Abstract
Accumulating evidence implicates hippocampal neurogenesis in the pathophysiology of depression. Psychosocial stress reduces neurogenesis in rodents, whereas chronic treatment with antidepressants increases neurogenesis and blocks the effects of stress. The effects of stress and antidepressant treatment on hippocampal neurogenesis parallel behavioral changes in animal models. Moreover, ablating hippocampal neurogenesis renders antidepressants inactive in behavioral paradigms used to model antidepressant response and anxiety-like behavior in mice. In humans, monoamine-modulating antidepressants demonstrate clinical efficacy in treating depression and anxiety, which are often precipitated by psychosocial stress. This review examines the mounting evidence that stress and antidepressant treatment regulate neurogenesis in animals. Special attention is paid to the cellular and molecular mechanisms by which this regulation takes place. An analysis of current animal models used to study response to stress and antidepressants indicates the importance of modeling chronic treatment, which reflects both changes in neurogenesis and clinical response. Exploring responses of hippocampal neurogenesis to experimental challenges in appropriate animal models should delineate the role of adult-born neurons in hippocampal physiology. Focusing on neurogenic response to experimental paradigms of stress and antidepressant treatment is particularly interesting for understanding the pathophysiology of major depressive disorder.
Keywords: Antidepressants, hippocampus, mouse models, neurogenesis, stress
The possibility that neuronal cells are continually added to the adult brain was intensely rejected by the scientific community (for review, see Gross 2000) despite accumulating evidence to the contrary (Altman and Das 1965a, 1965b; Kaplan 1981, 1985; Kaplan and Hinds 1977). In a series of elegant experiments, Nottebohm and colleagues demonstrated that new neurons were added to the adult avian brain (Burd and Nottebohm 1985; Goldman and Nottebohm 1983). The investigators further reported that the adult-born neurons made functional connections and responded to sound, an environmental input (Paton and Nottebohm 1984). Following the discovery that new neurons were added to the avian brain, much speculation ensued regarding the possibility that a similar phenomenon may occur in mammals. Newer techniques to detect DNA replication and thus cell division were combined with immunohistochemistry to confirm that cells born in the brains of adult rodents and other mammals can assume a neuronal phenotype. The next natural question was whether adult neurogenesis also occurred in humans. In a seminal study, Erickson and colleagues demonstrated that neurogenesis occurs in adult humans by identifying new neurons, which incorporated the thymidine analog bromodeoxyuridine (BrdU), in the hippocampi of deceased cancer patients who were receiving BrdU to assess tumor progression (Eriksson et al 1998). Once adult neurogenesis was described in all mammal species surveyed, investigators began to focus on identifying the physiological role of adult-born neurons. Some clues to the normal function of neurogenesis came from an observation that although robust, adult neurogenesis is exclusively restricted to the subependymal cells of the ventricular system (SVZ) and the subgranular zone of the dentate gyrus in the hippocampus (SGZ) in rodents. In humans, adult neurogenesis has been conclusively demonstrated only in the hippocampus (Eriksson et al 1998). The normal function of adult neurogenesis must reflect the normal function of the brain structures to which it is restricted. Given that hippocampus is the primary area of neurogenesis in humans, much effort focused on the function of new neurons in this structure.
Stress and Depression Affect the Hippocampus
The hippocampus has been firmly established to play a critical role in learning and memory. It also plays an important role in the brain’s response to psychosocial stress by regulating the release of hypothalamic corticotropin-releasing factor (CRF). Under stressful conditions, the paraventricular nucleus of the hypothalamus secretes CRF, which stimulates release of adrenocorticotropic hormone (ACTH) by the anterior pituitary. ACTH then stimulates release of glucocorticoids by the adrenal glands. Glucocorticoids have wide-ranging metabolic effects throughout the body. In the brain, prolonged exposure to experimental paradigms of psychosocial stress results in glucocorticoid-dependent reduction in hippocampal volume, dendritic arborization, and neurogenesis (McEwen 2001). Hippocampal neurons express receptors for glucocorticoids (McEwen et al 1968) suggesting that the effect of glucocorticoids on the hippocampus may be direct. In a regulatory feedback loop, inhibitory afferents from the hippocampus suppress hypothalamic release of CRF. Therefore, the hippocampus serves as both a target and a regulator of the brain’s response to stress.
