Abstract
Background:
Platelet concentrates are extensively utilized in the medical and dental field to promote tissue regeneration. The profusion of endogenous growth factors in platelets α-granules transmit their use for enhanced wound healing. However, little attention has been given to study their antimicrobial potential. This study was conducted to assess the antibacterial and antifungal property of platelet-rich fibrin (PRF) and PRF matrix (PRFM).
Materials and Methodology:
Blood samples were obtained from 16 participants, PRF and PRFM were processed as per the protocol prescribed by Choukroun et al. and Lucarelli et al., respectively. The susceptibility test against microbiota in the root canal and Candida albicans was assessed through minimum inhibition zone by agar diffusion technique.
Results:
PRF showed an effective antibacterial property, however, did not perform well against C. albicans strains. PRFM did not show any antibacterial or antifungal properties.
Conclusions:
The antibacterial efficacy of PRF may prove beneficial when used in the revascularization procedure of immature necrotic teeth.
Keywords: Antimicrobial property, oral Candida albicans, platelet rich fibrin, platelet rich fibrin matrix, root canal flora
INTRODUCTION
Endodontic regenerative procedures are biologically based procedures which includes regeneration of pulp-like tissue, more idealistically the dentin-pulp complex, affected coronal dentin following a carious exposure or trauma; and regeneration of pathological root resorption in cervical, middle, or apical areas.[1] Since two decades, an increased understanding of the physiological therapeutic role of platelets in wound healing has led to the use of platelet concentrates (PCs) as remedial tools for regenerative protocols.[2]
Platelet-rich fibrin (PRF) is the secondgeneration of PCs. It was introduced by Choukroun et al. in 2000. It contains platelets and growth factors (GFs) in the form of fibrin membrane. It is prepared from the patient's own blood and is free from any anticoagulant. These PCs contains biologically active protein that accelerates the wound healing and in addition, promotes a angiogenesis, tissue repair, however, causes moderate inflammation, and an immune response.[3] The binding of these proteins with a developing fibrin mesh or to the extracellular matrix can create chemotactic gradients aiding the recruitment of stem cells, thereby, stimulating cell migration, differentiation, and this promotes repair and regeneration. There is a novel concept in PCs called PRF matrix (PRFM) which is processed using higher gravitational force without the use of bovine thrombin.[4] It is obtained after two sets of centrifugation and is polymerized using calcium chloride. PRFM lacks leukocytes and is made of a dense fibrin network. The density of the fibrin matrix makes it suitable for regenerative endodontic treatment modalities.
Pulp revascularization procedures are being researched on to manage immature teeth for a decade. However, cases with pulpal and periapical infection usually do not respond favorably to revascularization procedures due to inability to completely disinfect the canal.[5] Microorganisms that leads to endodontic infections are mainly of low virulence. Their pathogenesis and endurance are influenced by the release of lipopolysaccharide, toxins, and the synthesis of enzymes. Pulpal and periapical lesions are associated with a mixed microbiota, consisting of aerobic, anaerobic, Gram-positive, and negative microorganisms.[6]
The application of PRF as a potential scaffold for regenerative endodontic protocol has been documented in previous literature. However, the literature survey reveals limited studies assessing antibacterial and antifungal properties of PRF and PRFM in the field of regenerative endodontics. Hence, the aim of this study was to evaluate the antibacterial and antifungal effects of PRF and PRFM against root canal microflora.
MATERIALS AND METHODOLOGY
This study protocol and procedures were accepted by Institutional Ethics committee in accordance with the Helsinki Declaration, 1975 and as revised in 2008. A written informed consent was taken from blood donors and patients from whom root canal samples were collected.
Donors
Blood samples were obtained from sixteen adults, age ranging from 25 to 45 years. They were systemically healthy, nonsmokers with no symptoms of infection or on antibiotics at least 3 months prior to experiments. A total volume of 10 ml of blood was collected from each donor. 5 ml was used for PRF and 5 ml for PRFM preparation. Samples of PRF and PRFM divided into two groups' anti-bacterial and antifungal group. Eight samples of PRF and PRFM were used antibacterial group and eight samples of PRF and PRFM were used in anti-fungal group. Metapex was used a control group.
