Abstract
Alzheimer’s disease (AD) is an incurable neurodegenerative disease that is more prevalent in women. The increased risk of AD in women is not well understood. It is well established that there are sex differences in metabolism and that metabolic alterations are an early component of AD. We utilized a cross-species approach to evaluate conserved metabolic alterations in the serum and brain of human AD subjects, two AD mouse models, a human cell line, and two Caenorhabditis elegans AD strains. We found a mitochondrial complex I-specific impairment in cortical synaptic brain mitochondria in female, but not male, AD mice. In the hippocampus, Polβ haploinsufficiency caused synaptic complex I impairment in male and female mice, demonstrating the critical role of DNA repair in mitochondrial function. In non-synaptic, glial-enriched, mitochondria from the cortex and hippocampus, complex II-dependent respiration increased in female, but not male, AD mice. These results suggested a glial upregulation of fatty acid metabolism to compensate for neuronal glucose hypometabolism in AD. Using an unbiased metabolomics approach, we consistently observed evidence of systemic and brain metabolic remodeling with a shift from glucose to lipid metabolism in humans with AD, and in AD mice. We determined that this metabolic shift is necessary for cellular and organismal survival in C. elegans, and human cell culture AD models. We observed sex-specific, systemic, and brain metabolic alterations in humans with AD, and that these metabolite changes significantly correlate with amyloid and tau pathology. Among the most significant metabolite changes was the accumulation of glucose-6-phosphate in AD, an inhibitor of hexokinase and rate-limiting metabolite for the pentose phosphate pathway (PPP). Overall, we identified novel mechanisms of glycolysis inhibition, PPP, and tricarboxylic acid cycle impairment, and a neuroprotective augmentation of lipid metabolism in AD. These findings support a sex-targeted metabolism-modifying strategy to prevent and treat AD.
Keywords: Mitochondria, Metabolism, Oxidative phosphorylation, Pentose phosphate pathway, Glycolysis, Tricarboxylic acid cycle, Ketone, Lipid, Glucose-6-phosphate, Hexokinase
Introduction
Alzheimer’s disease (AD) is the most prevalent age-related neurodegenerative disease and there are no efficacious treatments. Two-thirds of individuals diagnosed with AD are women [21], although whether these differences are simply due to a greater longevity among females compared to males is a subject of debate [49]. Independent of differences in longevity, there are likely to be sex differences in risk factors, pathogenesis, and underlying disease mechanisms [24, 81]. For example, prior studies have indicated that female AD patients have a higher burden of amyloid plaques and neurofibrillary tangles [8, 42], a more amnestic presentation of cognitive symptoms [42], and differences in glucose metabolism compared to male AD patients [54, 67]. However, the metabolic mechanisms and implicated cellular pathways that may be associated with increased AD risk in females are unknown. It has been known for decades that systemic metabolism differs between men and women [85]. For example, during endurance exercise tests in a clinical laboratory setting, male study participants derived 97% of energy for exercise from carbohydrates (glucose) and proteins (amino acids) while females derived 62% of energy for exercise from fat [41]. Brain glucose hypometabolism precedes cognitive impairment and has been demonstrated in human AD patients by positron emission topography (PET) neuroimaging and metabolomic approaches by several laboratories [21, 61]. These reports suggest that intrinsic sex differences in metabolism may contribute to the higher prevalence of AD among women.
Mitochondrial dysfunction is a central hallmark of nearly all neurodegenerative diseases including AD [52, 72]. Despite this consensus, the mitochondrial mechanisms implicated in AD remain unclear. Several reports have suggested different sites along the mitochondrial electron transport chain (ETC) as the site of dysfunction. However, these reports are inconsistent, with some studies reporting complex I (NADH dehydrogenase) dysfunction [1, 51], while others report that complex IV (cytochrome c oxidase) is the site of mitochondrial dysfunction [39, 72]. One report from a laboratory with mitochondrial expertise reported no consistent mitochondrial bioenergetic impairment in three different transgenic mouse models of AD [71]. As mitochondrial function is known to differ between males and females [15, 16, 88] and in a cell-type-specific manner [19] we sought to determine if the inconsistencies in reported mechanisms underlying mitochondrial dysfunction in AD could be due to differences in biological sex, brain region, brain cell-type and DNA repair proficiency using a comprehensive mitochondrial bioenergetics approach. We further investigated conserved metabolic mechanisms of AD using a cross-species approach in humans, mice, C. elegans, and human cells.
The Bohr lab has previously shown that human AD patients have a decrease in the essential base excision repair (BER) DNA polymerase beta (Polβ) [84], and that Polβ is present in brain, but not liver, mitochondria [59, 73]. These data suggest that Polβ has important mitochondrial functions in the brain that may contribute to AD pathophysiology. To evaluate the role of Polβ deficiency in brain mitochondrial function and AD, we utilized a DNA repair deficient (Polβ heterozygous) mouse and crossed it with the well-characterized 3×Tg model of AD (3×β) [32, 74]. Importantly, these mice have neuronal cell death and recapitulate the mitochondrial transcriptional changes observed in human AD [74]. In this study, we utilized four genotypes: wild-type (WT), Polβ ±, 3×, and 3×β mice to determine the putative contribution of Polβ deficiency to mitochondrial health in AD. The sites of mitochondrial ETC inhibition in cortical and hippocampal synaptic and non-synaptic mitochondria were evaluated to test the hypothesis that Polβ deficiency contributes to mitochondrial dysfunction, and that the underlying mechanisms of mitochondrial dysfunction contribute to increased female risk in AD.
Herein, we report sex-dependent mitochondrial and metabolic mechanisms in murine AD models that may explain differences in risk for AD in women. We identify a female-specific mitochondrial complex I impairment in cortical synaptosomes and a metabolic switch from glucose to fatty acid metabolism in non-synaptic mitochondria and systemically, in plasma, which is more pronounced in female compared to male AD mice. To assess the role and conservation of this metabolic switch in AD across species we utilized human cell culture and C. elegans models of AD that indicate the switch to fatty acid metabolism is a compensatory neuroprotective metabolic mechanism in AD. Furthermore, we observe striking similarities in patterns of metabolic remodeling in mice and humans with AD that provide insight into mechanisms underlying glucose hypometabolism and mitochondrial dysfunction in AD. Overall, these data suggest significant sex differences in mitochondrial mechanisms and metabolic responses to AD, which may explain AD risk and differing disease pathways in females compared to males. Furthermore, we identify specific sites of metabolic impairment within glycolysis and the pentose phosphate pathway in mice and humans that likely contribute to glucose hypometabolism and oxidative stress in AD and represent targets for preventive and therapeutic interventions.
Materials and methods
Mice
Age-matched (18-month old) male and female adult mice were used in all experiments with littermate controls used when possible. Mice were maintained on a standard NIH diet ad libitum in a 12-h light/dark cycle. All mice were housed in the National Institute on Aging (NIA), Baltimore. The animals were group housed where possible. All animal experiments were performed using protocols approved by the appropriate institutional animal care and use committee of the NIA. The original AD line was generated as described previously [74] and have been extensively behaviorally characterized [32].
C. elegans
Standard C. elegans strain maintenance procedures were followed. Nematodes were maintained at 15 °C on normal growth media (NGM) and OP50 bacteria. C. elegans were synchronized via egg lay at 20 °C. The following strains were used in this study. N2: wild-type, CL2355: Pan-neuronal expression of human amyloid β peptide; dvIs50 [Psnb-1Abeta 1–42; P mtl-2GFP] I, BR5270: Pan-neuronal overexpression of the F3 pro aggregation fragment of the human Tau protein with K280 deleted (line A); byIs161[prab-3F3ΔK280; pmyo-2mCherry], and BR5271: an-neuronal overexpression of the F3 pro aggregation fragment of the human Tau protein with K280 deleted and two isoleucines to proline substitutions in the hexapeptide motifs of the repeat region (line A), which abrogate tau aggregation; byIs162 [rab-3p:F3ΔK280 I277P I380P + myo-2p::mCherry]. L4-stage synchronized nematodes were placed on NGM plates containing 100 μM etomoxir or equal volumes of DMSO (vehicle control). All strains were maintained at 20 °C for survival experiments with the exception of CL2355, which were cultured at 25 °C due to the temperature-sensitive regulation of amyloid expression. Viability was determined 6 days following drug treatment and percent survival was calculated. Worms unresponsive to a light touch with a platinum wire were considered dead. Experiments were performed in triplicate (3 plates of n = 20 nematodes/plate) and were repeated in at least two independent experiments.
Cell culture
Human H4 neuroglioma cells expressing wild-type or the M146V mutated Presenelin-1 (PS1) protein under a doxycycline-dependent promotor were cultured as described [18]. Cells were cultured in DMEM with Zeocin and Blasticidin. PS1 expression was induced via the addition of doxycycline (1 μg/mL) for 72 h before the addition of carnitine palmitoyl-transferase (CPT) inhibitor etomoxir (40 μM) for 8 h prior to fixation with 4% paraformaldehyde–PBS solution and immunofluorescence staining for cleaved caspase-3 (Novusbio MAB835, 2.5 μg/mL). Z-stack images were acquired on Confocal images (z-series) were captured with Zeiss LSM 880 inverted laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) using a 40 × /1.3 Plan-NeoFluar oil-immersion objective at 1 μm z-step intervals with lateral pixel dimensions of 0.22 μm. Captured z-series were imported and analyzed using FIJI-ImageJ.
Human U2OS osteosarcoma cells were cultured in DMEM containing 10% fetal bovine serum and 1 mM pyruvate. 3–5 175 cm2 cell culture plates of U2OS cells were utilized for mitochondrial isolation for glucose-6-phosphate induced hexokinase dissociation experiments. 10–20 μg of mitochondria were incubated in MAS buffer with pyruvate (5 mM) and malate (2.5 mM) with the indicated dose of glucose-6-phosphate (0–500 mM) for 30 min at 37 °C before centrifugation of mitochondria at 17,000×g. Supernatant was aspirated and the mitochondrial pellet was resuspended in 1 × Laemelli buffer containing β-mercaptoethanol for western blot analysis.
Mitochondrial isolation
Brain tissues were rapidly collected and rinsed in ice cold mitochondrial isolation buffer (MS: 225 mM mannitol, 75 mM sucrose, 5 mM HEPES, 1 mM EGTA, 0.1% fatty acid-free BSA, pH 7.4 with KOH at 4 °C). Cortex and hippocampus were homogenized in 10 mL of MS buffer manually using ten strokes of a Potter–Elvehjem teflon tissue grinder pestle in a 15 ml Dounce Homogenizer (VWR). Resulting homogenate was centrifuged at 2200×g for 3 min at 4 °C. The supernatant was removed and kept on ice and the pellet was resuspended in 5 ml MS and centrifuged at 2200×g. Resulting supernatants were pooled and centrifuged at 22,000×g for 10 min. The pellet, containing primarily free (non-synaptic) mitochondria plus synaptosomes, was then resuspended in 3.5 mL of MS buffer containing 15% Percoll®. Synaptic and non-synaptic mitochondria were separated via centrifugation through a 24–40% Percoll® gradient as described [17, 40]. Synaptosomes were permeabilized to permit substrate influx via a 3 min incubation with 0.001% digitonin (Calbiochem). Non-synaptic and synaptic mitochondria were washed with 10 mL of fresh MS buffer and pelleted at 22,000×g for 10 min. This pellet was transferred to a 1.5 mL Eppendorf tube and centrifuged at 17,000×g for 10 min. This final pellet was resuspended in 50–100 μl of MS buffer without EGTA and protein content measured by bicinchoninic acid assay (Thermo Fisher).
