. L., Chen S. M., Li H., Xing H. Y., Wang F. Quality of life and self-care in elderly patients with cardiovascular diseases: the effect of a Traditional Chinese Medicine health educational intervention. Applied Nursing Research. 2017;38:134–140. doi: 10.1016/j.apnr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Xiao J. B., Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Current Medicinal Chemistry. 2015;22(1):23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 16.Jian X., Liu Y., Zhao Z., Zhao L., Wang D., Liu Q. The role of traditional Chinese medicine in the treatment of atherosclerosis through the regulation of macrophage activity. Biomedicine & Pharmacotherapy. 2019;118 doi: 10.1016/j.biopha.2019.109375. [DOI] [PubMed] [Google Scholar]
- 17.Hong X. The main components of antioxidant activity of traditional Chinese medicine and their free radical scavenging effect. Foreign medicine (traditional Chinese Medicine Division) 2003;3:150–153. [Google Scholar]
- 18.An F., Wang S., Yuan D., Gong Y., Wang S. Attenuation of oxidative stress of erythrocytes by plant-derived flavonoids, orientin and luteolin. Evidence-Based Complementary and Alternative Medicine. 2016;2016:8. doi: 10.1155/2016/3401269.3401269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H. T., Kim J. H., Heo W., et al. Explosively puffed ginseng ameliorates ionizing radiation-induced injury of colon by decreasing oxidative stress-related apoptotic cell execution in mice. Journal of Medicinal Food. 2019;22(5):490–498. doi: 10.1089/jmf.2018.4293. [DOI] [PubMed] [Google Scholar]
- 20.Chei C. L., Loh J. K., Soh A., Yuan J. M., Koh W. P. Coffee, tea, caffeine, and risk of hypertension: the Singapore Chinese Health Study. European Journal Of Nutrition. 2018;57(4):1333–1342. doi: 10.1007/s00394-017-1412-4. [DOI] [PubMed] [Google Scholar]
- 21.Qing Y., Dong X., Hongli L., Yanhui L. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomedicine & Pharmacotherapy. 2018;105:1050–1053. doi: 10.1016/j.biopha.2018.06.088. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z., Ye B., Dai Z., et al. Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes. Molecular Medicine Reports. 2015;11(6):4678–4684. doi: 10.3892/mmr.2015.3322. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzon dos Santos J., de Quadros A. S., Weschenfelder C., Garofallo S. B., Marcadenti A. Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients. 2020;12(3) doi: 10.3390/nu12030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shokr H., Dias I., Gherghel D. Microvascular function and oxidative stress in adult individuals with early onset of cardiovascular disease. Scientific Reports. 2020;10(1):p. 4881. doi: 10.1038/s41598-020-60766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata K. Prevention of endothelial dysfunction and cardiovascular disease by n-3 fatty acids-inhibiting action on oxidative stress and inflammation. Current Pharmaceutical Design. 2020;26(30):3652–3666. doi: 10.2174/1381612826666200403121952. [DOI] [PubMed] [Google Scholar]
- 26.Suen J., Thomas J., Kranz A., Vun S., Miller M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: a systematic review. Healthcare. 2016;4(3):p. 69. doi: 10.3390/healthcare4030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Rawi N. H., Shahid A. M. Oxidative stress, antioxidants, and lipid profile in the serum and saliva of individuals with coronary heart disease: is there a link with periodontal health? Minerva Stomatol. 2017;66(5):212–225. doi: 10.23736/S0026-4970.17.04062-6. [DOI] [PubMed] [Google Scholar]
- 28.Pignatelli P., Menichelli D., Pastori D., Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiologia Polska. 2018;76(4):713–722. doi: 10.5603/KP.a2018.0071. [DOI] [PubMed] [Google Scholar]
- 29.Bellanti F., Romano A. D., Lo Buglio A., et al. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas. 2018;109:6–12. doi: 10.1016/j.maturitas.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Banks W. A. A spectrum of topics for 2019: advances in neuroinflammation, oxidative stress, obesity, diabetes mellitus, cardiovascular disease, autism, exosomes, and central nervous system diseases. Current Pharmaceutical Design. 2020;26(1):1–5. doi: 10.2174/138161282601200225102049. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Rodríguez P., Ramiro-Cortijo D., Reyes-Hernández C. G., López de Pablo A. L., González M. C., Arribas S. M. Implication of oxidative stress in fetal programming of cardiovascular disease. Frontiers in Physiology. 2018;9:p. 602. doi: 10.3389/fphys.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C., Huang Y. Chinese herbal medicine on cardiovascular diseases and the mechanisms of action. Frontiers in Pharmacology. 2016;7 doi: 10.3389/fphar.2016.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., He T., Han S., et al. The role of traditional chinese medicine in the regulation of oxidative stress in treating coronary heart disease. Oxidative Medicine and Cellular Longevity. 2019;2019:13. doi: 10.1155/2019/3231424.3231424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S.-Y., Gao Y., Sun J.-Y., et al. Traditional chinese medicine: role in reducing β-Amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer's disease. Frontiers in Pharmacology. 2020;11:p. 497. doi: 10.3389/fphar.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senoner T., Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11(9):p. 2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B., Xu B., Zhao H., et al. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Molecular Medicine Reports. 2018;18(1):973–980. doi: 10.3892/mmr.2018.9024. [DOI] [PubMed] [Google Scholar]
- 37.Farrokhian A., Raygan F., Soltani A., et al. The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob Proteins. 2019;11(1):133–142. doi: 10.1007/s12602-017-9343-1. [DOI] [PubMed] [Google Scholar]
