Abstract
Mineral oil is often used as a clinical trial placebo. Pharmaceutical-grade mineral oil consists of a mixture of saturated hydrocarbons, with a purity and chemical structure that differs substantially from food-grade or technical-/industrial-grade mineral oils. Interest in mineral oil was piqued by suggestions that a portion of the substantially positive results of the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) might be attributable to the theoretical negative effects of mineral oil rather than being due to the clinical benefits of icosapent ethyl. The objective of this review was to explore possible mineral oil safety and efficacy effects and contextualize these findings in light of the REDUCE-IT conclusions. A literature search identified studies employing mineral oil placebos. Eighty studies were identified and relevant data extracted. Adverse events associated with mineral oil were generally gastrointestinal and consistent with use as a lubricant laxative. Changes in triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, high-sensitivity C-reactive protein, and other biomarkers were inconsistent and generally not statistically significant, or clinically meaningful with mineral oil, as were changes in blood pressure. There was no consistent evidence that mineral oil in the amounts used in the REDUCE-IT or Effect of Vascepa on Progression of Coronary Atherosclerosis in Patients With Elevated Triglycerides on Statin Therapy (EVAPORATE) trials affects absorption of essential nutrients or drugs, including statins. These results were then considered alongside publicly available data from REDUCE-IT. Based on available evidence, mineral oil does not appear to impact medication absorption or efficacy, or related clinical outcomes, and, therefore, does not meaningfully affect study conclusions when used as a placebo at the quantities used in clinical trials.
Keywords: Mineral oil, Placebo, LDL-cholesterol, Triglycerides, C-reactive protein, HMG CoA statins
Introduction
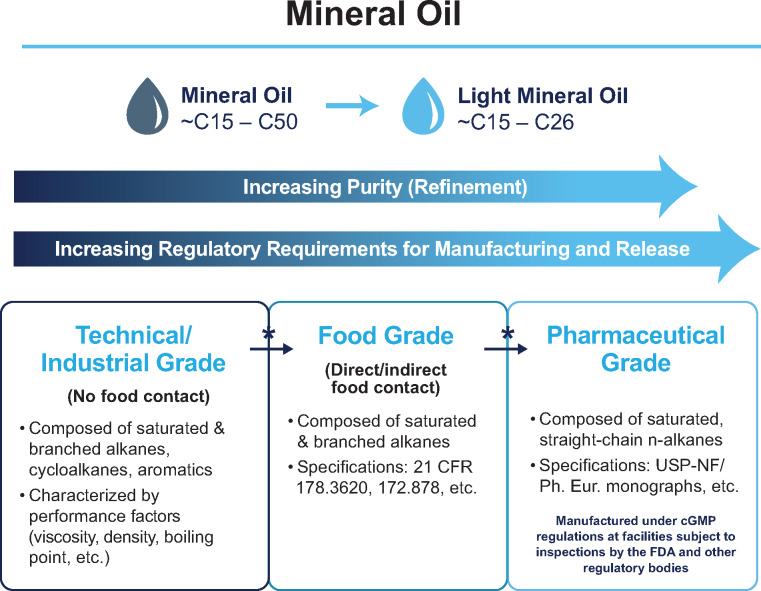
Mineral oil encompasses a wide variety of colourless, odourless mixtures of higher alkanes from a mineral source. Technical- or industrial-grade mineral oil is considered crude, with purification leading to food and pharmaceutical grades. Refined mineral oil is composed of a mixture of high–molecular-weight, saturated, iso-, and cyclo-hydrocarbons.1 When refined into saturated and branched alkanes, mineral oil is considered to be food grade (Figure 1). Food-grade mineral oils, at least in small amounts, are ‘generally recognized as safe’ (‘GRAS’) food additives by the Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), and the European Commission (EC).2–4 In large doses, food-grade mineral oil has been used to treat constipation, primarily in children.5,6 Food-grade mineral oil can be further refined and hydrogenated to remove impurities, and these straight-chain n-alkanes, when manufactured under current Good Manufacturing Practices at facilities subject to inspections by the FDA and other regulatory bodies, are considered pharmaceutical grade (Figure 1).7
Figure 1.
Mineral oil grade classifications.7,29,30 C, carbon atoms; CFR, Code of Federal Regulations; cGMP, Current Good Manufacturing Practices; FDA, United States Food and Drug Administration; Ph Eur, European Pharmacopoeia; USP-NF, United States Pharmacopoeia–National Formulary. Asterisks and arrows represent refinement/hydrogenation processing steps: removal of impurities such as aromatic compounds, unsaturated compounds, and nitrogen- or sulfur-containing compounds.
An ideal placebo is inert; however, omega-3 fatty acid (OM3 FA) intervention trials often employ biologically active oils. Saturated, monounsaturated, and omega-6 polyunsaturated fatty acids found in various ratios in olive, corn, safflower, sunflower, and coconut oils may impact various cardiovascular (CV) risk parameters differentially, including blood lipids, glucose metabolism, blood pressure, and inflammatory pathways. Use of these active comparators can make the detection of an effect from OM3 FA intervention more or less likely depending on the choice of placebo.
Due to its inert properties, highly refined, pharmaceutical-grade mineral oils are commonly used as trial placebos, especially when studying agents formulated in oil-based tablets/capsules or agents that are oils, including OM3 FAs (Tables 1 and 2). In 2015, the EC amended European Union regulations to add pharmaceutical-grade mineral oils to the list of substances/active ingredients that do not pose a risk.4 Pharmaceutical-grade mineral oil was used as a placebo for icosapent ethyl (IPE), a highly purified and stable ethyl ester form of eicosapentaenoic acid (EPA), in the Multi-Center, Placebo Controlled, Randomized, Double-Blind, 12-Week Study with an Open-Label Extension (MARINE), Effect of AMR101 (Ethyl Icosapentate) on Triglyceride Levels in Patients on Statins With High Triglyceride Levels (ANCHOR), Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), and Effect of Vascepa on Progression of Coronary Atherosclerosis in Patients With Elevated Triglycerides on Statin Therapy (EVAPORATE) trials.8–13
Table 1.
Reported biomarker changes in mineral oil placebo groups from cardiovascular disease and diabetes studies
| Author | n a | Dose and mineral oil used | Patient population | Statins | Duration of treatment | TG (% change, BL to EOT) | LDL-C (% change, BL to EOT) | Non–HDL-C (% change, BL to EOT) | HDL-C (% change, BL to EOT) |
|---|---|---|---|---|---|---|---|---|---|
| Bhatt et al.,8 2019 | 4090 | 4 g/day, mineral oil | Aged ≥45 years with established CV disease or aged ≥50 years with diabetes and ≥1 additional CV risk factor | Required; 63.0% moderate intensity; 30.0% high intensity | Median 4.9 years | −6.5% | +10.5% | +4.6% | +5.0% |
| Bays et al.,9 2011 | 75 | 4 g/day, LLP | TG ≥500 mg/dL and ≤2000 mg/dL | Allowed | 12 weeks | +9.7% | −3.0% | +7.8% | 0% |
| Ballantyne et al.,10 2012 | 227 | 4 g/day, not specified | TG ≥200 and <500 mg/dL; LDL-C ≥40 mg/dL and <100 mg/dL | Required | 12 weeks | +5.9% | +8.8% | +9.8% | +4.8% |
| Kabir et al.,37 2007 | 14 | 3 g/day, paraffin oil | Type 2 diabetes mellitus; without HTG | Allowed (n = 5) | 8 weeks | +10.5% | 0% | NR | +7.1% |
| Gholamhosseini et al.,38 2015 | 31 | Edible paraffin | Men with CV disease | 84% in placebo group on statin | 8 weeks | −11.2% | +6.7% | NR | +12.2% |
| Hosseini et al.,39 2013 | 35 | 5 mL/day, mineral oil | Type 2 diabetes mellitus | NR | 2 months | +6.0% | +3.0% | NR | +3.9% |
| Mortazavi et al.,40 2018 | 23 | 4 g/day, edible paraffin | Male subjects aged 45–55 years with CV disease and ≥50% occlusion in 1 coronary artery | NR | 8 weeks | Decreased 21.59 mg/dL | Increased 9.43 mg/dL | NR | Increased 3.81 mg/dL |
| Golzari et al.,41 2019 | 18 | 2 g/day, edible paraffin | Diabetes for ≥1 year, aged 35–50 years, and taking antidiabetic drugs for ≥3 months | NR | 8 weeks | +3.1% | +3.4% | NR | +0.4% |
| Agh et al.,42 2017 | 21 | Edible paraffin |
Men with CAD, ≤50% stenosis in ≥1 major coronary artery in the last 3 months, and BMI ≤30 kg/m2 Individuals using warfarin excluded from participation |
Statin therapy in 92% (does not refer specifically to the control arm) | 8 weeks | −11.5% | +9.2% | NR | +27.9% |
| Mazaherioun et al.,43 2017 | 44 | 2.7 g, edible paraffin | Men aged >30 years and premenopausal women aged >30 years; BMI 25–40 kg/m2 with type 2 diabetes | NR | 10 weeks | +3.0% | +1.7% | NR | −3.9% |
| Ramezani et al.,44 2018 | 20 | 4 soft gel edible paraffin capsules/day | Coronary vascular disease |
NR Patients not taking fibrates |
8 weeks | −8.9% | +8.5% | NR | +3.0% |
BL, baseline; BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; EOT, end of treatment; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NR, not reported; OM3, omega-3 fatty acid; TG, triglycerides.