Mitochondrial respirometry
Mitochondrial respiration was measured on a 96XFe Seahorse extracellular flux analyzer adapted from previously reported methods [63]. Mitochondria were suspended in respiration buffer (MAS: 70 mM sucrose, 220 mM mannitol, 10 mM KH 2PO4, 5 mM MgCl 2, 2 mM HEPES, 1 mM EGTA and 0.2% (w/v) fatty acid-free BSA, pH 7.2 at 37 °C) and plated in 96-well Seahorse plates before centrifugation at 2200×g for 10 min at 4 °C. 5 μg of mitochondria were plated in triplicate for complex I (pyruvate 10 mM, malate 2.5 mM)- and complex II (10 mM succinate, 2 μM rotenone)-dependent coupling experiments followed by injections of (A) ADP (5 mM), (B) oligomycin (2.5 μM), (C) FCCP (4 μM), (D) Antimycin A (2 μM) + rotenone (1 μM). 2.5 μg of mitochondria per well were plated in triplicate for maximal electron flow experiments (10 mM pyruvate, 2.5 mM malate, 4 μM FCCP) measurements followed by injections of (A) rotenone (2 μM), (B) Succinate (10 mM), (C) Antimycin A (2 μM), (D) TMPD (100 μM) + Ascorbate (10 mM). The mean of triplicates from each experiment was used to quantify each respiratory state. Experiments with negative oxygen consumption values or wells where mitochondria detached form the plate were excluded from analysis. Respiratory control ratios were calculated as state 3 (ADP simulated) divided by state 4 (oligomycin) respiration. Mix-wait-measure protocols have been previously described [63]. Remaining mitochondrial samples were treated with protease and phosphatase inhibitor cocktail (Cell signaling) and stored at − 80 °C for further analysis.
Untargeted mouse plasma metabolomics
Blood was collected in EDTA blood collection tubes (BD scientific) and centrifuged at 2200×g for 10 min. Plasma supernatant (60 μl) was aliquoted into sterile Eppendorf tubes, snap frozen in liquid nitrogen and stored at − 80 °C before analysis. ALEX-CIS GC-TOF mass spectrometry was performed at the NIH West Coast Metabolomics Center (UC Davis, Davis, CA). Data are acquired using the following chromatographic parameters, with more details to be found in [23]. Column: Restek corporation Rtx-5Sil MS (30 m length × 0.25 mm internal diameter with 0.25 μm film made of 95% dimethyl/5%diphenylpolysiloxane), Mobile phase: Helium, Column temperature: 50–330 °C Flow-rate: 1 mL min−1, Injection volume: 0.5 μL, Injection: 25 splitless time into a multi-baffled glass liner, Injection temperature: 50 °C ramped to 250 °C by 12 °C s−1, Oven temperature program: 50 °C for 1 min, then ramped at 20 °C min−1 to 330 °C, held constant for 5 min. Raw data files are preprocessed directly after data acquisition and stored as ChromaTOF-specific *.peg files, as generic *.txt result files and additionally as generic ANDI MS *.cdf files. ChromaTOF vs. 2.32 is used for data preprocessing without smoothing, 3 s peak width, baseline subtraction just above the noise level, and automatic mass spectral deconvolution and peak detection at signal/noise levels of 5:1 throughout the chromatogram. Apex masses are reported for use in the BinBase algorithm. Result *.txt files are exported to a data server with absolute spectra intensities and further processed by a filtering algorithm implemented in the metabolomics BinBase database. Data were analyzed with Metaboanalyst v4.0 [11] following log transformation to fit a gaussian distribution and statistically analyzed using One-way ANOVA with Tukey HSD post hoc test with FDR = 0.05 for multiple comparisons. Metabolite set enrichment (MSE) pathway analysis was used to determine pathway alterations in WT vs. AD mice. Partial least squares discriminant analysis (PLS-DA) was used for descriptive visualization of genotypes and identification of VIP metabolites. n = 4–6 mice/group.
Cardiac perfusion and brain slice preparation
Mice were anesthetized with isoflurane prior to transcardial perfusion with heparinized PBS (1U heparin/mL PBS) followed by 4% paraformaldehyde. Brains were cryostat sectioned at 20 μM thickness on a cryostat (Leica) in a 1:12 series and stored in cryoprotectant solution (66 mM NaH2PO4, 190 mM Na2HPO4, 870 mM sucrose, 30% ethylene glycol, and 1.25 mM povidone) at − 20 °C. 6 serial brain Sections (240 microns apart) representing the entire brain were mounted on Superfrost® slides (Fisher Scientific).
Oil Red O staining for lipid droplets
Following a 5-min equilibration in 60% isopropanol, tissue was exposed to Oil Red O (Sigma) working solution (30 ml:20 ml of Oil Red O Stain, Isopropanol in distilled water) for 1 h at room temperature. The sections were washed three times in 60% isopropanol for one minute and cover-slipped with mounting media containing DAPI (ProLong Gold Antifade Reagent with DAPI, Life technologies).
Images of CA3 hippocampi were captured in at least 2 sections containing the CA3 region of the hippocampus under brightfield at 20 × magnification on a Zeiss Axioimager M2 microscope. Lipid droplets were calculated for each image in Image J software using the particle analysis tool with the following thresholds (color: 60–255, Saturation:100–255, brightness: 0–229). Lipid droplet counts from each brain section were calculated and averaged per animal (n = 4 animals/group).
Thioflavin-S staining for amyloid beta
Amyloid beta plaques were detected by Thioflavin-S staining. Slides were first permeabilized in PBS + 0.1% Triton then given a 1-min equilibration in 75% isopropanol. Tissue was then exposed to a modified version of thioflavin-S staining technique. Traditional thioflavin-S staining was previously modified by [70] by adding pretreatments with KMnO4 for 4 min followed by 1% sodium borohydride and treatments with NaOH, H2O2, and acetic acid were abolished. And a post-treatment step in 3xPBS for 30 min at 4 °C was added to alleviate photobleaching. In our preliminary optimization trials, we tested dilutions of 0.05%, 0.025%, and 0.0125% Thioflavin-S to minimize autofluorescence. To manage non-specific lipofuscin autofluorescence, we added a TrueBlack® (Biotium, VWR) blocking step. We found that using 0.0125% Thioflavin-S and exposing tissue for 3 min to TrueBlack® was the most optimal procedure and was used for the remainder of the study. The mounted slides were treated with 0.25% KMnO4 potassium permanganate for 4 min on a shaker. Slides are then washed with distilled water followed by treatment in 1% sodium borohydride for 5 min on the rocker and washed again with distilled water. Slides are then stained with 0.0125% thioflavin-S in 50% ethanol in the dark for 8 min. This step was followed by differentiation in two changes of 80% ethanol for 10 s each and three washes in large volumes of distilled water. Slides were then incubated in 3 × PBS at 4C for 30 min, then briefly rinsed with distilled water and cover slipped with mounting media containing DAPI (ProLong Gold Antifade Reagent with DAPI, Life technologies).
Images of subiculum subregions were captured using Zeiss Axiomager M2 Microscope at 10 × magnification under autofluorescence setting. Amyloid beta plaques were calculated for each image in Image J software using the particle analysis tool with the following thresholds (color: 0–85, Saturation: 229–225, brightness: 50–255). To avoid non-specific particle detection, particle measurements was set to size 50-infinity and circumference of 0–1. Amyloid beta counts from each brain section were calculated and averaged per animal (n = 4 animals/group). Results are depicted in supplemental figure S2, online resource.
Western blotting
Cortical brain homogenates were solubilized in 1 × RIPA buffer containing phosphatase and protease inhibitor cocktail (Cell Signaling). 20 μg of protein were resuspended in 1×Laemelli buffer with β-mercaptoethanol and loaded on AnyKD® gels (BioRad) for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE). Proteins were transferred to 0.45 μM pore size Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) using the Transblot Turbo® semi-dry transfer system (BioRad). Membranes were blocks in 5% milk in PBS + 0.1% Tween-20 (PBST, BioRad) for 1 h at room temperature prior to overnight incubation with primary antibodies at 4 °C. See STAR methods table for antibody information. Western blots of isolated synaptic mitochondrial fractions (5 μg/lane) were run as above. Actin was used for the loading control and normalization for total cortical lysates, and mitochondrial housekeeping proteins VDAC or TOM20 were used for normalization in isolated mitochondrial samples. ImageLab version 5.1 (BioRad) was used for all densitometry analyses.
Preclinical statistical analysis
Statistical analysis of more than two groups was performed by one or two-way Analysis of Variance (ANOVA) with the indicated post hoc test for multiple comparisons and sample size (n) per group detailed in each corresponding figure legend. In instances where numerous measurements were assessed a two-step correction for multiple comparisons using the Benjamini, Krieger and Yekutieli post hoc test was employed with a false discovery rate (FDR) set to 0.05. GraphPad v6.0 was used from ANOVA analysis and histogram graphs. For mouse metabolomics analysis, Metaboanalyst v4.0 [87] was used with a Tukey HSD post hoc test with FDR = 0.05. For all statistical analysis, p/q values of < 0.05 were considered significant.
Human data and analyses
Human blood and brain metabolomics
The National Institute on Aging’s (NIA) Baltimore Longitudinal Study of Aging (BLSA) is one of the longest running scientific studies of human aging in the US [22]. This observational study began in 1958 and includes longitudinal clinical, radiological and laboratory evaluations on community dwelling volunteer participants. Participants are assessed every two years and starting in 2003, participants older than 80 years are assessed annually. Written informed consent was obtained at each visit for all BLSA participants. The BLSA study protocol has ongoing approval from the Institutional Review Board of the National Institute of Environmental Health Science, National Institutes of Health. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee 2009–074 “Early Markers of Alzheimer’s Disease (BLSA)”, Institutional Review Board number 2009–074 and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Blood tissue samples
Blood tissue samples (serum) in this study were from a subset of participants from the case–control study. Demographic characteristics of the sample (at baseline) are included in supplemental Table S2, online resource. Study design details have been published previously [9]. Briefly, the case–control study included participants who converted to AD (converters) and matched participants who remained cognitively normal (non-converters). The baseline visit was defined as approximately 5 years prior to age of onset of AD clinical symptoms for converters and matched visits for non-converters. Both converters and non-converters were cognitively normal at baseline. The follow-up visit was defined as at the age of onset of clinical symptoms for converters and matched visits for non-converters. As detailed previously, we minimized the effect of long storage times by excluding samples with methionine sulfoxide concentrations greater than 5 μM [80].
Blood serum samples were collected from BLSA participants at the NIA Clinical Research Unit in Harbor Hospital, Baltimore. Details on collection and processing have been published previously [9]. Briefly, venous blood samples were collected between 6 and 7 AM following an overnight fast. Serum samples were aliquoted into 0.5 mL volume in Nunc cryogenic tubes and stored at − 80 °C until further use. Samples were not subject to any freeze–thaw cycles prior to metabolomic assays.
Brain tissue samples
Brain tissue samples in this study were from a subset of participants from the autopsy program of the BLSA [55]. Demographic characteristics of the sample are included in supplemental Table S3, online resource. The autopsy program of the BLSA was initiated in 1986 and has been described previously [55]. Postmortem brains were examined by an expert neuropathologist to assess AD pathology. As described previously, the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) and Braak criteria were used to assess severity of AD pathology based on neuritic plaques [50] and neurofibrillary tangles [6], respectively. Participants indicated as AD had clinical symptoms of AD (i.e., cognitive impairment) during life (see below for description of how cognitive status was determined) and AD pathology at autopsy. Participants indicated as Control (CON) had no clinical symptoms during life and no AD pathology at autopsy. For analyses exploring associations with pathology, we additionally included the asymptomatic Alzheimer’s disease group (ASY) that has been described in detail previously. Participants indicated as ASY had no clinical symptoms of AD during life however had AD pathology at autopsy. The brain region selected a priori for this study was from the inferior temporal gyrus (ITG), sampled to represent a brain region vulnerable to early pathology accumulation. A sterile 4-mm-diameter tissue punch was extracted from the cortical surface of the brain tissue regions, which were stored at − 80 °C.
Determining cognitive status
Procedures for determining cognitive status in the BLSA have been described in detail previously [38]. Briefly, cognitive status was considered at consensus diagnosis conferences after each evaluation where participants underwent a battery of neuropsychological tests. Longitudinal participant data reviewed during case conference included cognitive assessments, medication history, self-reported diagnoses of comorbid medical conditions, neuroimaging data, and laboratory evaluation for reversible causes of cognitive impairment (e.g., serum TSH and B12 levels). Dementia diagnosis was based on the Diagnostic and Statistical Manual (DSM)-III-R criteria [58]. AD diagnosis was based on the National Institute of Neurological and Communication Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [48].
Metabolomic profiling
Quantitative and targeted metabolomics assays were performed on blood and brain tissue samples using capillary electrophoresis time-of-flight mass spectrometry (CE-TOF MS) by Human Metabolome Technologies (HMT), Inc., Tsuruoka, Japan and flow injection analysis-tandem mass spectrometry and liquid chromatography-tandem mass spectrometry by Biocrates. Detailed methods using both BIOCRATES [68, 80] and HMT [30] have been described previously. Metabolites in this study were chosen a priori from the larger list of metabolites generated by both platforms.