- 38.Patel J., al Rifai M., Scheuner M. T., et al. Basic vs more complex definitions of family history in the prediction of coronary heart disease: the multi-ethnic study of atherosclerosis. Mayo Clinic Proceedings. 2018;93(9):1213–1223. doi: 10.1016/j.mayocp.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ala-Korpela M. The culprit is the carrier, not the loads: cholesterol, triglycerides and apolipoprotein B in atherosclerosis and coronary heart disease. International Journal of Epidemiology. 2019;48(5):1389–1392. doi: 10.1093/ije/dyz068. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Mehta J. L. Role of oxidative stress in coronary heart disease. Indian heart journal. 2004;56(2):163–173. [PubMed] [Google Scholar]
- 41.van Rosendael A. R., Bax J. J., Arbab-Zadeh A. Noninvasive assessment of coronary atherosclerosis by cardiac computed tomography for risk stratifying patients with suspected coronary heart disease. Journal of cardiovascular computed tomography. 2019;13(5):235–241. doi: 10.1016/j.jcct.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Yang X., Li Y., Li Y., et al. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Frontiers in Physiology. 2017;8:p. 600. doi: 10.3389/fphys.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juni R. P., Duckers H. J., Vanhoutte P. M., Virmani R., Moens A. L. Oxidative stress and pathological changes after coronary artery interventions. Journal Of The American College of Cardiology. 2013;61(14):1471–1481. doi: 10.1016/j.jacc.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 44.Cervantes G. K., Llanas-Cornejo D., Husi H. CVD and oxidative stress. Journal of Clinical Medicine. 2017;6(2) doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed T., Raza S. H., Maryam A., et al. Ginsenoside Rb1 as a neuroprotective agent: a review. Brain Research Bulletin. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Li S., Tang S. H., Liu J. L., Su J., He F. Y. Ginseng prescription rules and molecular mechanism in treating coronary heart disease based on data mining and integrative pharmacology. China journal of Chinese materia medica. 2018;43(7):1303–1309. doi: 10.19540/j.cnki.cjcmm.20180115.006. [DOI] [PubMed] [Google Scholar]
- 47.El‐Bialy B. E. S., Abd Eldaim M. A., Hassan A., Abdel‐Daim M. M. Ginseng aqueous extract ameliorates lambda-cyhalothrin-acetamiprid insecticide mixture for hepatorenal toxicity in rats: role of oxidative stress-mediated proinflammatory and proapoptotic protein expressions. Environmental Toxicology. 2020;35(2):124–135. doi: 10.1002/tox.22848. [DOI] [PubMed] [Google Scholar]
- 48.Li C. Y., Yang P., Jiang Y. L., et al. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomedicine & Pharmacotherapy. 2020;125:p. 109913. doi: 10.1016/j.biopha.2020.109913. [DOI] [PubMed] [Google Scholar]
- 49.Chen S., Li X., Wang Y., et al. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion‑induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Molecular Medicine Reports. 2019;19(5):3633–3641. doi: 10.3892/mmr.2019.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J. M., Jiang J., Jamaluddin M. S., Liang Z., Yao Q., Chen C. Ginsenoside Rb1 blocks ritonavir-induced oxidative stress and eNOS downregulation through activation of estrogen receptor-beta and upregulation of SOD in human endothelial cells. International Journal of Molecular Sciences. 2019;20(2) doi: 10.3390/ijms20020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jianming T., Zheng S., Guo W., Jinmei Y., Longyun L. Protective effect of ginsenoside Rg2 on myocardial apoptosis induced by myocardial ischemia-reperfusion injury in rats. Chinese Pharmacological Bulletin. 2011;4, article 4802004 [Google Scholar]
- 52.Kang H. J., Huang Y. H., Lim H. W., et al. Stereospecificity of ginsenoside Rg2 epimers in the protective response against UV-B radiation-induced oxidative stress in human epidermal keratinocytes. Journal of Photochemistry and Photobiology B: Biology. 2016;165:232–239. doi: 10.1016/j.jphotobiol.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 53.Qin Q., Lin N., Huang H., et al. Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;Volume 12:1091–1103. doi: 10.2147/DMSO.S208989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y., Chu S. F., Zhang Z., et al. Ginsenoside Rg1 prevents acetaminophen-induced oxidative stress and apoptosisviaNrf2/ARE signaling pathway. Journal of Asian Natural Products Research. 2019;21(8):782–797. doi: 10.1080/10286020.2018.1504024. [DOI] [PubMed] [Google Scholar]
- 55.Xie X., Zhao R., Shen G. X. Influence of delphinidin-3-glucoside on oxidized low-density lipoprotein-induced oxidative stress and apoptosis in cultured endothelial cells. Journal of agricultural and food chemistry. 2012;60(7):1850–1856. doi: 10.1021/jf204461z. [DOI] [PubMed] [Google Scholar]
- 56.Jin X., Chen M., Yi L., et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Molecular Nutrition & Food Research. 2014;58(10):1941–1951. doi: 10.1002/mnfr.201400161. [DOI] [PubMed] [Google Scholar]
- 57.Zeng W. C., Jia L. R., Zhang Y., et al. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. Journal of Food Science. 2011;76(2):C318–C323. doi: 10.1111/j.1750-3841.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- 58.Shi X., Liu D., Zhang J., et al. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complementary and Alternative Medicine. 2016;16(1):p. 245. doi: 10.1186/s12906-016-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Salam S., Hashmi S. Myocardial ischemia reperfusion injury: apoptotic, inflammatory and oxidative stress role of galectin-3. Cellular Physiology and Biochemistry. 2018;50(3):1123–1139. doi: 10.1159/000494539. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Xia Z., Chen Y., Qi D., Zheng H. Mechanism and therapies of oxidative stress-mediated cell death in ischemia reperfusion injury. Oxidative Medicine and Cellular Longevity. 2018;2018:2. doi: 10.1155/2018/2910643.2910643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radical Biology and Medicine. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 62.Jing S. Q., Wang S. S., Zhong R. M., et al. Neuroprotection of Cyperus esculentus L. orientin against cerebral ischemia/reperfusion induced brain injury. Neural Regeneration Research. 2020;15(3):548–556. doi: 10.4103/1673-5374.266063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C., Cai C., Zheng X., Sun J., Ye L. Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Bioscience, Biotechnology, and Biochemistry. 2020;84(4):774–779. doi: 10.1080/09168451.2019.1702871. [DOI] [PubMed] [Google Scholar]