Number of participants in the mineral oil placebo group.
Table 2.
Reported effect on lipid parameters in mineral oil placebo groups from non-CV disease and diabetes studies
| Author | n a | Dose and mineral oil used | Patient population | Statins | Duration of treatment | TG | LDL-C | Non–HDL-C | HDL-C |
|---|---|---|---|---|---|---|---|---|---|
| De Truchis et al.,45 2007 | 62 | 2 g/day, paraffin oil | HIV patients on antiviral therapy; BL TG >200 mg/dL and <1000 mg/dL | Patients taking lipid-lowering drugs excluded | 8 weeks | +6.4% | NR | NR | +8.08% |
| Horrobin et al.,46 1991 | 10 | 4 g/day over 7 days, liquid paraffin | Healthy adults | NR | 10 days | No change | NR | NR | NR |
| Lemos et al.,47 2012 | 60 | 2 g/day, mineral oil | Terminal renal failure undergoing chronic haemodialysis | NR | 120 days | +12.5% | −6.1% | NR | +1.7% |
| Emsley et al.,48 2008 | 33 | 2 g/day, medicinal liquid paraffin | Schizophrenia or schizoaffective disorder and coexistent tardive dyskinesia | NR | 12 weeks | −13.3% | 0% | NR | −7.1% |
| Fogaça et al.,49 2011 | 11 | NA, paraffin | Alcohol-dependent patients | NR | 90 days | NR | NS | NR | NS |
| Mohammadi et al.,50 2012 | 31 | 2 g/day, liquid paraffin | Women with polycystic ovary syndrome | NR | 8 weeks | −4.8% | 0% | NR | −0.9% |
| Peet and Horrobin,51 2002 | 6 | 4 g/day; liquid paraffin | Schizophrenia; all six patients on clozapine | NR | 12 weeks | +15.8% | NR | NR | NR |
| Yang et al.,52 1999 | 18 | 5 g/day, paraffin oil | Atopic dermatitis | NR | 1 month | −1.8% | +2.4% | NR | +3.6% |
| 4 months | 0% | +1.0% | +6.4% | ||||||
| Nogueira et al.,53 2016 | 28 | 4 mL/day, mineral oil | Non-alcoholic steatohepatitis | NR | 6 months |
BL: 148.6 mg/dL EOT: NR P = NS |
BL: 116.6 mg/dL EOT: NR P = NS |
NR |
BL: 52.1 mg/dL EOT: NR P = NS |
| Allain et al.,54 2009 | 8 | 3 g/day, paraffin oil | Healthy volunteers | NR | 6 weeks | No significant change | No significant change | NR | No significant change |
| Mirmasoumi et al.,55 2018 | 30 | 1 g/day, liquid paraffin | Polycystic ovary syndrome | NR | 12 weeks | +8.6% | +3.1% | +1.9% | |
| Paixao et al.,56 2017 | 22 | 2 g/day, mineral oil | Breast cancer patients | NR | 30 days | −16% | −3.1% | NR | +2.1% |
| Mejia-Montilla et al.,57 2018 | 85 | Mineral oil capsule | Women with polycystic ovary syndrome and vitamin D deficiency | NR | 12 weeks | +0.8% | +1.7% | NR | −3.4% |
| Ghorbanihaghjo et al.,58 2012 | 43 | 1 g, paraffin pearl in the fasted state | Adult females diagnosed with RA and on a fixed therapeutic schedule for ≥2 months before study entry | Patients taking lipid-lowering drugs excluded | 3 months | NR | NR | NR |
BL: 38.6 mg/dL EOT: 38.2 mg/dL |
| Kremer et al.,59 1985 | 21 | 1.8 g/day, non-digestible paraffin wax | Rheumatoid arthritis | NR | 12 weeks | +18.5% | NR | NR | NR |
| Ferreira et al.,60 2019 | 80 | 5 oral drops of placebo (1% powdered lemon flavour, 0.2% ethylene-diamine- tetraacetic acid, liquid flavour qs, and liquid petrolatum qsp in 20 mL) | Post-menopausal women aged 50–65 years | NR | 9 months | +1.6% | −2.2% | NR | −0.4% |
| Rashidmayvan et al.,61 2019 | 22 | 1 g/day, paraffin oil | Patients aged 20–60 years with non-alcoholic fatty liver disease | NR | 8 weeks | −1.0% | −1.0% | NR | +1.7% |
BL, baseline; CV, cardiovascular; EOT, end of treatment; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NR, not reported; NS, not significant; TG, triglycerides.
Number of participants in the mineral oil placebo group.
As highlighted previously, REDUCE-IT reported a significant reduction in major adverse CV events (MACE) by 25%, including a 20% reduction in CV death with IPE. REDUCE-IT randomized statin-treated patients with low-density lipoprotein cholesterol (LDL-C) controlled below 100 mg/dL, but persistently elevated triglyceride (TG) levels of 135–499 mg/dL, to receive EPA (in the form of IPE) 4 g/day (2 g twice daily) or mineral oil placebo 2 g twice daily and demonstrated substantial reduction in the MACE primary endpoint [hazard ratio (HR): 0.75; 95% confidence interval (CI): 0.68–0.83; P < 0.001].8,14,15 While reduction in TG levels may contribute to overall outcomes, they likely did not contribute substantially to the degree of CV risk reduction observed.16,17 Preclinical and clinical studies support IPE reducing CV events through multiple mechanisms of action, including several that are not fully understood.18,19 However, a few critics have suggested that the observed reduction in CV risk was not only due to positive effects of IPE but also due to negative effects from the pharmaceutical-grade mineral oil control, a supposition based on small elevations in some lipid levels and inflammatory markers in the placebo arm.20 The objective of this review is to critically investigate evidence of biological activity, or lack thereof, of mineral oil and review publicly available data from REDUCE-IT.
Methods
This review is based upon a literature search of several databases conducted in October 2019 including: Biomed Central, clinicaltrials.gov, EMBASE, FDA.gov, Google Scholar, Medline/Medline Plus, and PubMed. Additional searches included: Biological Abstracts, Davis’ Drug Guide, Drug Delivery Systems, DrugInteractionInfo.org, Enhancement in Drug Delivery, Inchem.org, Medscape, Merck Index, Micromedex/Mayoclinic.com, patents (via Google Scholar), Toxnet.nlm.nih.gov/Hazardous Substances Data Bank, and Up-to-Date.
Key search terms employed included: ‘mineral oil’, ‘paraffin oil’, ‘liquid paraffin’, ‘liquid petrolatum’, and ‘edible paraffin’. In addition, the following search terms were combined with the key search terms: ‘placebo’/‘control’, ‘plasma lipids’/‘lipoproteins’ (and related terms), ‘statin’, ‘safety’, ‘toxicity’, ‘pharmacokinetics’, ‘reactivity’, ‘laxative’, ‘stool softener’, ‘gastric motility’, ‘generally recognized as safe’/‘GRAS’, and ‘absorption’. Literature searches were performed in the databases mentioned using various combinations of the search terms listed above. Results were not restricted by publication date and both adult and paediatric titles were screened in an attempt to capture the universe of literature on mineral oil.