Statistical analysis of human samples
Metabolites included in analyses were chosen a priori based on metabolic alterations identified in mouse AD models. All analyses were performed in the total sample and then separately in male and female samples.
For blood and brain analyses, similar to prior work [80], all metabolites with > 30% of missing values (indicated as lower than the limit of detection (LOD) were excluded. For the remaining metabolites, all missing values were imputed as LOD/2. For blood metabolites specifically, all values were then natural log transformed, and outlier values outside 1.5 times the interquartile range (1.5 × IR) were excluded.
For blood analyses within the case–control study, we explored whether blood metabolite concentration discriminated between converters and non-converters using logistic regression models controlling for sex (in total sample only), age and race at baseline and follow-up visits. Odds ratios, 95% confidence intervals, and p-values were reported for all models.
For brain analyses, similar to prior studies [2], we first explored whether brain tissue concentrations of metabolites differed between AD and CON groups using the nonparametric Kruskal–Wallis test. We then explored whether metabolite concentration was associated with severity of AD pathology (i.e. CERAD and Braak scores) using spearman rank correlation tests. Participants included AD, CON and ASY groups. P values were reported for all models. Stata 13.0 was used for all analyses and visualizations.
Results
Cortical synaptic mitochondrial complex I impairment in female AD mice
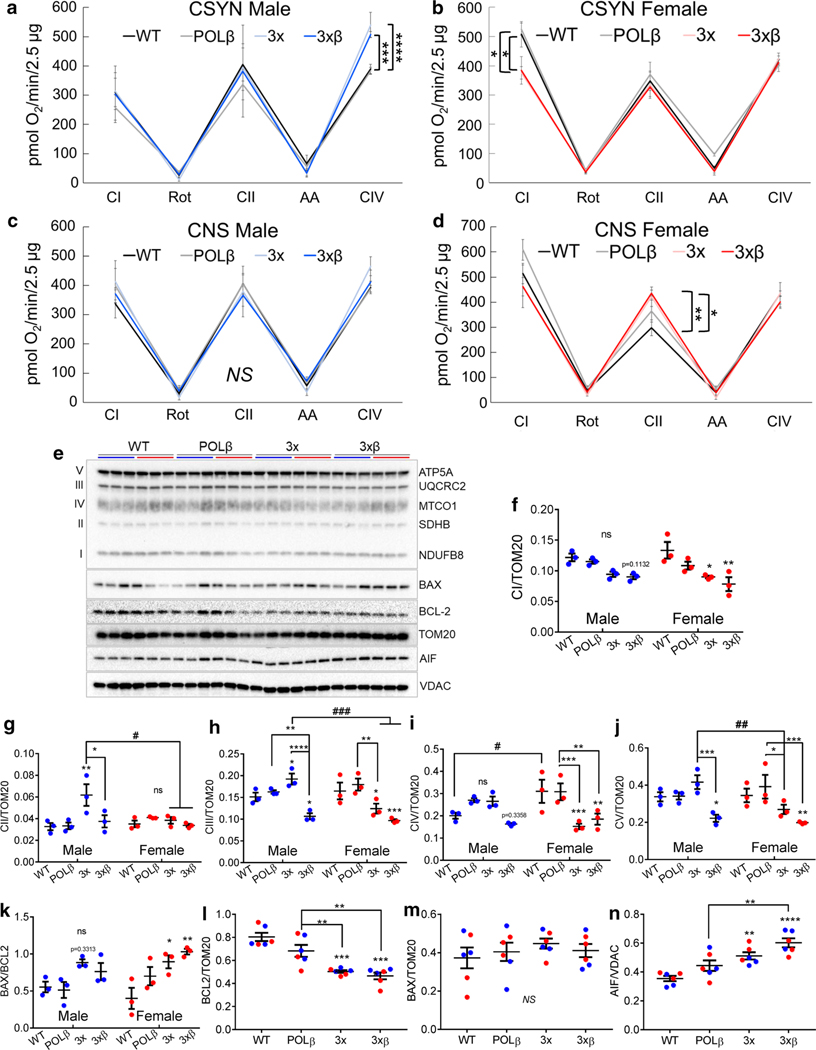
Surprisingly, male cortical synaptosomes showed no change in mitochondrial complex I (CI) function, but an increase in mitochondrial complex IV function in 3× and 3×β AD mice compared to WT mice (Fig. 1a) indicating a mitochondrial compensation to dysregulated brain energy metabolism in the male AD brain. Conversely, we observed that CI function is significantly decreased in cortical synaptic mitochondria from female 3 × and 3×β synaptic mitochondria compared to WT mice (Fig. 1b). Concomitant with the downregulation of CI function in cortical synaptosomes from females, we observed that non-synaptic (astrocyte-enriched) complex II (CII) mitochondrial function is increased compared to female WT mice (Fig. 1d). Interestingly, we did not find any difference in cortical non-synaptic mitochondrial respiration from male mice (Fig. 1c) nor significant changes in state 3, state 4 respiration, or respiratory control ratios under complex I or complex II substrates, with the exception of decreased state 3 respiration in hippocampal synaptic mitochondria from female 3×β mice (supplemental Table S1, online resource). Notably, female wild-type mice have higher rates of respiration for nearly all respiratory parameters measured (Fig. 1c, d, supplemental Table S1, online resource) despite similar levels of mitochondrial protein isolated (supplemental Fig. S1d, online resource). This may be due to a significant elevation of the master regulator of mitochondrial biogenesis, PGC1α, in female mouse cortex compared to males, independent of genotype (p = 0.026, supplemental Fig. S1 e, f online resource). Notably, PGC1α has been demonstrated to decline as a function of dementia in human AD subjects [60]. Taken together, these data indicate a female susceptibility to neuronal mitochondrial dysfunction and suggests an augmentation of fatty acid metabolism in astrocytes via the donation of reducing equivalents through succinyl-CoA, thus fueling succinate dehydrogenase (CII) for cerebral energy production. These findings are consistent with astrocytes playing the primary role in fatty acid and ketone metabolism in the brain [3, 29].
Fig. 1.
Female AD mice have mitochondrial respiratory impairment and increased apoptotic protein expression in the cortex. a–d Maximal electron flow through complex I under malate (2 mM) and pyruvate (10 mM) substrates, complex II with succinate (10 mM) and rotenone (2 μM), and complex IV with TMPD (100 μM) + Ascorbate (10 mM) in male (blue; left) and female (red; right) cortical synaptic (CSYN) and non-synaptic (CNS) brain mitochondria n = 4–6/group/ One-way ANOVA with Benjamini, Krieger and Yekutieli post hoc test for multiple comparisons p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. WT, NS = non-significant. e–j Western blot of cortical synaptic mitochondrial oxidative phosphorylation proteins, 2-Way-ANOVA with Benjamini, Krieger and Yekutieli post hoc test for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. sex-matched WT, unless indicated by brackets. #p < 0.05, ##p < 0.01, ###p < 0.001, significant effect of sex, n = 3/group
We then evaluated protein abundance of representative ETC subunits and BH3-only proteins involved in cell death pathways (Fig. 1e). Consistent with mitochondrial respiratory deficits, we found significant decreases in representative complex I subunit NDUFB8 expression in female, but not male, cortical synaptosomes from 3 × and 3×β AD mice (Fig. 1f), and an increase in complex II succinate dehydrogenase subunit B (SDHB) and complex III subunit UQCRC2 in male, but not female 3 × AD mice. In fact, we observe a significant decrease in UQCRC2 and cytochrome oxidase subunit MTCO1 in female, but increased protein abundance in male, 3 × AD mice (Fig. 1g–i). In cortical synaptosomes from 3×β AD mice, we found a decreased of UQCRC2 and complex V subunit ATP5A in both male and female mice (Fig. 1h, j), while MTCO1 levels were significantly decreased only in female 3xβ AD mice (Fig. 1i). Together, these results indicate that female AD mice are uniquely susceptible to cortical mitochondrial complex I dysfunction, and that male AD mice increase cytochrome oxidase activity and the expression of both nuclear and mitochondrially encoded ETC subunits to compensate for metabolic perturbations in AD.
Since the bioenergetic impairments in the female cortical synaptosomes may relate to the previously reported increased rate of cortical neurodegeneration observed in human female AD subjects [34], we evaluated levels of apoptotic proteins in cortical synaptic mitochondria. We observed a significant increase in BAX/BCL2 ratio in female, but not male cortical synaptosomes (Fig. 1k), despite a decrease in anti-apoptotic BCL2 in both male and female AD synaptosomes (Fig. 1l), and no significant change in BAX levels (Fig. 1m). Additionally, we find a significant increase in apoptosis inducing factor (AIF) in 3 × and 3×β cortical synaptosomes (Fig. 1n). AIF is best recognized for its role in cell death. However, it also plays substantial roles in redox homeostasis and can promote electron transport to coenzyme Q [20]. Therefore, increased AIF abundance may reflect a physiological adaptive mechanism to bypass complex I impairment in AD. Together these data suggest differing mechanisms of mitochondrial metabolic alterations and cell death signaling pathways that could contribute to the higher rates of cortical atrophy observed in women with AD [34].
Polβ deficiency causes mitochondrial complex I impairment in hippocampal synaptosomes
The hippocampus is a critical structure for learning and memory that progressively degenerates in both male and female AD subjects [61, 65]. DNA base excision repair (BER) capacity decreases in AD [84]. We therefore sought to determine the mechanisms of mitochondrial dysfunction and the role of BER deficiency in the hippocampus of AD mice. Synaptosomes isolated from the hippocampus displayed a CI-dependent respiratory impairment in both male (Fig. 2a) and female (Fig. 2b) POLβ mice, and a significant decline in CI function in 3×β mice in female mice with a similar trend in male 3×β mice (p = 0.12) (Fig. 2a, b). These results demonstrate the surprising result that the 3× AD mice lack hippocampal synaptic mitochondrial dysfunction and provide evidence for a critical role of DNA POLβ in hippocampal neuronal metabolism. The lack of sex differences in hippocampal synaptic mitochondrial dysfunction is consistent with neurodegeneration of the hippocampus as a universal hallmark of AD in men and women [65]. Similar to the cortex, we observe no differences among genotypes in male non-synaptic mitochondria from the hippocampus (Fig. 2c), but an increase in CII-mediated respiration in non-synaptic hippocampal mitochondria from female 3×β mice (Fig. 2d). We also observe a significant decrease in complex IV-dependent respiration in hippocampal non-synaptic mitochondria from female 3×β mice (Fig. 2d) indicating brain region and cell-type-specific sites of mitochondrial impairment in AD, which may explain the aforementioned disparate reports of underlying ETC impairments in AD.
Fig. 2.
Polymerase β deficiency results in CI impairment in hippocampal synaptosomes and upregulated CII activity in female non-synaptic hippocampal mitochondria. a–d Maximal electron flow through complex I under malate (2 mM) and pyruvate (10 mM) substrates, complex II with succinate (10 mM) and rotenone (2 μM), and complex IV with TMPD (100 μM) + Ascorbate (10 mM) in male (blue; left) and female (red; right) hippocampal synaptic (HSYN) and non-synaptic (HNS) brain mitochondria n = 4–6/group/One-way ANOVA with Benjamini, Krieger and Yekutieli post hoc test for multiple comparisons p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. vs. WT, NS = non-significant. e–j Western blot of cortical synaptic mitochondrial oxidative phosphorylation proteins, two-way ANOVA with Benjamini, Krieger and Yekutieli post hoc test for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. sex-matched WT, unless indicated by brackets. #p < 0.05, ##p < 0.01, ###p < 0.001, significant effect of sex, n = 3/group
We further evaluated ETC protein expression in hippocampal synaptosomes (Fig. 2e). Consistent with complex I-mediated impairment in mitochondrial function, we observed a significant decrease in NDUFB8 expression in POLβ and 3×β hippocampal synaptosomes from female mice (Fig. 2f). Interestingly, despite the same functional deficit observed in both male and female mice, we did not observe altered NDUFB8 levels in male hippocampal synaptosomes (Fig. 2f), possibly indicating sex-dependent complex I subunit dysfunction responsible for respiratory impairment. The SDHB subunit of complex II was downregulated in male POLβ, 3×, and 3×β mice, while in females, it was decreased in 3 × and 3×β mice (Fig. 2g). Complex III ETC subunit UQCRC2 was decreased in female POLβ, 3×, and 3×β female hippocampal synaptosomes, and unchanged in males, resulting in significantly higher levels of UQCRC2 in male POLβ and 3×β hippocampal synaptosomes compared to females (Fig. 2h). Similarly, we found that female WT mice have significantly higher CIV subunit MTCO1 than WT male mice (Fig. 2i), with a significant decline in POLβ, 3×, and 3×β mice, in both males and females (Fig. 2i). ATP synthase (CV) subunit ATP5A also demonstrated sex-dependent alterations in 3×β mice, with a decreased abundance only observed in female 3×β mice (Fig. 2j). These results further suggest sex-dependent mitochondrial adaptations to AD pathology. Similar to our observations in the cortex, the deficit in CI-mediated respiration in synaptic mitochondria concomitant with an increase in CII-mediated respiration in non-synaptic mitochondria suggests a switch to fatty-acid or ketone metabolism in astrocytes to provide metabolic support to neighboring neurons that cannot efficiently metabolize glucose. We sought to further investigate this possibility, while also determining if this metabolic switch was restricted to the brain, or whether it was reflective of systemic metabolic impairments in AD as has been postulated [53].
