Abstract
Background
Preterm birth is a major determinant of adverse health consequences, and early term births are also associated with increased risk of various outcomes. In light of climate change, the effect of ambient temperature on earlier delivery is an important factor to consider. Several studies have focused on associations of ambient air temperature (Ta) on preterm birth, but few have examined associations with early term births.
Aims
To investigate the association of prenatal exposure to Ta with preterm birth (< 37 completed gestation weeks) and with early-term birth (< 39 completed gestation weeks) in a semi-arid climate.
Methods
All singleton deliveries at the Soroka Medical Center from the Southern district of Israel, with estimated conception dates between May 1, 2004 and March 31, 2013 (N=62,547) were linked to prenatal Ta estimates from a spatiotemporally resolved model, with daily 1 km resolution. We used time-dependent Cox regression models with weekly mean Ta throughout gestation, adjusted for calendar month and year of conception, ethnicity, census-level socio-economic status and population density.
Results
Ta was positively associated with late preterm birth (31+0/7 – 36+6/7 weeks), with increased risk in the upper Ta quintile as compared to the third quintile, hazard ratio (HR) = 1.31, 95% confidence interval (CI) = 1.11 −1.56. Ta also associated with early term birth (37+0/6 – 38+6/7), with increased risk in the upper Ta quintile as compared to the third quintile, HR = 1.24, 95% CI = 1.13 – 1.36.
Conclusion
Exposure to high ambient temperature during pregnancy is associated with a higher risk of preterm and early term birth in southern Israel.
Keywords: Ambient temperature, preterm birth, early term
2. Introduction
Preterm birth is a major determinant of adverse health consequences, both short and long term, and the biggest contributor to the infant mortality rate (Lawn et al., 2006; Romero et al., 2006). Often, the specific cause of preterm birth is unclear. However, there are known risk factors of different types – genetic, behavioral, socioeconomic and environmental. Of interest is the inverse association between maternal cortical releasing hormone (CRH) and the timing of delivery also known as placental clock (Romero et al., 2006).
Recent studies have shown that early term births, defined as between 37 and 39 weeks of gestation, are also associated with higher risks of infant death and neonatal morbidity compared with full term births (gestation weeks 40–41) (Helle et al., 2016; Zhang and Kramer, 2009). Early term births are associated with long term inferior cognitive outcomes when compared with full term births, and although the absolute risk is smaller than for preterm births, early term births account for about 15–30% of all births (Delnord et al., 2018) and therefore even small risks can constitute a substantial public health burden.
In recent years, the influence of ambient near-surface air temperature (Ta) on adverse pregnancy outcome was studied. Several recent reviews reported that most, but not all, studies supported an association between high Ta and preterm birth (Carolan-Olah and Frankowska, 2014; Linn B. Strand et al., 2011; Zhang et al., 2017). However, the variability in results indicates the need for more studies. One source of variability is the exposure assessment method. Previous studies have typically used data from air temperature stations. Only a few used spatio-temporal model-predicted Ta, which provides a more accurate measure (Kloog et al., 2015; Sun et al., 2019). Another source of variability is the different climates being studied. Definitions of extreme hot and extreme cold can vary greatly across geographic areas. Studies are needed in diversified populations and climate zones in order to further understand the association between Ta and early delivery.
The timing of exposure used in the studies also varied: average Ta over the entire pregnancy (Guo et al., 2018; Ha et al., 2017b), trimester average (Zheng et al., 2018), immediately preceding the birth (Avalos et al., 2017; Cox et al., 2016; Mathew et al., 2017; Vicedo-Cabrera et al., 2015) were some of the windows used. Alternatively, some of the studies were focused on extreme short-term exposure or heat waves only (Auger et al., 2014; Dadvand et al., 2011; Wang et al., 2013). In order to avoid bias, studies of premature birth should consider that pregnancies have varying lengths, and that the chances of delivery increase with increasing gestational age. Considering entire pregnancy exposure for preterm births compared to ongoing pregnancies with exposure limited to the same length of gestation (Guo et al., 2018; Ha et al., 2017a) would avoid these biases, but would result in a substantial loss of statistical power.
