ABSTRACT
Epithelial-Mesenchymal Transition (EMT) and angiogenesis are crucial events for development of aggressive and often fatal Oral Squamous Cell Carcinomas (OSCCs). Both promote cancer progression and metastasis development, but while the former induces the loss of E-cadherin expression and, hence cadherin switching; the latter produces hematic blood vessel neo-formation and contribute to OSCC cell growth, tumor mass development, and dissemination. Cyclooxygenase-2 (COX-2) has an important role, not only in angiogenic mechanisms, but also in favoring cancer invasion. Indeed it decreases the expression of E-cadherin and leads to phenotypic changes in epithelial cells (EMT) enhancing their carcinogenic potential. Our aim is to evaluate the interplay between E-cadherin cytoplasmic delocalization, COX-2 up-regulation and COX-2 induced neo-angiogenesis in 120 cases of OSCC. We have analyzed the distribution and the number of neo-formed endothelial buds surrounding infiltrating cells that express COX-2, as well as the neo-formed vessels in chronic inflammatory infiltrate, which surround the tumor. A double immunostaining method was employed in order to verify co-localization of endothelial cell marker (CD34) and COX-2. IHC has also been used to assess E-cadherin expression. Our data demonstrate that the OSCC cells, which lose membranous E-cadherin staining, acquiring a cytoplasmic delocalization, overexpress COX-2. Moreover, we find a new CD34+ vessel formation (sprouting angiogenesis). Only basaloid type of OSCC showes low level of COX-2 expression together with very low level of neo-angiogenesis and consequent tumor necrosis. The well-known anti-metastatic effect of certain COX-2 inhibitors suggests that these molecules might have clinical utility in the management of advanced cancers.
KEYWORDS: Prostaglandins, Cox-2, E-cadherin, CD-34, OSCC, neo-angiogenesis, TMA, tumor microenvironment
Introduction
Oral Squamous Cell Carcinoma (OSCC), the sixth most frequent malignant tumor, with its distinct patterns of presentation and its complex and heterogeneous behaviors, can be considered one of the most greater public health problems in the world.1
Epithelial-Mesenchymal Transition (EMT) is a key-event in promoting neoplastic progression and metastasis development. Loss of E-cadherin expression and, hence, cadherin switching are two crucial events involved in EMT phenomenon.2 In malignant tumors the mechanisms of E-cadherin down-regulation are numerous and different: inherited and somatic mutations, loss of heterozygosity (LOH), aberrant protein processing, epigenetic silencing, increased endocytosis and proteolysis. We have previously showed that low E–Cadherin expression and/or its delocalization from membrane to cytoplasm, likely due to the hypermethylation of CDH1 promoter or to the increased expression of EGFR, is a negative prognostic factor in OSCC.3
The induction of transcriptional repressors, such as Snail, SLUG and ZEB family members, by certain microRNAs (miR-200 and miR-205), is able to determine EMT and the enrolment/action of “mesenchymal” cadherins.4 Above all that, other mechanisms are involved in E-cadherin down-regulation during EMT. It is plausible that local environmental factors (such as hypoxia and local inflammatory changes mediated by tumor–stromal interactions), extracellular molecules, cytokines or soluble growth factors can affect the expression of transcriptional E-cadherin repressors and induce EMT.5–7
Prostaglandin endoperoxide synthase 2, also known as cyclooxygenase 2 (COX-2), a key enzyme inducible in response to proinflammatory cytokines and growth factors, catalyzes the conversion of arachidonic acid to prostaglandins (including prostaglandin E2 (PGE2)) and other eicosanoids. COX2 has been found over-expressed in a great variety of human malignancies, including OSCC.8–11 At molecular level COX-2 participates in cancer invasion and metastasis by decreasing the expression of E-cadherin and leading to phenotypic changes in epithelial cells that could enhance their carcinogenic potential.11
It is also well known that COX-2 plays an important angiogenic role in the tumor microenvironment, regulating both neoplastic and endothelial cell biology. In particular, it can impact on neo-angiogenesis in different ways: (a) releasing active proangiogenic proteins; (b) producing molecule as TXA2, PGI2, PGE2 that directly stimulate endothelial cell migration and angiogenesis in vivo; (c) determining the inhibition of endothelial cell apoptosis and enhancing tumor cell survival, by Bcl-2 stimulation or PI3 K-Akt activation. The versatile contribution of COX-2 in the angiogenic pathway makes it an ideal target for pharmacological selective inhibitors. Interestingly, COX-2 inhihitors has been successfully used in modulation of E-cadherin expression with significant down-regulation of angiogenetic factors and microvessel density in solid tumors over-expressing COX-2. Given the safety, the tolerability and the potent anti-angiogenic properties of COX-2 inhibitors, the combination of these molecules with standard chemotherapy and radiation therapy leads up additive benefit in clinical patient management.12,13
The relationship between cyclooxygenase-2 (COX-2) and angiogenesis, as determination of microvessel density (MVD), has been also investigated in OSCC.14 Our previous report has shown a statistical association between COX-2 and prognostic characteristics of OSCC.15
We will evaluate the interplay between E-cadherin cytoplasmic delocalization, COX-2 up-regulation and COX-2 induced neo-angiogenesis in 120 cases of OSCCs included in a Tissue Microarray (TMA), focusing on the distribution and the number of neo-formed endothelial buds surrounding COX-2 expressing infiltrating cells, as well as the neo-formed vessels in chronic inflammatory infiltrate accompanying the tumor. A double immunostaining method was employed in order to verify co-localization of endothelial cell marker (CD34) and COX-2.