Upregulation of systemic fatty acid metabolism in AD mice
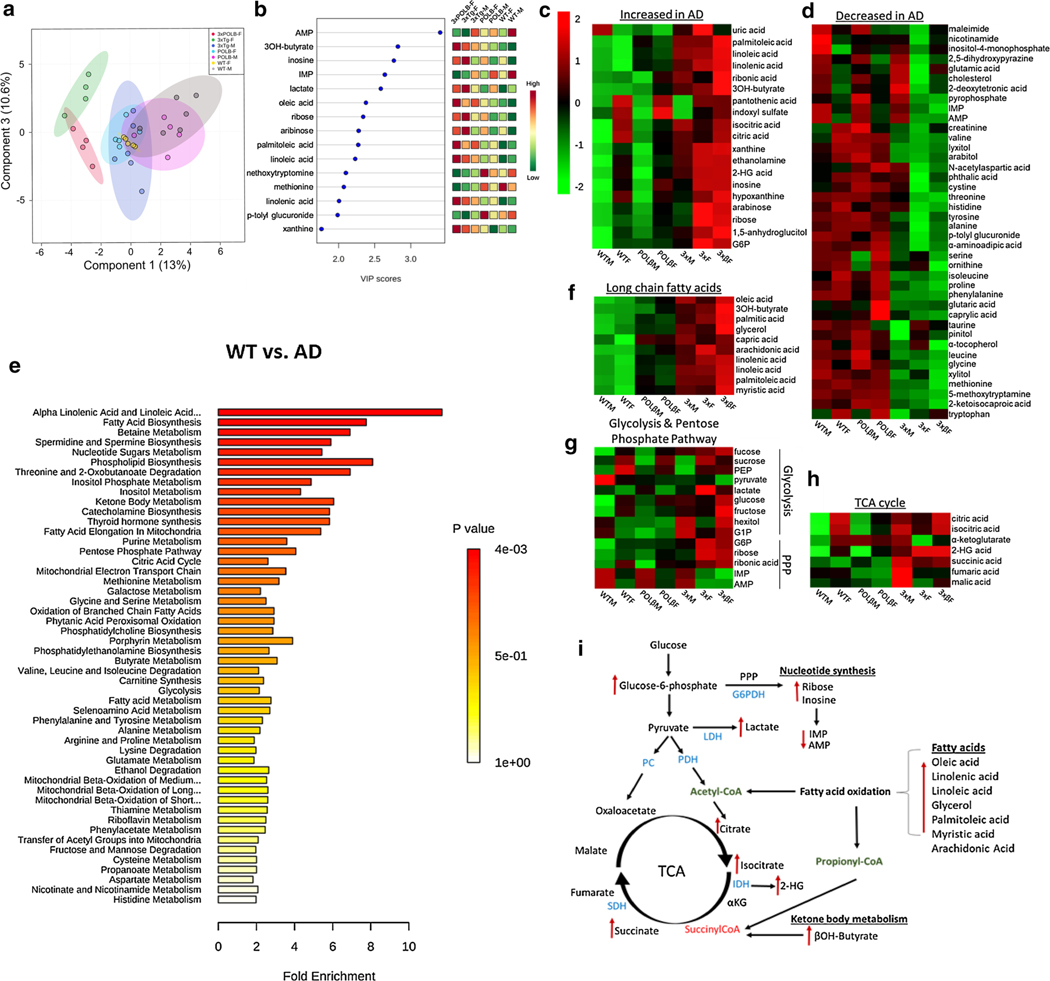
To investigate whether biological sex impacts systemic metabolism and further understand mechanisms of systemic metabolic remodeling in AD, we performed an unbiased gas chromatography time-of-flight (GC-TOF) metabolomics analysis of plasma from AD mice. Phenotypically, we observed that the males are significantly fatter than females in the 3 × and 3×β AD mice (Fig. S1a, online resource), indicating major sex differences in systemic metabolism. This evidence suggests that metabolic dysregulation is a central feature of AD and that intrinsic metabolic differences between men and women may contribute to the increased risk of AD in females. Partial least squares discriminant analysis (PLS-DA) demonstrated a significant separation between male and female AD mice (Fig. 3a) indicating that biological sex significantly impacts systemic metabolism. Furthermore, results confirmed an upregulation of fatty acids and the principal ketone body 3OH-butyrate, which was more pronounced in female than male AD mice (Fig. 3b, c, f). Moreover, we found a marked accumulation of TCA cycle intermediates citrate, isocitrate, and 2-hydroxyglutarate indicating a lack of flux through isocitrate dehydrogenase and α-ketoglutarate dehydrogenase [37, 83] (Fig. 3h). Consistent with a deficit in glucose metabolism, the accumulation of glucose-6-phosphate (G6P) has been reported to impair the enzyme activity of hexokinase [43], the rate-limiting step of glucose metabolism (Fig. 3g) [31]. Furthermore, G6P is the rate-limiting substrate of the pentose phosphate pathway (PPP) that is critical for the generation of NADPH and the subsequent detoxification of damaging free oxygen radicals [57], thus connecting glucose hypometabolism and oxidative stress commonly observed in AD. Metabolite set enrichment (MSE) pathway analysis indicated that affected metabolic pathways include: alpha linolenic and linoleic acid metabolism, fatty acid biosynthesis, betaine metabolism, spermidine and spermine biosynthesis, nucleotide sugars metabolism, phospholipid biosynthesis, threonine and 2-oxobutanoate degradation, inositol metabolism, ketone body metabolism, catecholamine biosynthesis, fatty acid elongation in mitochondria, purine metabolism, PPP, TCA cycle, and the mitochondrial ETC (Fig. 3e). The summary of metabolite pathway alterations is depicted in Fig. 3i. These results demonstrate that a systemic shift from glycolysis to lipid metabolism is evident in AD mice and that distinct sites of glycolytic, TCA cycle, and PPP inhibition likely play a role in the metabolic alterations implicated in AD etiology.
Fig. 3.
Systemic dysfunction of glycolysis, pentose phosphate, TCA cycle pathways and upregulation of fatty acid metabolism in plasma of AD mice. a Partial least squares discriminant analysis (PLS-DA) of plasma metabolic data and identified VIP metabolites (b). Heat map of upregulated (c) and downregulated (d) plasma metabolites in AD vs. WT mice. e Metabolite set enrichment (MEA) pathway analysis of WT vs. AD mice. f Upregulation of long-chain fatty acids (g) Glycolysis and Pentose phosphate pathway alterations, h TCA cycle metabolites and i summary of significant metabolite changes in metabolic pathways indicated by red arrows (right). Data analyzed with Metaboanalyst v4.0 [87] following log transformation to fit a gaussian distribution and analyzed using One-way ANOVA with Tukey HSD post hoc test with 0.05 FDR for multiple comparisons, n = 4–6 mice/group
Upregulation of lipolysis and ketolysis enzymes in the cortex of AD mice
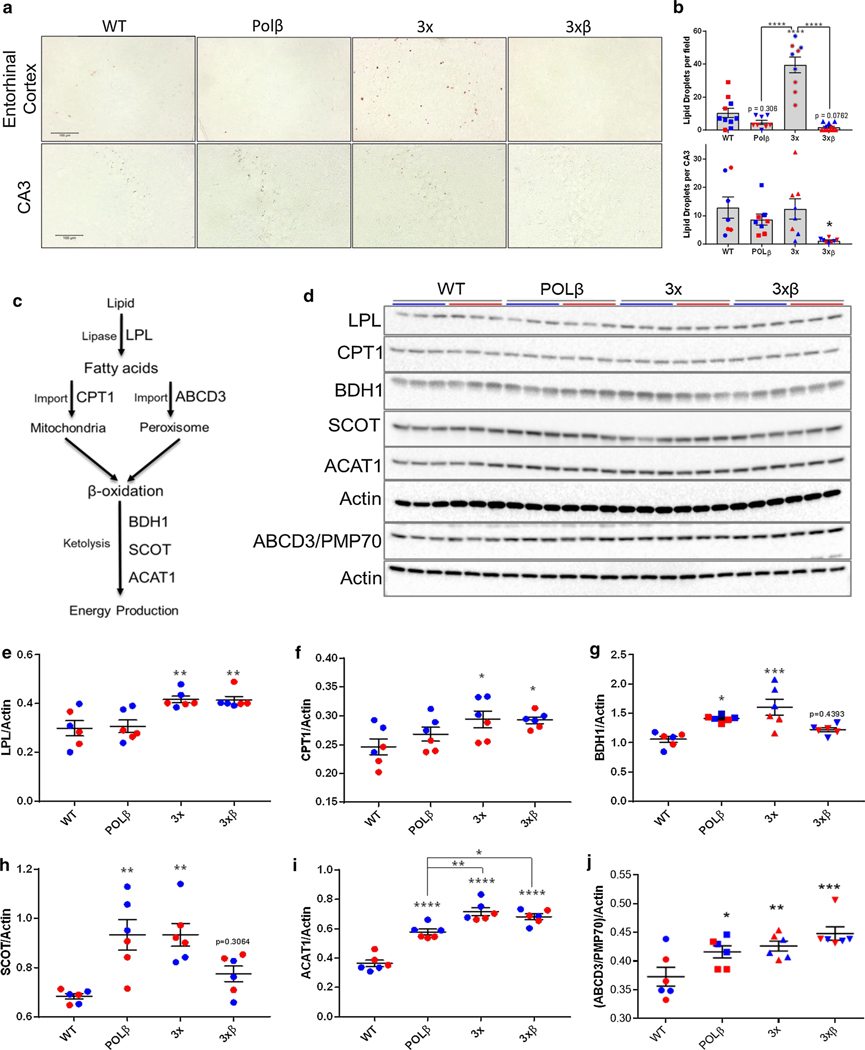
To investigate whether fatty acid metabolism and ketone utilization (ketolysis) are also increased in the brain of AD mice, we evaluated brain lipid droplet abundance by Oil Red O staining (Fig. 4a). Consistent with the increased CII-mediated respiration (Fig. 1d, Fig. 2d) and systemic augmentation of fatty acid metabolism observed in AD mice (Fig. 3), we found a striking depletion of lipid droplet stores in both the entorhinal cortex and the hippocampal CA3 region of 3×β mice (Fig. 4a, b). Lipid oxidation requires several steps to liberate free fatty acids from lipid stores, import fatty acids into the mitochondrial matrix for β-oxidation, and the formation of ketone bodies for acetyl-CoA and ATP synthesis. We, therefore, investigated the protein levels of enzymes critically involved in these pathways in the cortex of AD mice (Fig. 4c, d). We have previously observed a marked increase in lipoprotein lipase (LPL) expression throughout the brain of 3×β mice utilizing a spatial transcriptomics approach (Bohr lab, unpublished observations). Indeed, we observed increased protein levels of lipoprotein lipase (LPL) (Fig. 4e) and carnitine palmitoyltransferase-1 (CPT-1) expression in AD mice (Fig. 4f). CPT-1 is critical for the import of fatty acids into the mitochondria for β-oxidation [5]. Studies on CPT-1 activity in human AD patients are limited, and have thus far not found significant differences in activity [46]. However, we have observed previously that fatty acid metabolism is altered in postmortem human AD brain [68]. Peroxisomes are also known to coordinate beta oxidation of fatty acids [25]. We determined that peroxisomal fatty acid transport protein ABCD3/PMP70 [28, 82] was also upregulated in AD mouse cortex (Fig. 4d, j). Moreover, increased expression of downstream ketolysis enzymatic machinery succinyl-CoA:3-ketoacid CoA transferase (SCOT) (Fig. 4g), beta hydroxybutyrate dehydrogenase (BDH1) (Fig. 4h), and acetyl-CoA acetyltransferase (ACAT1) (Fig. 4i) further support an increase in the cerebral mobilization of fatty acids and the upregulation of ketone formation (ketogenesis) for energy metabolism and ATP generation, likely to compensate for glucose hypometabolism in AD.