The current study population is from the Southern District of Israel, characterized by a semi-arid climate zone with long, hot summers. Many of the women in our population are of lower socioeconomic status and may be more susceptible to heat. Two previous studies in this region examined the trends and rates of preterm delivery: One study found that the incidence of preterm delivery actually precedes sharp changes in maximum temperature by three days (Yackerson et al., 2008), and the other one found significant seasonal variation in preterm incidence, with the highest incidence during the summer period (Walfisch et al., 2017). Our aim in the current study is to examine associations between Ta and risk of preterm and early term births in this region.
3. Methods
3.1. Study population
Our study examines the association of Ta with preterm and early term birth. The study population included all singleton live births in Soroka University Medical Center (SUMC) with estimated conception dates between May 1, 2004 and March 31, 2013, of women from the Southern District of Israel who are Clalit Health Services members. We have limited the study population by conception dates (as determined in hospital, based on date of last menstrual period, if available, and ultrasonographic measures), in order to avoid a fixed cohort bias (Linn Beate Strand et al., 2011). SUMC is the only hospital providing tertiary services to the residents of the southern region of the country, which include the largest obstetrical emergency department in the country. Clalit Health Services is the largest health care provider in the area, covering approximately 70% of the population in the South. Under the National Health Insurance Law, every Israeli citizen is entitled to health care services and has the right to choose from four similar health maintenance organizations without limitations (Ministry of Health, 2015). Births missing data on gestational age were excluded (n = 72), resulting in a total of N=62,547 singleton births included in this study.
3.2. Exposure data
Ta is usually measured by sensors inside meteorological monitoring stations that provide very accurate data. However, they can be sparsely distributed in urban areas, thus not capturing most of the inter-city spatial variability of Ta. In addition, the monitors tend to be located in urban areas thus leaving spatial coverage gaps across rural areas which leads to increased exposure misclassification. Satellite based measurements provide daily surface temperature (Ts) data with high spatial and temporal resolution. We used a hybrid model that generated estimates of average daily Ta at 1 km resolution across Israel. For generating the daily estimates, we used linear mixed effect models, IDW (inverse distance weighted) interpolations and thin plate splines (using a smooth nonparametric function of longitude and latitude) to first calibrate between Ts and Ta in those locations where we have available data for both and used that calibration to fill in neighboring cells without surface monitors or missing Ts. Out-of-sample ten-fold cross validation (CV) was used to quantify the accuracy of our predictions. Our model performance was excellent for both days with and without available Ts observations for both Aqua and Terra (CV Aqua R2 results for min 0.966, mean 0.986, and max 0.967; CV Terra R2 results for min 0.965, mean 0.987, and max 0.968) (Rosenfeld et al., 2017). Average Ta values over the entire study period are shown in a map in Supplemental Fig. 1. Maternal residence was geocoded according to address at the date of birth and Ta during each gestation week was assessed by averaging mean daily Ta over that week using the exposure model, date of birth and gestational age.
3.3. Statistical methods
We used time dependent Cox proportional hazard regression models to estimate associations between exposure to Ta and risk of preterm birth, as well as early term birth. We considered gestational age (by week) as time-to-event data, and weekly mean Ta as a time dependent exposure variable. Mean weekly Ta was categorized into five quintiles, with the middle category set as the reference. Additional models also considered mean weekly Ta as a continuous variable modeled with natural splines with a priori knots located in Ta tertile breaks (16.8 and 24.18 °C), to observe a more detailed exposure-response curve. The exposure examined in the main models was the exposure during the week of birth. In addition, in order to account for a delayed effect, we also considered two-week moving average Ta exposure.
We separately analyzed the risk of birth at three distinct stages of pregnancy: Early preterm (23 – 30+6/7 weeks), late preterm (31+0/7 – 36+6/7 weeks), and early term (37+0/7 – 38+6/7 weeks). In each of these models, only the relevant population at risk and relevant exposure periods were considered. For example, in the model analyzing the risk of birth during the gestational period of 31+0/6 – 36+6/7 weeks, exposure and births during those weeks only were considered, with the population being all births with gestational age ≥31. Similarly, all pregnancies that have reached complete 37 weeks (term) are included in the early term (37+0/7 – 38+6/7) analysis, with censoring occurring at complete 39 weeks.