We have chosen the sialomucin CD34 as endothelial cell marker because it stains endothelial cells in human tumor stroma;16 in particular, it stains cells with migration capability;17 furthermore, it is also a marker of lymphatic angiogenesis in human tumors.18
Results
Tumor samples from 120 patients affected by OSCCs were analyzed and the clinic-pathological characteristics of the studied population were reported in Table 1.
Table 1.
Clinico-pathological data of the studied population (120 cases).
| N. | % | ||
| Sex | M | 85 | 70,8 |
| F | 35 | 29.2 | |
| Age | Mean | 67.3 ± 11.0 | |
| Range | 31–92 | ||
| Tumor localization | Tongue | 59 | 49.2 |
| Floor of Mouth | 14 | 11.7 | |
| Trigonus | 10 | 8.3 | |
| Gingiva | 7 | 5.8 | |
| Lip | 3 | 2.5 | |
| Mascella | 3 | 2.5 | |
| Oral cavity n.s. | 3 | 2.5 | |
| Mandibula | 2 | 1.7 | |
| Tongue and Floor | 2 | 1.7 | |
| Posterior tongue, floor of the mouth and trigonous | 1 | 0.8 | |
| Monoblocco | 1 | 0.8 | |
| n.s. | 15 | 12.5 | |
| Histologic classification of tumors | Conventional keratinizing OSCC | 110 | |
| Verrucous SCC | 9 | ||
| Basaloid non keratinizing OSCC | 1 | ||
| Rxt and/or Chm | Rxt | 70 | 58.3 |
| Chm | 28 | 23.3 | |
| Rxt + Chm | 70 | 58.3 | |
| No Therapy | 15 | 12.5 | |
| n.s. | 35 | 29.2 | |
| Grade | G1 | 23 | 19.2 |
| G2 | 61 | 50.8 | |
| G3 | 29 | 24.2 | |
| G1/G2 | 1 | 0.8 | |
| G2/G3 | 4 | 3.3 | |
| n.s. | 2 | 1.7 | |
| Tumor dimension | Mean | 2.73 ± 1.26 cm | |
| Range | 0.3–6.0 cm | ||
| Deep invasion | Mean | 11.17 ± 4.73 mm | |
| Range | 0.9–24 mm | ||
Abbreviations: n.: number of cases; n.s.: not specified.
Tumor dimension (as evaluated in TNM staging) and deep invasion
There was positive statistical correlation between large size of tumors (T) and degree of deep invasion as evaluated by means ± SD of distances in mm measured between surface layer and deeper infiltrating cancerous cells, as shown in Table 2. In particular, at two extremes of tumor size of TNM classification T1 cases had a mean value of 9.21 ± 3.72 mm in depth while T4 tumors reached 13.5 ± 5.73 mm (p =.02). This positive correlation is confirmed by Pearson’s test performed on tumor dimension and deep invasion (p < .001; Pearson’s R: +.44).
Table 2.
Tumor dimension (as evaluated in TNM staging) and deep invasion (with post-hoc Scheffé test for all pairwise comparisons).
| Group | N | Deep invasion (mean ± SD) | CI 95% | Min | Max | Different (P < 0,05) from factor |
|---|---|---|---|---|---|---|
| T1 | 21 | 9.21 ± 3.72 | 7.622–10.807 | 2.0 | 16.0 | T4 |
| T2 | 52 | 10.69 ± 4.37 | 9.502–11.879 | 0.9 | 24.0 | |
| T3 | 20 | 12.1 ± 4.78 | 10.006–14.194 | 5.0 | 20.0 | |
| T4 | 20 | 13.5 ± 5.73 | 10.990–16.010 | 4.0 | 22.0 | T1 |
Abbreviations: SD – standard deviation. All values are in millimeters.
E-cadherin expression in OSCC
The immunoreactivity of E-cadherin was examined in all OSCCs and in the corresponding normal oral epithelium of the oral cavity. As already reported in our previous work performed using whole sections of OSCCs normal epithelium showed strong membranous E-cadherin expression;3 on the other hand, heterogeneous areas of immunoreactivity varying in percentage value, intensity and/or subcellular localization were observed in tumor tissues. In tumor cells there were a loss of membranous staining and an increase of E-cadherin cytoplasm levels, especially in poorly differentiated, aggressive and proliferative areas, on the other hand well differentiated tumors kept the membranous E-cadherin staining.3 In the whole, we prevalently observed a cytoplasmic E-cadherin delocalization, that was statistically significant compared to the membrane localization of corresponding normal peritumoural oral epithelium (p < .05). Immunohistochemical results were statistically correlated with the clinico-pathological findings (sex, age, tumor maximum size, inflammatory infiltrate surrounding the tumor mass, tumor infiltration of surgical margins, tumor stage and histological differentiation) and evaluated by univariate analysis.
E-cadherin and clinic-pathological parameters
First of all, we noted a significant correlation between sex and E-cadherin delocalization, in deep (P = .006) and in superficial level of invasion (P = .011). In particular, men have higher cytoplasmic expression of E-cadherin than women (Table 3).
Table 3.
Correlation between E-cadherin and clinic-pathological parameters.
| Female | Male | Significance | |
|---|---|---|---|
| E-cadherin (C) in deep invasion | 10 | 30 | P =.006 |
| E-cadherin (C) in superficial invasion | 20 | 30 | P =.011 |
All values are medians.Mann-Whitney U test.
Moreover, we observed:
a positive correlation between tumor dimension and E-cadherin delocalized cytoplasmic expression, both in deep and in superficial margin of invasion (Spearman’s R: +.156; p < .05 in deep invasion; Spearman’s R: +.186; p = .032);
a positive correlation between E-cadherin delocalized cytoplasmic expression and disease-related exitus (Pearson’s R: +.283; p = .011).