Human imaging studies have suggested that structural hippocampal changes underlie the pathophysiology of major depressive disorder (MDD). Multiple studies have demonstrated that hippocampal volume is decreased in patients with MDD (Videbech and Ravnkilde 2004). Moreover, volumetric changes reflect the actual number of days that individuals have been depressed with prolonged symptomatic periods corresponding to smaller hippocampi (MacQueen et al 2003; Sheline et al 1996, 1999). Presence of hippocampal microscopic structural changes observed in animal models of stress was speculated to underlie the decrease in hippocampal volume observed in MDD (McEwen 1999). Moreover, stress-induced changes in plasticity and volume are prevented by antidepressant treatment in animals (Czeh et al 2001). Finally, some patients with depression exhibit dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis (reviewed in Holsboer 2000). Together, these data indicate that the hippocampus is affected by stress and depression.
Interaction of Stress Response and Monoamine Regulation in the Genetics of Major Depressive Disorder
Recent genetic studies suggest that the roles of stress and monoamines in MDD converge. Monoamine modulation has become the mainstay of antidepressant treatment. Inhibitors of the serotonin transporter are currently used for the treatment of all depressive and anxiety disorders. Genetic studies revealed a functional polymorphism resulting in transcriptional changes in the serotonin transporter gene (Lesch et al 1996). In a compelling study, Caspi and colleagues demonstrated that the polymorphism, which results in decreased transcription, conferred risk for MDD only in people who had suffered multiple adverse life events (Caspi et al 2003). Caspi’s finding was recently replicated in another cohort (Kendler et al 2005). Thus, psychosocial stress modified the ability of a genetic variability in synaptic serotonin to confer risk for MDD. In animal studies, mice deficient in the serotonin transporter gene demonstrate an altered physiological response to stress (Armando et al 2003; Tjurmina et al 2004).
Genetic differences in the stress response also modify behavioral effects of monoamine modulators. Binder and colleagues demonstrated that a functional polymorphism in FKBP5, predicted a more rapid response to antidepressant treatment with modulators of monoamines (Binder et al 2004). FKBP5 is a glucocorticoid receptor chaperone binding protein and the polymorphism resulted in increased intercellular FKBP5 protein. The FKBP5 polymorphism also predicted a higher corticotropin release following suppression of ACTH with dexamethasone in the depressed patients, thus indicating that the polymorphism is involved in the response to stress by the HPA axis. Elevated ACTH and cortisol and defects in the ability to suppress ACTH with dexamethasone are reliable findings in some patients with MDD (Holsboer 2000). In this example, a genetic variant in stress response predicts how monoamine modulation affects mood. Mice with dysfunctional glucocorticoid receptors demonstrate an altered physiologic response to chronic monoamine modulation and provide a window into the mechanism of how the two systems interact (Vinet et al 2004). Taken together, these studies indicate that the mechanisms of stress response and monoamine regulation interact: environmental manipulations of one system can modulate the genetic contributions of the other system to depression.
Psychosocial Stress and Monoamines Interact in Regulating Hippocampal Plasticity
Much of the evidence for convergence between stress and monoamines in animal models of depression comes from studies on the hippocampal response to stress and antidepressants. Chronic stress results in decreased transcription of the brain-derived neurotrophic factor (BDNF), whereas chronic treatment with monoamine modulators results in increased BDNF transcription. Both chronic stress and chronic treatment with monoamine modulators have opposing effects on two types of hippocampal plasticity. Structural plasticity of the hippocampus involves reorganization of synapses and changes in dendritic arborization in CA3 and CA1 (McEwen 2001). Cellular plasticity involves neurogenesis in the subgranular zone of the dentate gyrus. Exposure of animals to numerous experimental paradigms of mental stress decreases hippocampal neurogenesis. Physical restraint (Pham et al 2003), immobilization (Vollmayr et al 2003), and foot shock (Malberg and Duman 2003; Vollmayr et al 2003) all decrease neurogenesis in rats. Exposure to paradigms of social subordination results in a decrease in neurogenesis in marmosets (Gould et al 1998), tree shrews (Czeh et al 2001; Gould et al 1997; van der Hart et al 2002), and rats (Czeh et al 2002). Chronic exposure of rats to inescapable shock decreases cellular proliferation in the dentate gyrus and results in prolonged time before the animals escape when an escape route is provided (increased escape latency; Malberg and Duman 2003). In the same study, chronic treatment with the serotonin reuptake inhibitor (SRI) fluoxetine reversed the stress-induced changes in both hippocampal neurogenesis and behavioral phenotypes. Reversal of the chronic stress-induced decrease in neurogenesis has also been noted with the monoamine reuptake inhibitor moclobemide (Li et al 2004) and the serotonin reuptake hancer tianeptine (Czeh et al 2001). Chronic treatment with tianeptine also reversed the stress-induced decrease in hippocampal volume, dendritic arborization, and BDNF release (Czeh et al 2001; Magarinos et al 1999). It is not clear why treatment with the serotonin reuptake enhancer results in similar hippocampal changes as treatment with serotonin reuptake inhibitors. One possibility is that compensatory molecular changes in response to chronic treatment may be similar after either chronic inhibition or enhancement of serotonin transport. Another possibility is that the drugs modulate hippocampal plasticity by a serotonin-independent mechanism. To this end regulation of glutamatergic transmission by tianeptine has been described (Kole et al 2002). Thus, antidepressants modulate synaptic monoamines, increase both cellular and structural plasticity within the hippocampus, and reverse the effects of stress.