We used a Directed Acyclic Graph (DAG) (Greenland et al., 1999; Textor et al., 2017) to identify the minimum set of factors that we need to adjust for in our model, in order to eliminate potential confounding of the association between Ta and early delivery (Supplemental Fig. 2). Accordingly, we added to the model calendar conception month and calendar year, ethnicity (Jewish / non-Jewish), census level socioeconomic status (SES, as a proxy for individual SES) and population density. Information for the latter two variables was obtained from the Israel Central Bureau of Statistics (ICBS, 2019) and each one was categorized into five levels.
Possible effect modifications by ethnicity, maternal age or child sex were explored by models stratified by these factors. Sensitivity analyses were performed by fitting models that included only those for whom addresses were geocoded at the house or street level or were in small rural areas as well as models that excluded newborns who were small for gestational age. All analyses were conducted in R, version 3.6.1 (R Core Team, 2018), with the survival models fitted and tested for violations of proportionality assumption (by interaction terms with time) using the survival package (Therneau T, 2015). Continuous models were fitted using the function ns() (formerly in R package splines), and plotted using R package Greg (Gordon and Seifert, 2020).
4. Results
Our analysis included 62,547 singleton births, with mean gestational age of 38.9 weeks, 7.8% preterm births, and 10.6% of the preterm births before 31 completed weeks of gestation (Table 1). Our population is composed of 40% Jewish and 51% male newborns, with entire pregnancy Ta ranged 12.6 – 29.1°C, with mean of 19.8 (±12.6) °C. The weekly mean Ta ranged 4.6 – 36.5°C.
Table 1.
Characteristics of the Study Population (N = 62,547)
| Characteristic | % (Number)/ Mean (SD) |
|---|---|
| Gestational Age (weeks) | 39 (2.0) |
| Birth Weight (gr) | 3179 (521) |
| Maternal Age | 28.8 (5.7) |
| Ethnicity | |
| Jewish | 40% (25,120) |
| Non-Jewish | 60% (37,427) |
| Female gender | 49% (30,600) |
| Low Birth Weight (< 2500 gr) | 7.9% (4966) |
| Preterm delivery | 7.8% (4852) |
| Early preterm (23 – 30+6/7 weeks) | 0.82% (516) |
| Late preterm (31 – 36+6/7 weeks) | 6.9% (5336) |
| Early term delivery (weeks 37 – 38+6/7) | 25% (15,630) |
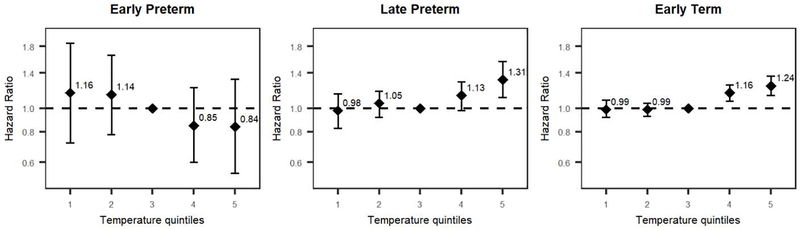
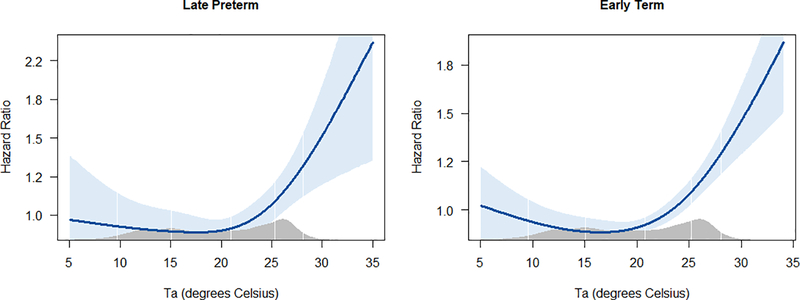
We observed no association between Ta and risk of birth before 31 completed weeks (Fig 1, left). Among late preterm deliveries, however, preterm birth was associated with higher Ta, with HR = 1.31 (95% CI = 1.11– 1.56) and HR = 1.13 (95% CI = 0.98– 1.29) for the 5th and 4th Ta quintiles respectively, in comparison to the middle quintile (Fig. 1, center). Heat was also associated with early term birth, with exposure to highest Ta quintile (HR = 1.24, 95% CI = 1.13 – 1.36) as well as the 4th Ta quintile (HR = 1.16, 95% CI = 1.07 – 1.25) (Fig. 1, right). Further analysis of the risk of late preterm or early term birth with Ta as a continuous variable is shown in Fig. 2.