Platelet-rich fibrin preparation
The following PRF preparation was adopted from the protocol by Dohan et al.[7] A total volume of 5 ml of blood was collected in vacutainer and centrifuged (Manual Centrifugation Machine, eTek) at 400 g for 15 min. After centrifugation, the PRF clot was removed from the tube using sterile tweezers, and is separated from the RBC base using scissors. PRF was then immediately transferred to the culture dish.
Platelet rich fibrin matrix preparation
A total volume of 5 ml of intravenous blood was collected into the blood collection tube coated with 3.2% sodium citrate solution used as an anticoagulant. Each tube was vortexed several times to confirm proper mixing of the blood and anticoagulant. The first centrifugation (Manual Centrifugation Machine, eTek) was done at 1100 g for 6 min. The result was the separation of the whole blood into its three basic components: red blood cells, platelet concentration, and plateletpoor plasma. The platelet concentration and platelet-poor plasma transferred into a vacutainer containing calcium chloride - (0.25 mL CaCl2 1M) under sterile conditions. Second centrifugation was operated at 4500 g for 25 min. A translucent, yellow-white platelet-fibrin matrix, with an inner diameter of (33 mm) which is equal to the bottle (33 mm) formed at the bottom was retrieved. This fibrin matrix so obtained is termed PRFM and was immediately transferred to the culture dish.
Microbial culturing
Collection of root canal samples
The microbial samples from the root canal were collected under strict asepsis. Under rubber dam isolation, the surface of the tooth was disinfected with 30% hydrogen peroxide for 1 min followed by 3% Sodium Hypochlorite (Venson's India, India) and neutralization was performed using 5% sodium thiosulfate (NICE Chemicals), to avoid interference with the bacteriological sampling. Access was prepared and the root canal was irrigated using sterile saline, only to moisten the canal before sample selection. Samples were taken from each canal using sterile Kfiles (MANI) and paper points by inserting it into the root canal for 1 min. Samples were obtained from the main canal in single-rooted teeth and largest canal or canal with periapical radiolucency in multi-rooted teeth. The collected sample was immediately (within 4 h) transported in a test tube containing peptone water, to microbial culture laboratory. It was incubated for 24 h.
Bacterial culturing
Following incubation samples were cultured on moistfree blood agar culture plate. A heated sterile platinum wire loop was used to streak the sample on the blood agar plate. The agar plate was then incubated for 2–3 days. If any macroscopic changes (cream-colored, raised colonies) seen indicated the growth of microorganisms.
Fungal culturing
After incubation, the samples were streaked on the Sabouraud's dextrose agar plate using a sterile platinum loop followed by which the culture media was subsequently kept at 37°C for 2 days. Any macroscopic changes (cream-colored, raised, smooth, and butyrous colonies) seen indicating the growth of fungi, was further subjected to gram staining and germ tube test to detect the presence or absence of Candida albicans.
The susceptibility tests of platelet-rich fibrin and platelet-rich fibrin matrix
The susceptibility test of root canal microflora and C. albicans to PRF and PRFM was tested by agar diffusion method. Each agar plate was then labeled and divided into three halves with the help of a marker on the bottom of the plate for PRF and PRFM and Metapex (MetaBiomed, Union Dental, and New Delhi, India) as a positive control. Incubation wells (4 × 4 mm) were created for placing PRF, PRFM, and Metapex for proper diffusion as shown in the figure given below. Cultures were evaluated after 2 days and the zones of inhibition for each substance used against root canal isolates [Figure 1] and C. albicans [Figure 2] were recorded using a transparent ruler. The mean value of zone of inhibition was observed in relation to PRF, PRFM, and Metapex and statistical analysis was done usingc IBM SPSS software (SPSS 20, SPSS Inc., Chicago, IL, USA). The mean values were analyzed using Kruskal–Wallis in SPSS software. Post hoc Mann–Whitney test was applied to check the statistical difference between the groups.
Figure 1.