Identified articles were searched manually by two reviewers for use of mineral oil as placebo in clinical trials, reported changes in blood lipids, use as a laxative, safety, inert properties, and drug interactions. Articles were screened for relevance by title, abstract, and then full text. Only articles describing oral administration of mineral oil were included. Outputs from these searches formed the basis of this review. Citations of relevant articles were screened and added if not otherwise captured by the literature searches. Select REDUCE-IT outcomes were then considered to contextualize the findings from this literature review.
The intent of this review was to provide a broad overview of relevant literature describing mineral oil and its biological properties, with a focus on its impact on CV parameters and outcomes. There was variability in the study populations based on a variety of parameters, such as age (adult vs. paediatric) and region. There was also variability in the type of study (preclinical vs. clinical vs. manufacturing). Within the clinical studies, variation in endpoints existed due to different therapeutic areas and even within the same therapeutic area. Given the degree of heterogeneity, no one data synthesis standard could be applied. As such, relevant findings of each study were extracted, synthesized, and contextualized, including REDUCE-IT findings when appropriate, in a narrative fashion rather than a systematic review or meta-analysis. REDUCE-IT pre-specified and post hoc analyses assessing the potential impact of the mineral oil on efficacy and safety conclusions were summarized and discussed in conjunction with the broad literature overview on mineral oil.
Results
In total, 281 articles were screened. This review identified 80 studies that used some form of mineral oil as a placebo.
Mineral oil—use as a laxative
We found that the only reported biological activity of mineral oil was as a lubricant (i.e. non-stimulant) laxative.21 Mineral oil treats constipation at doses of 15–45 mL, markedly higher than placebo doses.5,22 As a lubricant laxative, mineral oil coats the bowel and stool mass with a waterproof film that retards water absorption, leading to retention of moisture in the stool. This softens the stool, easing its passage.6,23 Mineral oil, as a laxative, has no direct effect on gastric motility, affecting colonic motility secondarily.24,25
Safety of mineral oil
Our literature review reported a lack of safety concerns with mineral oil across 80 cited studies. Adverse events (AEs) were generally categorized as unrelated to study treatment. The most common AEs attributed to mineral oil were abdominal pain and distention and watery stools, which are likely related to its laxative properties.22 Other AEs include pneumonia secondary to aspiration6,26 and various complications, including an autoimmune syndrome and death, following illegal direct injection of mineral oil as a dermal filler.27,28
Pharmaceutical-grade mineral oils are composed of saturated hydrocarbons that are highly refined to achieve extremely low levels of aromatic hydrocarbon impurities and to ensure elimination of carcinogenicity and compliance with international pharmacopoeia monographs (Figure 1).7 Reports of mineral oil toxicity are related to non–pharmaceutical-grade mineral oil and mainly due to peroxidation of aromatic and saturated hydrocarbon impurities rather than the mineral oil itself.31
In pharmacokinetic studies, mineral oil was undetectable in blood samples.32 Some studies showed mineral oil bioaccumulation in humans as a result of exposure to industrial-/technical- or food-grade mineral oils, which have been detected in fat, mesenteric lymph nodes, liver, and spleen, with lower levels in the lung, kidney, brain, and heart.33 These mineral oils are derived from food, cosmetics, release agents, lubricating oils, dust binders, packaging materials, and environmental contamination.1,3 Bioaccumulation of industrial-/technical- or food-grade mineral oil has been reported with longer durations of oral exposure, particularly for n-alkanes with 20–40 carbons.34 However, similar to above, these findings do not apply to pharmaceutical-grade mineral oil.
Concerns regarding long-term toxicity of mineral oil due to its accumulation in the liver are largely derived from animal studies that have been extrapolated to humans.7 For example, liver granulomas related to mineral oil have been observed in F-344 rats with exposure, although such granulomas have not been reported in humans.35,36 This response may be specific to this strain of rat and may not be relevant to humans, who respond to hydrocarbons more similarly to rat strains that do not produce granulomas.35,36 Evidence from decades of clinical use support safety of alkanes from mineral oils in humans.7
Lack of effects on blood lipids
Of the 80 studies identified that used mineral oil, only 28 reported changes in blood lipids or blood pressure and are summarized in Tables1–3. Table 1 includes 11 studies in which patients had CV disease (CVD) and/or diabetes,8–10,37–44 while Table 2 includes 17 studies conducted in healthy volunteers or patients with non-CV conditions, such as schizophrenia, non-alcoholic steatohepatitis, renal disease, polycystic ovary syndrome, rheumatoid arthritis, HIV infection, alcohol dependence, and cancer.45–61
In studies with mineral oil arms, changes in TG levels and lipids, in general, were mixed and inconsistent. In trials conducted in patients with CVD or diabetes, TG levels changed by a range of −16% to +18.5%; six studies showed an increase in TG levels9,10,37,39,41,43 and five showed a decrease (Table 1).8,38,40,42,44 The greatest change in TG level (18.5%) was reported in a study of 21 patients with rheumatoid arthritis.59
Low-density lipoprotein cholesterol changes from baseline in these trials ranged from −6.1% to 9.2% (Tables 1 and2).8–10,37–44High-density lipoprotein cholesterol (HDL-C) changes from baseline were generally small and ranged from −7.1% to +27.9% (Table 1).8–10,37–44
The only study in which mineral oil placebo was associated with a statistically, but not clinically, significant change was REDUCE-IT (>8000 patients), in which the median TG level increased from 216 mg/dL to 221 mg/dL at the end of year 1 (P < 0.001), a 2.2% increase which, in turn, was significantly different than the 18.3% reduction from baseline seen with IPE at year 1 (P < 0.001).8 A statistically significant 10.2% (7.0 mg/dL) increase was observed in LDL-C values in the statin-controlled placebo group at the end of year 1 (P < 0.001) vs. a 3.1% (2.0 mg/dL) increase in the IPE group (P < 0.001). In REDUCE-IT, there was minimal association of outcomes to increases or decreases in LDL-C or TG levels during follow-up.16
The remaining studies were relatively small. Only the ANCHOR study had >100 patients taking a mineral oil regimen.10 Six of the CV studies reported on statin use; the remainder did not document statin use (Table 1).8–10,37,38,42 In the non-CV studies summarized in Table 2, the changes in TG, LDL-C, and HDL-C levels ranged from −16% to +19%, −6% to +3%, and −9% to +9%, respectively.45–61 The only non-CV study in which any of these changes was statistically significant was a 12% increase in TG levels in terminal renal failure patients on chronic haemodialysis.47 Statin use was not reported in 19 of 21 non-CV studies and was prohibited in the other 2 studies.45,58
Lack of effect on inflammatory markers
Of the 80 trials identified that used mineral oil, 16 reported high-sensitivity C-reactive protein (hsCRP) levels ranging from −15.9% to +33.3%; levels were increased in 10 studies,8,40,42,53,55,56,62–64 decreased in 5 studies,47,50,61,65,66 and unchanged in 1 study.67 As with lipid parameters, most changes were inconsistent and not reported as statistically significant.