Figure 1.
Associations of exposure to ambient air temperature (Ta), taken as weekly mean, during the periods of 23 – 30+6/7 (left), 31 – 36+6/7 (center) and 37 – 38+6/7 weeks of pregnancy, with preterm birth or early term birth, modeled using Cox proportional hazard regressions. All models were adjusted for year of conception, month of conception, ethnicity, population density and socioeconomic status. There were N=62,547 pregnancies at risk of delivery from 23 – 30+6/7 weeks and 516 events; N=62,031 pregnancies at risk of delivery from 31 – 36+6/7 weeks and 4,336 events; and N=57,695 pregnancies at risk of delivery and 15,630 events. Ta was modeled as a categorical variable using quintiles within the following ranges (°C): 4.6–13.5 (1), 13.6–17.2 (2), 17.3–22.1 (3), 22.2–25.4 (4), 25.6– 36.5 (5). The third quintile (mild temperatures) was set as reference.
Figure 2.
Association between Ta exposure in gestational age periods of 31 – 36+6/7 weeks and 37 – 38+6/7 weeks and risk of early birth with Ta modeled as natural spline. The Blue band represents 95% confidence interval and the gray area at the bottom of each graph represents the Ta distribution in the study population.
An analysis of the association of Ta with early delivery using two-week moving average exposure instead of 1-week exposure yielded very similar results (Supplemental Fig. 3). A sensitivity analysis excluding newborns who were small for gestational age yielded slightly stronger results for association of Ta and late preterm birth, but similar results for early term birth (Supplemental Fig. 4). A second sensitivity analysis excluding newborns living in urban areas for whom residential addresses were geocoded at the locality level only, did not alter the main results (Supplemental Fig. 5). Models stratified by newborn sex, ethnicity and maternal age are reported in supplemental fig. 6, 7 and 8–9, respectively. These models do not show strong evidence of effect modification by any of these factors. However, a modification of the Ta effect on late preterm birth by sex is suggested, with the effect of heat being stronger in females than in males.
5. Discussion
This study provides further evidence that short term exposure to high ambient temperatures is associated with an increased risk of late preterm birth and to a lesser extent for early term delivery. These results are consistent with most studies examining associations of Ta and preterm delivery (Carolan-Olah and Frankowska, 2014; Zhang et al., 2017).However, only few studies did not limit their analysis to a preterm outcome, and examined associations of heat and gestational age for term pregnancies as well. A study from Chicago found no association of a heat wave with gestational age (Porter et al., 1999). Another study from Brisbane, Australia detected a significant association of heat with late preterm birth only, but not early term (Strand et al., 2012a). Finally, a study from Barcelona demonstrated an association of heat with gestational age for the entire pregnancy, but did not examine whether this association is different in different periods of gestation (Dadvand et al., 2011). These studies, and especially the Australian birth cohort, had relatively large sample sizes, but it is possible that the lack of associations in these studies is a result of crude exposure assessment methods based on several meteorological stations, without a spatio-temporal model that would have reduce random exposure error.