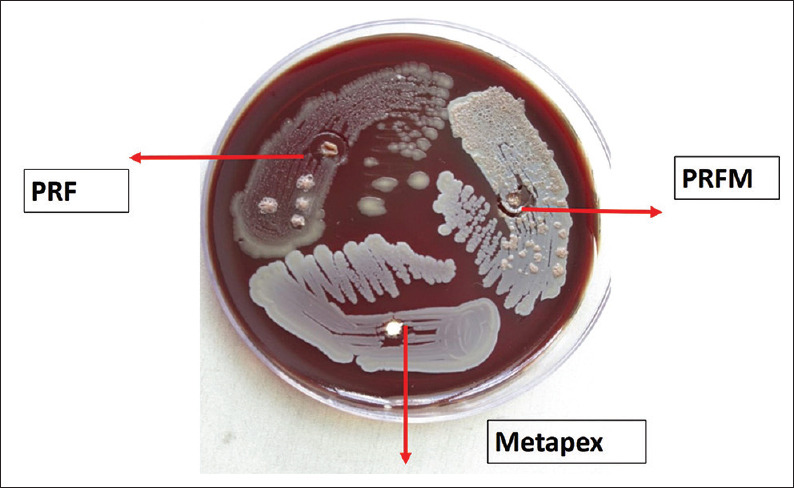
Antibacterial activity
Figure 2.
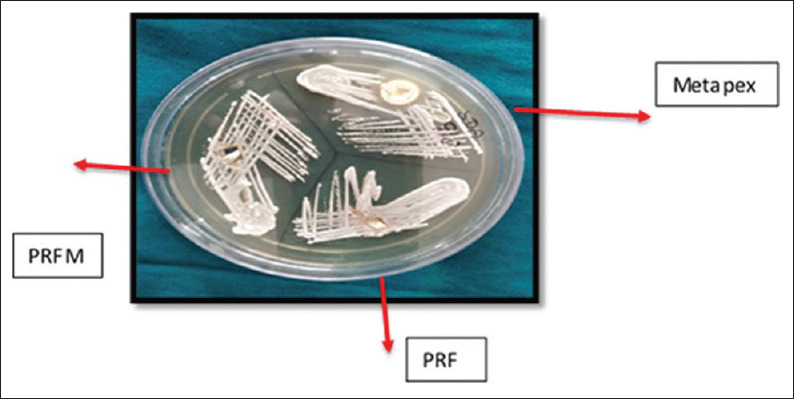
Antifungal activity
RESULTS
Table 1 shows the antifungal scores among the groups. The highest antifungal scores were seen in metapex - 12 ± 1.30 followed by PRF group - 1.50 ± 0.53 and PRFM group - 0.38 ± 0.51. Kruskal–Wallis test was applied to check the statistical difference among the groups and there was statistically significant difference seen among the groups (P = 0.00).
Table 1.
Comparison of the mean distribution of the antifungal scores among the groups using Kruskal-Wallis
| n | Minimum-maximum | Mean | SD | P | |
|---|---|---|---|---|---|
| PRF | 8 | 1-2 | 1.50 | 0.535 | 0.00* |
| PRFM | 8 | 0-1 | 0.38 | 0.518 | |
| Metapex | 8 | 10-13 | 12.00 | 1.309 |
*Significant. SD: Standard deviation, PRFM: Platelet-rich fibrin matrix, PRF: Platelet-rich fibrin
Table 2 shows the antibacterial scores among the groups. The highest antibacterial scores were seen in Metapex - 10.50 ± 1.8 followed by PRF group - 4.25 ± 0.88 and PRFM group - 1.25 ± 0.46. Kruskal–Wallis test was applied to check the statistical difference among the groups and there was statistically significant difference seen among the groups (P = 0.00).
Table 2.
Comparison of the mean distribution of the antibacterial scores among the groups using Kruskal-Wallis
| n | Minimum-maximum | Mean | SD | P | |
|---|---|---|---|---|---|
| PRF | 8 | 3-5 | 4.25 | 0.886 | 0.00* |
| PRFM | 8 | 1-2 | 1.25 | 0.463 | |
| Metapex | 8 | 7-13 | 10.50 | 1.852 |
*Significant. SD: Standard deviation, PRFM: Platelet-rich fibrin matrix, PRF: Platelet-rich fibrin
Post hoc Mann–Whitney test was applied to check the statistical difference between the groups as shown in Table 3. There was a statistical significant difference seen between PRF and PRFM; PRF and Metapex, PRFM and Metapex with respect to antifungal and antibacterial scores (P ≤ 0.05).