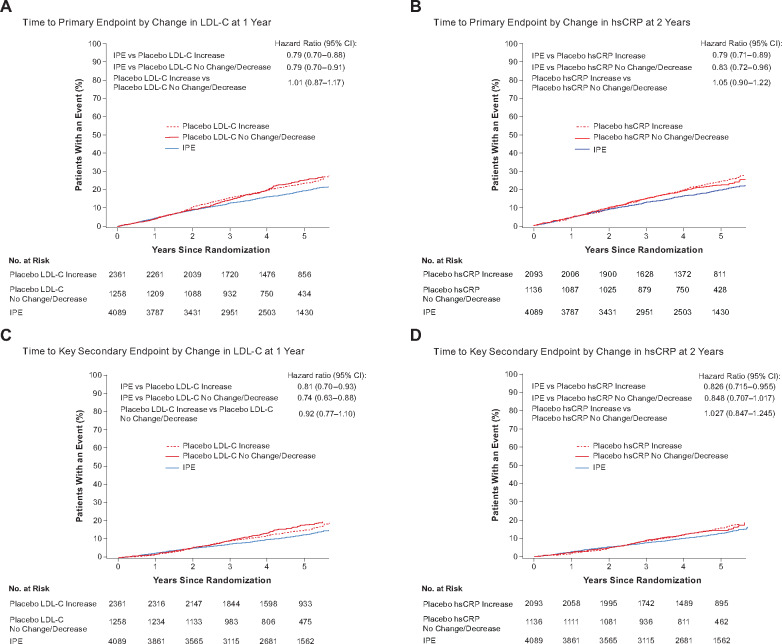
A statistically significant increase in hsCRP level was reported in REDUCE-IT (from 2.1 mg/L at baseline to 2.8 mg/L) after 2 years and at the last visit (P < 0.001), compared with a decrease in the IPE arm (from 2.2 mg/L to 1.8 mg/L; P = 0.04 after 2 years).8 hsCRP is subject to high intra- and inter-individual variability and is heavily influenced by transient conditions, such as minor trauma, inflammatory processes, and infections.68 However, concentrations were measured only at three time points in REDUCE-IT (baseline, year 2, and last visit)8; given that hsCRP values are highly variable, clinical significance of these observations is unlikely, particularly in statin-treated patients. Formal analyses of a theoretical mineral oil effect evaluated during an FDA Advisory Committee showed that hsCRP changes had little to no impact on the primary and secondary composite endpoints of REDUCE-IT (Figure 2, Supplementary material online, Table S1).16 Inconsistent increases and decreases in other inflammatory markers across a range of conditions, including interleukin (IL)-6,56,62,66,69,70 IL-1β,56,61,70,71 IL-10,69 tumour necrosis factor-α,37,56,61,69,70,72 intercellular adhesion molecule 1,62 monocyte chemoattractant protein-1,71 and immunoglobulin E52 have been reported. However, these changes have been small and in general, the clinical significance of hsCRP changes in CV outcome trials is unclear.
Figure 2.
Time to key primary and key secondary composite endpoint for icosapent ethyl and placebo by low-density lipoprotein cholesterol (A and C) and hsCRP (B and D) increase or no change/decrease (intent-to-treat population). The icosapent ethyl and placebo groups were compared regarding increases and no change/decreases in low-density lipoprotein cholesterol or high-sensitivity C-reactive protein levels for key REDUCE-IT primary and key secondary composite endpoints. Changes in these biomarkers did not influence time to primary and key secondary endpoints. CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; IPE, icosapent ethyl; ITT, intent-to-treat; LDL-C, low-density lipoprotein cholesterol. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. The key secondary end point was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.
Lack of effect on blood pressure
As with blood lipids, most trials identified through our literature searches did not report on-study blood pressure within mineral oil arms. Trials that did report blood pressure are summarized in Table 3, and include seven studies.40–42,54,60,61,73 More than half of the studies were conducted in CV or high-risk patients, and, similar to blood lipids, results were highly variable. For systolic blood pressure, reported changes ranged from a 0.3 mmHg decrease to a 2.5 mmHg increase.
Table 3.
Reported effects on blood pressure in studies with mineral oil placebo groups
| Author | n a | Dose and mineral oil used | Patient population | Statins | Duration of treatment | SBP | DBP | MAP |
|---|---|---|---|---|---|---|---|---|
| Mortazavi et al.,40 2018 | 23 | 4 g/day, edible paraffin | Male subjects aged 45–55 years with CV disease and ≥50% occlusion in 1 coronary artery | NR | 8 weeks | Decreased 0.29 mmHg | Increased 0.24 mmHg | NR |
| Golzari et al.,41 2019 | 18 | 2 g/day, edible paraffin | Diabetes for ≥1 year, aged 35–50 years, and taking antidiabetic drugs for ≥3 months | NR | 8 weeks |
BL: 124.11 mmHg EOT: 124.89 mmHg |
BL: 80.00 mmHg EOT: 80.00 mmHg |
BL: 94.70 mmHg EOT: 94.96 mmHg |
| Agh et al.,42 2017 | 21 | Edible paraffin |
Men with CAD, ≤50% stenosis in ≥1 major coronary artery in the last 3 months, and BMI ≤30 kg/m2 Individuals using warfarin excluded from participation |
Statin therapy in 92% (does not refer specifically to the control arm) | 8 weeks |
BL: 125.43 mmHg Change: −0.28 mmHg |
BL: 77.86 mmHg Change: +0.23 mmHg |
NR |
| Allain et al.,54 2009 | 8 | 3 g/day, paraffin oil | Healthy volunteers | NR | 6 weeks | NR | NR | Increased from 87.0 to 90.4 mmHg during hyperinsulinemic clamp; P < 0.05 |
| Ferreira et al.,60 2019 | 80 | 5 oral drops of placebo (1% powdered lemon flavour, 0.2% ethylene-diamine-tetraacetic acid, liquid flavour qs, and liquid petrolatum qsp in 20 mL) | Post-menopausal women aged 50–65 years | NR | 9 months |
BL: 136.5 mmHg EOT: 139.0 mmHg P = NS |
BL: 81.0 mmHg EOT: 81.3 mmHg P = NS |
NR |
| Rashidmayvan et al.,61 2019 | 22 | 1 g/day, paraffin oil | Patients aged 20–60 years with non-alcoholic fatty liver disease | NR | 8 weeks |
BL: 125.45 mmHg EOT: 127.77 mmHg P = NS |
BL: 79.45 mmHg EOT: 77.59 mmHg P = NS |
NR |
| Mozaffari-Khosravi et al.,73 2015 | 20 | 1 oral capsule pure liquid paraffin per week | Aged 25–50 years with definite diagnosis of hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg) and presence of vitamin D deficiency | NR | 8 weeks |
BL: 145.1 mmHg EOT: 146.0 mmHg |
BL: 93.1 mmHg EOT: 94.1 mmHg |
BL: 110.4 mmHg EOT: 111.4 mmHg |
BL, baseline; BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; DBP, diastolic blood pressure; EOT, end of treatment; MAP, mean arterial pressure; NR, not reported; OM3, omega-3 fatty acid; SBP, systolic blood pressure.
Number of participants in the mineral oil placebo group.
Lack of absorption interference
Mineral oil’s activity as a laxative (coating the bowel and stool with waterproof film) has been postulated by some to have a theoretical impact on absorption of drugs and essential nutrients. It has been suggested that mineral oil may interfere with the absorption of vitamins A, D, E, and K, although the evidence of this is generally weak and contradictory.74 Although a few have warned about the possibility of interference by mineral oil on absorption of drugs and nutrients, this appears to be based on theoretical concern rather than actual data, and the risk is likely clinically insignificant.26,75 Mineral oil likely has no clinically significant impact on absorption of vitamins such as A, E, and K.21,26 Studies have shown that fatty acids or vitamin D3 formulated in a nanoemulsion delivery system of indigestible mineral oil is less bioavailable than a similar emulsion of digestible corn oil.76,77 The relevance of these findings to absorption of fat-soluble vitamins and drugs not formulated in a nanoemulsion is not clear, as a vitamin D intervention study using pure liquid paraffin as a placebo found no reduction in vitamin D levels in the placebo group.73
Databases of drug–drug interactions provide no evidence of an interaction between mineral oil and statins or other medications.78,79 A recent analysis compared plaque morphology changes in the mineral oil placebo arms of two coronary plaque studies; the EVAPORATE trial, which used the same mineral oil placebo as REDUCE-IT, compared with a non-mineral oil placebo used in another prospective randomized trial (Garlic 5).80 The two trials were of similar design, with coronary computed tomography angiography performed in EVAPORATE at baseline and 9 months, and in Garlic 5 at baseline and 12 months. Although populations in the two trials were slightly different, the study did not observe any difference in progression of coronary plaque volumes between mineral oil placebo (EVAPORATE) and cellulose-based placebo (Garlic 5) in multivariable analysis, with virtually identical rates. In addition, a recent meta-analysis by the US Department of Defense, including 21 studies of 869 patients, concluded that, at most, the mineral oil quantities used in REDUCE-IT raised LDL-C levels by <5 mg/dL.81
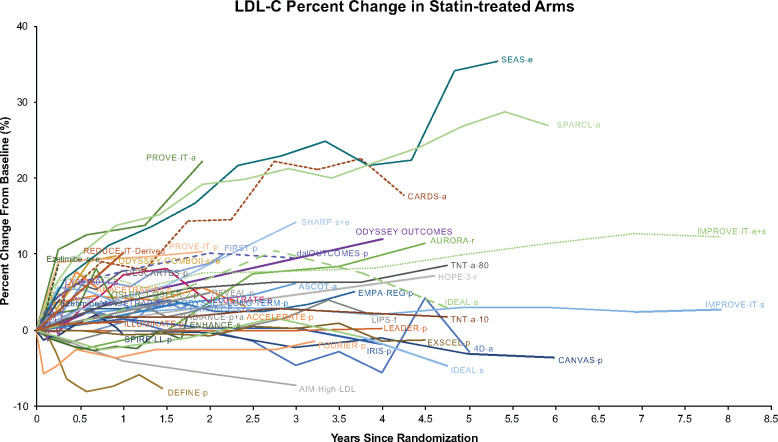
Analyses prepared for an FDA public Advisory Committee showed that LDL-C levels did not influence time to primary and secondary endpoints in REDUCE-IT (Figure 2). Similar findings were demonstrated with changes with other biomarkers (Supplementary material online, Table S1). The FDA concluded that statin absorption interference was unlikely if the statin is administered separately from mineral oil, and regardless of administration timing, had little impact on the overall conclusions of REDUCE-IT.82 An analysis of LDL-C percentage changes in CV outcome trials with statin-treated cohorts from 2003 to 2019 showed that 79% of studies reported increases in LDL-C after statin stabilization similar to those observed in REDUCE-IT placebo patients (Figure 3).