The association of high ambient temperature and late preterm birth seemed stronger in females, a finding that is consistent with a similar tendency found in a study from Belgium (Cox et al., 2016). Studies have shown that male sex is an independent risk factor for preterm birth with the risk being strongest for very short duration pregnancies and declining up to the 36th week, where it essentially disappears (Di Renzo et al., 2007; Zeitlin, 2002). Therefore, it is possible that there are other major causes for male preterm birth during 31+0/6 – 36+6/7 weeks of gestation that weaken the relative observed effect of Ta in males during this period. Fetal sex has been shown to modify associations of various stressors with fetal survival and other pregnancy outcomes (Catalano et al., 2013, 2006; Fukuda et al., 2014; Wainstock et al., 2015). However, it is also possible that the differences we observed in the associations between female and male fetuses are a result of random variability and limited sample size.
We did not find an association of Ta with preterm birth during the period of 23+0/7 – 30+6/7 weeks of gestation. This result is contrary to a previous report, which had a much larger sample size, suggesting that Ta exposure during this period increases the risk of preterm birth (He et al., 2015). We may have lacked the power with our sample size to detect such an association since the number of births in earlier weeks was much smaller. An alternative explanation is that there are different underlying mechanisms leading to early preterm deliveries, such as intra-amniotic infection or inflammation, vascular placental diseases and others (Romero et al., 2014).
The mechanism by which exposure to extreme heat may cause early delivery is not clear. One suggested theory is that pregnant women may become dehydrated with extreme heat exposure, which could decrease uterine blood flow and increase secretion of antidiuretic hormones and oxytocin, which would induce labor (Stan et al., 2002). However, this has only been seen in animal models. Another proposed explanation is that heat stress, like other stresses, causes elevated corticotropin-releasing hormone concentrations which has been found to be associated with preterm labor (Smith and Nicholson, 2007). High Ta may also change the pregnant woman’s exposure to other factors, possibly through changing her behavior. For example, changes in Ta may change the fraction of time spent indoors (and this by itself will change exposure to various indoor and ambient air pollutants and to air conditioning), physical activity, nutrition and other factors that may mediate the effect of Ta. Inspection of such mechanisms may be explored in future studies that track the behavior of pregnant women when Ta changes.
Our study has the advantage of being based on nonselective population data. SUMC serves the entire population in the region and captures most of the births. It is a diverse population culturally and socioeconomically. We included 70% of all deliveries taking place at SUMC over a 9-year period, limiting only by health fund which should not introduce bias. Another strength of our study is that we used a spatially refined, satellite-based prediction model to more accurately assess exposure compared to using data from weather stations. Air temperature stations do not adequately capture the variability within urban areas which may have heat islands, possibly leading to increased exposure misclassification. These stations also have limited coverage, particularly in rural areas. Use of a prediction model did not restrict us to populations near monitoring sites which may not be representative of the population as a whole.
Additionally, we analyzed the association using a survival approach which has been recommended for the study of the association between time dependent variables and preterm birth (S. O’Neill et al., 2003; Strand et al., 2012b). In this way we compared fetuses of the same gestational age and avoided bias caused by different probabilities of giving birth at different gestational ages. We also looked at various stages of pregnancy and separately for male and female fetuses, suggesting a non-uniform association throughout pregnancy and by sex that should be reproduced in future studies.
Our study has several limitations. Unfortunately, we do not have information on stillbirths in this population. This may result in live-birth bias (Liew et al., 2015; Raz et al., 2018), a type of selection bias that is related to the exposure in question affecting the chances of live birth. For this bias to act, Ta must increase the risk of stillbirth as well as preterm birth. Indeed, there is some evidence for associations of high Ta and risk of stillbirth (Li et al., 2018; Wang et al., 2019). In addition, for this bias to act there must be some other common cause of stillbirth and preterm birth that is not considered in our study, e.g., genetic factors. If this bias occurs, it is expected to preferentially deplete pregnancies that were exposed to higher Ta from our births samples, and thus bias our results towards the null. Since stillbirths is more common before week 32 (MacDorman et al., 2015), the earlier period may be more susceptible to this bias. In addition, we do not have information on maternal habits and behavior during pregnancy. The usage of Ta from a spatiotemporal model, however (unlike, for example, personal monitoring), generates exposure assessment that are based only on timing and address, and are thus independent of maternal behavior. Therefore, our results cannot be confounded by such variables (Weisskopf and Webster, 2017).
