Abstract
Antimalarial drugs (e.g. chloroquine and its close structural analogues) were developed primarily to treat malaria; however, they are beneficial for many dermatological, immunological, rheumatological and severe infectious diseases, for which they are used mostly today. Chloroquine and hydroxychloroquine, two of the most fascinating drugs developed in the last 50 years, are increasingly recognized for their effectiveness in myriad non-malarial diseases. In advanced research, chloroquine and hydroxychloroquine have been shown to have various immunomodulatory and immunosuppressive effects, and currently have established roles in the management of rheumatic diseases, lupus erythematosus (different forms) and skin diseases, and in the treatment of different forms of cancer. Recently, chloroquine analogues have also been found to have metabolic, cardiovascular, antithrombotic and antineoplastic effects. This review is concerned with the lysosomotropic, anti-inflammatory and immunomodulatory mechanisms of chloroquine, hydroxychloroquine, quinacrine and related analogues, and the current evidence for both their beneficial effects and potential adverse manifestations in various diseases.
Keywords: hydroxychloroquine, quinacrine, SLE, therapies, lysosomotropic actions, toxicity profiles
Introduction
Historical perspective and development of chloroquine analogues
The first natural antimalarial agent, quinine, derived from the bark of the cinchona tree, helped to shape today's world by making it possible to live in tropical countries despite lethal tropical malaria. Chemical synthesis of chloroquine analogues originated in the work of Paul Ehrlich's group, who treated malaria patients in 1891 with methylene blue, a synthetic dye that is selectively absorbed by the parasites causing malaria. This was the first synthetic drug used for the treatment of human malaria. Subsequently, an analogue of methylene blue was synthesized by replacing one methyl group with a basic side chain, which significantly improved antimalarial activity. This positive result eventually led in 1925 to the synthesis of pamaquine. The next event was the attachment of the basic side chain of pamaquine to several different heterocyclic ring systems, leading to the synthesis of the acridine derivative quinacrine (having an extra benzene ring and thus an acridine nucleus when compared with chloroquine). Investigation of the structure of quinacrine led to the discovery of two chloroquine analogues, sontoquine and primaquine, which showed excellent antimalarial activity. Studies on these compounds then led to the discovery of resochin. This compound was ignored for a decade, since it was initially thought to be too toxic for clinical use. However, its toxicological properties were re-examined and it was found to be safe for human subjects. The Wehrmacht in North Africa used a resochin formulation (‘sontoquine’); it was captured during World War II by the Americans, and this led to the synthesis of chloroquine.1,2 From earlier empirical studies, it became clear that chloroquine was one of the most effective agents, and the study of further structural variations led to the discovery of hydroxychloroquine (which differs from chloroquine only by a hydroxyl group), which proved to be 3-fold less toxic.3 The historical developmental events are summarized in Figure 1. Although chloroquine has been abandoned for prophylaxis in most countries due to the resistance of the pathogens Plasmodium falciparum and Plasmodium vivax, chloroquine analogues are still used in Korea, China, Turkey, Mexico, Paraguay, etc., for the prophylactic treatment of malaria.4–6 A milestone in the fortunes of chloroquine analogues occurred during World War II; millions of soldiers took antimalarial prophylaxis, and observations indicated that antimalarial treatment improved the soldiers' rashes and inflammatory arthritis. This led to the first trial that showed the efficacy of quinacrine in systemic lupus erythematosus (SLE). Similar observations opened the door for regular treatment of patients with rheumatoid arthritis (RA) and SLE with chloroquine analogues. Nowadays, chloroquine analogues are used for the treatment of other rheumatic disorders, as well as a wide variety of dermatological, immunological, cancerous and infectious diseases.7
Figure 1.
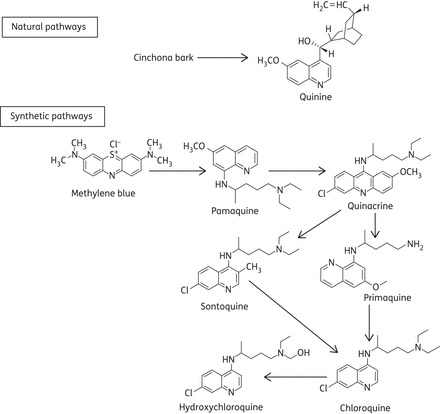
Historical developmental pathways of chloroquine analogues.
Pharmacokinetic considerations
Chloroquine analogues are water soluble and almost completely absorbed from the gastrointestinal tract. Both chloroquine and hydroxychloroquine reach the peak plasma concentration 4–12 h after an individual dose and achieve equilibrium plasma levels after 4–6 weeks of constant daily dosing, although there is considerable inter-individual variation. The half-lives of chloroquine and hydroxychloroquine are prolonged, ranging between 40 and 50 days. Chloroquine analogues have strong affinities for blood constituents, particularly thrombocytes and granulocytes, which reduces the plasma concentrations. In addition, a major fraction of chloroquine analogues in the plasma is bound to plasma proteins, mainly albumin and α-acid glycoprotein, and also avidly bound to several tissues in the body when given at therapeutic doses. As a result, excretion of chloroquine analogues is quite slow. Although small amounts are excreted in bile, sweat and saliva, the major elimination route of chloroquine analogues is via the renal system, and elimination may thus be affected by the pH of urine.8–10
Indications for chloroquine analogues
Chloroquine analogues have been shown to have potent beneficial effects in many non-malarial diseases. For practical purposes, the indications for chloroquine analogues can be summarized in several ways (Table 1). The current evidence for applications of chloroquine, hydroxychloroquine and, to a lesser extent, quinacrine is discussed below.
Table 1.
Summary of indications for chloroquine analogues
| FDA-approved and FDA-labelled indications |
| Malaria (except resistant P. falciparum and P. vivax causing malaria) |
| Lupus erythematosus in different forms, such as discoid, systemic; also effective in pregnant SLE patients |
| RA, act as first-line disease-modifying antirheumatic drugs |
| Chloroquine analogues in clinical research trials |
| Lupus erythematosus (discoid and cutaneous) in different adjunct therapies |
| RA in combination with other drugs |
| Psoriatic arthritis |
| Prostatic cancer |
| Additional research trials |
| Local metastatic melanoma, chronic lymphocytic leukaemia and diffuse large B cell lymphoma |
| Unapproved but first-line uses include |
| PCT and chronic ulcerative stomatitis |
| Hepatic amoebic abscess |
| Refractory chronic urticaria135,136 |
| Quinacrine is used as an effective contraception137 |
| Second- and third-line treatments |
| Non-infectious skin diseases such as dermatomyositis, sarcoidosis, polymorphous light eruption and disseminated granuloma annulare |
| Miscellaneous conditions |
| Sjögren's syndrome, granuloma annulare, erosive lichen planus, frontal fibrosing alopecia,138 necrobiosis lipoidica, chronic actinic dermatitis, actinic reticuloid, actinic prurigo, epidermolysis bullosa, Kikuchi–Fujimoto disease, graft-versus-host disease, chronic erythema nodosum, morphea and systemic sclerosis, pemphigus vulgaris, pemphigus foliaceus and pemphigoid gestationis139 |
| Chloroquine analogues and current research |
| Bone diseases, different forms of cancers, hyperglycaemia, dyslipidaemia, thrombosis and severe infectious diseases |
| Chloroquine analogues as investigational drugs |
| AIDS and severe acute respiratory syndrome (SARS) |
| Human prion diseases (CJD) and LAM |
Lupus erythematosus (LE)
Chloroquine analogues prevent lupus flares clinically and increase the long-term survival of patients with systemic SLE, cutaneous LE (CLE) or discoid LE.11–14 These drugs are also effective for the treatment of lupus patients who are pregnant, for neonates with lupus, or lupus patients who also have other diseases such as osteonecrosis and inflammatory bowel disease.15–18 In patients with SLE, chloroquine and hydroxychloroquine improve certain systemic manifestations, such as arthralgia, myalgia, serositis and haematological abnormalities. Recently, prolactinoma and recurrent granulomatous mastitis in SLE patients have been successfully treated with hydroxychloroquine.19 In patients with CLE, a combination of hydroxychloroquine and quinacrine is more appropriate as initiation therapy than hydroxychloroquine or chloroquine monotherapy and improves the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score and the response rate in non-responders.20,21 Hydroxychloroquine is beneficial for patients with membranous lupus nephritis.12 Furthermore, the use of hydroxychloroquine in SLE patients is associated with improved overall survival, decreased accrual of damage and lowered rates of infections.