Figure 3.
Overview of the low-density lipoprotein cholesterol percentage changes observed in statin-treated cohorts from recent (published since 2003) cardiovascular outcome trials and other long-term studies that reported at least two statin-treated low-density lipoprotein cholesterol measurements over time, along with a comparison to low-density lipoprotein cholesterol changes in the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT). All plots represent statin-only arms, as represented by placebo (p) or statin (r = rosuvastatin, s = simvastatin, a = atorvastatin) or +e (ezetimibe), depending on the study. 4D, Die Deutsche Diabetes Dialyse Studie; ACCELERATE, Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes; AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; ASCOT, Anglo-Scandinavian Cardiovascular Outcomes Trial; AURORA, A Study to Evaluate the Use of Rosuvastatin in Subjects On Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events; CANVAS, Canagliflozin Cardiovascular Assessment Study; CARDS, Collaborative Atorvastatin Diabetes Study; dalOUTCOMES, Effects of the Cholesterol Ester Transfer Protein Inhibitor Dalcetrapib in Patients with Recent Acute Coronary Syndrome; DEFINE, Determining the Efficacy and Tolerability of Cholesteryl Ester Transfer Protein (CETP) Inhibition with Anacetrapib; DESCARTES, Durable Effect of PCSK9 Antibody Compared with Placebo Study; EMPA-REG, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes; ENHANCE, Effect of Ezetimibe Plus Simvastatin vs. Simvastatin Alone on Atherosclerosis in the Carotid Artery; EXSCEL, Exenatide Study of Cardiovascular Event Lowering; FIRST, Evaluation of Choline Fenofibrate (ABT-335) on Carotid Intima-Media Thickness (cIMT) in Subjects with Type IIb Dyslipidaemia with Residual Risk in Addition to Atorvastatin Therapy; FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; HOPE-3, Heart Outcomes Prevention Evaluation-3; IDEAL, Incremental Decrease in End Points Through Aggressive Lipid-Lowering; ILLUMINATE, Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events; ILLUSTRATE, Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation; IMPROVE-IT, Improved Reduction of Outcomes: Vytorin Efficacy International Trial; IRIS, Insulin Resistance Intervention After Stroke; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; LIPS, Lescol Intervention Prevention Study; ODYSSEY-COMBO-II, Efficacy and Safety of Alirocumab vs. Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolaemia; ODYSSEY OUTCOMES: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab; OSLER-1, Open Label Study of Long Term Evaluation Against LDL-C Trial; PROVE-IT, Pravastatin or Atorvastatin Evaluation and Infection Therapy; RADIANCE, Rating Atherosclerotic Disease Change by Imaging With a New CETP Inhibitor; REVEAL, Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification; SEAS, Simvastatin Ezetimibe in Aortic Stenosis; SHARP, Study of Heart and Renal Protection; SPARCL, Stroke Prevention by Aggressive Reduction in Cholesterol Levels; SPIRE, Studies of PCSK9 Inhibition and the Reduction of Vascular Events; TNT, Treating to New Targets.
Discussion
This comprehensive review found no consistent pattern of changes in lipid levels and inflammatory markers in patients given mineral oil. Even in those studies where statistically significant changes were reported, changes were generally small and were of no clinical significance. No relevant safety concerns, including CV AEs, have been identified with oral administration of mineral oil, including in children receiving a high volume to treat constipation.6,22,26
There appears to be no effect on CV outcomes of the observed LDL-C increase in placebo patients in REDUCE-IT, and such a change in statin-controlled patients is not expected to be predictive of CV outcomes. Even if one were to explore a theoretical increase in CV risk due to the observed increase in LDL-C within the placebo group, the 5 mg/dL difference between the placebo and treatment groups would be estimated to increase the risk of CVD events by 2–3% in the placebo group,83–87 which cannot account for the overall robust and consistent REDUCE-IT findings, including a 25% relative risk reduction in the primary endpoint, and a 32% reduction in total ischaemic events.88,89 The REDUCE-IT results are also in agreement with the observed risk reduction in the JELIS study, which did not administer a mineral oil placebo within the control group.8,90 The independent Data and Safety Monitoring Committee (DMC) of REDUCE-IT met regularly and monitored changes in biomarkers in the placebo arm, and changes in LDL, TGs, and hsCRP in the placebo arm were not significantly associated with CV outcomes.91
Consistent with the REDUCE-IT DMC’s findings, the FDA independent analysis concluded that the small degrees of changes in biomarkers could not account for the 25% relative risk reduction seen in REDUCE-IT, with the ultimate conclusion that such small theoretical impacts, if real, would not change the overall study conclusions.82 This conclusion is in agreement with our review of the mineral oil literature.
Finally, it is worth noting that examination of the rates of treatment-emergent AEs between IPE and mineral oil placebo in REDUCE-IT showed no significant differences, whether a very sensitive or very specific definition was used (Table 4).8 In fact, the actual rates were almost identical in the two treatment arms. Thus, in the overall trial, the drug was tolerated as well as and as safely as the placebo. Importantly, this also means that the placebo was tolerated as well and as safely as the drug, arguing against any clinically meaningful toxicity from the mineral oil placebo.
Table 4.
Treatment-emergent adverse events from REDUCE-IT
| Icosapent ethyl (n = 4089) | Placebo (n = 4090) | P-value a | |
|---|---|---|---|
| Patients with at least one TEAE,bn (%) | 3343 (81.8) | 3326 (81.3) | 0.63 |
| Serious TEAE | 1252 (30.6) | 1254 (30.7) | 0.98 |
| TEAE leading to withdrawal of study drugc | 321 (7.9) | 335 (8.2) | 0.60 |
| Serious TEAE leading to withdrawal of study drugc | 88 (2.2) | 88 (2.2) | 1.00 |
| Serious TEAE leading to deathd | 94 (2.3) | 102 (2.5) | 0.61 |
Note: A treatment-emergent adverse event (TEAE) is defined as an event that first occurs or worsens in severity on or after the date of dispensing study drug and within 30 days after the completion or withdrawal from study. Percentages are based on the number of patients randomized to each treatment group in the safety population. Events that were positively adjudicated as clinical endpoints are not included. From Ref.8 Copyright ©2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
P value from Fisher’s exact test.
All TEAEs are coded using the Medical Dictionary for Regulatory Activities version 20.1.
Withdrawal of study drug excludes patients who were off drug in study for 30 days or more, and restarted study drug.
The most common serious TEAEs leading to death by system organ class were neoplasms (1.1%); infections and infestations (0.4%); respiratory, thoracic, and mediastinal disorders (0.2%); cardiac disorders (0.2%); and vascular disorders (0.1%). No serious TEAEs leading to death by system organ class were statistically significant across treatment groups except for cardiac disorders, which occurred in 3 (0.1%) of icosapent ethyl patients and 15 (0.4%) of placebo patients (P = 0.008).
Limitations
This review has several limitations. It is likely that not all studies using a mineral oil placebo were identified, as many do not describe the composition of placebo in detail. Nonetheless, a lack of data reporting the use of mineral oil likely suggests little to no clinical impact of mineral oil in such studies. For all studies identified, mineral oil was the placebo (except for paediatric constipation studies); as a result, no placebo-controlled trials reported the clinical and biochemical effects of mineral oil. Nonetheless, the substantial use in clinical studies, with a general lack of AEs being associated with mineral oil, suggests a lack of safety concerns.