Table 3.
Post hoc (Mann-Whitney)
| Antifungal | Antibacterial | |||
|---|---|---|---|---|
| Mean difference | P | Mean difference | P | |
| PRF versus PRFM | 1.12 | 0.003* | 3.00 | 0.00* |
| PRF versus Metapex | −10.5 | 0.001* | −6.25 | 0.001* |
| PRFM versus Metapex | −11.62 | 0.001* | −9.25 | 0.00* |
*P set significant at 0.05/3=0.016. PRFM: Platelet-rich fibrin matrix, PRF: Platelet-rich fibrin
DISCUSSION
Over the last two decades, the regenerative potential of PCs has been studied extensively. However, there is very limited literature on the antimicrobial efficacy of PCs. Existing scientific evidence suggests that platelets may play multiple roles in antimicrobial host defense.[8] The mechanism of antimicrobial activity of PC is not well understood. Platelets are capable of binding, aggregating, and internalizing microorganisms, which enhances the clearance of pathogens from the bloodstream. Platelets also participate in antibody-dependent cell cytotoxicity functions to destroy protozoal pathogens, and finally, platelets release an array of potent antimicrobial peptides.[9,10]
Activated platelets could release various GFs and platelet microbicidal proteins.[11] These are a complex mixture of platelets, leukocytes, and plasma. The plasma contains complement, an integral variable of the immune system which could play role in the antimicrobial activity.
In the present study, PRF inhibited the root canal microflora after 2 days of incubation period. However, PRFM was not able to inhibit these bacteria. This could be since PRF is a matrix of autologous fibrin, in which a large number of platelets and leukocyte cytokines are embedded intrinsically during centrifugation leading to their progressive release over time as the network of fibrin disintegrates.[12,13] According to Badade et al., calcium chloride in PRFM is added for activation of platelets, which may be the reason for the contribution of the antibacterial activity in PRFM,[14] on the contrary, our study, observed no significant antimicrobial effect of PRFM as shown in Graph 1 and therefore, the role of calcium chloride remains insignificant.
Graph 1.
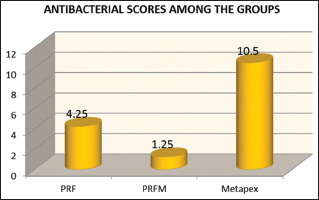
Antibacterial activity of platelet-rich fibrin, plateletrich fibrin matrix, and metapex
For the first time, the antimicrobial activity of PRFM was tested. The leukocyte concentration and handling characteristics of PRF and PRFM vary, PRFM lacks leukocytes. Leukocytes have an intrinsic role in immune response and antimicrobial potential. Insignificant antimicrobial activity of PRFM could be attributed to the absence of leukocytes. Bielecki et al. made an extensive study on the impact of leukocyte in PCs and their role in wound healing and immune response. Elements of neutrophils such as polymorphonuclear neutrophilic granulocytes granule proteins, heparin-binding protein, cathepsin G, cathelicidin, Calprotectin, defensins, phospholipase A2, and eosinophils are effective immune mediators.[15] Hence, the antibacterial activity of PC is not due to platelets alone but due to the complex mixture of cellular component, fibrin matrix, and plasma proteins. The role of these components in PC requires extensive research.
In previous studies, single bacterial species were tested which showed susceptibility to PCs such as PRF and PRP.[16] Root canal harbors diverse endodontic flora which differs in their metabolism which affects the disinfection treatment instituted. Hence, it is imperative to evaluate susceptibility to clinical microbiota rather than an isolated single strain. Hence, in our study, we used clinical strain and mixed microbiota which is more clinically relevant.