RA
Both chloroquine and hydroxychloroquine inhibit antigen presentation in dendritic cells, cytokine production in macrophages, and calcium and Toll-like receptor (TLR) signalling in B, T and other immune cells. Thus, chloroquine analogues have become the most commonly prescribed drugs in the treatment of many rheumatic diseases, including RA, palindromic arthritis, psoriatic arthritis and juvenile idiopathic arthritis.22–25 In RA, hydroxychloroquine is usually a component of medication combinations, including triple-drug therapy with methotrexate and sulfasalazine, a regimen that has been advocated as a safe, well-tolerated alternative to more expensive biological therapies. In the early stages of RA, chloroquine analogues reduce cardiovascular risks. Hydroxychloroquine also confers significant improvement in the symptoms of mild to moderate knee osteoarthritis, rheumatoid vasculitis and non-gout joint deposition diseases.26–28
Anticancer strategies
The incorporation of chloroquine, hydroxychloroquine, quinacrine and other chloroquine analogues, such as 8-hydroxyquinoline,29,30 in chemotherapeutic regimens has become a therapeutic approach in oncology, because of their inhibitory actions on lysosomes or acceleration of the radio-sensitizing effects of some chemotherapeutic drugs used concomitantly with radiotherapy.31,32 Therefore, chloroquine analogues are taken into consideration in clinical trials with radiotherapy and chemotherapy. The use of chloroquine analogues has been focused on the treatment of highly aggressive and metastatic cancers, including relapsed leukaemias, melanoma, osteosarcoma and cancers of the head and neck, brain, lung, breast, ovary, prostate and pancreas, as well as gastrointestinal cancers, which remain incurable in the clinic in spite of aggressive therapy.31,33 In these cases, chloroquine analogues influence the potential biological effects of different cancer cells, such as by inhibiting cell growth and/or inducing cell death by autophagy-dependent modulation.34–36 Some of these studies have used relatively high drug concentrations, doubling the usual dose in patients with SLE. While these doses have low levels of toxicity, especially in the setting of life-threatening illness, more efficient drug delivery systems, such as the use of targeted nanoparticles, have been proposed as methods of enhancing the efficacies of these agents. Several clinical trials using combinations of chloroquine analogues with different therapeutic agents for cancers are currently being carried out.35 The results of these clinical trials are likely to be helpful in determining the directions of research on chloroquine analogue-mediated cancer treatments.
Contemporary cases of dermatological disorders
Porphyria cutanea tarda (PCT)
In PCT, reactive oxygen species are produced and damage the skin, resulting in severe mucosal erosions and epidermal friability and blistering. Repeated phlebotomy is the mainstay treatment for PCT. Nevertheless, in many of the cases in which patients do not respond this procedure is contraindicated. In these cases, a low-dose regimen of chloroquine analogues gives favourable results without untoward reactions.37,38
Chronic ulcerative stomatitis (CUS)
CUS is characterized by a course of painful ulceration in the oral mucosa of older patients, caused by the interaction of antinuclear antibodies with squamous epithelia. Lesions associated with CUS are refractory to local and systemic corticosteroids, but treatment with chloroquine analogues lead to remission and so these agents are the first-line treatment for CUS.1,39
Dermatomyositis
Chloroquine analogues are effective in the treatment of dermatomyositis (childhood or juvenile form). The improvement in muscle strength in patients receiving chloroquine analogues as adjunct therapies is (at least in part) a consequence of the improvement of skin manifestations.40,41
Sarcoidosis
It is reported that quinacrine improves cutaneous sarcoidosis. The beneficial outcome of chloroquine in the medication of pulmonary sarcoidosis was discovered in 1960. Since then, many reports have confirmed the effectiveness of chloroquine analogues in the treatment of subcutaneous, pulmonary and osseous sarcoidosis.42–44
Sjögren's syndrome
Hydroxychloroquine is of benefit in patients with primary Sjögren's syndrome.45–47 Hydroxychloroquine lowers serum B-cell activating factor (BAFF) levels and improves the atherogenic index in primary Sjögren's syndrome.48
Lichen planus
Chloroquine analogues cure cutaneous lichen planus of the nails, oral mucosa and lower lip, and lichen planopilaris.49–51 The efficacy of hydroxychloroquine has also been proved in the treatment of oral lichen planus, in which it acts by lowering the up-regulated expression of regulatory T cells (Tregs).52
Miscellaneous
This section summarizes some diseases in which chloroquine analogues are used randomly, the scientific evidence being insufficient and limited to isolated case reports. The use of chloroquine analogues is recommended in patients with disseminated granuloma annulare that does not respond well to topical corticosteroids or in whom corticosteroids cannot be used due to the extent of the lesions.53 Chloroquine analogues (particularly hydroxychloroquine) are effective alternatives for the long-term treatment of some photosensitive disorders, such as chronic actinic dermatitis and actinic reticuloid.54 Chloroquine analogues inhibit the development of graft-versus-host disease (GVHD) by suppressing T cell responses to foreign minor and major histocompatibility complex (MHC) antigens and alterations in T cell cytokine production.55 Hydroxychloroquine also prevents acute GVHD in patients who have received bone marrow transplantation.56
Chloroquine analogues and current research
Bone diseases
Administration of chloroquine or hydroxychloroquine reportedly results in a slowing or even arrest of joint destruction as well as increased bone mineral density (BMD) in RA and SLE patients.57 We and other groups have also investigated the direct inhibitory effects of chloroquine on osteoclast function58 and the differentiation and bone-forming activity of osteoblasts.59 In these studies, chloroquine suppressed the bone-resorbing activity of osteoclasts by inhibition of acidification in lysosomes, as well as osteoclast differentiation in vitro and in vivo. These studies demonstrate the importance of the effect of chloroquine analogues on the function as well as the differentiation of osteoclast-mediated bone diseases, including osteoporosis as well as RA.
Hyperglycaemia
The hypoglycaemic effect of chloroquine analogues increases insulin sensitivity in patients with insulin resistance.60,61 A clinical trial in type II diabetic patients who were treated with a short course of chloroquine showed a significant improvement in glucose tolerance. Hydroxychloroquine also emerges as a well-tolerated therapeutic option for type II diabetic mellitus. When hydroxychloroquine was combined with insulin for the treatment of diabetes mellitus, glycated haemoglobin decreased significantly compared with patients receiving placebo, and the insulin dose had to be reduced by 30% in the hydroxychloroquine group.62 The anti-diabetic mechanism of chloroquine analogues involves decreases in insulin clearance and degradation rates and an increase in the secretion of C-peptide.63
Anti-lipidaemic effects
Chloroquine analogues have plasma lipid-lowering effects in RA, SLE, dyslipidaemia and diabetes mellitus that are therapeutically relevant due to the increased risks of premature atherosclerosis in these diseases.11,21,64,65 Treatment with hydroxychloroquine reduces the levels of cholesterol, triglycerides and LDL irrespective of concomitant steroid administration, diet or weight. In fact, dyslipidaemias are very frequent in SLE and certainly play a pivotal role in the 50-fold greater risk of developing coronary artery disease. Coronary diseases are important causes of mortality in SLE patients.66 Mechanisms responsible for altered lipid profiles with chloroquine analogue treatment include a significant increase in lipid clearance rate and up-regulation of LDL receptors.
Coagulopathy and thrombosis
Hydroxychloroquine prevents significant thromboembolic events in the postoperative period following total hip arthroplasty or during pregnancy.11,67,68 Several studies indicate that chloroquine analogues have an effect in the prevention of thrombotic phenomena.69 The antithrombotic effect of chloroquine analogues has been attributed to a range of mechanisms, including reduction in red blood cell aggregation, inhibition of platelet aggregation and adhesion, reduction in blood viscosity and enhancement of antiplatelet activity.70,71 Chloroquine and hydroxychloroquine exert beneficial effects in pulmonary arterial hypertension (PAH), pulmonary haemosiderosis and common variable immunodeficiency (CVID) granulomatous disease.72–74 Chronic hydroxychloroquine treatment reduces hypertension, endothelial dysfunction and organ damage in patients with severe lupus.75 These studies demonstrate the direct impact of chloroquine analogues on cardiac patient care.