- 64.Liu L., Wu Y., Huang X. Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy. European Journal of Pharmacology. 2016;776:90–98. doi: 10.1016/j.ejphar.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y., Kong X., Yin H., Zhang W., Wang W. Effect of hawthorn leaf flavonoids in dehydroepiandrosterone-induced polycystic ovary syndrome in rats. Pathobiology. 2019;86(2-3):102–110. doi: 10.1159/000493895. [DOI] [PubMed] [Google Scholar]
- 66.Min Q., Bai Y., Zhang Y., et al. Hawthorn leaf flavonoids protect against diabetes-induced cardiomyopathy in rats via PKC-alpha signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2017;2017:8. doi: 10.1155/2017/2071952.2071952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu L., Wang L., Yingfeng Z. Effects of Salvia miltiorrhiza and hawthorn total flavonoids on blood lipid, superoxide dismutase and malondialdehyde in hyperlipidemic rats. Chinese medicine guide. 2014;11(20):9–12. [Google Scholar]
- 68.Wang Z., Cai J., Fu Q., et al. Anti-inflammatory activities of compounds isolated from the rhizome of Anemarrhena asphodeloides. Molecules. 2018;23(10) doi: 10.3390/molecules23102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao Z. D., Xu F. Q., Wu D. L., Zhang W., Huang Q. A new benzophenone isolated from fibrous roots of Anemarrhena asphodeloides. Zhongguo Zhong Yao Za Zhi. 2019;44(7):1392–1396. doi: 10.19540/j.cnki.cjcmm.20190118.004. [DOI] [PubMed] [Google Scholar]
- 70.Liao Z. D., Chen F. F., Xu F. Q., et al. One new benzophenone and one new 1,3-diphenylpropane from the fibrous roots of Anemarrhena asphodeloides Bge. and their cytotoxicity. Natural Product Research. 2019. pp. 1–7. [DOI] [PubMed]
- 71.Kaname N., Zhang J., Meng Z., et al. Effect of timosaponin E1 and E2 on superoxide generation induced by various stimuli in human neutrophils and on platelet aggregation in human blood. Clinica Chimica Acta. 2000;295(1-2):129–140. doi: 10.1016/S0009-8981(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 72.Xie Q., Zhao H., Li N., Su L., Xu X., Hong Z. Protective effects of timosaponin BII on oxidative stress damage in PC12 cells based on metabolomics. Biomedical Chromatography. 2018;32(10):p. e4321. doi: 10.1002/bmc.4321. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Dan Y., Yang D., et al. The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2014;153(1):42–60. doi: 10.1016/j.jep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Karimi N., Monfared A. S., Haddadi G. H., et al. Radioprotective effect of hesperidin on reducing oxidative stress in the lens tissue of rats. International Journal of Pharmaceutical Investigation. 2017;7(3):149–154. doi: 10.4103/jphi.JPHI_60_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dhanya R., Jayamurthy P. In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochemistry and Function. 2020;38(4):419–427. doi: 10.1002/cbf.3478. [DOI] [PubMed] [Google Scholar]
- 76.Welbat J. U., Naewla S., Pannangrong W., Sirichoat A., Aranarochana A., Wigmore P. Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochemical Pharmacology. 2020;178, article 114083 doi: 10.1016/j.bcp.2020.114083. [DOI] [PubMed] [Google Scholar]
- 77.Mahmoud A. M., Mohammed H. M., Khadrawy S. M., Galaly S. R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chemico-Biological Interactions. 2017;277:146–158. doi: 10.1016/j.cbi.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Hu X., Jiang H. ERS-PERK signaling pathway-mediated Nrf2/ARE-HO-1 axis: a novel therapeutic target for attenuating myocardial ischemia and reperfusion injury. International Journal of Cardiology. 2016;203:779–780. doi: 10.1016/j.ijcard.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Li X., Hu X., Wang J., et al. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. International Journal of Molecular Medicine. 2018;42(4):1917–1924. doi: 10.3892/ijmm.2018.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whelton P. K., Campbell N. R., Lackland D. T., et al. Strategies for prevention of cardiovascular disease in adults with hypertension. The Journal of Clinical Hypertension. 2020;22(2):132–134. doi: 10.1111/jch.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McManus R., Constanti M., Floyd C. N., Glover M., Wierzbicki A. S. Managing cardiovascular disease risk in hypertension. Lancet. 2020;395(10227):869–870. doi: 10.1016/S0140-6736(20)30048-9. [DOI] [PubMed] [Google Scholar]
- 82.Miller F. J. Hypertension and mitochondrial oxidative stress revisited: sirtuin 3, the improved "antioxidant". Circulation Research. 2020;126(4):453–455. doi: 10.1161/CIRCRESAHA.120.316567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ou M., Zhao H., Ji G., Zhao X., Zhang Q. Long noncoding RNA MALAT1 contributes to pregnancy-induced hypertension development by enhancing oxidative stress and inflammation through the regulation of the miR-150-5p/ET-1 axis. Faseb Journal. 2020;34(5):6070–6085. doi: 10.1096/fj.201902280R. [DOI] [PubMed] [Google Scholar]
- 84.Kim C. H., Vaziri N. D. Hypertension promotes integrin expression and reactive oxygen species generation by circulating leukocytes. Kidney International. 2005;67(4):1462–1470. doi: 10.1111/j.1523-1755.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 85.Cifuentes M. E., Pagano P. J. Targeting reactive oxygen species in hypertension. Current opinion in nephrology and hypertension. 2006;15(2):179–186. doi: 10.1097/01.mnh.0000214776.19233.68. [DOI] [PubMed] [Google Scholar]
- 86.Lassegue B., Griendling K. K. Reactive oxygen species in hypertension; an update. American Journal of Hypertension. 2004;17(9):852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Dikalov S. I., Nazarewicz R. R., Bikineyeva A., et al. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxidants & redox signaling. 2014;20(2):281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forman H. J., Torres M., Fukuto J. Redox signaling. Molecular and Cellular Biochemistry. 2002;234/235(1):49–62. doi: 10.1023/A:1015913229650. [DOI] [PubMed] [Google Scholar]