COX-2 expression in OSCC
We observed an overall over-expression of COX-2 in OSCCs (Table 4), with a mean percentage of 63.63%, a mean intensity of 1.64 and a score of 120.21. Furthermore, OSCCs often showed a discrete number of neo-formed vessels (CD34-positive) surrounding cord of cancerous cells overexpressing COX-2 with evidence of new vessels formation from preexisting vessels (sprouting angiogenesis) (Figure 1).
Table 4.
Expression of COX-2 and CD34 in OSCCs.
| Mean | Std Dev | Min | Max | |
|---|---|---|---|---|
| COX-2 (percentage) | 63.63 | 35.27 | 0 | 100 |
| COX-2 (intensity) | 1.64 | 0.92 | 0 | 3 |
| COX-2 (score) | 120.21 | 99.84 | 0 | 300 |
| CD-34 Large microvessels | 4.61 | 5.14 | 0 | 30 |
| CD-34 Small microvessels | 32.79 | 25.13 | 0 | 114 |
| CD-34 Total microvessels | 37.40 | 25.23 | 1 | 120 |
Figure 1.
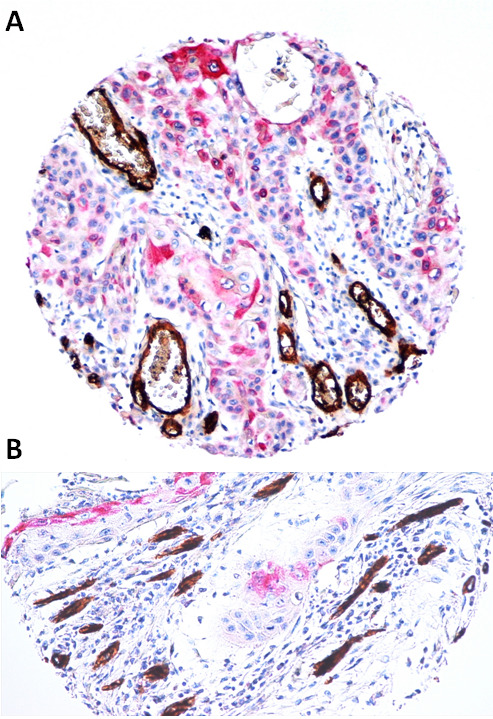
Sprouting angiogenesis surrounding COX-2 positive oral squamous cell carcinoma in deep invasion. 1a, 1b. Two representative OSCCs with over-expression of COX-2, that is accompanied by the formation of new CD-34-positive vessels (LSAB-HRP, nuclear counterstaining with hematoxylin; original magnification x100).
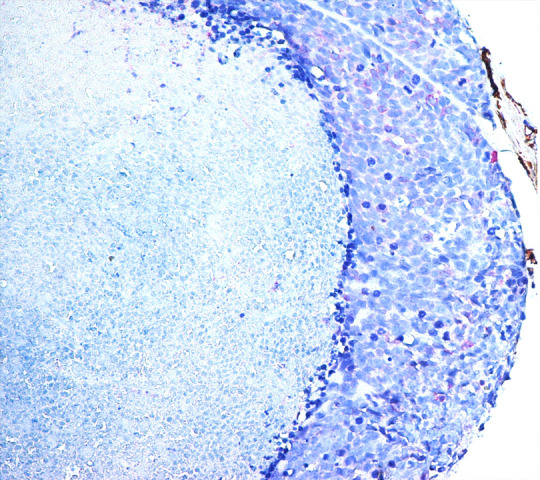
Only a case of basaloid type of OSCCs showed low level of COX-2 expression together with very low level of neoangiogenesis and consequent tumor necrosis (Figure 2).
Figure 2.
Basaloid oral cancer. COX-2 showed low level of expression in basaloid type OSCC together with very low level of neoangiogenesis and consequent tumor necrosis (LSAB-HRP, nuclear counterstaining with hematoxylin; original magnification x200).
No evidence of linear correlation between COX-2 and deep invasion of tumor (p > .05; Pearson’s R) has been reported.
COX-2 and CD34 relationship in phlogistic infiltrates accompanying tumor invasion
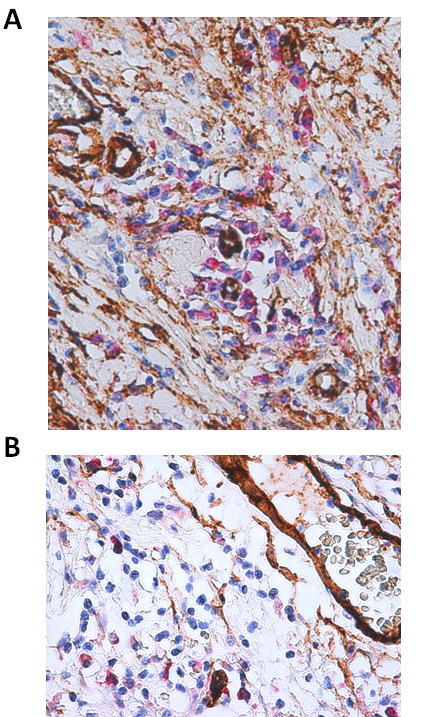
Phlogistic cells in OSCCs expressed high level of COX-2 and were observed closely spaced to CD34 positive endothelial cells in micro-areas of deep invasion, in this way contributing to neoplastic neoangiogenesis (Figure 3).
Figure 3.