Chloroquine analogues as investigational drugs in microbial infections
Chloroquine analogues have been found to be effective against bacterial infections such as endocarditis76 and Q fever,77 parasitic infections such as giardiasis78 and viral infections such as Ebola virus disease,79 hepatitis C virus-related arthritis80 and chikungunya.81 Chloroquine analogues are being or have been used in clinical trials as investigational antiretroviral agents in humans with HIV-1/AIDS (registration numbers NCT01650558, NCT02004314 and NCT01067417). Combined treatment with hydroxychloroquine, hydroxyurea and didanosine in antiretroviral-naive HIV patients decreased viral replication and increased the CD4 count.82 Human corona virus (hCoV) threatened to cause a pandemic of SARS. Chloroquine was shown to inhibit the replication and spread of corona virus in vitro and to prevent infection with hCoV in newborn mice, and this shows promise as a potential therapy for this resistant virus.83,84 Human prion diseases are characterized clinically by cognitive, neuropsychiatric and motor dysfunction. The most common form of prion disease is sporadic Creutzfeldt–Jakob disease (CJD), which affects ∼1–2 people per million annually worldwide. The accumulation of the pathogenic form of prion protein is a pivotal event in prion diseases. The chloroquine analogue quinacrine inhibits not only the accumulation of pathogenic prion protein but also the conversion of normal cellular prion protein to disease-associated forms. Clinical trials of quinacrine in patients with CJD are in progress.85,86 Lymphangioleiomyomatosis (LAM) is associated with cystic lung destruction and lymphatic and kidney tumours and predominantly affects premenopausal women. Inhibition of autophagy with chloroquine analogues results in a decreased LAM cell load in the lungs and improvement in pulmonary function.87
Chloroquine analogues and antiphospholipid syndrome (APS)
The APS is a systemic autoimmune disorder characterized by recurrent thrombosis and/or pregnancy morbidity occurring in patients with persistent antiphospholipid antibodies (aPL). There is ample evidence of the protective effects of hydroxychloroquine in primary obstetrical APS.88,89
Mechanisms of action
Although it would be aesthetically pleasing to ascribe all therapeutic effects to a single mode of action, this is not the case with the actions of chloroquine analogues. There is certainly more than one mechanism for the actions of these drugs, and some of them are discussed here.
Rationales for lysosomotropic amines
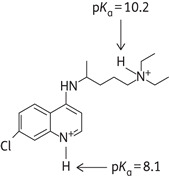
Chloroquine is a diprotic weak base (pKa1 = 8.1, pKa2 = 10.2 at 37°C) that can exist in both protonated and unprotonated forms (Figure 2 and Table 2). Unprotonated chloroquine can diffuse freely and rapidly across the membranes of cells and organelles to acidic cytoplasmic vesicles (late endosomes and lysosomes). Once protonated, chloroquine is trapped in the acidic organelles (lysosomes) and can no longer freely diffuse out. Therefore, chloroquine analogues are known as lysosomotropic agents (i.e. they are taken up selectively into lysosomes).90 Lysosomes are cellular compartments containing acid hydrolases that digest several macromolecules. For optimal activity of hydrolases, pH is maintained at ∼5.0 by the action of lysosomal H+-ATPases.91 As more H+ ions are pumped into the acidic vesicle by the ATP-dependent pumps of lysosomal H+-ATPases, more chloroquine will diffuse from the cell's cytoplasm into the acidic vesicle to cause partition according to the difference between two pH gradients. This leads to an irreversible accumulation of chloroquine in lysosomes to >100-fold excess concentration and causes an elevation of pH due to trapping of H+ ions by chloroquine.87 Hydroxychloroquine, a related lysosomotropic amine, appears to be very similar to chloroquine in its effect on cellular function. Thus, chloroquine analogues interfere with lysosomal acidification, which in turn inhibits proteolysis, chemotaxis, phagocytosis and antigen presentation. As a result, cells are not able to proceed with endocytosis, exosome release and phagolysosomal fusion in an orderly manner.92
Figure 2.
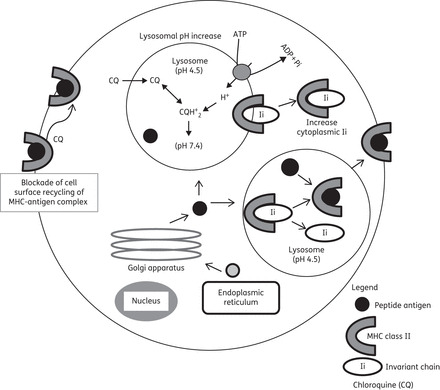
Major inhibitory roles of chloroquine in lysosomes. Intralysosomal pH is increased by chloroquine. Note that the raised pH is not involved in the antimalarial mechanism of action.
Table 2.
Percentages of protonated forms of chloroquine at different pHs
| |||
|---|---|---|---|
| pH | % CQ | % CQH+ | % CQH22+ |
| 5.0 | 0.000 | 0.079 | 99.919 |
| 5.5 | 0.000 | 0.252 | 99.747 |
| 6.0 | 0.000 | 0.794 | 99.205 |
| 6.4 | 0.000 | 1.971 | 98.028 |
| 6.9 | 0.002 | 5.979 | 94.017 |
| 7.4 | 0.026 | 16.739 | 83.234 |
| 8.4 | 1.039 | 66.095 | 32.865 |
Neutral (CQ), mono-protonated (CQH+) and di-protonated (CQH22+) forms of chloroquine are included. The percentages are calculated using the Hendersen–Hasselbalch equation, with pKa values of 8.1 for the quinoline nitrogen and 10.2 for the side chain diethylamine nitrogen.
As antigen processing is an acidic, pH-dependent phenomenon, chloroquine turns off the process of antigen presentation by decreasing the number of autoantigenic peptides appearing on the cell surface. Because autoantigenic peptides have low affinity for self-MHC, elevation of pH in the acidic compartments selectively decreases the loading of autoantigenic self-peptides, while leaving intact the response to exogenous peptides with higher affinity. This decreased amount of self-peptide–MHC complex on antigen-presenting macrophages and other target cells results in decreased stimulation of CD4+ T cells. Thus, the production of a series of cytokines by both T cells and antigen-presenting cells also decreases. The increased pH induced by chloroquine in lysosomes also causes decreased activities of the aspartyl protease cathepsin D and the cysteine protease cathepsin B, which are responsible for early and late cleavage of invariant chains from the MHC class II molecule, respectively (Figure 2). Inhibition of antigen presentation by chloroquine appears to primarily affect professional antigen-presenting cells such as dendritic cells, B cells and macrophages, which have MHC class II-enriched compartments.1,21,93
Anti-inflammatory and immunomodulatory effects
Chloroquine analogues have well-recognized anti-inflammatory and immunomodulatory actions,21 but their specific mechanisms in individual diseases are not clear. The major proposed mechanisms of actions of chloroquine analogues are summarized in Table 3 and Figure 3.
Table 3.
Anti-inflammatory and immunomodulatory actions of chloroquine analogues
|
Figure 3.
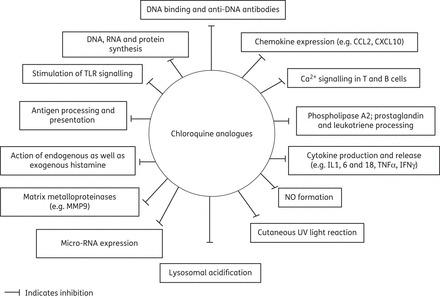
Major anti-inflammatory and immunomodulatory effects of chloroquine analogues.
Anticancer effects
The anticancer mechanisms of chloroquine analogues are more complex, with many potential cellular targets. The most common approach in cancer therapy is the inhibition of autophagy and sensitization of malignant cells to radiation and chemotherapeutic agents by chloroquine analogues.31,32 A number of clinical trials are in progress; the results obtained so far indicate that the use of chloroquine analogues may lead to changes in cancer therapeutic strategies.35 The lysosomotropic properties of chloroquine analogues are their most important characteristics for alteration of the malignant progression of cancer cells. Chloroquine accumulates preferentially in lysosomes and raises intralysosomal pH, which in turn increases the permeability and volume of lysosomes. The increased intralysosomal pH produced by chloroquine analogues may not be sufficient to cause cellular damage specifically in tumour cells at therapeutically achievable concentrations. However, the analogues can damage tumour cells when lysosomal permeability is also increased by radiation, which causes the release of proteolytic enzymes and damages cellular functional proteins, including plasma membrane-associated proteins (Figure 4). Thus, chloroquine analogues sequester and modify many important cell membrane constituents (ceramides in lysosomes), thus limiting plasma membrane repair and recycling. These events are therapeutically useful.94
Figure 4.
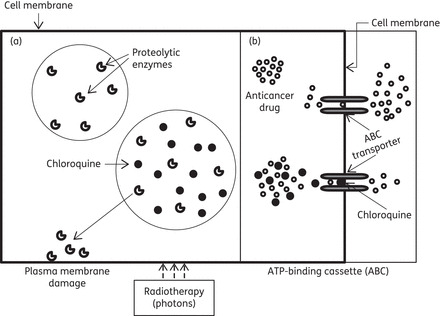
Mechanisms of anticancer actions of chloroquine analogues. (a) Radiosensitizing effect: membrane-damaging proteolytic enzymes are released and lysosomal permeability is increased as a result of radiation and the effect of chloroquine. (b) Chemosensitizing effect: anticancer drug extrusion is prevented via blockade of ABC transporters with chloroquine, and intracellular drug availability is increased and cells damaged.