For the first time, antifungal efficacy was tested. C. albicans is the most common fungi isolated from the root canal. C. albicans circumvent the immune system because it harbors certain characteristic properties such as adherence to surface, thigmotropism (i.e., contact sensing by candidal hyphae to find intracellular junctions or microscopic breaks on mucosal surfaces), phenotypic switching, and secretion of a degenerative enzyme known as “aspartyl protease” that degrades dentinal collagen.[17] The increased rate of protease activity of C. albicans in comparison with other candida species also suggests its higher virulence characteristic. C. albicans also has an ability to grow on the dentinal surfaces in the absence of oral tissue fluids and penetrates into the dentinal tubules by its own various growth forms (hyphae and blastophores).[18] Sen et al. suggested that Candida is considered as dentinophilic microorganism.[19] It is for this reason that the sample in the present study was obtained by filing the dentinal walls of the canals using K file.
The results of the present study showed that calcium hydroxide was more effective on C. albicans than PRF and PRFM as shown in Graph 2. These results could be attributed to its ability to inactivate the cytotoxic effects of the endotoxin released by microorganisms. The second reason could be due to the usage of calcium hydroxide in a paste form (DENTOCAL) that could have acted as a backup reservoir for the medicament thus enhancing its efficacy. However, PRF and PRFM did not show any antifungal properties.
Graph 2.
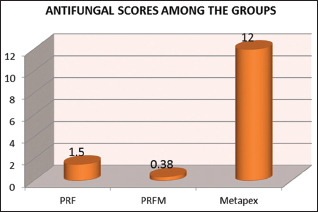
Antifungal activity of platelet-rich fibrin, plateletrich fibrin matrix and metapex
Thereby, to conclude, within the limitations of the study PRFM had no demonstrable antibacterial or antifungal efficacy. PRF demonstrated antibacterial activity against root canal isolates but had no antifungal efficacy. This antibacterial property might be a valuable adjunct when using PRF for regenerative procedures in endodontics. Both PRF and PRFM need to be analyzed for antimicrobial properties for longer duration and various other clinical strains with larger sample size. Furthermore, molecular mechanisms behind the antimicrobial property of these bio-scaffold need to be investigated in depth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16:284–93. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Lucarelli E, Beretta R, Dozza B, Tazzari PL, O'Connel SM, Ricci F, et al. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13–23. doi: 10.22203/ecm.v020a02. [DOI] [PubMed] [Google Scholar]
- 5.Fouad AF, Verma P. Healing after regenerative procedures with and without pulpal infection. J Endod. 2014;40:S58–64. doi: 10.1016/j.joen.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 6.de Mendonça Cavalcante A, Soares NM, Santos IC, de Azevedo Ximenes EA, da Silva MA, Junior KA. Assessment of microbiota in root canals with pulp necrosis by means of Gram test. Afr J Microbiol Res. 2018;12:508–11. [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Karde PA, Sethi KS, Mahale SA, Khedkar SU, Patil AG, Joshi CP. Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2017;21:97–101. doi: 10.4103/jisp.jisp_201_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–22. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 10.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–33. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeaman MR, Tang YQ, Shen AJ, Bayer AS, Selsted ME. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–31. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toffler M, Toscano N, Holtzclaw D, Corso MD, Ehrenfest DD. Introducing Choukroun's platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Adv Clin Dent. 2009;1:21–30. [Google Scholar]
- 13.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Badade PS, Mahale SA, Panjwani AA, Vaidya PD, Warang AD. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res. 2016;27:300–4. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- 15.Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr Pharm Biotechnol. 2012;13:1153–62. doi: 10.2174/138920112800624373. [DOI] [PubMed] [Google Scholar]
- 16.Fabbro MD, Bortolin M, Taschieri S, Ceci C, Weinstein RL. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets. 2016;27:276–85. doi: 10.3109/09537104.2015.1116686. [DOI] [PubMed] [Google Scholar]
- 17.Waltimo TM, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Top. 2004;9:66–78. [Google Scholar]
- 18.Chandra SS, Miglani R, Srinivasan MR, Indira R. Antifungal efficacy of 5.25% sodium hypochlorite, 2% chlorhexidine gluconate, and 17% EDTA with and without an antifungal agent. J Endod. 2010;36:675–8. doi: 10.1016/j.joen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Sen BH, Safavi KE, Spångberg LS. Growth patterns of Candida albicans in relation to radicular dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:68–73. doi: 10.1016/s1079-2104(97)90298-5. [DOI] [PubMed] [Google Scholar]