Fig. 4.
Increased mitochondrial and peroxisomal fatty acid metabolism in AD mouse cortex. a Alterations in lipid droplet abundance by oil red O staining in the entorhinal cortex (top) and CA3 region of the hippocampus (bottom) demonstrate a decline in lipid droplet abundance in 3×β mice (b) One-way ANOVA with Tukey post hoc test for multiple comparisons. *p < 0.05, ****p < 0.0001 vs. WT unless indicated by brackets. c, d Investigation of enzymatic lipolysis/ketolysis pathways indicate an increase in lipoprotein lipase (e), carnitine palmitoyltransferase-1 (CPT-1) (f), 3OH-butyrate dehydrogenase (BDH1) (g), succinyl-CoA:3-ketoacid CoA transferase (SCOT) (h), and acetyl-CoA acetyltransferase 1 (ACAT1) (i). One-way ANOVA with Dunnett’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs WT, unless indicated by brackets
Increased fatty acid metabolism is a beneficial compensatory response
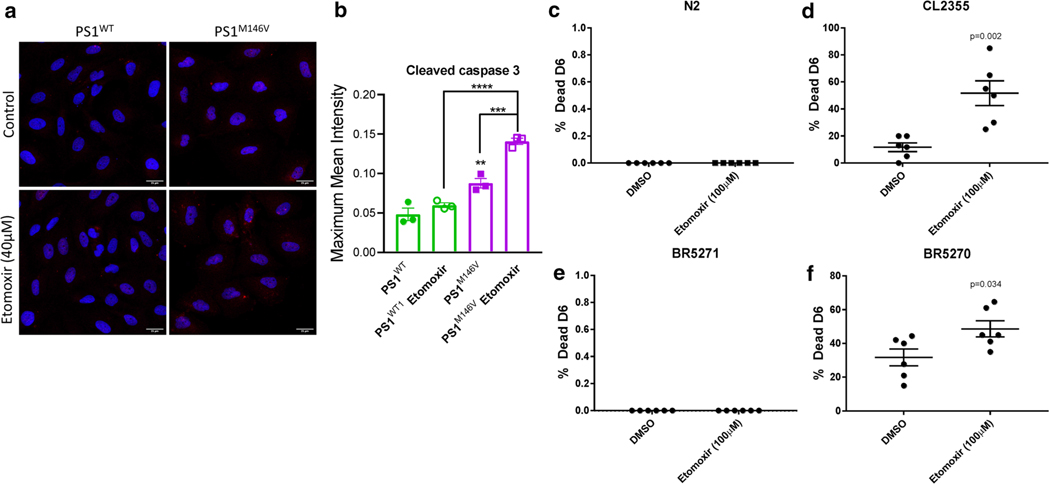
To determine if the augmentation of lipid metabolism pathways observed in the plasma and brain of AD mice is a detrimental or beneficial metabolic response, we utilized human H4 neuroglioma cells expressing wild-type (PS1WT) and the human AD-linked presenilin mutation (PS1M146V) and C. elegans strains expressing neuronal human amyloid beta or tau. To test the importance of fatty acid metabolism in these systems, we treated these cells/nematodes with etomoxir (40 μM/100 μM, respectively), a CPT-1 inhibitor, that inhibits mitochondrial fatty acid transport and, therefore, β-oxidation [27]. We determined that PS1 M146V cells activated caspase-3-dependent cell death pathways following CPT-1 inhibition, whereas PS1WT expressing cells did not display an increase in active caspase-3 in response to etomoxir treatment (Fig. 5a, b). These data indicate that the augmentation of fatty acid metabolism observed in AD is an adaptive metabolic switch to maintain adequate energy production necessary for cell survival. To investigate the cross-species conservation of the metabolic switch to fatty acid oxidation in vivo, we utilized C. elegans AD strains treated with etomoxir and assayed nematode viability. We found that AD strains expressing neuronal amyloid beta (CL2355) or tau (BR5270) had a significant decrease in viability following etomoxir treatment (Fig. 5d, f), whereas wild-type (N2) and non-aggregating neuronal tau (BR5271) expressing C. elegans survival were unaffected by the CPT-1 inhibition (Fig. 5c, e). These results further support the concept that the increased fatty acid metabolism observed in AD is a compensatory physiologic adaptation to maintain adequate cellular energy production and prevent neurodegeneration. Encouraged by the conservation of metabolic mechanisms in mice, C. elegans and human cell culture AD models, and the possible relevance to patterns of neurodegeneration in humans with AD, we sought to investigate if similar metabolic alterations are also evident in serum and brain tissue of AD patients.
Fig. 5.
Inhibition of fatty acid metabolism is detrimental to cellular and organismal survival in AD models. a representative images of active caspase-3 immunofluorescence following 8 h of exposure to vehicle (DMSO control) or CPT-1 inhibitor etomoxir (40 μM) with quantified fluorescent intensity of active caspase-3 (b). Quantification of percent C. elegans survival following etomoxir treatment from L4 stage to day 6 in wild-type (N2) (c), neuronal expressing amyloid β strain (CL2355) (d), neuronal expressing non-aggregating tau (BR5271) (e), or aggregating neuronal tau (BR5270) (f), p-values from 2-tailed unpaired student’s t-tests are shown on corresponding graphs
Sex-dependent systemic metabolic alterations in preclinical and clinical AD serum
The Baltimore Longitudinal Study of Aging (BLSA) is a prospective cohort study of community dwelling participants that began in 1958 [22]. Detailed clinical and cognitive evaluations, including laboratory, radiological, and neurological evaluations were conducted every two years, and for participants older than 80 years, every year. A subset of the overall BLSA study included a case–control cohort [9, 80] of converters (cognitively normal individuals at baseline who convert to incident AD at follow-up; denoted as AD in figures) and non-converters (cognitively normal individuals at baseline who remain cognitively normal at follow-up; denoted as CON in figures) [9] with blood serum data at baseline (i.e. preclinical AD stage) and follow-up (clinical AD stage). We tested whether the metabolite alterations observed in AD mouse models were also altered in human serum within this case–control cohort of AD (n = 107, male n = 48, female n = 59, supplemental Table S2, online resource) and CON (n = 119, male n = 64, female n = 55, Table S2, online resource). Importantly, several metabolites identified in mouse models of AD (Fig. 3) were also altered in a sex-specific manner in the serum of AD subjects.
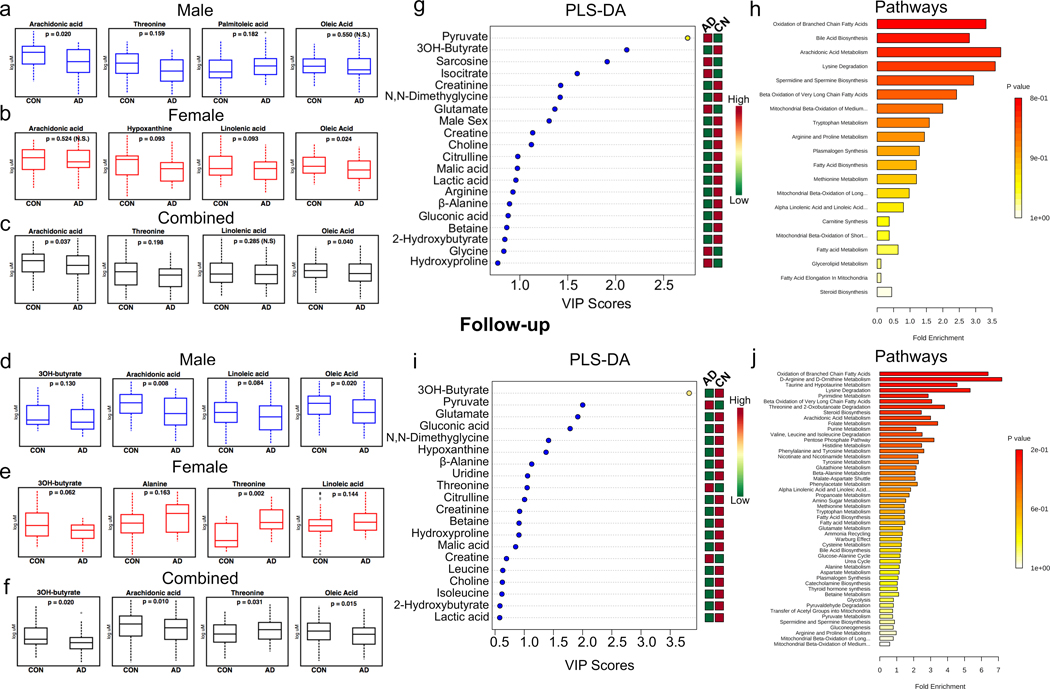
At baseline in AD (i.e. preclinical AD stage) compared to CON males, we observed significantly lower plasma arachidonic acid levels (p = 0.020), and a trend toward lower threonine (p = 0.159) and higher palmitoleic acid (p = 0.182), with no change in oleic acid (p = 0.550, Fig. 6a). Conversely, at baseline in AD compared to CON females, we observed significantly lower oleic acid (p = 0.024), and a trend toward lower levels of linolenic acid (p = 0.093) and hypoxanthine (p = 0.093) with no change in arachidonic acid (p = 0.524, Fig. 6b). These results suggest sex-dependent systemic metabolic alterations in humans who are asymptomatic, but will eventually express clinical symptoms of AD. Importantly, when these results are not sex stratified, in AD compared to CON, we observe a significantly lower arachidonic acid (p = 0.037) and oleic acid (p = 0.040), and a trend toward lower levels of threonine (p = 0.198) (Fig. 6c) indicating the importance of sex-stratified analyses in studies of AD.
Fig. 6.