The ATP-binding cassette (ABC) family of transmembrane proteins and the multidrug resistance proteins extrude chemotherapeutic drugs from targeted cancer cells. This drug-extruding activity contributes to drug resistance in cancer, and expression of one or more ABC proteins and multidrug resistance proteins is often up-regulated during anticancer chemotherapy. In chemosensitization, chloroquine inhibits anticancer drug extrusion by blocking transporters of the ABC family and multidrug resistance proteins. Thus, chloroquine modulates the tumour response to anticancer drugs (Figure 4). Chloroquine analogues, used at clinically achievable concentrations, are also known to sensitize cells to radiation and anticancer drugs.95 Chloroquine enhances the radiosensitizing effects of some chemotherapeutic drugs used concomitantly with radiotherapy by increasing lysosomal permeability, by releasing membrane-damaging proteolytic enzymes or by inhibiting ABC-mediated drug extrusion (Figure 4). The other main actions of chloroquine analogues responsible for most intracellular actions are (i) the molecular intercalation of chloroquine into DNA96 and (ii) the inhibition of lysosomal enzymes, particularly phospholipase A2.97 Configurational changes in nucleic acids render neoplastic cells more susceptible to the cytotoxic effects of radiotherapy as well as chemotherapy. Therefore, potentiation of the effects of chloroquine analogues is taken into consideration in clinical trials with radiotherapy and chemotherapy.
Adverse effects
There are relatively few adverse effects at the standard doses of chloroquine analogues that are used for the prophylaxis of malaria and other systemic diseases. However, acute toxicity of chloroquine analogues is encountered most frequently when therapeutic or high doses are administered very rapidly by parenteral routes. The most serious complications of chloroquine analogues are retinopathy, cardiomyopathy, neuromyopathy and myopathy. The estimated frequency and reversibility of these complications is given in Table 4.
Table 4.
Major adverse effects of chloroquine analogues
| Organ/system | Major adverse effects | Duration of therapy/overdose | Reversibility | Estimated frequency |
|---|---|---|---|---|
| Sensory systems | ||||
| eyes | keratopathy | higher dose and/or prolonged periods | irreversible | low incidence |
| retinopathy | irreversible | |||
| ears | ototoxicity | higher dose and/ or prolonged periods | reversible | rare |
| tongue and nose | disturbances of taste and smell | long time | reversible | report on only one patient |
| Gastrointestinal system | gastrointestinal discomfort | therapeutic doses | reversible | most common |
| Cutaneous system | pruritus | short time | reversible | most common, 1% of patients |
| photosensitivity | short time | reversible | ||
| Nervous system | neuromyopathy | higher doses | reversible | several reports suggested more common |
| seizures, psychosis | prolonged periods | |||
| Musculoskeletal system | myopathy | 5–7 months | reversible | occasionally |
| Cardiovascular system | conduction disorders cardiomyopathy | long term/high dose | irreversible | occasionally |
| Haematological system | haemolysis | long term/high dose | irreversible | less common |
| leucopenia | reversible | less common | ||
| aplastic anaemia | reversible | (1/50 000) | ||
| Respiratory system | alveolitis | overdose | reversible | rare |
| Urinary tract system | impaired renal function | long time | reversible | occasional |
| Immunological system | allergic contact dermatitis | short time | reversible | report on a patient |
| Metabolism | hypoglycaemia | long time | reversible | several reports less common |
| Other | hearing loss | long time | irreversible | very rare |
Long time, >1 year; short time, <1 year; reversible, relief of symptoms on withdrawal of treatment; irreversible, permanent loss.
Sensory systems
Eyes
Chloroquine and its congeners can cause two typical adverse effects in the eyes: keratopathy and retinopathy. Both of these effects are associated with the administration of the drugs over long periods of time. Chloroquine-induced keratopathy is limited to the corneal epithelium, where high concentrations of the drug are usually used. The retinopathy encountered with the prolonged use of chloroquine analogues is a much more serious clinical problem and can lead to irreversible damage to the retina and loss of vision. The hallmark feature of hydroxychloroquine toxicity is bilateral pigmentary retinopathy.98,99 At an early stage in hydroxychloroquine-induced retinal disease, patients may often be asymptomatic despite having subtle paracentral scotomas. Later in the disease, patients may develop a ‘bull's eye’ maculopathy, characterized by a ring of retinal pigment epithelium (RPE) in the macular area closer to the fovea, which is often accompanied by paracentral and central scotomas.100,101 End-stage hydroxychloroquine toxicity leads to widespread RPE and retinal atrophy with a loss of central, peripheral and night vision.102 The incidence of retinopathy associated with the use of chloroquine analogues is low, as long as the dose does not exceed the therapeutic doses and the medication is used for <10 years in patients with normal renal function. Quinacrine does not cause retinopathy. Other adverse effects on the eyes include rhegmatogenous retinal detachment and bitemporal hemianopsia in association with chloroquine retinopathy. Diplopia and impaired accommodation are also observed even at lower doses in some patients. The best current opinion seems to be to avoid retinopathy by using doses of hydroxychloroquine not exceeding 6.5 mg/kg/day, with periodic checking of renal and hepatic function.103
Ears
Besides their well-known retinal toxicity, chloroquine analogues are suspected to be associated with ototoxicity. There are reports suggesting sensorineural hearing loss, tinnitus, a sense of imbalance and cochleovestibular manifestations.104,105 The reversibility of chloroquine ototoxicity is debatable, but there is a suggestion that such complications can be corrected if the medication is stopped and appropriate therapy, with steroids and plasma expanders, is instituted.
Cardiovascular system
Cardiac side effects of chloroquine analogues are rarely reported, but in some cases can be severe and irreversible. Conduction disturbances (bundle-branch block, atrioventricular block), cardiomyopathy (often with hypertrophy and congestive heart failure) are the major toxic effects.106–109 A case report suggested that chloroquine cardiotoxicity manifested suddenly as atrioventricular block with QT(c) interval prolongation and short torsade de pointes.109 Symptoms like syncope and Stokes–Adams attacks and signs of cardiac failure can also occur. Acute intoxication can cause fatal cardiovascular collapse and/or respiratory failure.
Gastrointestinal system
Gastrointestinal discomfort is the most common reaction in patients receiving chloroquine analogues, although the discomfort is usually mild and can be managed by dose reduction. The other common gastrointestinal events are nausea, vomiting and diarrhoea. Overdoses of the analogues can cause vomiting. Stomatitis with buccal ulceration has occasionally been reported with the analogues. Less frequent gastrointestinal effects include anorexia, abdominal distress, abnormal liver function and transaminitis.110 Hepatotoxicity, which is uncommon with either chloroquine or proguanil, has been reported after the use of a fixed-dose combination of chloroquine and proguanil.111
Cutaneous system
The most common dermatological adverse event associated with chloroquine analogues is pruritus.112 It is much more common in darker-skinned people, in whom chloroquine binds to melanin in the skin. Recent case reports have suggested that pigmentary changes of the skin and mucous membranes develop during the course of chloroquine therapy for connective tissue disorders. Chloroquine analogues induce hyperpigmentation and longitudinal melanonychia in older patients. Quinacrine causes darker pigmentation than chloroquine and hydroxychloroquine.113,114 Prolonged use of chloroquine may occasionally cause lichenoid skin eruptions, bullous skin eruption, skin lesions (including epidermal necrolysis) and bleaching of the hair.115 Chloroquine can turn the nail bed blue–brown and the nail itself may develop longitudinal stripes. Photosensitivity and photoallergic dermatitis have been seen, particularly during prolonged therapy with high doses of the analogues. A near fatal case of Stevens–Johnson syndrome has been reported after treatment with an analogue.116 Chloroquine therapy can also cause vitiligo,117 pemphigus118 and severe cutaneous necrotizing vasculitis.119
Nervous system
Chloroquine analogues can cause a marked neuromyopathy, characterized by slowly progressive weakness, particularly with long-term use or standard doses in elderly people. Chloroquine can also cause seizures in patients with epilepsy and SLE.120 Convulsion has also been reported in patients in whom chloroquine is part of a prophylactic regimen; the condition is reversible if the analogues are withdrawn. The many mental changes attributed to chloroquine analogues include agitation, aggressiveness, confusion, personality changes, loss of memory, psychotic symptoms and depression.121,122 Hallucinations have also been reported after hydroxychloroquine treatment for erosive oral lichen planus.51
Musculoskeletal system
Chloroquine analogues occasionally cause a myopathy associated with muscle weakness, and reduced or absent tendon reflexes. Severe vacuolar myopathy has also been reported with hydroxychloroquine.123
Haematological system
Haematological side effects of chloroquine analogues are uncommon. Rare instances of haemolysis and blood dyscrasias have been reported. Haemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, aplastic anaemia and leucopenia have been recorded with chloroquine analogues, particularly quinacrine at higher doses. Leucopenia, agranulocytosis and the occasional case of thrombocytopenia have been noted.124 There is some evidence that myelosuppression is dose-dependent. Liver function and blood tests, particularly complete blood counts, should be performed monthly at the start of therapy and at least every 4–6 months throughout treatment.