Interplay between COX-2 over-expression in phlogistic cells and CD-34 positive endothelial cell at invasive front of OSCCs. Photo 2a, COX-2 expressing phlogistic cells captured in deep invasion of OSCC contribute to neoplastic neoangiogenesis inducing CD34 positive endothelial cells (LSAB-HRP, nuclear counterstaining with hematoxylin; original magnification x200). Photo 2b Higher magnification of micro- and macro vessels CD34 positive and COX-2 positive inflammatory cells (LSAB-HRP, nuclear counterstaining with hematoxylin; original magnification x400).
COX-2 and CD34 relationship in deep infiltrating OSCC
Large microvessels expressed CD34 with a mean percentage of 4.61; higher level of CD34 stained endothelial cells have been observed in small microvessels (32.79%) (Table 4).
Moreover, we found a positive linear correlation between COX-2 score (% x intensity) and CD-34 expressing small microvessels (p = .034; Pearson’s R: 0.246), also considering the total amount of small and large microvessels (p = .031; Pearson’s R: 0.251) (Figure 4a-b) (Table 5).
Figure 4.
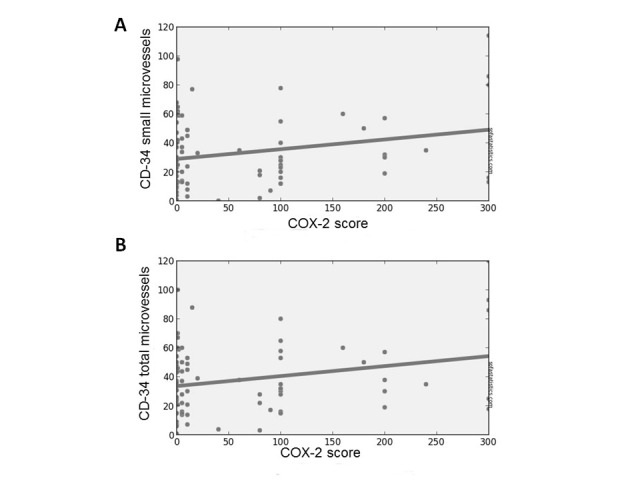
Positive correlation between COX-2 score and small microvessels (a), and between COX-2 score and total microvessels (b) (P < .05).
Table 5.
Correlation between COX-2 score and CD34 expressing vessels.
| Two-tailed p | Pearson’s R | |
|---|---|---|
| COX-2 score and CD-34 small microvessels | 0.034 | 0.246 |
| COX-2 score and CD-34 total microvessels | 0.031 | 0.251 |
Predictors of survival
Then, we have studied predictors of survival throughout Cox proportional hazards regression, analyzing the effect of several risk factors on survival. This analysis was conducted on COX-2 expression; small, large and total microvessels; chemiotherapy and radiotherapy. The overall model fit was significant. Only the variable “large microvessels” was found to significantly contribute to the prediction of survival time (p = .019; Exp(b) = 1.102; 95% CI of Exp(b) = 1,0163 to 1,1957) (Figure 5).
Figure 5.
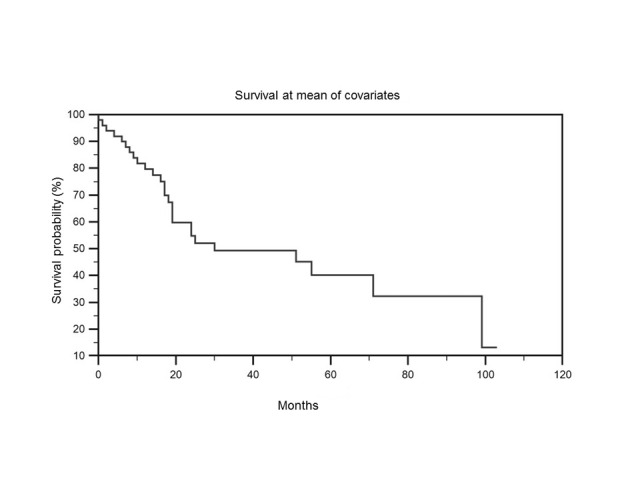
Survival at mean of covariates. The variable “large microvessels” was found to significantly contribute to the prediction of survival time (p =.019).
Discussion and conclusions
Angiogenesis is crucial for development of aggressive and often fatal cancers.19,20 Progression of OSCC is characterized by loco-regional infiltration and regional lymph-node metastases, without significant distant metastases (very low frequency).21 Therefore, hematic blood vessel formation may contribute to OSCC cell growth and tumor mass development, whereas lymphatic vessel neo-angiogenesis may contribute to tumor dissemination.22 Since this is clinically significant in this study we have analyzed some crucial aspects of the invasion microenvironment, in particular the relationship between COX-2 expression by malignant cells and inflammatory cells and formation of new vessels.
During the formation of a network of new vessels, the phenomenon, called sprouting angiogenesis, occurs.23–28 It involves highly specialized cells of hematopoietic origin that can be distinguished in two types: a) stalk cells that maintain a proliferative phenotype and contribute to forming a vascular lumen, and b) tip cells that have low proliferative potential and high power of migration. The tip cells, which are CD34+, guide the process of invasion through the stroma, in fact they extend filopodia and migrate from a preexisting vascular structure toward a microenvironment full of angiogenic growth factors.29 In particular, in our study we have demonstrated that the OSCC cells hyper-express COX-2 and the expression of this protein is strongly associated with the ability to attract tip cells; in turn, this phenomenon leads to a considerable statistically significant increase of micro-vessels, budded around the cords and droplets of malignant cells spreading at invasion front of tumors.