Sex-dependent serum metabolic alterations in early onset AD patients. a Male (blue) non-converters/ cognitively normal controls (CON, n = 64) and converters/ Alzheimer’s (AD, n = 48) subject’s plasma levels of arachidonic acid, oleic acid, threonine and palmitoleic acid. b Female (red) cognitively normal controls (CON, n = 55) and Alzheimer’s (AD, n = 59) subject’s relative plasma levels of arachidonic acid, oleic acid, linolenic acid, and hypoxanthine. c Sex combined analysis of CON (n = 119) and AD (n = 107) samples arachidonic acid, oleic acid, methionine, and d Partial lease squares discriminant analysis (PLS-DA) important metabolites for discrimination between CON and AD groups. e Metabolite set enrichment (MSE) pathway analysis. f Follow-up plasma levels of male (blue) and g) female (red) metabolites. h Sex combined analysis of plasma metabolite changes at follow-up. i Partial lease squares discriminant analysis (PLS-DA) important metabolites for discrimination between CN and AD groups at follow-up. j Metabolite set enrichment (MSE) pathway analysis. Statistical analysis was performed with Stata 13.0. PLS-DA and MSE analysis were performed with MetaboAnalyst V4.0
At the follow-up visit where converters expressed AD clinical symptoms, we observed similar changes to baseline measures, with a more pronounced reliance on ketone body metabolism. In male AD compared to CON, we observed significantly lower arachidonic acid (p = 0.008), oleic acid (p = 0.020), and a trend toward lower 3OH-butyrate (p = 0.130) and linoleic acid (p = 0.084) (Fig. 6d). In female AD compared to CON, we observed higher threonine (p = 0.002), and a trend toward for higher alanine (p = 0.163) and linoleic acid (p = 0.144), with a trend toward lower 3OH-butyrate (0.062) (Fig. 6e). In the combined male and female analysis, we observed significantly lower 3OH-butyrate (p = 0.020), oleic acid (p = 0.015), arachidonic acid (p = 0.010) and higher threonine (p = 0.031) (Fig. 6f). Overall, these data suggest a progressive systemic deterioration of lipids and ketone bodies in response to a metabolic shift away from glucose oxidation that differs substantially between male and female AD subjects. It is important to note that in a prior paper using only BIOCRATES data in a similar cohort, we reported on specific amino acids that discriminated between converter and non-converter samples [9]. We then asked which metabolites in the entire data set (not restricted to the metabolic alterations observed in AD mice) could be utilized to separate individuals who would remain cognitively normal and those that would progress to AD using a partial least squares discriminant analysis (PLS-DA). Interestingly, the top 2 results of the top 20 depicted were an accumulation of pyruvate and depletion of 3OH-butyrate in AD vs. CON subjections (Fig. 6g). These results suggest an initial impairment of mitochondrial oxidative phosphorylation leading to the accumulation of pyruvate, the terminal carbon source for acetyl-CoA generation by glycolysis, and a switch to ketone body metabolism as demonstrated by the depletion of the primary ketone body, 3OH-butyrate in AD (Fig. 6g). We then determined which metabolic pathways were altered by performing metabolite set enrichment (MSE) pathway analysis between converters and non-converters. Results indicated that the top altered pathways are indeed consistent with an increase in fatty acid oxidation including: oxidation of branch-chain fatty acids, bile acid biosynthesis, arachidonic acid metabolism, lysine degradation, spermidine and spermine biosynthesis, β-oxidation of very long-chain fatty acids, mitochondrial β-oxidation of medium chain fatty acids, and tryptophan metabolism (Fig. 6h). PLS-DA analysis at the follow-up time-point revealed that the top metabolites differing between individuals remaining cognitively normal and progressing to AD were indeed the depletion of plasma 3OH-butyrate, followed by the accumulation of pyruvate (Fig. 6i), consistent with a progressive deterioration of mitochondrial oxidative phosphorylation and metabolic remodeling toward lipid metabolism. MSE pathway analysis revealed an increased oxidation of branched chain fatty acids, D-arginine and D-ornithine metabolism, taurine and hypotaurine metabolism, lysine degradation, pyrimidine metabolism, β-oxidation of long-chain fatty acids, threonine and 2-oxobutanoate degradation, steroid biosynthesis, arachidonic acid metabolism, folate, purine, valine, leucine and isoleucine degradation and PPP metabolism (Fig. 6j). These altered pathways are remarkably similar to those observed in our mouse models of AD, and further suggest progressive sex-dependent systemic metabolic remodeling from glucose to fatty acid metabolism in response to mitochondrial dysfunction.
Sex-dependent metabolic alterations in autopsy confirmed human AD brain tissue
To investigate whether the systemic metabolic alterations observed in AD mice and humans were relevant within the brain, we analyzed brain tissue metabolomics from the inferior temporal gyrus (ITG) within a subset of participants from the autopsy program of the BLSA [55]. The BLSA autopsy subsample is not significantly different from the overall BLSA cohort in terms of rates of dementia and clinical stroke [26]. Similar to our findings in serum, we identified sex-dependent metabolic alterations in similar pathways in AD (n = 16, male n = 8, female n = 8, supplemental Table S3, online resource) compared to cognitively normal (CN; n = 13, male n = 10, female n = 3, supplemental Table S3, online resource) samples as well as associations with neuritic plaques (Consortium to Establish a Registry for Alzheimer’s disease (CERAD) scores) and neurofibrillary tangles (Braak scores), hallmarks of AD pathology [6, 50] (Fig. 7).
Fig. 7.
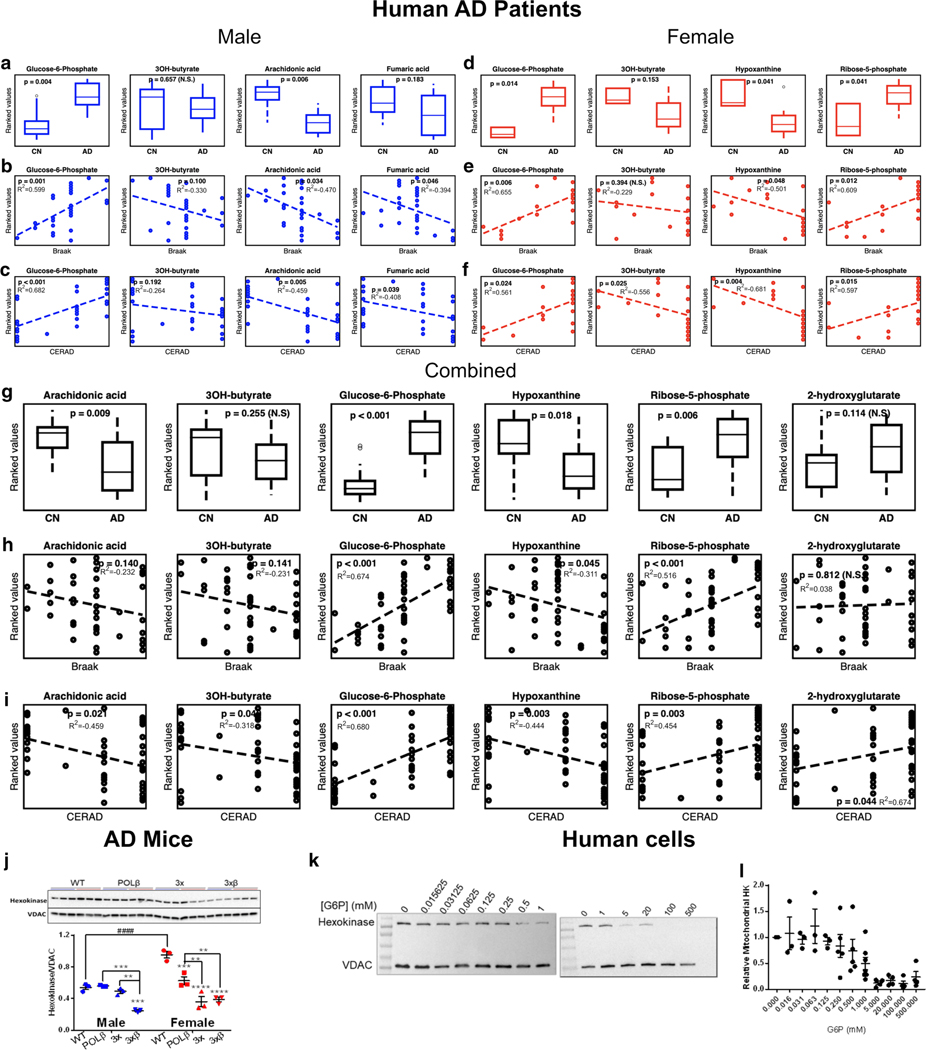
Brain metabolite changes and correlations to pathology scores in human AD patients. Relative metabolite changes in inferior frontotemporal gyrus (IFTG) of male (a) and female (d) cognitively normal controls (CN, male n = 10, female n = 3) and Alzheimer’s disease (AD, male n = 8, female n = 8) subjects. Correlations of metabolite changes to Braak and CERAD pathology scores of male (b, c, respectively) and female (e, f, respectively) AD subjects. g Sex combined metabolite changes of CON (n = 13) and AD (n = 16) samples and h correlations to Braak and CERAD (i) pathology scores. j Western blot of mitochondrial hexokinase expression and quantification in hippocampal synaptic mitochondria and quantification. 2-Way ANOVA with Tukey’s post hoc test for multiple comparisons. **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. WT, unless indicated by brackets. ####p < 0.0001 effect of sex as indicated by brackets. k Representative western blots demonstrating glucose-6-phosphate (G6P) concentrations dissociate hexokinase (HK) from mitochondria in a concentration-dependent manner. j Quantification of relative mitochondrial HK following 30 min G6P incubation, each dot represents an independent reaction from 3–5 independent biological experiments
Similar to findings in serum, in males with AD compared to CN—and not in females—we observed significantly lower arachidonic acid (p = 0.006), and a trend toward lower fumaric acid (p = 0.183) (Fig. 7a). We observed significant correlations in males with Braak and CERAD scores, respectively: Arachidonic acid (p = 0.034, p = 0.005) and fumaric acid (p = 0.046, p = 0.039) were negatively correlated with neurofibrillary tangles (Fig. 7c). In females with AD compared to CN, we observed significantly lower hypoxanthine (p = 0.041), a purine derivative and breakdown product involved in nucleotide salvage [64], and increased ribose-5-phosphate (p = 0.041) (Fig. 7d), suggestive of PPP nucleotide synthesis impairment. Importantly, these changes were also significantly correlated in females with Braak and CERAD scores, respectively: hypoxanthine (p = 0.048, p = 0.004) and 3OH-butyrate (p = 0.394, p = 0.025) were negatively correlated and ribose-5-phosphate (p = 0.012, p = 0.015) was positively correlated with neurofibrillary tangles (Fig. 7e, f). In combined male and female analysis, we observed higher G6P (p < 0.001), ribose-5-phosphate (p = 0.006) and a non-significant trend toward increased 2-hydroxyglutarate (p = 0.114), and lower levels of arachidonic acid (p = 0.009), hypoxanthine (p = 0.018), and a non-significant trend toward lower 3OH-butyrate (p = 0.255) between AD and CN samples (Fig. 7g). These metabolite alterations significantly correlated with Braak and CERAD pathology scores, respectively, as follows: 3OH-butyrate (p = 0.141, p = 0.04), arachidonic acid (p = 0.14, p = 0.021), ribose-5-phosphate (p < 0.001, p = 0.003), hypoxanthine (p = 0.045, p = 0.003), and 2-hydroxyglutyrate (p = 0.812, p = 0.044) (Fig. 7h, i). It is noteworthy that three metabolites (arachidonic acid, 3OH-butyrate, and 2-hydroxyglutarate) significantly correlate with amyloid pathology (CERAD), but not tau pathology (Braak) suggesting differing metabolic alterations conferred by amyloid and tau pathology.
The most striking finding was the significantly higher G6P in brain tissue in both males and females with AD, and combined analysis (p = 0.004, p = 0.014, p < 0.001, respectively) (Fig. 7a, d, g). As previously mentioned, G6P is the product of the initiating and rate-limiting enzymatic step of glycolysis, the phosphorylation of glucose following cellular uptake to G6P by hexokinase (HK). G6P is also the rate-limiting substrate for the PPP, which was identified in MSE pathway analysis in both mouse plasma (Fig. 3e) and human (Fig. 6j) serum. Furthermore, G6P was significantly positively correlated with Braak and CERAD scores in both males and females, and combined analysis (Braak: p = 0.001, p = 0.006, p < 0.001, respectively; CERAD: p < 0.001, p = 0.024, p < 0.001, respectively) (Fig. 7b, c, e, f, h, i). Overall, these observations suggest that impairments in glycolysis and the PPP may involve an impairment in G6P metabolism and downstream impairments of purine biosynthesis and mitochondrial energy production in the brain of both male and female AD subjects.
Increasing [glucose-6-phosphate] decreases mitochondrial hexokinase
G6P accumulation has been reported to impair glycolysis via a feedback inhibition mechanism on HK enzymatic activity by dissociating HK from the mitochondrial voltage-dependent anion channel (VDAC) [43]. In a prior paper using BLSA brain autopsy data [2], we reported increased brain tissue hexose concentrations as well as reduced glycolytic flux—i.e. reduced HK phosphofructokinase, and pyruvate kinase activity. Our results here support these prior findings suggesting that in both mice and humans with AD, HK dysfunction and the accumulation of G6P, a branch point between the oxidative PPP and glycolysis, likely represents a critical step affected by AD pathogenesis. Since we observed striking similarities in these pathways in mice and humans with AD, we sought to investigate whether the isolated synaptic mitochondria from the mouse hippocampus had altered levels of HK. We observed a significant decrease in synaptic HK levels in male 3×β mice, and in female POLβ, 3×, and 3×β mice (Fig. 7j) suggesting that the accumulation of G6P may contribute to neuronal glucose hypometabolism in the hippocampus of AD mice. Interestingly, we also observed that female WT mice have significantly higher expression of HK in hippocampal synaptosomes (Fig. 7j), which may reflect intrinsic sex differences in glucose metabolism as has been reported [33].