- 89.Forman H. J., Ursini F., Maiorino M. An overview of mechanisms of redox signaling. Journal of Molecular And Cellular Cardiology. 2014;73:p. 2-9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuzkaya N., Weissmann N., Harrison D. G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. Journal Of Biological Chemistry. 2003;278(25):22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 91.Xia N., Daiber A., Forstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. British journal of pharmacology. 2017;174(12):1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y. R., Li S., Lin C. C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44(1):69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 93.Hor S. L., Teoh S. L., Lim W. L. Plant polyphenols as neuroprotective agents in Parkinson's disease targeting oxidative stress. Current Drug Targets. 2020;21(5):458–476. doi: 10.2174/1389450120666191017120505. [DOI] [PubMed] [Google Scholar]
- 94.Chong M. F., Macdonald R., Lovegrove J. A. Fruit polyphenols and CVD risk: a review of human intervention studies. British Journal of Nutrition. 2010;104(S3) Supplement 3:S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 95.Tain Y. L., Lee W. C., Wu K., Leu S., Chan J. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Molecular Nutrition & Food Research. 2018;62, article e1800066 doi: 10.1002/mnfr.201800066. [DOI] [PubMed] [Google Scholar]
- 96.Chen H. E., Lin Y. J., Lin I. C., et al. Resveratrol prevents combined prenatal N(G)-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: interplay between nutrient-sensing signals, oxidative stress and gut microbiota. Journal of Nutritional Biochemistry. 2019;70:28–37. doi: 10.1016/j.jnutbio.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Petrovski G., Gurusamy N., Das D. K. Resveratrol in cardiovascular health and disease. Annals of the New York Academy of Sciences. 2011;1215(1):22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 98.Xia N., Strand S., Schlufter F., et al. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Miyazaki R., Ichiki T., Hashimoto T., et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(7):1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 100.Mao X., Gu C., Chen D., Yu B., He J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget. 2017;8(46):81649–81661. doi: 10.18632/oncotarget.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Negishi H., Xu J. W., Ikeda K., Njelekela M., Nara Y., Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. Journal of Nutrition. 2004;134(1):38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 102.San Cheang W., Ngai C. Y., Tam Y. Y., et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Scientific reports. 2015;5(1, article 10340) doi: 10.1038/srep10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang M., Guan Y., Xu J., et al. Evaluating the protective mechanism of panax notoginseng saponins against oxidative stress damage by quantifying the biomechanical properties of single cell. Analytica Chimica Acta. 2019;1048:186–193. doi: 10.1016/j.aca.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 104.Pan C., Huo Y., An X., et al. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vascular Pharmacology. 2012;56(3-4):150–158. doi: 10.1016/j.vph.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y., Dong J., Liu P., et al. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. British journal of pharmacology. 2014;171(13):3171–3181. doi: 10.1111/bph.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y., Yang Z., Wei L., Ma X., Zhenping W. Research progress of saponins in Panax notoginseng and their pharmacological effects. Chinese herbal medicine. 2015;46(9):1381–1392. [Google Scholar]
- 107.Ma X., Chen Z., Wang L., et al. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by berberine. Frontiers in Pharmacology. 2018;9:p. 782. doi: 10.3389/fphar.2018.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cui H. X., Hu Y. N., Li J. W., Yuan K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxidative Medicine and Cellular Longevity. 2018;2018:13. doi: 10.1155/2018/8930374.8930374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Z., Jiang T., Lu Q., et al. Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cellular and Molecular Neurobiology. 2020;40(4):587–602. doi: 10.1007/s10571-019-00756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lan J., Zhao Y., Dong F., et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. Journal of Ethnopharmacology. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 111.Tian H., Kang Y. M., Gao H. L., et al. Chronic infusion of berberine into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation via the ROS/Erk1/2/iNOS pathway. Phytomedicine. 2019;52:216–224. doi: 10.1016/j.phymed.2018.09.206. [DOI] [PubMed] [Google Scholar]
- 112.El-Sheakh A. R., Ghoneim H. A., Suddek G. M., Ammar E. Attenuation of oxidative stress, inflammation, and endothelial dysfunction in hypercholesterolemic rabbits by allicin. Canadian Journal of Physiology and Pharmacology. 2016;94(2):216–224. doi: 10.1139/cjpp-2015-0267. [DOI] [PubMed] [Google Scholar]
- 113.Hong Y., Nan B., Wu X., Yan H., Yuan Y. Allicin alleviates acrylamide-induced oxidative stress in BRL-3A cells. Life Sciences. 2019;231, article 116550 doi: 10.1016/j.lfs.2019.116550. [DOI] [PubMed] [Google Scholar]
- 114.García-Trejo E., Arellano-Buendía A. S., Argüello-García R., et al. Effects of allicin on hypertension and cardiac function in chronic kidney disease. Oxidative Medicine and Cellular Longevity. 2016;2016:13. doi: 10.1155/2016/3850402.3850402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu S., He Y., Shi J., et al. Allicin attenuates myocardial ischemia reperfusion injury in rats by inhibition of inflammation and oxidative stress. Transplantation proceedings. 2019;51(6):2060–2065. doi: 10.1016/j.transproceed.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 116.Chen S., Tang Y., Qian Y., et al. Allicin prevents H2O2-induced apoptosis of HUVECs by inhibiting an oxidative stress pathway. BMC complementary and alternative medicine. 2014;14(1):p. 321. doi: 10.1186/1472-6882-14-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rashid K., Chowdhury S., Ghosh S., Sil P. C. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochemical Pharmacology. 2017;143:140–155. doi: 10.1016/j.bcp.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 118.Daverey A., Agrawal S. K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience. 2016;333:92–103. doi: 10.1016/j.neuroscience.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 119.Ali B. H., Al‐Salam S., Al Suleimani Y., et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic & Clinical Pharmacology & Toxicology. 2018;122(1):65–73. doi: 10.1111/bcpt.12817. [DOI] [PubMed] [Google Scholar]