Many of the studies identified were small and not designed or powered to evaluate possible effects of mineral oil (or the active comparator) on lipids and inflammatory markers. Although REDUCE-IT is a comparatively larger study, it too was not powered to compare differences in biomarkers. As such, although small changes in biomarkers may be statistically significant, their clinical significance is debatable. These studies covered a range of clinical conditions, so not all data included lipid levels and inflammatory markers. The studies in this analysis included a range of mineral oil formulations and intakes ranging from 1 to 5 g/day, over varying lengths of administration, which potentially complicated data interpretation.
Conclusions
The preponderance of evidence identified in this review confirms that mineral oil is essentially inert, with no systemic effects in humans when taken orally, other than a lubricating laxative effect in the gastrointestinal tract. While some changes in select biomarkers were reported in REDUCE-IT patients randomized to mineral oil placebo, similar increases in lipid biomarkers within statin-treated patients have been seen in other contemporary lipid CV outcome trials, and importantly, no clinical impact of such biomarker changes in the REDUCE-IT placebo group was observed. Prespecified and post hoc analyses of REDUCE-IT support that on-treatment EPA levels, not choice of placebo, overwhelmingly accounted for the robust REDUCE-IT clinical findings.16 Multiple analyses by distinct and independent groups conclude that even if theoretical mineral oil effects were real, such effects would be small and would not impact study conclusions or the robustness of the CV event risk reduction observed in REDUCE-IT.
Supplementary material
Supplementary material is available at European Heart Journal Supplements online.
Funding
Editorial assistance for this article, limited to copy editing, collation of co-author comments, and reference formatting, was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Amarin Pharma, Inc., Bridgewater, NJ. The first draft was written by Dr Olshansky. The authors would like to thank Steve Ketchum, PhD and Craig Granowitz, MD, PhD from Amarin Pharma, Inc for their regulatory contributions and literature analysis concerning mineral oil. This paper was published as part of a supplement supported by an unrestricted educational grant from Amarin Pharma, Inc.
Conflict of interest
B.O. reports serving as the Amarin DMC chair, Boehringer Ingelheim GLORIA AF-US co-coordinator, Sanofi Aventis consultant, and Respicardia consultant. M.J.B. has received grant support and is on the speaker’s bureau for Amarin Pharma, Inc. S.P., L.J., C.C., A.G., R.J., and R.D. are stock shareholders and employees of Amarin Pharma, Inc. D.L.B. serves as the Chair and International Principal Investigator for REDUCE-IT, with research funding from Amarin to Brigham and Women’s Hospital. D.L.B. discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, and Takeda. The other authors have no conflict of interest to declare.
Data availability
No new data were generated or analysed in support of this research.
Supplementary Material
References
- 1. Aguilar F, Charrondiere UR, Dusemund B, Galtier P, Gilbert J, Gott DM, Grilli S, Guertler R, Koenig J, Lambré C, Larsen J‐C, Leblanc J‐C, Mortensen A, Parent‐Massin D, Pratt I, Rietjens IMCM, Stankovic I, Tobback P, Verguieva T, Woutersen RA. Scientific opinion on the use of high viscosity white mineral oils as a food additive. EFSA J 2009;7:1387. [Google Scholar]
- 2.Food Additive Status List. https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (24 October2019).
- 3.Alexander J, Benford D, Boobis AR, Ceccatelli S, Cottrill B, Cravedi J-P, Domenico AD, Doerge D, Dogliotti E, Edler L, Farmer P, Filipič M, Fink‐Gremmels J, Fürst P, Guérin T, Knutsen HK, Machala M, Mutti A, Rose M, Schlatter JR, van Leeuwen R. Scientific opinion on mineral oil hydrocarbons in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 2012;10:2704. [Google Scholar]
- 4.European Commission. COMMISSION REGULATION (EU) 2015/1608 of 24 September 2015 amending Annex IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for capric acid, paraffin oil (CAS 64742-46-7), paraffin oil (CAS 72623-86-0), paraffin oil (CAS 8042-47-5), paraffin oil (CAS 97862-82-3), lime sulphur and urea in or on certain products https://op.europa.eu/en/publication-detail/-/publication/0aec504d-6354-11e5-9317-01aa75ed71a1/language-en (22 July 2020).
- 5. Sharif F, Crushell E, O'Driscoll K, Bourke B.. Liquid paraffin: a reappraisal of its role in the treatment of constipation. Arch Dis Child 2001;85:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gal-Ezer S, Shaoul R.. The safety of mineral oil in the treatment of constipation–a lesson from prolonged overdose. Clin Pediatr 2006;45:856–858. [DOI] [PubMed] [Google Scholar]
- 7.Mineral Oils Are Safe for Human Health? https://www.concawe.eu/wp-content/uploads/2017/10/DEF_C_MM_digital-1.pdf (22 July 2020).
- 8. Bhatt DL, Steg G, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM.. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 9. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN.. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 2011;108:682–690. [DOI] [PubMed] [Google Scholar]
- 10. Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN.. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984–992. [DOI] [PubMed] [Google Scholar]
- 11. Budoff MJ, Muhlestein JB, Bhatt DL, Le VT, May HT, Shaikh K, Shekar C, Kinninger A, Lakshmanan S, Roy S, Tayek J, Nelson JR.. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499mg/dl) on statin therapy (EVAPORATE study) [abstract] Presented at Annual Scientific Sessions of the American Heart Association. Philadelphia, PA, 2019. [Google Scholar]
- 12. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR.. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol 2018;41:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Budoff MJ, Muhlestein JB, Bhatt DL, Le Pa VT, May HT, Shaikh K, Shekar C, Kinninger A, Lakshmanan S, Roy S, Tayek J, Nelson JR.. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results. Cardiovasc Res 2020;doi:10.1093/cvr/cvaa184. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL. REDUCE-IT: residual cardiovascular risk in statin-treated patients with elevated triglycerides: now we can REDUCE-IT! Eur Heart J 2019;40:1174–1175.30982067 [Google Scholar]
- 15. Boden WE, Bhatt DL, Toth PP, Ray KK, Chapman MJ, Luscher TF.. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: why these results usher in a new era in dyslipidaemia therapeutics. Eur Heart J 2020;41:2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatt DL, Miller M, Steg G, Brinton EA, Jacobson TA, Ketchum SB, Juliano RA, Jiao L, Doyle RT Jr, Copland C, Dunbar RL, Granowitz C, Martens FMAC, Budoff M, Nelson JR, Mason RP, Libby P, Ridker P, Tardiff J-C, Ballantyne CM, EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl-intervention trial. Presented at the American College of Cardiology 2020 annual meeting (virtual). Available at: https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=062fc9e4b3a74a9fb1c196a35dad8f3b (18 May2020). [Google Scholar]
- 17. Mason RP, Libby P, Bhatt DL.. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020;40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson JR, True WS, Le V, Mason RP.. Can pleiotropic effects of eicosapentaenoic acid (EPA) impact residual cardiovascular risk? Postgrad Med 2017;129:822–827. [DOI] [PubMed] [Google Scholar]
- 19. Nelson JR, Raskin S.. The eicosapentaenoic acid: arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad Med 2019;131:268–277. [DOI] [PubMed] [Google Scholar]
- 20. Kastelein JJP, Stroes ESG.. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med 2019;380:89–90. [DOI] [PubMed] [Google Scholar]
- 21. Clark JH, Russell GJ, Fitzgerald JF, Nagamori KE.. Serum beta-carotene, retinol, and alpha-tocopherol levels during mineral oil therapy for constipation. Am J Dis Child 1987;141:1210–1212. [DOI] [PubMed] [Google Scholar]
- 22. Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG.. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2016;doi: 10.1002/14651858.CD009118.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laxative (oral route) http://www.mayoclinic.com/health/drug-information/DR602359 (5 September 2012).