Metabolism
Therapeutic doses of chloroquine analogues can cause hypoglycaemia.125 Convulsion is more common in hypoglycaemic children. Although hydroxychloroquine has been used to treat PCT, there are some reports that it can also worsen porphyria in SLE patients.126
Others
Chloroquine-induced impaired renal function has occasionally been reported. Allergic contact dermatitis followed by severe asthma has occurred in a patient (60 years old) with hydroxychloroquine exposure.127 It is reported that induction or exacerbation of psoriasis is observed in lichen planopilaris (a 40-year-old female) and psoriatic arthritic (a 37-year-old primigravida) patients treated with hydroxychloroquine.128 An acute gluteal abscess after an injection of chloroquine has also been reported.129 Acute generalized exanthematous pustulosis has been reported in Canada as an adverse reaction to hydroxychloroquine.130
Pregnancy and infants of treated women
Chloroquine inactivates DNA and crosses the placenta in animals. Caution is generally advised with respect to the use of chloroquine analogues during pregnancy, but there are no reports available to date of complications in mothers or their newborn infants after treatment with chloroquine during pregnancy and lactation.11,24,88 During lactation, the amount of hydroxychloroquine transferred to the infant seems to be negligible and does not confer a risk of toxicity to the infant.24
Conclusion
Chloroquine analogues have been credited with saving the lives of thousands of patients with malaria. Since the first use of chloroquine analogues nearly a century ago, their effectiveness has been increasingly recognized in nearly all major branches of medicine, including immunology, oncology, haematology, dermatology, cardiology and severe infectious diseases such as AIDS and SARS. Although these drugs are not FDA approved for several therapies, rheumatologists, dermatologists and other professionals have recognized their effectiveness for various pathologies in their specialities. To date, chloroquine analogues have established roles in the treatment of SLE, RA, osteoarthritis, cancers and various skin diseases (e.g. lichen planus and Sjögren's syndrome). There are also currently many clinical trials studying the effects of chloroquine analogues in various diseases, such as malignant neoplasms of the lung, breast, prostate, pancreas and colon, melanoma, renal cell carcinoma, multiple myeloma, influenza, HIV infection, and the metabolic syndrome (Table 5). To investigate the roles of these analogues in a wide variety of diseases, a number of molecular modifications, such as the prodrug131 and metabolomic approaches,132 have been used with the aims of improving their pharmacokinetic and pharmacodynamic properties, reducing undesirable side effects, costs and drug sensitivities. However, the exact mechanisms of action of the analogues and their effectiveness in these diseases remain to be demonstrated. Despite their benefits and their current use in >70 countries, chloroquine analogues remain unavailable for clinical use to treat patients in Japan due to a series of lawsuits as a result of the retinal toxicity of chloroquine in the 1970s. However, because of their use as the standard of care worldwide, clinical trials of hydroxychloroquine for SLE in Japan have recently been started.133,134 Our understanding of the history of chloroquine analogues suggests that the appropriate use of an efficacious therapy will soon lead to an era of improvement of patient care, survival and quality of life for many patients.
Table 5.
Selected examples of clinical trials of chloroquine analogues
| Status | Condition | Phase | Intervention | Trial ID |
|---|---|---|---|---|
| Recruiting | SLE | II | mycophenolate mofetil; HCQ or CQ; prednisone | NCT01946880 |
| Recruiting | RA | III | tocilizumab; DMARD (HCQ or CQ) | NCT01941940 |
| Recruiting | RA | III | DMARD (HCQ or CQ); tocilizumab | NCT01941095 |
| Completed, has results | RA, insulin resistance | III | HCQ | NCT01132118 |
| Completed | osteoarthritis | IV | GS and CS; (GS and CS)+PS or/and CQ | NCT00805519 |
| Recruiting | colorectal adenocarcinoma | I/II | QC; capecitabine | NCT01844076 |
| Recruiting | metastatic renal cell carcinoma | I/II | HCQ; IL2 | NCT01550367 |
| Recruiting | pancreatic cancer | II | capecitabine; HCQ; proton or photon radiation therapy | NCT01494155 |
| Recruiting | sarcoma | II | sirolimus and HCQ | NCT01842594 |
| Active, not recruiting | prostate cancer | II | HCQ | NCT00726596 |
| Recruiting | renal cell carcinoma | I | HCQ | NCT01144169 |
| Active, not recruiting | pancreatic cancer | II | HCQ | NCT01273805 |
| Active, not recruiting | pancreatic cancer | I/II | HCQ; gemcitabine | NCT01128296 |
| Recruiting | brain metastasis | CQ | NCT01727531 | |
| Recruiting | pancreatic cancer | I | CQ; gemcitabine | NCT01777477 |
| Recruiting | intraductal carcinoma | I/II | CQ | NCT01023477 |
| Recruiting | advanced solid tumours | I | CQ; carboplatin; gemcitabine | NCT02071537 |
| Recruiting | small cell lung cancer | I | CQ | NCT01575782 |
| Recruiting | small cell lung cancer | I | CQ | NCT00969306 |
| Recruiting | advanced cancers | I | HCQ; sirolimus; vorinostat | NCT01266057 |
| Recruiting | HIV | CQ | NCT01650558 | |
| Recruiting | malaria | III | sulfadoxine/pyrimethamine; CQ | NCT01443130 |
| Recruiting | hepatitis C virus | IV | CQ | NCT02058173 |
| Unknown | influenza | II | CQ | NCT01078779 |
| Completed | HIV | CQ | NCT02004314 | |
| Active, not recruiting | metabolic syndrome X, overweight, hypertension, dyslipidaemias, pre-diabetic state | II | CQ | NCT00455325 |
| Completed | primary SS | III | HCQ | NCT00632866 |
| Active, not recruiting | pre-diabetes | IV | HCQ; sugar pill | NCT01326533 |
| Recruiting | LAM | I | sirolimus and HCQ | NCT01687179 |
| Completed | autoimmune diseases, SS, dry eye | III | HCQ | NCT01601028 |
| Recruiting | type 2 DM | HCQ | NCT02026232 | |
| Completed | Hashimoto's thyroiditis | HCQ | NCT01760421 | |
| Completed | pulmonary sarcoidosis | III | PS; HCQ+PS | NCT02200146 |
| Active, not recruiting | Hashimoto's thyroiditis | HCQ | NCT02126683 | |
| Recruiting | congenital heart block, neonatal lupus, autoantibody-associated heart block | II | HCQ | NCT01379573 |
| Recruiting | NSCLC; advanced NSCLC, recurrent NSCLC | II | paclitaxel; carboplatin; HCQ; bevacizumab | NCT01649947 |
| Recruiting | malignant solid tumour | I | HCQ; vorinostat | NCT01023737 |
| Recruiting | DM type 2 with hyperglycaemia | II | HCQ; pioglitazone | NCT02303405 |
| Recruiting | Crohn's disease | II | ciprofloxacin; doxycycline; HCQ; budesonide | NCT01783106 |
| Recruiting | PCT | II | HCQ; phlebotomy | NCT01573754 |
| Unknown | HIV infections | II | HCQ | NCT01067417 |
| Recruiting | APS | III | HCQ | NCT01784523 |
| Completed, has results | CJD | II | QC | NCT00183092 |
| Unknown | prion disease | QC | NCT00104663 |
CQ, chloroquine; CS, chondroitin sulphate; DM, diabetes mellitus; DMARD, disease-modifying antirheumatic drug; GS, glucosamine; HCQ, hydroxychloroquine; NSCLC, non-small cell lung cancer; PS, prednisolone; QC, quinacrine; SS, Sjögren's syndrome.
Transparency declarations
None to declare.
Acknowledgements
I would like to acknowledge M. Shinohara and H. Takayanagi (Department of Cell Signaling, Tokyo Medical and Dental University) for technical assistance with the literature search.