In summary, our study provides additional evidence for the possible effects of increasing global temperatures on human health. Given the rising concerns about climate change and the health problems associated with earlier birth, our research highlights the need for more awareness among health professionals, policy makers and pregnant women on the potential adverse effects of extreme temperature, even for term pregnancies, and further stresses the urgent need of climate change mitigation.
Supplementary Material
Highlights.
We studied the association between ambient temperature and early delivery in Southern Israel
High ambient temperature is associated with increased risk of late preterm and early term delivery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 References
- Auger N, Naimi AI, Smargiassi A, Lo E, Kosatsky T, 2014. Extreme heat and risk of early delivery among preterm and term pregnancies. Epidemiology 25, 344–350. 10.1097/EDE.0000000000000074 [DOI] [PubMed] [Google Scholar]
- Avalos LA, Chen H, Li D-K, Basu R, 2017. The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ. Health 16, 5 10.1186/s12940-017-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Malig B, Ostro B, 2010. High Ambient Temperature and the Risk of Preterm Delivery. Am. J. Epidemiol. 172, 1108–1117. 10.1093/aje/kwq170 [DOI] [PubMed] [Google Scholar]
- Carolan-Olah M, Frankowska D, 2014. High environmental temperature and preterm birth: A review of the evidence. Midwifery 30, 50–59. [DOI] [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Marks AR, Eskenazi B, 2006. Exogenous shocks to the human sex ratio : the case of September 11, 2001 in New York City 21, 3127–3131. 10.1093/humrep/del283 [DOI] [PubMed] [Google Scholar]
- Catalano R, Yorifuji T, Kawachi I, 2013. Natural selection in utero: Evidence from the great east japan earthquake. Am. J. Hum. Biol. 25, 555–559. 10.1002/ajhb.22414 [DOI] [PubMed] [Google Scholar]
- Cox B, Vicedo-Cabrera AM, Gasparrini A, Roels HA, Martens E, Vangronsveld J, Forsberg B, Nawrot TS, 2016. Ambient temperature as a trigger of preterm delivery in a temperate climate. J. Epidemiol. Community Health 70, 1191–1199. 10.1136/jech-2015-206384 [DOI] [PubMed] [Google Scholar]
- Dadvand P, Basagaña X, Sartini C, Figueras F, Vrijheid M, de Nazelle A, Sunyer J, Nieuwenhuijsen MJ, 2011. Climate extremes and the length of gestation. Environ. Health Perspect. 119, 1449–53. 10.1289/ehp.1003241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnord M, Mortensen L, Hindori-Mohangoo AD, Blondel B, Gissler M, Kramer MR, Richards JL, Deb-Rinker P, Rouleau J, Morisaki N, Nassar N, Bolumar F, Berrut S, Nybo Andersen AM, Kramer MS, Zeitlin J, 2018. International variations in the gestational age distribution of births: An ecological study in 34 high-income countries. Eur. J. Public Health 28, 303–309. 10.1093/eurpub/ckx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM, 2007. Does fetal sex affect pregnancy outcome? Gend. Med. 4, 19–30. 10.1016/S1550-8579(07)80004-0 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Ph D, Fukuda K, Shimizu T, Ph D, 2014. Climate change is associated with male : female ratios of fetal deaths and newborn infants in Japan. Fertil. Steril. 102, 1364–1370.e2. 10.1016/j.fertnstert.2014.07.1213 [DOI] [PubMed] [Google Scholar]
- Gordon M, Seifert R, 2020. R Package Greg. [Google Scholar]
- Greenland S, Pearl J, Robins JM, 1999. Causal Diagrams for Epidemiologic Research. Epidemiology. 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- Guo T, Wang Yuanyuan, Zhang H, Zhang Y, Zhao J, Wang Yan, Xie X, Wang L, Zhang Q, Liu D, He Y, Yang Y, Xu J, Peng Z, Ma X, 2018. The association between ambient temperature and the risk of preterm birth in China. Sci. Total Environ. 613–614, 439–446. 10.1016/j.scitotenv.2017.09.104 [DOI] [PubMed] [Google Scholar]
- Ha S, Zhu Y, Kim SS, Mendola P, Liu D, Sherman S, 2017a. Ambient Temperature and Early Delivery of Singleton Pregnancies. Environ. Health Perspect. 125, 453–459. 10.1289/EHP97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Zhu Y, Liu D, Sherman S, Mendola P, 2017b. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ. Res. 155, 394–400. 10.1016/j.envres.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J-R, Liu Y, Xia X-Y, Ma W-J, Lin H-L, Kan H-D, Lu J-H, Feng Q, Mo W-J, Wang P, Xia H-M, Qiu X, Muglia LJ, 2015. Ambient Temperature and the Risk of Preterm Birth in Guangzhou, China (2001–2011). Environ. Health Perspect. 124, 1100–6. 10.1289/ehp.1509778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle E, Andersson S, Häkkinen U, Järvelin J, Eskelinen J, Kajantie E, 2016. Morbidity and Health Care Costs After Early Term Birth. Paediatr. Perinat. Epidemiol. 30, 533–540. 10.1111/ppe.12321 [DOI] [PubMed] [Google Scholar]
- ICBS, 2019. Israel Central Bureau of Statistics [WWW Document].