- 24. Duke GE, Evanson OA.. Inhibition of gastric motility by duodenal contents in turkeys. Poult Sci 1972;51:1625–1636. [DOI] [PubMed] [Google Scholar]
- 25. Soares AC, Tahan S, Morais MB.. Effects of conventional treatment of chronic functional constipation on total and segmental colonic and orocecal transit times. J Pediatr (Rio J) 2009;85:322–328. [DOI] [PubMed] [Google Scholar]
- 26. Sood MR, Li BUK, Hoppin AG.. Chronic Functional Constipation and Fecal Incontinence in Infants and Children: Treatment https://www.uptodate.com/contents/chronic-functional-constipation-and-fecal-incontinence-in-infants-and-children-treatment?search=%E2%80%9CMineral%20oil%E2%80%9D%20%20placebo&source=search_result&selectedTitle=1∼44&usage_type=default&display_rank=1 (22 July 2020).
- 27. Yokote K, Niwa K, Hakoda T, Oh F, Kajimoto Y, Fukui T, Kim H, Noda Y, Lundstrom T, Yajima T.. Short-term efficacy (at 12 weeks) and long-term safety (up to 52 weeks) of omega-3 free fatty acids (AZD0585) for the treatment of Japanese patients with dyslipidemia - A randomized, double-blind, placebo-controlled, phase III study. Circ J 2020;84:994–1003. [DOI] [PubMed] [Google Scholar]
- 28. Vera-Lastra O, Medina G, Cruz-Domínguez MP, Ramírez GM, Blancas RBP, Amaro ALP, Martínez AV, Delgado JS, Jara LJ.. Autoimmune/inflammatory syndrome induced by mineral oil: a health problem. Clin Rheumatol 2018;37:1441–1448. [DOI] [PubMed] [Google Scholar]
- 29.CFR - Code of Federal Regulation Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (9 September2013).
- 30.Code of Federal Regulations Title 21: Part 172 Food additives permitted for direct addition to food for human consumption: Section 172.878 White Mineral Oil. https://www.ecfr.gov/cgi-bin/text-idx?SID=179b65479a1b617b5b814df1bd789878&mc=true&node=se21.3.172_1878&rgn=div8 (22 July 2020).
- 31. Ainsworth AJ, Fredrickson JR, Morbeck DE.. Improved detection of mineral oil toxicity using an extended mouse embryo assay. J Assist Reprod Genet 2017;34:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boogaard PJ, Goyak KO, Biles RW, van Stee LL, Miller MS, Miller MJ.. Comparative toxicokinetics of low-viscosity mineral oil in Fischer 344 rats, Sprague-Dawley rats, and humans–implications for an Acceptable Daily Intake (ADI). Regul Toxicol Pharmacol 2012;63:69–77. [DOI] [PubMed] [Google Scholar]
- 33. Barp L, Kornauth C, Wuerger T, Rudas M, Biedermann M, Reiner A, Concin N, Grob K.. Mineral oil in human tissues, Part I: concentrations and molecular mass distributions. Food Chem Toxicol 2014;72:312–321. [DOI] [PubMed] [Google Scholar]
- 34. Barp L, Biedermann M, Grob K, Blas YEF, Nygaard UC, Alexander J, Cravedi JP.. Accumulation of mineral oil saturated hydrocarbons (MOSH) in female Fischer 344 rats: comparison with human data and consequences for risk assessment. Sci Total Environ 2017;575:1263–1278. [DOI] [PubMed] [Google Scholar]
- 35. Adenuga D, Goyak K, Lewis RJ.. Evaluating the MoA/human relevance framework for F-344 rat liver epithelioid granulomas with mineral oil hydrocarbons. Crit Rev Toxicol 2017;47:750–766. [DOI] [PubMed] [Google Scholar]
- 36. Miller MJ, Lonardo EC, Greer RD, Bevan C, Edwards DA, Smith JH, Freeman JJ.. Variable responses of species and strains to white mineral oils and paraffin waxes. Regul Toxicol Pharmacol 1996;23:55–68. [DOI] [PubMed] [Google Scholar]
- 37. Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW.. Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–1679. [DOI] [PubMed] [Google Scholar]
- 38. Gholamhosseini S, Nematipour E, Djazayery A, Javanbakht MH, Koohdani F, Zareei M, Djalali M.. omega-3 fatty acid differentially modulated serum levels of IGF1 and IGFBP3 in men with CVD: a randomized, double-blind placebo-controlled study. Nutrition 2015;31:480–484. [DOI] [PubMed] [Google Scholar]
- 39. Hosseini MS, Mirkarimi SA, Amini M, Mohtashami R, Kianbakht S, Huseini F.. Effects of Nigella sativa L. seed oil in type II diabetic patients: a randomized, double-blind, placebo—controlled clinical trial. J Medicinal Plants 2013;12:93–99. [Google Scholar]
- 40. Mortazavi A, Nematipoor E, Djalali M, Keshavarz SA, Samavat S, Zarei M, Javanbakht MH.. The effect of omega-3 fatty acids on serum apelin levels in cardiovascular disease: a randomized, double-blind, placebo-controlled trial. Rep Biochem Mol Biol 2018;7:59–66. [PMC free article] [PubMed] [Google Scholar]
- 41. Golzari MH, Javanbakht MH, Ghaedi E, Mohammadi H, Djalali M.. Effect of eicosapentaenoic acid supplementation on paraoxonase 2 gene expression in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Clin Nutr Res 2019;8:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agh F, Mohammadzadeh Honarvar N, Djalali M, Nematipour E, Gholamhoseini S, Zarei M, Ansari S, Javanbakht MH.. Omega-3 fatty acid could increase one of myokines in male patients with coronary artery disease: a randomized, double-blind, placebo-controlled trial. Arch Iran Med 2017;20:28–33. [PubMed] [Google Scholar]
- 43. Mazaherioun M, Djalali M, Koohdani F, Javanbakht MH, Zarei M, Beigy M, Ansari S, Rezvan N, Saedisomeolia A.. Beneficial effects of n-3 fatty acids on cardiometabolic and inflammatory markers in type 2 diabetes mellitus: a clinical trial. Med Princ Pract 2017;26:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramezani A, Djalali M, Yosefinejad A. [ Changes in lipid profiles among patients with coronary vascular diseases treated with omega3 and vitamin E: a randomized control clinical trial]. J Mazandaran Univ Med Sci 2018;28:1–11. [Google Scholar]
- 45. De Truchis P, Kirstetter M, Perier A, Meunier C, Zucman D, Force G, Doll J, Katlama C, Rozenbaum W, Masson H, Gardette J, Melchior JC.. Reduction in triglyceride level with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J Acquir Immune Defic Syndr 2007;44:278–285. [DOI] [PubMed] [Google Scholar]
- 46. Horrobin DF, Ells KM, Morse-Fisher N, Manku MS.. The effects of evening primrose oil, safflower oil and paraffin on plasma fatty acid levels in humans: choice of an appropriate placebo for clinical studies on primrose oil. Prostaglandins Leukot Essent Fatty Acids 1991;42:245–249. [DOI] [PubMed] [Google Scholar]
- 47. Lemos JR, Alencastro MG, Konrath AV, Cargnin M, Manfro RC.. Flaxseed oil supplementation decreases C-reactive protein levels in chronic hemodialysis patients. Nutr Res 2012;32:921–927. [DOI] [PubMed] [Google Scholar]
- 48. Emsley R, Niehaus DJ, Oosthuizen PP, Koen L, Ascott-Evans B, Chiliza B, van Rensburg SJ, Smit RM.. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry Res 2008;161:284–291. [DOI] [PubMed] [Google Scholar]
- 49. Fogaça MN, Santos-Galduroz RF, Eserian JK, Galduroz JC.. The effects of polyunsaturated fatty acids in alcohol dependence treatment–a double-blind, placebo-controlled pilot study. BMC Clin Pharmacol 2011;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S.. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr 2012;21:511–518. [PubMed] [Google Scholar]
- 51. Peet M, Horrobin DF; E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 2002;36:7–18. [DOI] [PubMed] [Google Scholar]
- 52. Yang B, Kalimo KO, Mattila LM, Kallio SE, Katajisto JK, Peltola OJ, Kallio HP.. Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on atopic dermatitis. J Nutr Biochem 1999;10:622–630. [DOI] [PubMed] [Google Scholar]
- 53. Nogueira MA, Oliveira CP, Ferreira Alves VA, Stefano JT, Rodrigues LS, Torrinhas RS, Cogliati B, Barbeiro H, Carrilho FJ, Waitzberg DL.. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016;35:578–586. [DOI] [PubMed] [Google Scholar]
- 54. Allain G, Le Guen V, Lesven S, Mansourati J, Guerrero F, Kerspern H, Delarue J.. Effects of fish oil on metabolic alterations induced by carbohydrate overfeeding in healthy volunteers [abstract 534]. Diabetologia 2009;52(Suppl 1):S214–S215. [Google Scholar]
- 55. Mirmasoumi G, Fazilati M, Foroozanfard F, Vahedpoor Z, Mahmoodi S, Taghizadeh M, Esfeh NK, Mohseni M, Karbassizadeh H, Asemi Z.. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes 2018;126:222–228. [DOI] [PubMed] [Google Scholar]