References
- 1. Rodriguez-Caruncho C, Bielsa Marsol I. Antimalarials in dermatology: mechanism of action, indications, and side effects. Actas Dermosifiliogr 2014; 105: 243–52. [DOI] [PubMed] [Google Scholar]
- 2. Chen C. Development of antimalarial drugs and their application in China: historical review. Infect Dis Poverty 2014; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolf R, Wolf D, Ruocco V. Antimalarials: unapproved uses or indications. Clin Dermatol 2000; 18: 17–35. [DOI] [PubMed] [Google Scholar]
- 4. Price RN, von Seidlein L, Valecha N, et al. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14: 982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Souza NB, Carmo AML, da Silva AD, et al. Antiplasmodial activity of chloroquine analogs against chloroquine-resistant parasites, docking studies and mechanisms of drug action. Malar J 2014; 13: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC. Malaria Information and Prophylaxis. http://www.cdc.gov/malaria/travelers/country_table/a.html.
- 7. Mushtaque M, Shahjahan Reemergence of chloroquine (CQ) analogs as multi-targeting antimalarial agents: a review. Eur J Med Chem 2014; 90: 280–95. [DOI] [PubMed] [Google Scholar]
- 8. Moore BR, Page-Sharp M, Stoney JR, et al. Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob Agents Chemother 2011; 55: 3899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller F, König J, Glaeser H, et al. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother 2011; 55: 3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Hernandez I, Aguirre-Cruz L, Sotelo J, et al. Distribution of hydroxychloroquine in lymphoid tissue in a rabbit model for HIV infection. Antimicrob Agents Chemother 2014; 58: 584–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costedoat-Chalumeau N, Dunogué B, Morel N, et al. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014; 43: e167–80. [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 2011; 7: 718–29. [DOI] [PubMed] [Google Scholar]
- 13. Privette ED, Werth VP. Update on pathogenesis and treatment of CLE. Curr Opin Rheumatol 2013; 25: 584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wahie S, Meggitt SJ. Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. Br J Dermatol 2013; 169: 653–9. [DOI] [PubMed] [Google Scholar]
- 15. Peart E, Clowse ME. Systemic lupus erythematosus and pregnancy outcomes: an update and review of the literature. Curr Opin Rheumatol 2014; 26: 118–23. [DOI] [PubMed] [Google Scholar]
- 16. Morel N, Georgin-Lavialle S, Levesque K, et al. Neonatal lupus syndrome: literature review. Rev Med Interne 2014; 10.1016/j.revmed.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 17. Fajardo-Hermosillo LD, López-López L, Nadal A, et al. Multifocal osteonecrosis in systemic lupus erythematosus: case report and review of the literature. BMJ Case Rep 2013; 10.1136/bcr-2013-008980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katsanos KH, Voulgari PV, Tsianos EV. Inflammatory bowel disease and lupus: a systematic review of the literature. J Crohns Colitis 2012; 6: 735–42. [DOI] [PubMed] [Google Scholar]
- 19. Zhang LN, Shi TY, Yang YJ, et al. An SLE patient with prolactinoma and recurrent granulomatous mastitis successfully treated with hydroxychloroquine and bromocriptine. Lupus 2014; 23: 417–20. [DOI] [PubMed] [Google Scholar]
- 20. Chang AY, Piette EW, Foering KP, et al. Response to antimalarials in cutaneous lupus erythematosus a prospective analysis. Arch Dermatol 2011; 147: 1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace DJ, Gudsoorkar VS, Weisman MH, et al. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 2012; 8: 522–33. [DOI] [PubMed] [Google Scholar]
- 22. van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol 2009; 5: 531–41. [DOI] [PubMed] [Google Scholar]
- 23. Joshi P, Dhaneshwar SS. An update on disease modifying antirheumatic drugs. Inflamm Allergy Drug Targets 2014; 13: 249–61. [DOI] [PubMed] [Google Scholar]
- 24. Sammaritano LR, Bermas BL. Rheumatoid arthritis medications and lactation. Curr Opin Rheumatol 2014; 26: 354–60. [DOI] [PubMed] [Google Scholar]
- 25. Abdel MP, Figgie MP. Surgical management of the juvenile idiopathic arthritis patient with multiple joint involvement. Orthop Clin North Am 2014; 45: 435–42. [DOI] [PubMed] [Google Scholar]
- 26. Kingsbury SR, Tharmanathan P, Adamson J, et al. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis (HERO): study protocol for a randomized controlled trial. Trials 2013; 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol 2015; 27: 63–70. [DOI] [PubMed] [Google Scholar]
- 28. Pascart T, Richette P, Flipo RM. Treatment of nongout joint deposition diseases: an update. Arthritis 2014; 2014: 375202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Huang J, Wang X, et al. The eradication of breast cancer cells and stem cells by 8-hydroxyquinoline-loaded hyaluronan modified mesoporous silica nanoparticle-supported lipid bilayers containing docetaxel. Biomaterials 2013; 34: 7662–73. [DOI] [PubMed] [Google Scholar]
- 30. Barilli A, Atzeri C, Bassanetti I, et al. Oxidative stress induced by copper and iron complexes with 8-hydroxyquinoline derivatives causes paraptotic death of HeLa cancer cells. Mol Pharm 2014; 11: 1151–63. [DOI] [PubMed] [Google Scholar]
- 31. Vlahopoulos S, Critselis E, Voutsas IF, et al. New use for old drugs? Prospective targets of chloroquines in cancer therapy. Curr Drug Targets 2014; 15: 843–51. [DOI] [PubMed] [Google Scholar]
- 32. Kangwan N, Park JM, Kim EH, et al. Chemoquiescence for ideal cancer treatment and prevention: where are we now? J Cancer Prev 2014; 19: 89–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Farrill JS, Gordon N. Autophagy in osteosarcoma. Adv Exp Med Biol 2014; 804: 147–60. [DOI] [PubMed] [Google Scholar]
- 34. Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol 2014; 11: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duffy A, Le J, Sausville E, et al. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol 2014; 10.1007/s00280-014-2637-z. [DOI] [PubMed] [Google Scholar]
- 36. Dermawan JK, Gurova K, Pink J, et al. Quinacrine overcomes resistance to erlotinib by inhibiting FACT, NF-κB, and cell-cycle progression in non-small cell lung cancer. Mol Cancer Ther 2014; 13: 2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singal AK, Kormos-Hallberg C, Lee C, et al. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol 2012; 10: 1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez-Estrada A, Gomez-Morales LB, Garcia-Morillo JS. Sporadic porphyria cutanea tarda: treatment with chloroquine decreases hyperglycemia and reduces development of metabolic syndrome. Eur J Intern Med 2014; 25: e76–e77. [DOI] [PubMed] [Google Scholar]
- 39. Solomon LW, Neiders ME, Zwick MG, et al. Autoimmunity to deltaNp63alpha in chronic ulcerative stomatitis. J Dent Res 2007; 86: 826–31. [DOI] [PubMed] [Google Scholar]
- 40. Hornung T, Wenzel J. Innate immune-response mechanisms in dermatomyositis: an update on pathogenesis, diagnosis and treatment. Drugs 2014; 74: 981–98. [DOI] [PubMed] [Google Scholar]
- 41. Haro R, Revelles JM, Fariña Mdel C, et al. Wong's dermatomyositis: a new case and review of the literature. Int J Dermatol 2013; 52: 466–70. [DOI] [PubMed] [Google Scholar]
- 42. Vorselaars AD, Cremers JP, Grutters JC, et al. Cytotoxic agents in sarcoidosis: which one should we choose? Curr Opin Pulm Med 2014; 20: 479–87. [DOI] [PubMed] [Google Scholar]
- 43. Marchetti M, Baker MG, Noland MM. Treatment of subcutaneous sarcoidosis with hydroxychloroquine: report of 2 cases. Dermatol Online J 2014; 20: 21250. [PubMed] [Google Scholar]
- 44. Sparks JA, McSparron JI, Shah N, et al. Osseous sarcoidosis: clinical characteristics, treatment, and outcomes—experience from a large, academic hospital. Semin Arthritis Rheum 2014; 44: 371–9. [DOI] [PubMed] [Google Scholar]
- 45. Gottenberg JE, Ravaud P, Puéchal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014; 312: 249–58. [DOI] [PubMed] [Google Scholar]