- Kloog I, Melly SJ, Coull BA, Nordio F, Schwartz JD, 2015. Using Satellite-Based Spatiotemporal Resolved Air Temperature Exposure to Study the Association between Ambient Air Temperature and Birth Outcomes in Massachusetts. Environ. Health Perspect. 123, 1053–1058. 10.1289/ehp.1308075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Wilczynska-Ketende K, Cousens SN, 2006. Estimating the causes of 4 million neonatal deaths in the year 2000. Int. J. Epidemiol. 35, 706–718. 10.1093/ije/dyl043 [DOI] [PubMed] [Google Scholar]
- Li S, Chen G, Jaakkola JJK, Williams G, Guo Y, 2018. Temporal change in the impacts of ambient temperature on preterm birth and stillbirth: Brisbane, 1994–2013. Sci. Total Environ. 634, 579–585. 10.1016/j.scitotenv.2018.03.385 [DOI] [PubMed] [Google Scholar]
- Liew Z, Olsen J, Cui X, Ritz B, Arah OA, 2015. Bias from conditioning on live birth in pregnancy cohorts: An illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int. J. Epidemiol. 44, 345–354. 10.1093/ije/dyu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDorman MF, Reddy UM, Silver RM, 2015. Trends in Stillbirth by Gestational Age in the United States, 2006–2012. Obstet. Gynecol 10.1097/AOG.0000000000001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Mathur D, Chang AB, McDonald E, Singh GR, Nur D, Gerritsen R, 2017. Examining the Effects of Ambient Temperature on Pre-Term Birth in Central Australia. Int. J. Environ. Res. Public Health 14 10.3390/ijerph14020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, 2015. Rights of the insured under the National Health Insurance Law [WWW Document].
- Porter KR, Thomas SD, Whitman S, 1999. The relation of gestation length to short-term heat stress. Am. J. Public Health 89, 1090–1092. 10.2105/AJPH.89.7.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A language and environment for statistical computing.