- 120.Nakmareong S., Kukongviriyapan U., Pakdeechote P., et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn-Schmiedeberg's archives of pharmacology. 2011;383(5):519–529. doi: 10.1007/s00210-011-0624-z. [DOI] [PubMed] [Google Scholar]
- 121.Han J., Pan X. Y., Xu Y., et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2014;8(5):812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- 122.Li B., Chi R. F., Qin F. Z., Guo X. F. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: association with oxidative stress. Experimental Physiology. 2016;101(8):1050–1063. doi: 10.1113/EP085586. [DOI] [PubMed] [Google Scholar]
- 123.Sorescu D., Griendling K. K. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congestive Heart Failure. 2007;8(3):132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 124.Liu X., Tong Z., Chen K., Hu X., Jin H., Hou M. The role of miRNA-132 against apoptosis and oxidative stress in heart failure. Biomed Research International. 2018;2018:8. doi: 10.1155/2018/3452748.3452748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kiyuna L. A., Albuquerque R., Chen C. H., Mochly-Rosen D., Ferreira J. Targeting mitochondrial dysfunction and oxidative stress in heart failure: challenges and opportunities. Free Radical Biology and Medicine. 2018;129:155–168. doi: 10.1016/j.freeradbiomed.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J. M., Gall N. P., Grieve D. J., Chen M., Shah A. M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40(4):477–484. doi: 10.1161/01.HYP.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 127.Li Y., Zhu Z., Zhang T., Zhou Y. Ligustrazine attenuates inflammation and oxidative stress in a rat model of arthritis via the Sirt1/NF-κB and Nrf-2/HO-1 pathways. Archives of Pharmacal Research. 2019;42(9):824–831. doi: 10.1007/s12272-018-1089-0. [DOI] [PubMed] [Google Scholar]
- 128.Jia N., Qiao H., Zhu W., et al. Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. International Journal of Biological Macromolecules. 2019;132:213–221. doi: 10.1016/j.ijbiomac.2019.03.196. [DOI] [PubMed] [Google Scholar]
- 129.Cheng S., Zhang X., Feng Q., et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sciences. 2019;227:82–93. doi: 10.1016/j.lfs.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 130.Liu T., Yang F., Liu J., et al. Astragaloside IV reduces cardiomyocyte apoptosis in a murine model of coxsackievirus B3-induced viral myocarditis. Experimental Animals. 2019;68(4):549–558. doi: 10.1538/expanim.19-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Y., Qian X., Li J., et al. Astragaloside-IV protects H9C2(2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artificial cells, nanomedicine, and biotechnology. 2019;47(1):4172–4181. doi: 10.1080/21691401.2019.1687492. [DOI] [PubMed] [Google Scholar]
- 132.Garcia N., Zazueta C., Aguilera-Aguirre L. Oxidative stress and inflammation in cardiovascular disease. Oxidative Medicine and Cellular Longevity. 2017;2017:2. doi: 10.1155/2017/5853238.5853238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin J., Fang L., Li H., et al. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. European Journal of Pharmacology. 2019;859, article 172490 doi: 10.1016/j.ejphar.2019.172490. [DOI] [PubMed] [Google Scholar]
- 134.Mei M., Tang F., Lu M., et al. Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environmental toxicology and pharmacology. 2015;40(3):764–773. doi: 10.1016/j.etap.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 135.Lu Y., Li S., Wu H., et al. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. International Journal of Molecular Medicine. 2015;36(5):1223–1232. doi: 10.3892/ijmm.2015.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang H., Lai S., Wan Q., Qi W., Liu J. Astragaloside IV protects cardiomyocytes from anoxia/reoxygenation injury by upregulating the expression of Hes1 protein. Canadian Journal of Physiology and Pharmacology. 2016;94(5):542–553. doi: 10.1139/cjpp-2015-0457. [DOI] [PubMed] [Google Scholar]
- 137.Kinugawa S., Tsutsui H., Hayashidani S., et al. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circulation Research. 2000;87(5):392–398. doi: 10.1161/01.RES.87.5.392. [DOI] [PubMed] [Google Scholar]
- 138.Li X. Z., Ding Y. Z., Wu H. F., et al. Astragaloside IV prevents cardiac remodeling in the apolipoprotein E-deficient mice by regulating cardiac homeostasis and oxidative stress. Cellular Physiology And Biochemistry. 2018;44(6):2422–2438. doi: 10.1159/000486166. [DOI] [PubMed] [Google Scholar]
- 139.Ye Q., Zhang Y., Fu J., et al. Effect of ligustrazine on endometrium injury of thin endometrium rats. Evidence-Based Complementary and Alternative Medicine. 2019;2019:7. doi: 10.1155/2019/7161906.7161906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ding Y., Du J., Cui F., Chen L., Li K. The protective effect of ligustrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Human & Experimental Toxicology. 2019;38(10):1168–1177. doi: 10.1177/0960327119851260. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X., Dong H., Liu Y., Han J., Tang S., Si J. Tetramethylpyrazine partially relieves hypoxia-caused damage of cardiomyocytes H9c2 by downregulation of miR-449a. Journal of Cellular Physiology. 2019;234(9):15098–15107. doi: 10.1002/jcp.28151. [DOI] [PubMed] [Google Scholar]
- 142.Li Y., Song P., Zhu Q., et al. Liguzinediol improved the heart function and inhibited myocardial cell apoptosis in rats with heart failure. Acta Pharmacologica Sinica. 2014;35(10):1257–1264. doi: 10.1038/aps.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J., Yang S., Yuan Y. J. Integrated investigation of lipidome and related signaling pathways uncovers molecular mechanisms of tetramethylpyrazine and butylidenephthalide protecting endothelial cells under oxidative stress. Molecular BioSystems. 2012;8(6):1789–1797. doi: 10.1039/c2mb05510d. [DOI] [PubMed] [Google Scholar]