One study showed that while treatment with two SRIs reversed the effect of stress on cellular proliferation, there was no reversal of stress-induced changes in dendritic morphology (Magarinos et al 1999). The last result is the only report of a pharmacologic dissociation between the effect of chronic stress on hippocampal neurogenesis and dendritic arborization. It is still not clear whether the ability of antidepressants to modify dendritic complexity is important for the behavioral response. The ability of antidepressants to increase neurogenesis appears necessary for the behavioral response in some behavioral paradigms (Jiang et al 2005; Santarelli et al 2003).
Exposure of tree shrews to chronic stress also depresses hippocampal expression of genes important for proliferation and plasticity, whereas treatment with the tricyclic antidepressant clomipramine reverses stress-induced changes in gene expression (Alfonso et al 2004). These studies suggest an intriguing possibility that neurogenesis plays a central role in the interaction of stress and antidepressants in modulating hippocampal function. Taken together, the morphological, behavioral, and gene expression changes induced by experimental paradigms of stress, and reversal of these changes by treatment with antidepressants, support the possibility that stress and monoamines have converging mechanisms of action in the hippocampus (Figure 1). Accordingly, much emphasis has been placed on identifying the mechanisms by which stress and monoamines affect hippocampal neurogenesis.
Figure 1.
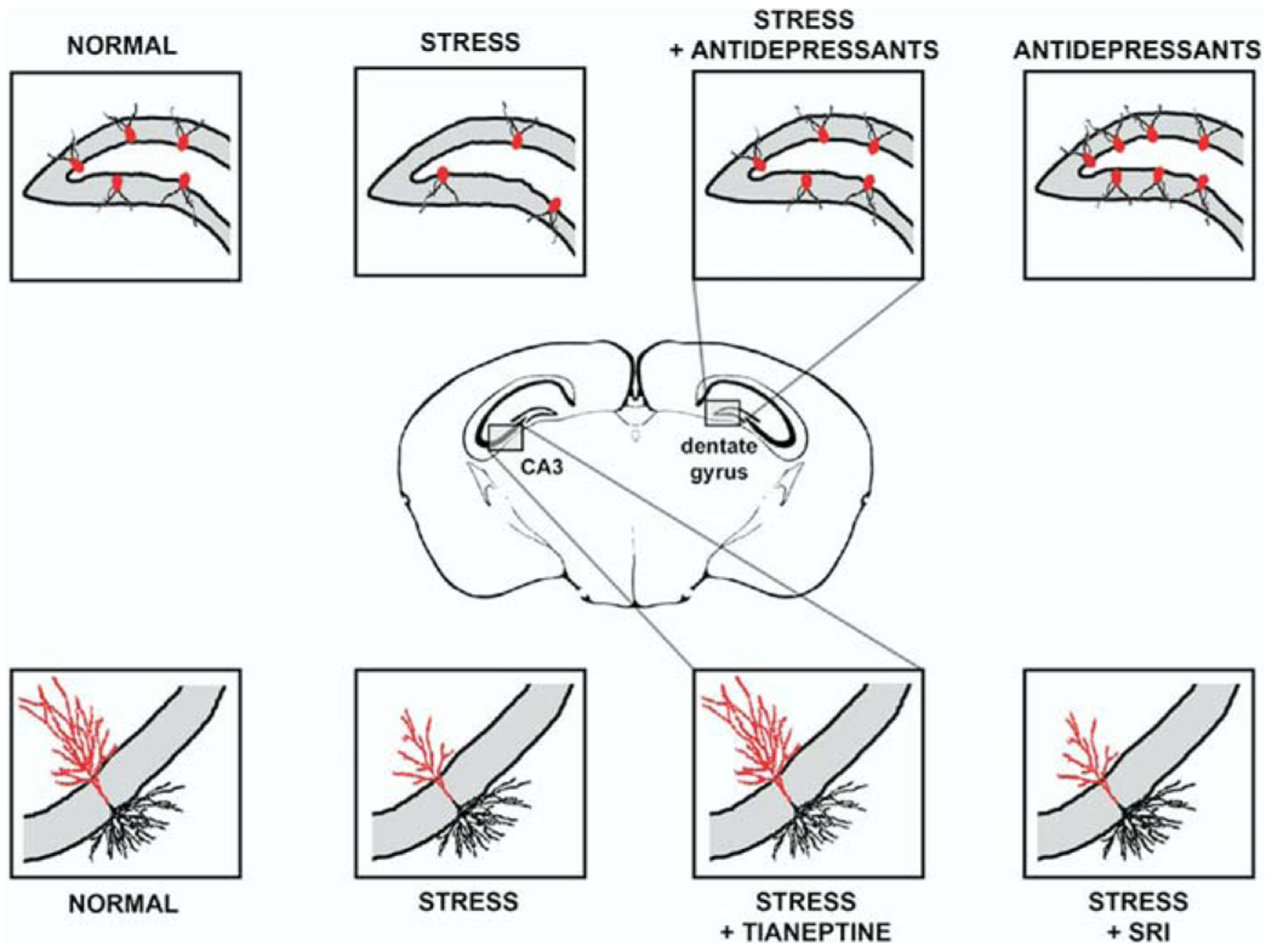
Hippocampal neurogenesis, stress, and antidepressant treatment: schematic representation of a coronal section through a mouse brain. The expanded views in the panels illustrate the dentate gyrus of the hippocampus with the cell bodies of young neurons (red) in the subgranular layer and dendritic projections traversing the granule cell layer. Baseline neurogenesis is enhanced by antidepressants, reduced by stress and the stress-induced reduction is reversed with antidepressant treatment. The expanded views in the bottom panels show a pyramidal neuron in CA3 with basal and apical (red) dendrites extending outside the pyramidal cell layer (gray). Stress reduces the baseline arborization of apical dendrites. Tianeptine, but not serotonin reuptake inhibitors, reverses the effect of stress on dendritic arborization.
Stress Hormones and Monoamines Regulate Adult Neurogenesis