COX-2 has been involved in the processes of formation of new blood vessels that support the viability of the cancerous cells (neo-angiogenesis).23–28 Elevated levels of COX-2 have been associated with increased levels of invasion in human tumors.29,30
In particular, in order to clarify the role of COX-2 in promoting tumoral angiogenesis of lymphatic vessels and lymph node metastasis in OSCC, Morita Y et al. studied the highly metastatic fluorescent labeled OSCC cell line SAS-LM3. They observed that SAS-LM3 tumors showed increased lymphangiogenesis, elevated expression of VEGF-C and COX-2 compared to parental SAS cells.31
Since the early observation by Chan G., et al. in 1999 regarding COX-2 up-regulation in squamous cell carcinoma of the head and neck region, numerous other scientific papers have highlighted the importance of prostanoids also in oral carcinogenesis.8,32–39
In a recent study, Seyedmajidi M et al. have demonstrated high levels of COX-2 expression in OSCC and dysplasia compared to normal mucosa; moreover, they have found a positive correlation between COX-2 expression and severity of dysplasia, supporting the thesis of a role of COX-2 in carcinogenesis and progression of premalignant lesion to malignancy.40
Our preliminary study in 45 OSCCs showed COX-2 expression in oral cancer cells in a percentage 77.8% of the examined cases.15 A further study based on Real-Time PCR expression of COX-1 and COX-2 transcripts in OSCCs showed that most of the tumor samples expressed at least one cyclooxygenase enzyme (COX-1 or COX-2 mRNA) with an inverse relationship between COX-1 and COX-2 in each sample; we also demonstrated that patients with tumors over-expressing COX-2 had a significantly worse overall survival when compared to those COX-2 under-expressed.41
It has postulated that there is a close link between inflammation, angiogenesis and tumor invasion since the angiogenetic factors are produced by inflammatory cells infiltrating and/or surrounding cancers such as mast cells, macrophages, and T lymphocytes, cells of the vascular repair, such as platelets and by tumor cells themselves.42–46 In fact in our study we have demonstrated the angiogenic property of OSCC cells themselves, overexpressing COX-2 and in this way determining new formed CD34-positive vessels (sprouting angiogenesis). Moreover, also phlogistic cells captured in deep tumoral invasion expressed high level of COX-2, inducing CD34+ endothelial cells and contributing to increase the neoplastic neo-angiogenesis (Figure 3).
Beside the well-known angiogenic role of COX-2, recent studies demonstrated that this molecule participates in cancer invasion and metastasis also by decreasing the expression of E-cadherin and leading to phenotypic changes in epithelial cells (EMT) that could enhance their tumorigenic potential. An inverse relationship between E-cadherin and COX-2 and its molecular mechanism in cancer cells was first described in non-small cell lung cancer (NSCLC).
Dohadwala M et al. demonstrated that PGE(2), in autocrine or paracrine fashion, modulates transcriptional repressors of E-cadherin and thereby regulates COX-2-dependent E-cadherin expression in NSCLC. In particular, Authors have shown that treatment of NSCLC cells with exogenous PGE(2) significantly decreased the expression of E-cadherin, whereas treatment of genetically modified COX-2-sense NSCLC cells (low E-cadherin expressing) with celecoxib led to increased E-cadherin expression.47
Similarly, Chen Z et al. suggested that in gastric cancer NF-κB and Snail could take part in COX-2-dependent modulation of E-cadherin expression.48
Other Authors demonstrated that significantly decreased expression of COX2, increased E-cadherin and apoptosis, decreased VEGF/Microvessel Density (MVD) and inhibited angiogenesis were observed in gastric cancer tissues from patients receiving Celecoxib compared to Surgery group.12
A similar effect of COX-2 inhibitors (able to reverse the EMT, restore E-cadherin expression and suppress the invasive potential) was also found in subsets of colon and bladder cancer cells.49–52
However, in oral cancer, neither the effect of COX-2 inhibitors on the regulation of E-cadherin has been examined, neither the molecular mechanisms, through which COX-2 regulates E-cadherin expression and function, have not yet been fully elucidated. Scientific literature reports only few studies.
St John MA et al., for the first time, have investigated interleukin-1β (IL-1β)-induced up-regulation of Snail leading to EMT in surgical specimens and HNSCC cell lines. Authors have shown an inverse relationship between COX-2 and E-cadherin. They have revealed that treatment of HNSCC cells with IL-1beta caused the down-regulation of E-cadherin expression, an increase in the mRNA expression of the transcriptional repressor Snail and up-regulation of COX-2 expression. This effect was blocked in the presence of COX-2 small hairpin RNA.53
Emi Segawa et al. have demonstrated that overexpression of COX-2 increased tumorigenicity and hematogenous metastasis via down-regulating E-cadherin and up-regulating CD44 expressions in KB cells.54 On the same wave, in our work we have observed an overall loss of E-cadherin membranous expression and an over-expression of COX-2 in OSCCs, that is accompanied by new formed CD34-positive vessels (sprouting angiogenesis). Only basaloid type of OSCCs showed low level of COX-2 expression together with very low level of neoangiogenesis and consequent tumor necrosis.
The positive correlation of metastatic potential and COX-2 overexpression indicates that COX-2 may become a target molecule for regulating metastases of oral cancer.
Recently, Fujii R et al. have suggested that the appropriately selective administration of certain COX-2 inhibitors may have an anti-metastatic effect by suppression of the EMT by restoring E-cadherin expression on the cell surface of the HNSCC cells, through the down-regulation of its transcriptional repressors.55
Finally, it has been believed that endothelial cells can determinate an environmental ground permissive of tumoral growth, angiogenesis, and invasion.56 The continued dependence on angiogenesis for early and late stages of tumorigenesis suggests that COX-2 inhibitors will have clinical utility in the management of advanced cancers.