- Raz R, Kioumourtzoglou MA, Weisskopf MG, 2018. Live-Birth Bias and Observed Associations Between Air Pollution and Autism. Am. J. Epidemiol. 187, 2292–2296. 10.1093/aje/kwy172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ, 2014. Preterm labor: One syndrome, many causes. Science (80-.). 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic J, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M, 2006. The preterm parturition syndrome. BJOG An Int. J. Obstet. Gynaecol. 113, 17–42. 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld A, Dorman M, Schwartz J, Novack V, Just AC, Kloog I, 2017. Estimating daily minimum, maximum, and mean near surface air temperature using hybrid satellite models across Israel. Environ. Res. 159, 297–312. [DOI] [PubMed] [Google Scholar]
- O’Neill S, M., Hertz-Picciotto I, Pastore LM, Weatherley BD, 2003. Have studies of urinary tract infection and preterm delivery used the most appropriate methods? Paediatr. Perinat. Epidemiol. 17, 226–233. 10.1046/j.1365-3016.2003.00499.x [DOI] [PubMed] [Google Scholar]
- Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P, 2013. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001–2010 . Environ. Int. 61, 77–87. 10.1016/J.ENVINT.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Smith R, Nicholson RC, 2007. Corticotrophin releasing hormone and the timing of birth. Front. Biosci 10.2741/2113 [DOI] [PubMed] [Google Scholar]
- Stan CM, Boulvain M, Pfister R, Hirsbrunner-Almagbaly P, 2002. Hydration for treatment of preterm labour, in: Stan CM (Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, Chichester, UK: 10.1002/14651858.CD003096 [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S, 2012a. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am. J. Epidemiol. 175, 99–107. 10.1093/aje/kwr404 [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S, 2012b. Maternal Exposure to Ambient Temperature and the Risks of Preterm Birth and Stillbirth in Brisbane, Australia. Am. J. Epidemiol. 175, 99–107. 10.1093/aje/kwr404 [DOI] [PubMed] [Google Scholar]
- Strand Linn B., Barnett AG, Tong S, 2011. The influence of season and ambient temperature on birth outcomes: A review of the epidemiological literature. Environ. Res. 111, 451–462. 10.1016/J.ENVRES.2011.01.023 [DOI] [PubMed] [Google Scholar]
- Strand Linn Beate, Barnett AG, Tong S, 2011. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med. Res. Methodol. 11, 49 10.1186/1471-2288-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA, 2019. Ambient temperature and preterm birth: A retrospective study of 32 million US singleton births. Environ. Int. 126, 7–13. 10.1016/j.envint.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH, 2017. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int. J. Epidemiol. dyw341. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Therneau T, 2015. A Package for Survival Analysis in S.
- Vicedo-Cabrera AM, Olsson D, Forsberg B, 2015. Exposure to seasonal temperatures during the last month of gestation and the risk of preterm birth in Stockholm. Int. J. Environ. Res. Public Health 12, 3962–78. 10.3390/ijerph120403962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainstock T, Shoham-vardi I, Glasser S, Anteby E, Lerner-geva L, 2015. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes 3890, 49–56. 10.3109/10253890.2014.974153 [DOI] [PubMed] [Google Scholar]
- Walfisch A, Kabakov E, Friger M, Sheiner E, 2017. Trends, seasonality and effect of ambient temperature on preterm delivery. J. Matern. Neonatal Med. 30, 2483–2487. 10.1080/14767058.2016.1253063 [DOI] [PubMed] [Google Scholar]
- Wang J, Tong S, Williams G, Pan X, 2019. Exposure to Heat Wave During Pregnancy and Adverse Birth Outcomes: An Exploration of Susceptible Windows. Epidemiology 30, S115–S121. 10.1097/EDE.0000000000000995 [DOI] [PubMed] [Google Scholar]
- Wang J, Williams G, Guo Y, Pan X, Tong S, 2013. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG An Int. J. Obstet. Gynaecol. 120, 1631–1641. 10.1111/1471-0528.12397 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Webster TF, 2017. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology, Epidemiology. 10.1097/EDE.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yackerson N, Piura B, Sheiner E, 2008. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J. Perinatol. 28, 707–711. 10.1038/jp.2008.69 [DOI] [PubMed] [Google Scholar]
- Zeitlin J, 2002. Fetal sex and preterm birth: are males at greater risk? Hum. Reprod. 17, 2762–2768. 10.1093/humrep/17.10.2762 [DOI] [PubMed] [Google Scholar]
- Zhang X, Kramer MS, 2009. Variations in Mortality and Morbidity by Gestational Age among Infants Born at Term. J. Pediatr. 154 10.1016/j.jpeds.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu C, Wang L, 2017. Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ. Pollut. 225, 700–712. 10.1016/j.envpol.2017.02.066 [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang W, Lu C, Norbäck D, Deng Q, 2018. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: Identifying windows of susceptibility during pregnancy. J. Therm. Biol. 74, 201–207. 10.1016/j.jtherbio.2018.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.