While these independent observations imply that G6P mediated mitochondrial dissociation of HK could contribute to the glucose hypometabolism commonly observed in AD, we wondered whether the accumulation of G6P could directly cause the mitochondrial dissociation of HK. To test this hypothesis, we isolated mitochondria from human U2OS cells and incubated them with increasing concentrations of G6P (15.6 μM–500 mM) while respiring on complex I substrates (pyruvate 5 mM and malate 2.5 mM) at 37 °C for 30 min prior to measuring levels of HK that remained bound to mitochondria. Indeed, we observe a dose-dependent dissociation of HK from VDAC, with ~ 50% HK dissociation when [G6P] approaches millimolar concentrations (Fig. 7k, l). These results establish that the accumulation of G6P observed in the brain of human AD subjects could cause impaired glycolytic flux via the mitochondrial dissociation of HK and consequent inhibition of HK activity [43].
Discussion
Alzheimer’s disease is the most prevalent age-related dementia. To date, the efficacy on anti-amyloid and anti-tau therapeutics have failed several large clinical trials demonstrating the importance of reconsidering the etiological mechanisms of AD to prevent or delay the progression of this devastating disease. Moreover, the higher prevalence of AD in women than men strongly suggest the need for a precision, sex-targeted therapeutic approach to combat AD. Metabolic alterations including cerebral glucose hypometabolism precede cognitive impairment by decades, and diabetes mellitus type II is a risk factor for developing AD [12, 47]. These observations suggest that brain and systemic metabolic remodeling occurs early and likely contribute to the etiology of AD.
In the present study, we utilized a cross-species approach to evaluate conserved metabolic alterations in two transgenic mouse models, human cell lines, two C. elegans AD strains, and two cohorts of human AD subjects. We consistently observed evidence of metabolic remodeling with a shift from glucose to lipid metabolism in all laboratory models and humans. Utilizing two mouse models of AD, we found cortical impairment of mitochondrial complex-I-dependent respiration in female synaptosomes, with no observable impairment, but rather an increased complex IV function, in cortical synaptosomes isolated from male AD mice (Fig. 1). Moreover, we identify a novel and important role for base excision DNA repair deficiency in hippocampal neuronal mitochondrial impairment (Fig. 2). In astrocyte-enriched non-synaptic mitochondria, we observed an increase in complex-II-dependent respiration implying an upregulation of astrocyte fatty acid metabolism that was subsequently supported by systemic increases in fatty acid and ketone metabolism pathways (Fig. 3), decreased brain lipid stores, and upregulated expression of lipolysis/ketolysis enzymes (Fig. 4). Importantly, we determined that this metabolic shift to lipid metabolism is a compensatory physiological adaptation essential for cellular and organismal survival using in vitro and in vivo laboratory models of AD (Fig. 5), conceivably as a mechanism to bypass impaired glucose metabolism. Subsequent evaluation of similar metabolic alterations in humans demonstrated a strikingly consistent alteration in glucose and lipid metabolism pathways in human AD serum (Fig. 6) and brain (Fig. 7). The majority of these alterations significantly correlate with Braak/CERAD pathology, further supporting the relevance of the identified metabolic alterations to AD pathogenesis (Fig. 7). A working model summary of these findings is depicted in Fig. 8. These findings are largely consistent with literature describing glucose hypometabolism and systemic metabolic syndromes predisposing individuals to develop AD. Together these data provide novel insight into the critical need for a sex-specific approach to therapeutic development for men and women affected by AD.
Fig. 8.
Working model. Glucose hypometabolism occurs with aging, possibly due to the accumulation of glycolysis intermediate glucose-6-phosphate, thereby causing feedback inhibition on rate-limiting glycolysis enzyme hexokinase via the dissociation of hexokinase from mitochondrial VDAC. This glucose hypometabolism is further exacerbated by a decline in synaptic complex I function in the cortex of female AD mice. Impaired glycolysis forces a metabolic shift toward fatty acid and ketone metabolism that corresponds with increased non-synaptic (astrocyte-enriched) mitochondrial complex II function of the cortex and hippocampus of female AD mice. In contrast, male synaptic complex I function is preserved, and augmented complex IV respiration is observed. Decreased mitochondrial function combined with impaired purine biosynthesis and polymerase β deficiency drives synaptic complex I impairment in the hippocampus contributing to energy failure and eventual hippocampal atrophy. These metabolic mechanisms of glucose hypometabolism likely contribute to an increased AD risk in woman compared to men
Sex differences in AD pathophysiology have become the subject of intense research. A recent study identifies sex and cell-type-specific transcriptional changes in human AD [44]. Sex differences in mitochondrial metabolism and cell death pathways have been described [15, 88]. In the present study, we contribute to this effort by identifying distinct sites of mitochondrial ETC inhibition in a sex, brain region, and cell-type-dependent manner. These results are consistent with a report demonstrating the rate of cortical atrophy is significantly faster in female and compared to male AD subjects [34]. These data support the notion that mitochondrial impairment is a significant driver of the progression of neurodegeneration in AD [72, 88]. Indeed, a recent study determined that impaired mitochondrial calcium homeostasis precedes tau and amyloid beta pathology in mice [35], further supporting that metabolic impairments precede the classical hallmarks of AD pathology. It is evident that there are distinct sex-specific mechanisms of metabolic impairment that likely contribute to the higher prevalence of AD in females, however, there are also likely shared mechanisms of metabolic impairment in males and females.
The underlying mechanisms of glucose hypometabolism have been reported to include decreased glucose uptake [2, 36, 66], insulin resistance [4, 45], and impaired pyruvate dehydrogenase activity [62, 69]. Consistent with our recent previous report of reduced HK activity [2], we expand on these findings with evidence of a novel mechanism that likely contribute to glucose hypometabolism mediated by feedback inhibition of HK by accumulating G6P. Importantly, G6P accumulation in the brain of human AD subjects and correlations to pathology were observed in both men and women, suggesting that this is a common mechanism of metabolic impairment in AD. We further establish a proof-of-principle that G6P can causally dissociate HK from mitochondria and estimate that cytoplasmic concentrations approaching millimolar values are required for HK inhibition. G6P is also the rate-limiting metabolite for the PPP, which is the principle source of cytoplasmic NADPH for endogenous antioxidant defense systems, and downstream nucleotide synthesis for DNA integrity maintenance. G6P, therefore, connects glucose hypometabolism, DNA repair and oxidative stress [31]. Interestingly, one study suggests that HK dissociation from VDAC is a signal to activate the NLRP3 inflammasome [86] and thus suggests that G6P accumulation could contribute to neuroinflammation in AD. The most obvious culprit for G6P accumulation is a deficiency in glucose-6-phosphate dehydrogenase (G6PDH), however, studies measuring G6PDH activity have yielded conflicting results [77, 79]. Further investigation is needed to determine the underlying mechanisms resulting in G6P accumulation in the AD brain.
More broadly, determining the trigger for the observed metabolic shift from glucose to lipid metabolism is of utmost importance. Studies have implicated circulating 17β estradiol levels as a putative explanation for why females may be at a higher risk for developing AD [73, 78], and that 17β estradiol contributes to sex differences in glucose and fatty acid metabolism [75]. Limitations of the current study include the lack of estradiol measurements within AD subjects as well as the reliance on postmortem brain tissue metabolite quantifications. Postmortem interval, although not significantly different between control and AD groups within this study (supplemental Table S3, online resource), undoubtedly alter the native metabolite concentrations during life. It is important to consider this caveat when interpreting results from postmortem tissues. Further investigation aimed at identifying the cause of the identified metabolic alterations in AD is warranted.
Cunnane and colleagues have recently demonstrated that while brain glucose metabolism declines with normal aging and more severely in AD, the capacity to metabolize ketone bodies, the brain’s only other energy source (during starvation or ketogenic diet), remains normal in older individuals and AD patients [10]. Ketone body utilization is linearly dependent on the plasma concentrations of major ketones acetoacetate and 3OH-hydroxybutyrate [13, 14]. These data are from human subjects on prolonged fasting regimens or a diet rich in medium chain triglyceride (MCT) ketone precursors. Indeed, several small clinical trials in patients with AD or mild cognitive impairment illustrate an exciting proof-of-principle that metabolic interventions can improve cognitive processing and perhaps alter AD disease trajectory [7, 56]. These therapeutic strategies were recently labeled “neuroketotherapeutics”, and have shown some efficacy in preventing cognitive decline in individuals with AD [76]. Our data support the advancement of metabolic interventions such as neuroketotherapeutics to bypass dysfunctional glucose metabolism in hopes of stalling or preventing AD pathogenesis.
Conclusions
The present study provides several novel insights supporting the need to consider sex-targeted metabolism-modifying therapeutic approaches to combat the increasing incidence of AD. We identify mechanisms of impaired brain mitochondrial function, glycolytic inhibition, and the natural physiological adaptive mechanisms upregulated to compensate for impaired cellular energy production in AD. These data argue for a refocusing of AD prevention on early metabolic changes, and describe the critical role of biological sex and DNA repair deficiency in driving neurodegeneration in AD. Targeting these pathways with a careful consideration of intrinsic metabolic differences between male and female physiology will provide a greater chance for developing efficacious therapies using a personalized medicine approach to prevent or delay the progression of AD. A substantial further effort is required to understand the metabolic underpinnings of AD etiology and pathogenesis.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes on Aging, National Institutes of Health. The authors are grateful to the Baltimore Longitudinal Study of Aging study participants and staff for their dedication to these studies. We would like to thank Kelly Palagia (West coast metabolomics center, UC Davis) for performing the GC-TOF plasma metabolomics and Drs. Jong-Hyuk Lee and Anthony Moore for their critical review of the manuscript.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00401-020-02152-8) contains supplementary material, which is available to authorized users.