- 46. Migkos MP, Markatseli TE, Iliou C, et al. Effect of hydroxychloroquine on the lipid profile of patients with Sjogren syndrome. J Rheumatol 2014; 41: 902–8. [DOI] [PubMed] [Google Scholar]
- 47. Wong JK, Nortley R, Andrews T, et al. Psychiatric manifestations of primary Sjögren's syndrome: a case report and literature review. BMJ Case Rep 2014; 10.1136/bcr-2012-008038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mumcu G, Bicakcigil M, Yilmaz N, et al. Salivary and serum B-cell activating factor (BAFF) levels after hydroxychloroquine treatment in primary Sjogren's syndrome. Oral Health Prev Dent 2013; 11: 229–34. [DOI] [PubMed] [Google Scholar]
- 49. Baibergenova A, Donovan J. Lichen planopilaris: update on pathogenesis and treatment. Skinmed 2013; 11: 161–5. [PubMed] [Google Scholar]
- 50. Fazel N. Cutaneous lichen planus: a systematic review of treatments. J Dermatolog Treat 2014; 9: 1–4. [DOI] [PubMed] [Google Scholar]
- 51. Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges 2013; 11: 981–91. [DOI] [PubMed] [Google Scholar]
- 52. Zhu Y, Li J, Bai Y, et al. Hydroxychloroquine decreases the upregulated frequencies of Tregs in patients with oral lichen planus. Clin Oral Investig 2014; 18: 1903–11. [DOI] [PubMed] [Google Scholar]
- 53. Masmoudi A, Abdelmaksoud W, Turki H, et al. Beneficial effects of antimalarials in the treatment of generalized granuloma annular in children. Tunis Med 2006; 84: 125–7. [PubMed] [Google Scholar]
- 54. Wolverton JE, Soter NA, Cohen DE. The natural history of chronic actinic dermatitis: an analysis at a single institution in the United States. Dermatitis 2014; 25: 27–31. [DOI] [PubMed] [Google Scholar]
- 55. Schultz KR, Su WN, Hsiao CC, et al. Chloroquine prevention of murine MHC-disparate acute graft-versus-host disease correlates with inhibition of splenic response to CpG oligodeoxynucleotides and alterations in T cell cytokine production. Biol Blood Marrow Transplant 2002; 8: 648–55. [DOI] [PubMed] [Google Scholar]
- 56. Khoury H, Trinkaus K, Zhang MJ, et al. Hydroxychloroquine for the prevention of acute graft-versus-host disease after unrelated donor transplantation. Biol Blood Marrow Transplant 2003; 9: 714–21. [DOI] [PubMed] [Google Scholar]
- 57. Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 2005; 14: 106–12. [DOI] [PubMed] [Google Scholar]
- 58. Al-Bari MAA, Shinohara M, Nagai Y, et al. Inhibitory effect of chloroquine on bone resorption reveals the key role of lysosomes in osteoclast differentiation and function. Inflamm Regen 2012; 32: 222–31. [Google Scholar]
- 59. Xiu Y, Xu H, Zhao C, et al. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest 2013; 124: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Solomon DH, Garg R, Lu B, et al. The effect of hydroxychloroquine on insulin sensitivity and lipid parameters in non-diabetic patients with rheumatoid arthritis: a randomized blinded cross-over trial. Arthritis Care Res (Hoboken) 2014; 66: 1246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Araiza-Casillas R, Diaz-Molina R, Gonzalez-Ortiz M, et al. Effects of hydroxychloroquine on insulin sensitivity and lipid profile in patients with rheumatoid arthritis. Rev Med Chil 2014; 141: 1019–25. [DOI] [PubMed] [Google Scholar]
- 62. Pareek A, Chandurkar NB, Thomas N, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin 2014; 30: 1257–66. [DOI] [PubMed] [Google Scholar]
- 63. Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 2007; 20: 160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hage MP, Al-Badri MR, Azar ST. A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther Adv Endocrinol Metab 2014; 5: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken) 2014; 66: 1619–26. [DOI] [PubMed] [Google Scholar]
- 66. Ward MM. Outcomes of hospitalizations for myocardial infarctions and cerebrovascular accidents in patients with systemic lupus erythematosus. Arthritis Rheum 2004; 50: 3170–6. [DOI] [PubMed] [Google Scholar]
- 67. Mar N, Kosowicz R, Hook K. Recurrent thrombosis prevention with intravenous immunoglobulin and hydroxychloroquine during pregnancy in a patient with history of catastrophic antiphospholipid syndrome and pregnancy loss. J Thromb Thrombolysis 2014; 38: 196–200. [DOI] [PubMed] [Google Scholar]
- 68. Arnaud L, Mathian A, Devilliers H, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev 2014; 14: 192–200. [DOI] [PubMed] [Google Scholar]
- 69. Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum 2010; 62: 863–8. [DOI] [PubMed] [Google Scholar]
- 70. Achuthan S, Ahluwalia J, Shafiq N, et al. Hydroxychloroquine's efficacy as an antiplatelet agent study in healthy volunteers: a proof of concept study. J Cardiovasc Pharmacol Ther 2014. pii:1074248414546324. [DOI] [PubMed] [Google Scholar]
- 71. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2008; 69: 20–8. [DOI] [PubMed] [Google Scholar]
- 72. Long L, Yang X, Southwood M, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type ii receptor degradation. Circ Res 2013; 112: 1159–70. [DOI] [PubMed] [Google Scholar]
- 73. Kurahara D, Morie M, Yamane M, et al. Pulmonary hemosiderosis in children with bronchopulmonary dysplasia. Case Rep Pediatr 2014; 2014: 876195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boursiquot JN, Gérard L, Malphettes M, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol 2013; 33: 84–95. [DOI] [PubMed] [Google Scholar]
- 75. Alkmim Teixeira R, Borba EF, Pedrosa A, et al. Evidence for cardiac safety and antiarrhythmic potential of chloroquine in systemic lupus erythematosus. Europace 2014; 16: 882–92. [DOI] [PubMed] [Google Scholar]
- 76. Fenollar F, Célard M, Lagier JC, et al. Tropheryma whipplei endocarditis. Emerg Infect Dis 2013; 19: 1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics 2014; 55: 409–13. [DOI] [PubMed] [Google Scholar]
- 78. Escobedo AA, Hanevik K, Almirall P, et al. Management of chronic Giardia infection. Expert Rev Anti Infect Ther 2014; 12: 1143–57. [DOI] [PubMed] [Google Scholar]
- 79. Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother 2015; 49: 196–206. [DOI] [PubMed] [Google Scholar]
- 80. Palazzi C, D'Amico E, D'Angelo S, et al. An update on the management of hepatitis C virus-related arthritis. Expert Opin Pharmacother 2014; 15: 2039–45. [DOI] [PubMed] [Google Scholar]
- 81. Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol 2014; 66: 319–26. [DOI] [PubMed] [Google Scholar]
- 82. Chauhan A, Khandkar M. Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination. Microb Pathog 2014; 78: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Keyaerts E, Li S, Vijgen L, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother 2009; 53: 3416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Forloni G, Artuso V, Roiter I, et al. Therapy in prion diseases. Curr Top Med Chem 2013; 13: 2465–76. [DOI] [PubMed] [Google Scholar]
- 86. Geschwind MD, Kuo AL, Wong KS, et al. Quinacrine treatment trial for sporadic Creutzfeldt-Jakob disease. Neurology 2013; 81: 2015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Taveira-DaSilva AM, Moss J. Optimizing treatments for lymphangioleiomyomatosis. Expert Rev Respir Med 2012; 6: 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mekinian A, Costedoat-Chalumeau N, Masseau A, et al. Obstetrical APS: is there a place for hydroxychloroquine to improve the pregnancy outcome? Autoimmun Rev 2015; 14: 23–9. [DOI] [PubMed] [Google Scholar]
- 89. Chaturvedi S, McCrae KR. Recent advances in the antiphospholipid antibody syndrome. Curr Opin Hematol 2014; 21: 371–9. [DOI] [PubMed] [Google Scholar]
- 90. Martin RE, Marchetti RV, Cowan AI, et al. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science 2009; 325: 1680–2. [DOI] [PubMed] [Google Scholar]
- 91. Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 2009; 10: 623–35. [DOI] [PubMed] [Google Scholar]
- 92. Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci 2007; 96: 729–46. [DOI] [PubMed] [Google Scholar]
- 93. Taherian E, Rao A, Malemud CJ, et al. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr Rheumatol Rev 2013; 9: 45–62. [DOI] [PubMed] [Google Scholar]