- 144.Qu B., Yuan L., Yang L., Li J., Lv H., Yang X. Polyurethane end-capped by tetramethylpyrazine-nitrone for promoting endothelialization under oxidative stress. Advanced Healthcare Materials. 2019;8(20, article e1900582) doi: 10.1002/adhm.201900582. [DOI] [PubMed] [Google Scholar]
- 145.Ni X., Wong S. L., Wong C. M., et al. Tetramethylpyrazine protects against hydrogen peroxide-provoked endothelial dysfunction in isolated rat aortic rings: implications for antioxidant therapy of vascular diseases. Evidence-Based Complementary and Alternative Medicine. 2014;2014:10. doi: 10.1155/2014/627181.627181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhan H. D., Zhou H. Y., Sui Y. P., et al. The rhizome of Gastrodia elata Blume - an ethnopharmacological review. Journal of Ethnopharmacology. 2016;189:361–385. doi: 10.1016/j.jep.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 147.Kim I. S., Choi D. K., Jung H. J. Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules. 2011;16(7):5349–5361. doi: 10.3390/molecules16075349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liang W. Z., Jan C. R., Hsu S. S. Cytotoxic effects of gastrodin extracted from the rhizome of Gastrodia elata Blume in glioblastoma cells, but not in normal astrocytes, via the induction of oxidative stress-associated apoptosis that involved cell cycle arrest and p53 activation. Food And Chemical Toxicology. 2017;107(Part A):280–292. doi: 10.1016/j.fct.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 149.Li Q., Niu C., Zhang X., Dong M. Gastrodin and isorhynchophylline synergistically inhibit MPP+-Induced oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3β pathways: involvement of Nrf2 nuclear translocation. ACS Chemical Neuroscience. 2018;9(3):482–493. doi: 10.1021/acschemneuro.7b00247. [DOI] [PubMed] [Google Scholar]
- 150.Han X., Xu J., Xu S., et al. Role of mitochondrial permeability transition pore in mediating the inhibitory effect of gastrodin on oxidative stress in cardiac myocytes in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(11):1306–1311. doi: 10.12122/j.issn.1673-4254.2018.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kawaguchi M., Takahashi M., Hata T., et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 152.Zhou D., Qu Z., Wang H., et al. The effect of hydroxy safflower yellow A on coronary heart disease through Bcl-2/Bax and PPAR-gamma. Experimental and Therapeutic Medicine. 2018;15(1):520–526. doi: 10.3892/etm.2017.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin X., Shi L., Chang F., Lu Y. Efficacy and safety of safflower yellow in early diabetic nephropathy: a meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2019;2019:10. doi: 10.1155/2019/8065376.8065376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ren C., Wang J., Xian B., et al. Transcriptome analysis of flavonoid biosynthesis in safflower flowers grown under different light intensities. PeerJ. 2020;8:p. e8671. doi: 10.7717/peerj.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jia Z., Liu Y., Su H., et al. Safflower extract inhibiting apoptosis by inducing autophagy in myocardium derived H9C2 cell. International Journal of Clinical and Experimental Medicine. 2015;8(11):20254–20262. [PMC free article] [PubMed] [Google Scholar]
- 156.Ma Z., Li C., Qiao Y., et al. Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. International Journal of Molecular Medicine. 2016;37(3):603–612. doi: 10.3892/ijmm.2016.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kelainy E. G., Ibrahim L. I., Ibrahim S. R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environmental Science and Pollution Research. 2019;26(31):31675–31684. doi: 10.1007/s11356-019-06099-6. [DOI] [PubMed] [Google Scholar]
- 158.Wang G., Dai G., Song J., et al. Lactone component from Ligusticum chuanxiong alleviates myocardial ischemia injury through inhibiting autophagy. Frontiers in Pharmacology. 2018;9:p. 301. doi: 10.3389/fphar.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng C. Y., Kao S. T., Lee Y. C. Ferulic acid exerts anti-apoptotic effects against ischemic injury by activating HSP70/Bcl-2- and HSP70/autophagy-mediated signaling after permanent focal cerebral ischemia in rats. The American journal of Chinese medicine. 2019;47(1):39–61. doi: 10.1142/S0192415X19500034. [DOI] [PubMed] [Google Scholar]
- 160.Chowdhury S., Ghosh S., Das A. K., Sil P. C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative Insult, Inflammation and autophagy. Frontiers in Pharmacology. 2019;10:p. 27. doi: 10.3389/fphar.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren X., Wang X., Yuan M., et al. Mechanisms and treatments of oxidative stress in atrial fibrillation. Current Pharmaceutical Design. 2018;24(26):3062–3071. doi: 10.2174/1381612824666180903144042. [DOI] [PubMed] [Google Scholar]
- 162.Amrita J., Mahajan M., Bhanwer A. J., Mohan G. Oxidative stress: an effective prognostic tool for an early detection of cardiovascular disease in menopausal women. Biochemistry research international. 2016;2016:7. doi: 10.1155/2016/6157605.6157605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sánchez-Sánchez M. A., Zepeda-Morales A. S., Carrera-Quintanar L., et al. Alliin, an Allium sativum nutraceutical, reduces metaflammation markers in DIO mice. Nutrients. 2020;12(3):p. 624. doi: 10.3390/nu12030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mohamed M. Z., Morsy M. A., Mohamed H. H., Hafez H. M. Paeonol protects against testicular ischaemia-reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia. 2020;52(6):p. e13599. doi: 10.1111/and.13599. [DOI] [PubMed] [Google Scholar]
- 165.Al-Taher A. Y., Morsy M. A., Rifaai R. A., Zenhom N. M., Abdel-Gaber S. A. Paeonol attenuates methotrexate-induced cardiac toxicity in rats by inhibiting oxidative stress and suppressing TLR4-induced NF-κB inflammatory pathway. Mediators of Inflammation. 2020;2020:10. doi: 10.1155/2020/8641026.8641026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J. H., Xiong Y.'a. Effect of paeonol on ischemic arrhythmia and miRNA-1 expression in myocardial cells of rats. Chinese Journal of experimental prescription. 2015;21(5):129–132. [Google Scholar]