We retain that further understanding the biological mechanisms regulating cell interaction and angioinhibitors molecular relationship may facilitate development of conventional chemotherapy and an effective and suitable anti-tumoral strategy.57,58 Angioinhibitory therapy may also be used to prevent acquired drug resistance in OSCCS.
Material and methods
Study population
A tissue microarray containing 120 OSCCs has been constructed. Methods to build this TMA and therapeutic criteria have been previously reported.59 To the aim of this work, we further specify that none patient have been treated with anti-angiogenetic factors. In brief, the source paraffin blocks were cored and a 0.6 mm cores (area: 0.28 mm2) transferred to the recipient block using Galileo TMA CK 3500 Tissue Microarrayer (ISE TMA Software, Integrated System Engineering). For each patient, two superficial and two deep samples have been cored.
Tissue microarray based double staining immunohistochemistry
The collection of cases was approved by ethics board of National Cancer Institute, Fondazione ‘G. Pascale’, Napoli, Italy. The source block was cored and a 0.6 mm core transferred to the recipient master block. Four cores from different areas (two representative of superficial and two deep invasion) of the same tissue block were arrayed for each case. All the donor cores were formatted into one recipient block. H&E staining of a 4-µm TMA section was used to verify all samples. Double immunostaining has been performed using monoclonal CD-34 and COX-2 antibodies. Primary Ab anti-E-cadherin has also been tested in all OSCC cases.
Primary antibodies were revealed by automated staining device (Leica BOND RX) using standard linked strepatavidin-biotin horseradish peroxidase (LSAB-HRP);60,61 and linked streptavidin biotin alkaline phosphatase (LSAB-AP) techniques performed at the same time. Immune-stained cells were detected in 4 high power fields (HPFs) at optical microscope (OLYMPUS BX53, at x200). Immune-stained spots were acquired by digital camera and analyzed by ISE TMA Software (Integrated System Engineering, Milan, Italy), and Cellsens V1.9® Olympus image analysis softwares. By CD-34 staining, we were able to count and measure diameters of micro-vessels ranging from to 2 micron to 20 micron. Neo-formed vessels have been considered all CD-34 positive endothelial buds measuring less than 20 microns and surrounding tumor cords and nests in deep infiltration spots as evaluated by computed image analysis. Then, we grouped all measureable vessels in two groups: a) small microvessels (measuring from 2 to 10 micron), and b) large microvessels (from 11 to 20 micron). As regard E-cadherin and COX-2 staining we evaluated percent of stained cells and intensity of immune-labeling detected in cancer cells by a continuous scale of values. Then, a total score has been calculated multiplying percentage by intensity, in order to obtain a continuous scale of values ranging from zero to 300. Furthermore, COX-2 positive leucocytes and macrophages cells have been counted in reactive phlogistic infiltrate surrounding OSCCs categorized according to Wada T. classification.62
Statistical analysis
All data were analyzed by MedCalc 12.2.1.0 (for Windows), SOFA Statistics 1.4.3 and R 2.11.1 (for Linux) statistical software, using Debian 7 and Windows Operating Systems. The delocalized E-cadherin was assessed as previously described.3 Differences between groups were determined using the one-way analysis of variance (ANOVA) and Scheffé test for pairwise comparisons. Pearson’s method and Point-biserial correlation coefficient were used to study linear correlation and to determine the relationship between COX-2 expression and neo-angiogenesis evaluated by CD-34 expression and between tumor dimension, tumor stage (as evalutated in TNM staging) and deep invasion. Finally, Cox proportional hazards regression was used to examine the effect of several risk factors on survival. Only p values < .05 were considered significant.
Acknowledgments
We thank the Oncology Italian Consortium (CIISO) and Prof Aldo De Micheli for financing the costs of publication of this article.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
List of abbreviation
- EMT
Epithelial-Mesenchymal Transition
- OSCCs
Oral Squamous Cell Carcinomas
- COX-2
Cyclooxygenase-2
- IHC
Immunohistochemistry
- LOH
loss of heterozygosity
- CDH1
Cadherin-1 gene
- EGFR
Epidermal Growth Factor receptor
- PGE2
prostaglandin E2
- TXA2
Thromboxane A2
- PGI2
Prostaglandin I2
- MVD
microvessel density
- TMA
Tissue Microarray
- LSAB-HRP
linked strepatavidin-biotin horseradish peroxidase
- LSAB-AP
streptavidin biotin alkaline phosphatase
- HPFs
high power fields
- VEGF-C
Vascular Enditelial Growth Factor-C
- NSCLC
non-small cell lung cancer
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M.. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Wells A. Yates C and Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25(6):621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannone G, Santoro A, Feola A, Bufo P, Papagerakis P, Lo Muzio L, Staibano S, Ionna F, Longo F, Franco R, et al. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Curr Cancer Drug Targets. 2014;14(2):115–127. doi: 10.2174/1568009613666131126115012. [DOI] [PubMed] [Google Scholar]