- 56. Paixao E, Oliveira ACM, Pizato N, Muniz-Junqueira MI, Magalhaes KG, Nakano EY, Ito MK.. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naive breast cancer patients: a randomized double-blind controlled trial. Nutr J 2017;16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mejia-Montilla J, Reyna-Villasmil E, Andrade-Albán M, Lozada-Meza M, Rodríguez-Cevallos M, Solís-Manzano A. [ Vitamin D supplementation and lipid profile in women with polycystic ovary syndrome and vitamin D deficiency]. Med Reprod Embriol Clin 2018;5:123–131. [Google Scholar]
- 58. Ghorbanihaghjo A, Kolahi S, Seifirad S, Rashtchizadeh N, Argani H, Hajialilo M, Khabazi A, Alizadeh S, Bahreini E.. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: a double-blind randomized controlled trial. Arch Iran Med 2012;15:549–552. [PubMed] [Google Scholar]
- 59. Kremer JM, Bigauoette J, Michalek AV, Timchalk MA, Lininger L, Rynes RI, Huyck C, Zieminski J, Bartholomew LE.. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet 1985;325:184–187. [DOI] [PubMed] [Google Scholar]
- 60. Ferreira PP, Cangussu L, Bueloni-Dias FN, Orsatti CL, Schmitt EB, Nahas-Neto J, Nahas EAP.. Vitamin D supplementation improves the metabolic syndrome risk profile in postmenopausal women. Climacteric 2020;23:24–31. [DOI] [PubMed] [Google Scholar]
- 61. Rashidmayvan M, Mohammadshahi M, Seyedian SS, Haghighizadeh MH.. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J Diabetes Metab Disord 2019;18:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN.. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 2013;13:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gharekhani A, Khatami MR, Dashti-Khavidaki S, Razeghi E, Noorbala AA, Hashemi-Nazari SS, Mansournia MA.. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol 2014;70:655–665. [DOI] [PubMed] [Google Scholar]
- 64. Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, Seyed Hosseini E, Haddad Kashani H, Karamali M, Asemi Z.. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab (Lond) 2017;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Belch JJ, Ansell D, Madhok R, O'Dowd A, Sturrock RD.. Effects of altering dietary essential fatty acids on requirements for non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a double blind placebo controlled study. Ann Rheum Dis 1988;47:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abdolahi M, Sarraf P, Javanbakht MH, Honarvar NM, Hatami M, Soveyd N, Tafakhori A, Sedighiyan M, Djalali M, Jafarieh A, Masoudian Y, Djalali M.. A novel combination of omega-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: a randomized clinical trial study. CNS Neurol Disord Drug Targets 2018;17:430–438. [DOI] [PubMed] [Google Scholar]
- 67. Bahadori B, Uitz E, Thonhofer R, Trummer M, Pestemer-Lach I, McCarty M, Krejs GJ.. Omega-3 fatty acids infusions as adjuvant therapy in rheumatoid arthritis. JPEN J Parenter Enteral Nutr 2010;34:151–155. [DOI] [PubMed] [Google Scholar]
- 68. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001;103:1813–1818. [DOI] [PubMed] [Google Scholar]
- 69. Gharekhani A, Khatami MR, Dashti-Khavidaki S, Razeghi E, Abdollahi A, Hashemi-Nazari SS, Mansournia MA.. Effects of oral supplementation with omega-3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. J Ren Nutr 2014;24:177–185. [DOI] [PubMed] [Google Scholar]
- 70. Tan A, Sullenbarger B, Prakash R, McDaniel JC.. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids 2018;132:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lotfi-Dizaji L, Mahboob S, Aliashrafi S, Vaghef-Mehrabany E, Ebrahimi-Mameghani M, Morovati A.. Effect of vitamin D supplementation along with weight loss diet on meta-inflammation and fat mass in obese subjects with vitamin D deficiency: a double-blind placebo-controlled randomized clinical trial. Clin Endocrinol (Oxf) 2019;90:94–101. [DOI] [PubMed] [Google Scholar]
- 72. Abdolahi M, Tafakhori A, Togha M, Okhovat AA, Siassi F, Eshraghian MR, Sedighiyan M, Djalali M, Mohammadzadeh Honarvar N, Djalali M.. The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients. Immunogenetics 2017;69:371–378. [DOI] [PubMed] [Google Scholar]
- 73. Mozaffari-Khosravi H, Loloei S, Mirjalili MR, Barzegar K.. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. Blood Press Monit 2015;20:83–91. [DOI] [PubMed] [Google Scholar]
- 74.Mineral Oil Drug Information: Summary of Interactions with Vitamins, Herbs and Foods. https://wa.kaiserpermanente.org/kbase/topic.jhtml?docId=hn-1156003 (22 July 2020).
- 75.Mineral Oil. https://www.drugguide.com/ddo/view/Davis-Drug-Guide/51506/all/mineral_oil.
- 76. Cho HT, Salvia-Trujillo L, Kim J, Park Y, Xiao H, McClements DJ.. Droplet size and composition of nutraceutical nanoemulsions influences bioavailability of long chain fatty acids and Coenzyme Q10. Food Chem 2014;156:117–122. [DOI] [PubMed] [Google Scholar]
- 77. Ozturk B, Argin S, Ozilgen M, McClements DJ.. Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem 2015;187:499–506. [DOI] [PubMed] [Google Scholar]
- 78.Mineral Oil Drug Interactions. http://www.drugs.com/drug-interactions/mineral-oil-index.html (18 October 2012).
- 79. cis-5,8,11,14,17-Eicosapentaenoic Acid Material Safety Data Sheet. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=44864&brand=FLUKA&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Finterface%3DProduct%2520No.%26term%3D44864%26lang%3Den%26region%3DUS%26focus%3Dproduct%26N%3D220003048%2B219853269%2B219853286%26mode%3Dmode%2520matchpartialmax (22 October 2012).
- 80. Lakshmanan S, Shekar C, Kinninger A, Dahal S, Onuegbu A, Cai AN, Hamal S, Birudaraju D, Roy SK, Nelson JR, Budoff MJ, Bhatt DL.. Comparison of mineral oil and non-mineral oil placebo on coronary plaque progression by coronary computed tomography angiography. Cardiovasc Res 2020;116:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Radel J, Pender D, Shah S.. Effect of mineral oil on plasma lipida: a meta-analysis [abstract]. J Am Coll Cardiol 2020;75:1908. [Google Scholar]
- 82.Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) Meeting Presentation. https://www.fda.gov/media/132767/download (22 July 2020).
- 83. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS.. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 84. Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, Demicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM Jr, Ridker PM, Kastelein JJ.. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA 2012;307:1302–1309. [DOI] [PubMed] [Google Scholar]
- 85. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R.. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 86. Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Raber L, Mach F, Windecker S.. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2018;39:1172–1180. [DOI] [PubMed] [Google Scholar]
- 87. Robinson JG, Smith B, Maheshwari N, Schrott H.. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005;46:1855–1862. [DOI] [PubMed] [Google Scholar]
- 88. Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg G, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif JC, Olshansky B, Chung MK, Gibson CM, Giugliano RP, Budoff MJ, Ballantyne CM; On behalf of the REDUCE-IT Investigators. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation 2020;141:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Gregson J, Pocock SJ, Ballantyne CM; The REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 90. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K.. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 91. Endocrinologic and Metabolic Drugs Advisory Committee Briefing Document: Vascepa® (icosapent ethyl;AMR101) REDUCE-IT® (Reduction of Cardiovascular Events with EPA—Intervention Trial) NDA Number: 202057 Amarin Backgrounder: US Food and Drug Administration; 2019. https://www.fda.gov/advisory-committees/november-14-2019-meeting-endocrinologic-and-metabolic-drugs-advisory-committee-meeting-announcement#event-materials.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.