- 167.Wu G., Zhou W., Zhao J., et al. Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal. Oncotarget. 2017;8(7):11621–11628. doi: 10.18632/oncotarget.14598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.Sun K., Bai Y., Zhao R., et al. Neuroprotective effects of matrine on scopolamine-induced amnesia via inhibition of AChE/BuChE and oxidative stress. Metabolic Brain Disease. 2019;34(1):173–181. doi: 10.1007/s11011-018-0335-y. [DOI] [PubMed] [Google Scholar]
- 169.Khan A., Shal B., Naveed M., et al. Matrine ameliorates anxiety and depression-like behaviour by targeting hyperammonemia-induced neuroinflammation and oxidative stress in CCl4 model of liver injury. Neurotoxicology. 2019;72:38–50. doi: 10.1016/j.neuro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 170.Xu G., Zhang W., Wang Z., Chen M., Shi B. Matrine regulates H2O2-induced oxidative stress through long non-coding RNA HOTAIR/miR-106b-5p axis via AKT and STAT3 pathways. Bioscience Reports. 2020;40(5) doi: 10.1042/BSR20192560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun K., Yang P., Zhao R., Bai Y., Guo Z. Matrine attenuates D-galactose-induced aging-related behavior in MiceviaInhibition of cellular senescence and oxidative stress. Oxidative Medicine and Cellular Longevity. 2018;2018:12. doi: 10.1155/2018/7108604.7108604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jingmei S., Simiao C., Bing C., et al. Effects of different proportions of chuanxiong and Sophora flavescens on active components and antioxidant activity. Chinese traditional medicine emergency. 2020;29(1):87–90. [Google Scholar]
- 173.Hu C., Zhang X., Wei W., et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharmaceutica Sinica B. 2019;9(4):690–701. doi: 10.1016/j.apsb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gustafsson A. B., Gottlieb R. A. Autophagy in ischemic heart disease. Circulation Research. 2009;104(2):150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maximilian B. L. Mitochondria in ischemic heart disease. Advances in Experimental Medicine and Biology. 2017;982:127–140. doi: 10.1007/978-3-319-55330-6_7. [DOI] [PubMed] [Google Scholar]
- 176.Bai Y. D., Yang Y. R., Mu X. P., et al. Hydrogen sulfide alleviates acute myocardial ischemia injury by modulating autophagy and inflammation response under oxidative stress. Oxidative Medicine and Cellular Longevity. 2018;2018:17. doi: 10.1155/2018/3402809.3402809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y., Liu F., Yang Y., et al. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. International Journal of Biological Macromolecules. 2014;68:209–214. doi: 10.1016/j.ijbiomac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 178.Liu D., Xu J., Qian G., et al. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. International Journal of Biological Macromolecules. 2018;108:350–359. doi: 10.1016/j.ijbiomac.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 179.Chen W., Ju J., Yang Y., et al. Astragalus polysaccharides protect cardiac stem and progenitor cells by the inhibition of oxidative stress-mediated apoptosis in diabetic hearts. Drug design, development and therapy. 2018;Volume 12:943–954. doi: 10.2147/DDDT.S155686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Awad A., Khalil S. R., Hendam B. M., Abd E. R., Metwally M., Imam T. S. Protective potency of Astragalus polysaccharides against tilmicosin- induced cardiac injury via targeting oxidative stress and cell apoptosis-encoding pathways in rat. Environmental Science and Pollution Research. 2020;27(17):20861–20875. doi: 10.1007/s11356-020-08565-y. [DOI] [PubMed] [Google Scholar]
- 181.Magder S. Reactive oxygen species: toxic molecules or spark of life? Critical Care. 2006;10(1):p. 208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ozbek N., Bali E. B., Karasu C. Quercetin and hydroxytyrosol attenuates xanthine/xanthine oxidase-induced toxicity in H9c2 cardiomyocytes by regulation of oxidative stress and stress-sensitive signaling pathways. General Physiology and Biophysics. 2015;34(4):407–414. doi: 10.4149/gpb_2015021. [DOI] [PubMed] [Google Scholar]
- 183.Prince P. S., Sathya B. Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. European Journal of Pharmacology. 2010;635(1-3):142–148. doi: 10.1016/j.ejphar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 184.Çevik Ö., Çadırcı S., Şener T. E., et al. Quercetin treatment against ischemia/reperfusion injury in rat corpus cavernosum tissue: a role on apoptosis and oxidative stress. Free radical research. 2013;47(9):683–691. doi: 10.3109/10715762.2013.814912. [DOI] [PubMed] [Google Scholar]
- 185.Edwards R. L., Lyon T., Litwin S. E., Rabovsky A., Symons J. D., Jalili T. Quercetin reduces blood pressure in hypertensive subjects. Journal of Nutrition. 2007;137(11):2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 186.Rhiu S., Chae M. K., Lee E. J., Lee J. B., Yoon J. S. Effect of tanshinone IIA in an in vitro model of Graves' orbitopathy. Investigative Ophthalmology & Visual Science. 2014;55(9):5900–5910. doi: 10.1167/iovs.14-14008. [DOI] [PubMed] [Google Scholar]
- 187.Yu L. Z., Shi C. R. Effect of tanshinone II(A) on expression of different components in renin-angiotensin system of left ventricles of hypertensive rats. Zhongguo Zhong Yao Za Zhi. 2014;39(8):1468–1472. [PubMed] [Google Scholar]