- 4.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, Portillo F, Nieto MA.. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 5.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visco V, Belleudi F, Marchese C, Leone L, Aimati L, Cardinali G, Kovacs D, Frati L, Torrisi MR. Differential response to keratinocyte growth factor receptor and epidermal growth factor receptor ligands of proliferating and differentiating intestinal epithelial cells. J Cell Physiol. 2004;200(1):31–44. doi: 10.1002/jcp.10385. [DOI] [PubMed] [Google Scholar]
- 7.D’Amici S, Ceccarelli S, Vescarelli E, Romano F, Frati L, Marchese C, Angeloni A. TNFα modulates Fibroblast Growth Factor Receptor 2 gene expression through the pRB/E2F1 pathway: identification of a non-canonical E2F binding motif. PLoS One. 2013;8(4):e61491. doi: 10.1371/journal.pone.0061491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 9.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, Chiarugi V, Masini E. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18:153–160. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- 11.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Ran J, Tang C, Wu J, Honghua L, Xingwen L, Chen N, Qiao L. Effect of celecoxib on E-cadherin, VEGF, microvessel density and apoptosis in gastric cancer. Cancer Biol Ther. 2007;6:269–275. doi: 10.4161/cbt.6.2.3629. [DOI] [PubMed] [Google Scholar]
- 13.Gately S, Kerbel R. Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res. 2003;37:179–192. [DOI] [PubMed] [Google Scholar]
- 14.Cao XP1, Zhang ST, Wu HY, Liu XJ, Zhang YY. Relationship between the expression of cyclooxygenase-2 and microvessel density in oral squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi. 2005;23(5):431–433. [PubMed] [Google Scholar]
- 15.Pannone G, Bufo P, Caiaffa MF, Serpico R, Lanza A, Lo Muzio L, Rubini C, Staibano S, Petruzzi M, De Benedictis M, et al. Cyclooxygenase-2 expression in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2004;17(3):273–282. doi: 10.1177/039463200401700307. [DOI] [PubMed] [Google Scholar]
- 16.Schlingemann RO, Rietveld FJR, Dewaal RMW, Bradley NJ, Skene AI, Davies AJS, Greaves MF, Denekamp J, Ruiter DJ. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990;62(6):690–696. [PubMed] [Google Scholar]
- 17.Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2012;15(1):151–163. doi: 10.1007/s10456-011-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiedler U, Christian S, Koidl S, Kerjaschki D, Emmett MS, Bates DO, Christofori G, Augustin HG. The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol. 2006;168(3):1045–1053. doi: 10.2353/ajpath.2006.050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain RK, Carmeliet PF. Vessels of death or life. Sci Am. 2001;285(6):38–45. doi: 10.1038/scientificamerican1201-38. [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ. Angiogenesis and cancer metastasis. Cancer J. 2000;6(Suppl 2):S134–41. [PubMed] [Google Scholar]
- 21.Woolgar AJ. Histopathological prognosticators in oral andoropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42:229–239. doi: 10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K.. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26(3–4):489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 26.Langer R, Conn H, Vacanti J, Haudenschild C, Folkman J. Control of tumor growth in animals by infusion of an angiogenesis inhibitor. Proc Natl Acad Sci U S A. 1980;77(7):4331–4335. doi: 10.1073/pnas.77.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkman J. Angiogenesis: initiation and control. Ann N Y Acad Sci. 1982;401:212–227. doi: 10.1111/j.1749-6632.1982.tb25720.x. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita Y, Hata K, Nakanishi M, Nishisho T, Yura Y, Yoneda T. Cyclooxygenase-2 promotes tumor lymphangiogenesis and lymph node metastasis in oral squamous cell carcinoma. Int J Oncol. 2012;41(3):885–892. doi: 10.3892/ijo.2012.1529. [DOI] [PubMed] [Google Scholar]
- 32.Sumitani K, Kamijo R, Toyoshima T, Nakanishi Y, Takizawa K, Hatori M, Nagumo M. Specific inhibition of cyclooxygenase-2 results in inhibition of proliferation of oral cancer cell lines via suppression of prostaglandin E2 production. J Oral Pathol Med. 2001;30(1):41–47. doi: 10.1034/j.1600-0714.2001.300107.x. [DOI] [PubMed] [Google Scholar]
- 33.Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res. 2003;9(5):1885–1897. [PubMed] [Google Scholar]
- 34.Ranelletti FO, Almadori G, Rocca B, Ferrandina G, Ciabattoni G, Habib A, Galli J, Maggiano N, Gessi M, Lauriola L. Prognostic significance of cyclooxygenase-2 in laryngeal squamous cell carcinoma. Int J Cancer. 2001;95(6):343–349. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol. 2002;33(7):708–714. doi: 10.1053/hupa.2002.125376. [DOI] [PubMed] [Google Scholar]
- 36.Peng JP, Su CY, Chang HC, Chai CY, Hung WC. Overexpression of cyclo-oxygenase 2 in squamous cell carcinoma of the hypopharynx. Hum Pathol. 2002;33(1):100–104. doi: 10.1053/hupa.2002.30187. [DOI] [PubMed] [Google Scholar]
- 37.Renkonen J, Wolff H, Paavonen T. Expression of cyclo-oxygenase-2 in human tongue carcinoma and its precursor lesions. Virchows Arch. 2002;440(6):594–597. doi: 10.1007/s00428-002-0616-y. [DOI] [PubMed] [Google Scholar]