- 94. Gewirtz DA. The autophagic response to radiation: relevance for radiation sensitization in cancer therapy. Radiat Res 2014; 182: 363–7. [DOI] [PubMed] [Google Scholar]
- 95. Szakacs G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006; 5: 219–34. [DOI] [PubMed] [Google Scholar]
- 96. Gurova K. New hopes from old drugs: revisiting DNA-binding small molecules as anticancer agents. Future Oncol 2009; 5: 1685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nosal R, Jancinova V. Cationic amphiphilic drugs and platelet phospholipase A(2) (cPLA2). Thromb Res 2002; 105: 339–45. [DOI] [PubMed] [Google Scholar]
- 98. Geamănu Pancă A, Popa-Cherecheanu A, Marinescu B, et al. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. J Med Life 2014; 7: 322–6. [PMC free article] [PubMed] [Google Scholar]
- 99. Stelton CR, Connors DB, Walia SS, et al. Hydrochloroquine retinopathy: characteristic presentation with review of screening. Clin Rheumatol 2013; 32: 895–8. [DOI] [PubMed] [Google Scholar]
- 100. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmol 2015; 122: 110–16. [DOI] [PubMed] [Google Scholar]
- 101. Ascaso FJ, Rodriguez NA, San Miguel R, et al. The “flying saucer” sign on spectral domain optical coherence tomography in chloroquine retinopathy. Arthritis Rheum 2013; 65: 2322. [DOI] [PubMed] [Google Scholar]
- 102. Guha S, Coffey EE, Lu W, et al. Approaches for detecting lysosomal alkalinization and impaired degradation in fresh and cultured RPE cells: evidence for a role in retinal degenerations. Exp Eye Res 2014; 126: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011; 118: 415–22. [DOI] [PubMed] [Google Scholar]
- 104. Bortoli R, Santiago M. Chloroquine ototoxicity. Clin Rheumatol 2007; 26: 1809–10. [DOI] [PubMed] [Google Scholar]
- 105. Coutinho MB, Duarte I. Hydroxychloroquine ototoxicity in a child with idiopathic pulmonary haemosiderosis. Int J Pediatr Otorhinolaryngol 2002; 62: 53–7. [DOI] [PubMed] [Google Scholar]
- 106. Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 2014; 30: 1706–15. [DOI] [PubMed] [Google Scholar]
- 107. Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol 2013; 35: 434–42. [DOI] [PubMed] [Google Scholar]
- 108. Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013; 2: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol 2006; 44: 173–5. [DOI] [PubMed] [Google Scholar]
- 110. van Beek MJ, Piette WW. Antimalarials. Dermatol Clin 2001; 19: 147–60. [DOI] [PubMed] [Google Scholar]
- 111. Bentsi-Enchill KO. Pigmentary skin changes associated with ocular chloroquine toxicity in Ghana. Trop Geogr Med 1980; 32: 216–20. [PubMed] [Google Scholar]
- 112. Onyeji CO, Ogunbona FA. Pharmacokinetic aspects of chloroquine-induced pruritus: influence of dose and evidence for varied extent of metabolism of the drug. Eur J Pharm Sci 2001; 13: 195–201. [DOI] [PubMed] [Google Scholar]
- 113. Sifuentes Giraldo WA, Grandal Platero M, de la Puente Bujidos C, et al. Generalized skin hyperpigmentation and longitudinal melanonychia secondary to treatment with hydroxychloroquine in systemic lupus erythematosus. Reumatol Clin 2013; 9: 381–2. [DOI] [PubMed] [Google Scholar]
- 114. Jallouli M, Frances C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case–control study. JAMA Dermatol 2013; 149: 935–40. [DOI] [PubMed] [Google Scholar]
- 115. Murphy M, Carmichael AJ. Fatal toxic epidermal necrolysis associated with hydroxychloroquine. Clin Exp Dermatol 2001; 26: 457–8. [DOI] [PubMed] [Google Scholar]
- 116. Leckie MJ, Rees RG. Stevens–Johnson syndrome in association with hydroxychloroquine treatment for rheumatoid arthritis. Rheumatology (Oxford) 2002; 41: 473–4. [DOI] [PubMed] [Google Scholar]
- 117. Martin Garcia RF, del R Camacho N, Sanchez JL. Chloroquine-induced, vitiligo-like depigmentation. J Am Acad Dermatol 2003; 48: 981–3. [DOI] [PubMed] [Google Scholar]
- 118. Ghaffarpour G, Jalali MHA, Yaghmaii B, et al. Chloroquine/hydroxychloroquine induced pemphigus. Int J Dermatol 2006; 45: 1261–3. [DOI] [PubMed] [Google Scholar]
- 119. Luong MS, Bessis D, Raison Peyron N, et al. Severe mucocutaneous necrotizing vasculitis associated with the combination of chloroquine and proguanil. Acta Dermatol Venereol 2003; 83: 141. [DOI] [PubMed] [Google Scholar]
- 120. Tristano AG, Falcon L, Willson M, et al. Seizure associated with chloroquine therapy in a patient with systemic lupus erythematosus. Rheumatol Int 2004; 24: 315–6. [DOI] [PubMed] [Google Scholar]
- 121. Kushlaf HA. Emerging toxic neuropathies and myopathies. Psychiatr Clin North Am 2013; 36: 209–18. [DOI] [PubMed] [Google Scholar]
- 122. Ghosh PS, Swift D, Engel AG. Teaching neuroimages: hydroxychloroquine-induced vacuolar myopathy. Neurology 2013; 80: e248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bolanos-Meade J, Zhou L, Hoke A, et al. Hydroxychloroquine causes severe vacuolar myopathy in a patient with chronic graft-versus-host disease. Am J Hematol 2005; 78: 306–9. [DOI] [PubMed] [Google Scholar]
- 124. Wozniacka A, McCauliffe DP. Optimal use of antimalarials in treating cutaneous lupus erythematosus. Am J Clin Dermatol 2005; 6: 1–11. [DOI] [PubMed] [Google Scholar]
- 125. Sharma N, Varma S. Unusual life-threatening adverse drug effects with chloroquine in a young girl. J Postgrad Med 2003; 49: 187. [PubMed] [Google Scholar]
- 126. Kutz DC, Bridges AJ. Bullous rash and brown urine in a systemic lupus erythematosus patient treated with hydroxychloroquine. Arthritis Rheum 1995; 38: 440–3. [DOI] [PubMed] [Google Scholar]
- 127. Hocker JM, Schmid H, Weiss M, et al. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry's disease: case report and review of the literature. Hum Pathol 2003; 34: 285–9. [DOI] [PubMed] [Google Scholar]
- 128. Gravani A, Gaitanis G, Zioga A, et al. Synthetic antimalarial drugs and the triggering of psoriasis—do we need disease-specific guidelines for the management of patients with psoriasis at risk of malaria? Int J Dermatol 2014; 53: 327–30. [DOI] [PubMed] [Google Scholar]
- 129. Adam I, Elbashir MI. Acute gluteal abscess due to chloroquine injection in Sudanese pregnant woman. Saudi Med J 2004; 25: 963–4. [PubMed] [Google Scholar]
- 130. Bailey K, McKee D, Wismer J, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: first case report in Canada and review of the literature. J Cutan Med Surg 2013; 17: 414–8. [DOI] [PubMed] [Google Scholar]
- 131. Davanco MG, Aguiar ACC, dos Santos LA, et al. Evaluation of antimalarial activity and toxicity of a new primaquine prodrug. PLoS One 2014; 9: e105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vincent IM, Barrett MP. Metabolomic-based strategies for anti-parasite drug discovery. J Biomol Screen 2014; pii:1087057114551519. [DOI] [PubMed] [Google Scholar]
- 133. Yamamoto T, Hiraiwa T. Beneficial effect of hydroxychloroquine on cutaneous lupus erythematosus in a Japanese girl. J Dermatol 2014; 41: 357–9. [DOI] [PubMed] [Google Scholar]
- 134. Yokogawa N, Kato Y, Sugii S, et al. Response to hydroxychloroquine in Japanese patients with systemic lupus erythematosus using the cutaneous lupus erythematosus disease area and severity index (CLASI). Mod Rheumatol 2012; 22: 249–55. [DOI] [PubMed] [Google Scholar]
- 135. Khan DA. Alternative agents in refractory chronic urticaria: evidence and considerations on their selection and use. J Allergy Clin Immunol Pract 2013; 1: 433–440.e1. [DOI] [PubMed] [Google Scholar]
- 136. Asero R, Tedeschi A, Cugno M. Treatment of refractory chronic urticaria: current and future therapeutic options. Am J Clin Dermatol 2013; 14: 481–8. [DOI] [PubMed] [Google Scholar]
- 137. Growe RG, Luster MI, Fail PA, et al. Quinacrine-induced occlusive fibrosis in the human fallopian tube is due to a unique inflammatory response and modification of repair mechanisms. J Reprod Immunol 2013; 97: 159–66. [DOI] [PubMed] [Google Scholar]
- 138. MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol 2012; 67: 955–61. [DOI] [PubMed] [Google Scholar]
- 139. Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther 2013; 26: 354–63. [DOI] [PubMed] [Google Scholar]