- 188.Gu Y., Liang Z., Wang H., et al. Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Experimental and Therapeutic Medicine. 2016;12(2):1147–1152. doi: 10.3892/etm.2016.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo Z., Yan M., Chen L., et al. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Experimental and Therapeutic Medicine. 2018;16(4):3333–3344. doi: 10.3892/etm.2018.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Han D., Wu X., Liu L., Shu W., Huang Z. Sodium tanshinone IIA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis. Scientific reports. 2018;8(1, article 15137) doi: 10.1038/s41598-018-33552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu H., Shi L., Qi G., Zhao S., Gao Y., Li Y. Gypenoside protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of mitogen-activated protein kinase mediated nuclear factor kappa B pathway in vitro and in vivo. Frontiers in Pharmacology. 2016;7:p. 148. doi: 10.3389/fphar.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang K., Zhang H., Luo Y., et al. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: insight into the ERalpha-mediated PI3K/Akt pathway. International Journal of Molecular Sciences. 2017;18(2) doi: 10.3390/ijms18020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Han X. Y., Wei H. B., Zhang F. C. Analysis of the inhibitory effect of gypenoside on Na(+), K (+)-ATPase in rats' heart and brain and its kinetics. Chinese Journal of Integrative Medicine. 2007;13(2):128–131. doi: 10.1007/s11655-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 194.Huang T. H., Tran V. H., Roufogalis B. D., Li Y. Gypenoside XLIX, a naturally occurring PPAR-alpha activator, inhibits cytokine-induced vascular cell adhesion molecule-1 expression and activity in human endothelial cells. European Journal of Pharmacology. 2007;565(1-3):158–165. doi: 10.1016/j.ejphar.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 195.Essawy A. E., Abdou H. M., Ibrahim H. M., Bouthahab N. M. Soybean isoflavone ameliorates cognitive impairment, neuroinflammation, and amyloid beta accumulation in a rat model of Alzheimer's disease. Environmental Science and Pollution Research. 2019;26(25):26060–26070. doi: 10.1007/s11356-019-05862-z. [DOI] [PubMed] [Google Scholar]
- 196.Jung Y. S., Kim Y. J., Kim A. T., et al. Enrichment of polyglucosylated isoflavones from soybean isoflavone aglycones using optimized amylosucrase transglycosylation. Molecules. 2020;25(1):p. 181. doi: 10.3390/molecules25010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lu M., Xie K., Huang K., et al. Effects of soybean isoflavone on metabolism of rat osteoblasts and cytokines in vitro. Journal of Food Science. 2020;85(4):1302–1306. doi: 10.1111/1750-3841.14986. [DOI] [PubMed] [Google Scholar]
- 198.Barbosa A. C., Lajolo F. M., Genovese M. I. Effect of free or protein-associated soy isoflavones on the antioxidant status in rats. Journal of the Science of Food and Agriculture. 2011;91(4):721–731. doi: 10.1002/jsfa.4242. [DOI] [PubMed] [Google Scholar]
- 199.Strom J. O., Theodorsson A., Theodorsson E. Mechanisms of estrogens' dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. International Journal of Molecular Sciences. 2011;12(3):1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roghani M., Vaez Mahdavi M. R., Jalali‐Nadoushan M. R., et al. Chronic administration of daidzein, a soybean isoflavone, improves endothelial dysfunction and attenuates oxidative stress in streptozotocin-induced diabetic rats. Phytotherapy Research. 2013;27(1):112–117. doi: 10.1002/ptr.4699. [DOI] [PubMed] [Google Scholar]
- 201.Bunbupha S., Pakdeechote P., Maneesai P., Prachaney P., Boonprom P. Carthamus tinctorius L. extract attenuates cardiac remodeling in L-NAME-induced hypertensive rats by inhibiting the NADPH oxidase-mediated TGF-beta1 and MMP-9 pathway. Annals Of Anatomy-Anatomischer Anzeiger. 2019;222:120–128. doi: 10.1016/j.aanat.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 202.Zhou M. X., Fu J. H., Zhang Q., Wang J. Q. Effect of hydroxy safflower yellow A on myocardial apoptosis after acute myocardial infarction in rats. Genetics and Molecular Research. 2015;14(2):3133–3141. doi: 10.4238/2015.April.10.24. [DOI] [PubMed] [Google Scholar]
- 203.Min J., Wei C. Hydroxysafflor yellow A cardioprotection in ischemia-reperfusion (I/R) injury mainly via Akt/hexokinase II independent of ERK/GSK-3beta pathway. Biomedicine & Pharmacotherapy. 2017;87:419–426. doi: 10.1016/j.biopha.2016.12.113. [DOI] [PubMed] [Google Scholar]
- 204.Hu T., Wei G., Xi M., et al. Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. International Journal of Molecular Medicine. 2016;38(1):83–94. doi: 10.3892/ijmm.2016.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li Y., Zhang H. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food & Function. 2017;8(8):2935–2944. doi: 10.1039/c7fo00342k. [DOI] [PubMed] [Google Scholar]
- 206.Chekalina N. I., Shut S. V., Trybrat T. A., et al. Effect of quercetin on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiadomosci lekarskie. 2017;70(4):707–711. [PubMed] [Google Scholar]
- 207.Chekalina N. I. Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiadomosci lekarskie. 2017;70, 2 part 2:286–291. [PubMed] [Google Scholar]
- 208.Yu M. L., Li S. M., Gao X., Li J. G., Xu H., Chen K. J. Sodium tanshinone II a sulfonate for coronary heart disease: a systematic review of randomized controlled trials. Chinese Journal of Integrative Medicine. 2020;26(3):219–226. doi: 10.1007/s11655-018-2556-7. [DOI] [PubMed] [Google Scholar]
- 209.Zhang H., Niu H., Yuan X., Chang J., Wang X. Trimetazidine combined with berberine on endothelial function of patients with coronary heart disease combined with primary hypertension. Experimental and Therapeutic Medicine. 2018;16(2):1318–1322. doi: 10.3892/etm.2018.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang L., Zhou G. B., Liu P., et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proceedings of the National Academy of Sciences. 2008;105(12):4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hao D. C., Xiao P. G. Network pharmacology: a Rosetta Stone for traditional Chinese medicine. Drug development research. 2014;75(5):299–312. doi: 10.1002/ddr.21214. [DOI] [PubMed] [Google Scholar]
- 212.Shi Y., Liu D., Yuan J., et al. Compound Danshen dripping pills prevented leptin deficiency-induced hepatic ER stress, stimulated autophagy, and improved insulin resistance of ob/ob mice. Evidence-Based Complementary and Alternative Medicine. 2020;2020:7. doi: 10.1155/2020/5368657.5368657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen G., Wei B., Wang J., et al. Shensongyangxin capsules for paroxysmal atrial fibrillation: a systematic review of randomized clinical trials. PLoS One. 2016;11(3, article e151880) doi: 10.1371/journal.pone.0151880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Williamson E. M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]