References
- 1.Adami PVM, Quijano C, Magnani N, Galeano P, Evelson P, Cassina A et al. (2017) Synaptosomal bioenergetic defects are associated with cognitive impairment in a transgenic rat model of early Alzheimer’s disease. J Cereb Blood Flow Metab 37:69–84. 10.1177/0271678X15615132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O et al. (2018) Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dement 14:318–329. 10.1016/J.JALZ.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auestad N, Korsak RA, Morrow JW, Edmond J (1991) Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 56:1376–1386. 10.1111/j.1471-4159.1991.tb11435.x [DOI] [PubMed] [Google Scholar]
- 4.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68:51–57. 10.1001/archneurol.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BONNEFONT J (2004) Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 25:495–520. 10.1016/j.mam.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 7.Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC (2019) Preliminary report on the feasibility and efficacy of the modified atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J Alzheimer’s Dis 68:969–981. 10.3233/JAD-180995 [DOI] [PubMed] [Google Scholar]
- 8.Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ et al. (2019) Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 76:542 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova R, Varma S, Simpson B, Kim M, An Y, Saldana S et al. (2016) Blood metabolite markers of preclinical Alzheimer’s disease in two longitudinally followed cohorts of older individuals. Alzheimer’s Dement 12:815–822. 10.1016/j.jalz.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellano C-A, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G et al. (2014) Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimer’s Dis 43:1343–1353. 10.3233/JAD-141074 [DOI] [PubMed] [Google Scholar]
- 11.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G et al. (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494. 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chornenkyy Y, Wang W-X, Wei A, Nelson PT (2019) Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol 29:3–17. 10.1111/bpa.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croteau E, Castellano CA, Fortier M, Bocti C, Fulop T, Paquet N et al. (2018) A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol 107:18–26. 10.1016/j.exger.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M et al. (2016) Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci 9:1–21. 10.3389/fnmol.2016.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demarest TG, McCarthy MM (2014) Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr 47:173–188. 10.1007/s10863-014-9583-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarest TG, Schuh RA, Waite EL, Waddell J, McKenna MC, Fiskum G (2016) Sex dependent alterations in mitochondrial electron transport chain proteins following neonatal rat cerebral hypoxic-ischemia. J Bioenerg Biomembr 48:591–598. 10.1007/s10863-016-9678-4 [DOI] [PubMed] [Google Scholar]
- 17.Demarest TG, Waite EL, Kristian T, Puche AC, Waddell J, McKenna MC et al. (2016) Sex-dependent mitophagy and neuronal death following rat neonatal hypoxia–ischemia. Neuroscience 335:103–113. 10.1016/j.neuroscience.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM et al. (2016) Extracellular vesicle-associated Aβ mediates trans-neuronal bioenergetic and Ca2+-handling deficits in Alzheimer’s disease models. Aging Mech Dis 2:16019 10.1038/npjamd.2016.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fecher C, Trovò L, Müller SA, Snaidero N, Wettmarshausen J, Heink S et al. (2019) Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci. 10.1038/s41593-019-0479-z [DOI] [PubMed] [Google Scholar]
- 20.Ferreira P, Villanueva R, Cabon L, Susin S, Medina M (2013) The Oxido-reductase Activity of the Apoptosis Inducing Factor: A Promising Pharmacological Tool? Curr Pharm Des 19:2628–2636. 10.2174/1381612811319140012 [DOI] [PubMed] [Google Scholar]
- 21.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A et al. (2018) Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol 14:457–469. 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L (2008) The baltimore longitudinal study of aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci 63:1416–1419. 10.1093/gerona/63.12.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y et al. (2008) Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J 53:691–704. 10.1111/j.1365-313X.2007.03387.x [DOI] [PubMed] [Google Scholar]
- 24.Fishman E (2017) Risk of developing dementia at older ages in the United States. Demography 54:1897–1919. 10.1007/s13524-017-0598-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen M, Lismont C, Walton P (2017) The peroxisome-mitochondria connection: How and why? Int J Mol Sci 18:1126 10.3390/ijms18061126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O’Brien R (2006) Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology 67:1363–1369. 10.1212/01.wnl.0000240285.89067.3f [DOI] [PubMed] [Google Scholar]
- 27.Gerondaes P, Alberti KGMM, Agius L (1988) Interactions of inhibitors of carnitine palmitoyltransferase I and fibrates in cultured hepatocytes. Biochem J 253:169–173. 10.1042/bj2530169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant P, Ahlemeyer B, Karnati S, Berg T, Stelzig I, Nenicu A et al. (2013) The biogenesis protein PEX14 is an optimal marker for the identification and localization of peroxisomes in different cell types, tissues, and species in morphological studies. Histochem Cell Biol 140:423–442. 10.1007/s00418-013-1133-6 [DOI] [PubMed] [Google Scholar]
- 29.Guzmán M, Blázquez C (2001) Is there an astrocyte–neuron ketone body shuttle? Trends Endocrinol Metab 12:169–173. 10.1016/S1043-2760(00)00370-2 [DOI] [PubMed] [Google Scholar]
- 30.Hirayama A, Nakashima E, Sugimoto M, Akiyama SI, Sato W, Maruyama S et al. (2012) Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem 404:3101–3109. 10.1007/s00216-012-6412-x [DOI] [PubMed] [Google Scholar]
- 31.Horecker BL (2002) The pentose phosphate pathway. J Biol Chem 277:47965–47971. 10.1074/jbc.X200007200 [DOI] [PubMed] [Google Scholar]
- 32.Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E et al. (2018) NAD + supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci 115:E1876–E1885. 10.1073/pnas.1718819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y, Xu Q, Li K, Zhu H, Qi R, Zhang Z et al. (2013) Gender differences of brain glucose metabolic networks revealed by FDGPET: evidence from a large cohort of 400 young adults. PLoS ONE 8:e83821. 10.1371/journal.pone.0083821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Weiner MW et al. (2010) Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol Aging 31:1463–1480. 10.1016/j.neurobiolaging.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadiya P, Kolmetzky DW, Tomar D, Di Meco A, Lombardi AA, Lambert JP et al. (2019) Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat Commun 10:3885 10.1038/s41467-01911813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalaria RN, Harik SI (1989) Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem 53:1083–1088. 10.1111/j.1471-4159.1989.tb07399.x [DOI] [PubMed] [Google Scholar]
- 37.Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH et al. (2016) Oncometabolite d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci 113:10436–10441. 10.1073/pnas.1601650113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A (2000) Age-specific incidence rates of Alzheimer’s disease: the baltimore longitudinal study of aging. Neurology 54:2072–2077. 10.1212/wnl.54.11.2072 [DOI] [PubMed] [Google Scholar]
- 39.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang L-J et al. (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59:776–779. 10.1111/j.1471-4159.1992.tb09439.x [DOI] [PubMed] [Google Scholar]
- 40.Kristian T (2010) Isolation of mitochondria from the CNS. Curr Protoc Neurosci 52:1–12. 10.1002/0471142301.ns0722s52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamont LS (2005) Gender differences in amino acid use during endurance exercise. Nutr Rev 63:419–422. 10.1301/nr.2005.dec.419-422 [DOI] [PubMed] [Google Scholar]
- 42.Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S et al. (2018) Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol 136:873–885. 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Kim CS, Kurbanov FT, Honzatko RB, Fromm HJ (1999) Dual mechanisms for glucose 6-phosphate inhibition of human brain hexokinase. J Biol Chem 274:31155–31159. 10.1074/jbc.274.44.31155 [DOI] [PubMed] [Google Scholar]
- 44.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ et al. (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570:332–337. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y et al. (2010) Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75:764–770. 10.1212/WNL.0b013e3181eee25f [DOI] [PubMed] [Google Scholar]
- 46.Maurer I, Zierz S, Möller HJ (1998) Carnitine acyltransferases are not changed in Alzheimer disease. Alzheimer Dis Assoc Disord 12:71–76. 10.1097/00002093-199806000-00003 [DOI] [PubMed] [Google Scholar]
- 47.McDade E, Bateman RJ (2017) Stop Alzheimer’s before it starts. Nature 547:153–155. 10.1038/547153a [DOI] [PubMed] [Google Scholar]
- 48.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of alzheimer’s disease: report of the NINCDS-ADRDA work group* under the auspices of department of health and human services task force on alzheimer’s disease. Neurology 34:939–944. 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 49.Mielke MM, Ferretti MT, Iulita MF, Hayden K, Khachaturian AS (2018) Sex and gender in Alzheimer’s disease—Does it matter? Alzheimer’s Dement 14:1101–1103. 10.1016/j.jalz.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 50.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al. (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486. 10.1212/wnl.41.4.479 [DOI] [PubMed] [Google Scholar]
- 51.Monteiro-Cardoso VF, Oliveira MM, Melo T, Domingues MRM, Moreira PI, Ferreiro E et al. (2014) Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. J Alzheimer’s Dis 43:1375–1392. 10.3233/JAD-141002 [DOI] [PubMed] [Google Scholar]
- 52.Moreira PI, Zhu X, Wang X, Lee H, Nunomura A, Petersen RB et al. (2010) Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta Mol Basis Dis 1802:212–220. 10.1016/J.BBADIS.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris JK, Honea RA, Vidoni ED, Swerdlow RH, Burns JM (2014) Is Alzheimer’s disease a systemic disease? Biochim Biophys Acta Mol Basis Dis 1842:1340–1349. 10.1016/j.bbadis.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I et al. (2017) Sex differences in Alzheimer risk. Neurology 89:1382–1390. 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O et al. (2009) Neuropathologic studies of the baltimore longitudinal study of aging (BLSA). J Alzheimer’s Dis 18:665–675. 10.3233/JAD-2009-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H et al. (2019) Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci Lett 690:232–236. 10.1016/j.neulet.2018.10.048 [DOI] [PubMed] [Google Scholar]
- 57.Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 14:5209–5215. 10.1002/j.1460-2075.1995.tb00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pichot P (1986) DSM-III: the 3d edition of the diagnostic and statistical manual of mental disorders from the American Psychiatric association. Rev Neurol (Paris) 142:489–99 [PubMed] [Google Scholar]
- 59.Prasad R, Çağlayan M, Dai D-P, Nadalutti CA, Zhao M- L, Gassman NR et al. (2017) DNA polymerase β: a missing link of the base excision repair machinery in mammalian mitochondria. DNA Repair (Amst) 60:77–88. 10.1016/j.dnarep.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD et al. (2009) PGC-1α expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol 66:352–361. 10.1001/archneurol.2008.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen RC, Jack CR Jr, Xu Y-C, Waring SC, O’Brien PC, Smith GE et al. (2012) Memory and MRI-based hippocampal volumes in aging and AD. Neurology 54:581–587. 10.1212/wnl.54.3.575 [DOI] [PubMed] [Google Scholar]
- 62.Rex Sheu K-F, Kim Y-T, Blass JP, Weksler ME (1985) An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer’s disease brain. Ann Neurol 17:444–449. 10.1002/ana.410170505 [DOI] [PubMed] [Google Scholar]
- 63.Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA et al. (2011) High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE. 10.1371/journal.pone.0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rolfes RJ (2006) Regulation of purine nucleotide biosynthesis: in yeast and beyond. Biochem Soc Trans 34:786–790. 10.1042/BST0340786 [DOI] [PubMed] [Google Scholar]
- 65.Seab JP, Jagust WJ, Wong STS, Roos MS, Reed BR, Budinger TF (1988) Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn Reson Med 8:200–208. 10.1002/mrm.1910080210 [DOI] [PubMed] [Google Scholar]
- 66.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P (1994) Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol 35:546–551. 10.1002/ana.410350507 [DOI] [PubMed] [Google Scholar]
- 67.Small GW, Kuhl DE, Riege WH, Fujikawa DG, Ashford JW, Metter EJ et al. (1989) Cerebral glucose metabolic patterns in Alzheimer’s disease. Arch Gen Psychiatry 46:527 10.1001/archpsyc.1989.01810060047008 [DOI] [PubMed] [Google Scholar]
- 68.Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O’Brien R et al. (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: a nontargeted metabolomic study. PLoS Med 14:e1002266. 10.1371/journal.pmed.1002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13:72–78. 10.1002/ana.410130116 [DOI] [PubMed] [Google Scholar]
- 70.Sun A, Nguyen XV, Bing G (2002) Comparative analysis of an improved thioflavin-S stain, Gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J Histochem Cytochem 50:463–472. 10.1177/002215540205000403 [DOI] [PubMed] [Google Scholar]
- 71.Choi SW, Gerencser AA, Ng R, Flynn JM, Melov S, Danielson SR et al. (2012) No consistent bioenergetic defects in presynaptic nerve terminals isolated from mouse models of Alzheimer’s disease. J Neurosci 32:16775–16784. 10.1016/j.freeradbiomed.2008.10.025.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimer’s Dis 20:S265–S279. 10.3233/JAD-2010-100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sykora P, Kanno S, Akbari M, Kulikowicz T, Baptiste BA, Leandro GS et al. (2017) DNA polymerase beta participates in mitochondrial DNA repair. Mol Cell Biol. 10.1128/MCB.00237-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D et al. (2015) DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res 43:943–959. 10.1093/nar/gku1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarnopolsky MA (2000) Gender differences in substrate metabolism during endurance exercise. Can J Appl Physiol 25:312–327. 10.1139/h00-024 [DOI] [PubMed] [Google Scholar]
- 76.Taylor MK, Swerdlow RH, Sullivan DK (2019) Dietary neuroketotherapeutics for Alzheimer’s disease: an evidence update and the potential role for diet quality. Nutrients 11:1910 10.3390/nu11081910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiwari M (2017) Glucose 6 phosphatase dehydrogenase (G6PD) and neurodegenerative disorders: mapping diagnostic and therapeutic opportunities. Genes Dis 4:196–203. 10.1016/j.gendis.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uchoa MF, Moser VA, Pike CJ (2016) Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front Neuroendocrinol 43:60–82. 10.1016/j.yfrne.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ulusu NN (2015) Glucose-6-phosphate dehydrogenase deficiency and Alzheimer’s disease: Partners in crime? The hypothesis. Med Hypotheses 85:219–223. 10.1016/j.mehy.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 80.Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM et al. (2018) Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. 10.1371/journal.pmed.1002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viña J, Lloret A (2010) Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-β peptide. J Alzheimer’s Dis 20:527–533. 10.3233/JAD-2010-100501 [DOI] [PubMed] [Google Scholar]
- 82.Violante S, Achetib N, van Roermund CWT, Hagen J, Dodatko T, Vaz FM et al. (2019) Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J 33:4355–4364. 10.1096/fj.201801498r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei X, Yang H, Liu Y, Yang Y, Wang P, Kim S-H et al. (2011) Oncometabolite 2-hydroxygulatatrate is a competitive inhibitor of a-KG dependent dioxygenase. Cancer Cell 19:17–30. 10.1016/j.ccr.2010.12.014.Xu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weissman L, Jo DG, Sørensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP et al. (2007) Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res 35:5545–5555. 10.1093/nar/gkm605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Widdowson EM (1976) The response of the sexes to nutritional stress. Proc Nutr Soc 35:175 10.2307/465275 [DOI] [PubMed] [Google Scholar]
- 86.Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML et al. (2016) Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166:624–636. 10.1016/j.cell.2016.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia J, Psychogios N, Young N, Wishart DS (2009) MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37:652–660. 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad USA 106:14670–14675. 10.1073/pnas.0903563106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.