- 38.Sudbø J, Ristimäki A, Sondresen JE, Kildal W, Boysen M, Koppang HS, Reith A, Risberg B, Nesland JM, Bryne M. Cyclooxygenase-2 (COX-2) expression in high-risk premalignant oral lesions. Oral Oncol. 2003;39(5):497–505. doi: 10.1016/S1368-8375(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 39.Itoh S, Matsui K, Furuta I, Takano Y. Immunohistochemical study on overexpression of cyclooxygenase-2 in squamous cell carcinoma of the oral cavity: its importance as a prognostic predictor. Oral Oncol. 2003;39(8):829–835. doi: 10.1016/S1368-8375(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 40.Seyedmajidi M, Shafaee S, Siadati S, Khorasani M, Bijani A, Ghasemi N. Cyclo-oxygenase-2 expression in oral squamous cell carcinoma. J Cancer Res Ther. 2014;10(4):1024–1029. doi: 10.4103/0973-1482.138205. [DOI] [PubMed] [Google Scholar]
- 41.Pannone G, Sanguedolce F, De Maria S, Farina E, Lo Muzio L, Serpico R, Emanuelli M, Rubini C, De Rosa G, Staibano S, et al. Cyclooxygenase isozymes in oral squamous cell carcinoma: areal-time RT-PCR study with clinic pathological correlations. Int J Immunopathol Pharmacol. 2007;20(2):317–324. doi: 10.1177/039463200702000211. [DOI] [PubMed] [Google Scholar]
- 42.Lucas T, Abraham D, Aharinejad S. Modulation of tumor associated macrophages in solid tumors. Front Biosci. 2008;13:5580–5588. doi: 10.2741/3101. [DOI] [PubMed] [Google Scholar]
- 43.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99(8):1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986;46:467–473. [PubMed] [Google Scholar]
- 46.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 47.Dohadwala M1, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66(10):5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z1, Liu M, Liu X, Huang S, Li L, Song B, Li H, Ren Q, Hu Z, Zhou Y, et al. COX-2 regulates E-cadherin expression through the NF-κB/Snail signaling pathway in gastric cancer. Int J Mol Med. 2013;32(1):93–100. doi: 10.3892/ijmm.2013.1376. [DOI] [PubMed] [Google Scholar]
- 49.Noda M, Tatsumi Y, Tomizawa M, Takama T, Mitsufuji S, Sugihara H, Kashima K, Hattori T. Effects of etodolac, a selective cyclooxygenase-2 inhibitor, on the expression of E-cadherin-catenin complexes in gastrointestinal cell lines. J Gastroenterol. 2002;37:896–904. doi: 10.1007/s005350200151. [DOI] [PubMed] [Google Scholar]
- 50.Bozzo F, Bassignana A, Lazzarato L, Boschi D, Gasco A, Bocca C, Miglietta A. Novel nitro-oxy derivatives of celecoxib for the regulation of colon cancer cell growth. Chem Biol Interact. 2009;182:183–190. doi: 10.1016/j.cbi.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Jang TJ, Cha WH, Lee KS. Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human bladder transitional cell carcinomas. Virchows Arch. 2010;457:319–328. doi: 10.1007/s00428-010-0943-3. [DOI] [PubMed] [Google Scholar]
- 52.Adhim Z, Matsuoka T, Bito T, Shigemura K, Lee KM, Kawabata M, Fujisawa M, Nibu K, Shirakawa T. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br J Cancer. 2011;105:393–402. doi: 10.1038/bjc.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St John MA, Dohadwala M, Luo J, Wang G, Lee G, Shih H, Heinrich E, Krysan K, Walser T, Hazra S, et al. 2009. Clin Proinflammatory mediators upregulate snail in head and neck squamous cell carcinoma. Cancer Res. 15(19):6018–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segawa E, Kishimoto H, Takaoka K, Noguchi K, Hashitani S, Sakurai K, Urade M. Promotion of hematogenous metastatic potentials in human KB carcinoma cells with overexpression of cyclooxygenase-2. Oncol Rep. 2010;24(3):733–739. doi: 10.3892/or_00000915. [DOI] [PubMed] [Google Scholar]
- 55.Fujii R, Imanishi Y, Shibata K, Sakai N, Sakamoto K, Shigetomi S, Habu N, Otsuka K, Sato Y, Watanabe Y, et al. Restoration of E-cadherin expression by selective Cox-2 inhibition and the clinical relevance of the epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2014;33:40. doi: 10.1186/1756-9966-33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitadai Y. Cancer-stromal cell interaction and tumor angiogenesis in gastric cancer. Cancer Microenviron. 2010;3(1):109–116. doi: 10.1007/s12307-009-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marina DD, Carmela R, Alfredo F, Maria PG. Anti-VEGF therapy in breast and lung mouse models of cancers. J of Biomed Biotechnol. 2011;2011:947–928. doi: 10.1155/2011/947928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franco R, Nicoletti G, Lombardi A, Di Domenico M, Botti G, Zito Marino F, Caraglia M. Current treatment of cutaneous squamous cancer and molecular strategies for its sensitization to new target-based drugs. Expert Opin Biol Ther. 2013;13(1):51–66. doi: 10.1517/14712598.2012.725720. [DOI] [PubMed] [Google Scholar]
- 59.Aquino G, Pannone G, Santoro A, Liguori G, Franco R, Serpico R, Florio G, De Rosa A, Mattoni M, Cozza V, et al. pEGFR-Tyr 845 expression as prognostic factors in oral squamous cell carcinoma: a tissue-microarray study with clinic-pathological correlations. Cancer Biol Ther. 2012;13(11):967–977. doi: 10.4161/cbt.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esposito F, Boscia F, Franco R, Tornincasa M, Fusco A, Kitazawa S, Looijenga LH, Chieffi P. Down-regulation of estrogen receptor-β associates with transcriptional coregulator PATZ1 delocalization in human testicular seminomas. J Pathol. 2011;224:110–120. doi: 10.1002/path.2846. [DOI] [PubMed] [Google Scholar]
- 61.Esposito F, Libertini S, Franco R, Abagnale A, Marra L, Portella G, Chieffi P. Aurora B expression in post-puberal testicular germ cell tumours. J Cell Phys. 2009;221:435–439. doi: 10.1002/jcp.21875. [DOI] [PubMed] [Google Scholar]
- 62.Wada T. Nature of mononuclear cell infiltrates in oral squamous cell carcinoma and their clinical significance. Wakayama Med Rep. 1989;30:103–117. [Google Scholar]