Recent evidence indicates that psychosocial stress decreases neurogenesis via activation of the HPA axis and stimulation of the glucocorticoid receptor (GR). Salivary levels of cortisol are increased rapidly after exposure to psychosocial stress in humans (Kirschbaum et al 1996). Because both glucocorticoids (GC) and mineralocorticoids (MC) are produced by the adrenal glands, experimental adrenalectomy has been used extensively to study the importance of GC and MC in hippocampal response to stress in rodents. Older studies demonstrated that adrenalectomy during the early postnatal period results in increased cellular proliferation in the brain (Yehuda et al 1989). More recent work revealed that treatment with corticosterone decreases proliferation of neuronal progenitors (Cameron and Gould 1994). Stimulation of GR is sufficient to achieve this effect in the hippocampus (Kim et al 2004). An elegant analysis of how adrenal hormones regulate neurogenesis revealed that in addition to proliferation, the hormones also regulate survival (Wong and Herbert 2004) and cell fate (Wong and Herbert 2006). Similar results were obtained by pharmacologic stimulation of either GR or MR (mineralocorticoid receptor), suggesting that adrenal hormones exert their effect through either receptor (Wong and Herbert 2005). Moreover, the ability of glucocorticoids to block neurogenesis appears to be dependent on stimulation of the N-methyl-D-aspartate (NMDA) receptor (Cameron et al 1998; Magarinos and McEwen 1995; reviewed in Nacher and McEwen 2006). Thus, stress can regulate neurogenesis at multiple stages and GR, MR, and NMDA are likely to mediate this effect. It is not yet clear whether GC act directly on neuronal progenitors or indirectly through stimulation of mature cells. Both mature neurons as well as neuronal progenitors express GR while MR is present only on mature cells (Cameron et al 1993; Garcia et al 2004). Regulation of progenitor proliferation is activity-dependent (Deisseroth et al 2004) and can result from stimulating mature cell inputs into the SGZ. Interestingly, immature neurons, which have unique physiological properties, do not express receptors for adrenal hormones (Cameron et al 1993; Garcia et al 2004). Thus, the effects of stress on neurogenesis are mediated by GC, which can act directly on neuronal progenitors or mature neurons to inhibit neurogenesis but can only indirectly affect the function of immature neurons. The role of MC in neurogenesis must be indirect.
Serotonin and norepinephrine both have positive roles in regulating hippocampal neurogenesis. To date, chronic treatment with SRIs, tricyclic antidepressants (TCAs), monoamine oxidase inhibitors, and electroconvulsive therapy (ECT) have all been shown to increase SGZ neurogenesis (reviewed in Duman 2004). Several lines of evidence support a role for serotonin and norepinephrine in regulating SGZ neurogenesis. Partial lesions of the dorsal and median raphe, obliterates serotonergic neurons that innervate the dentate gyrus and results in a reduction in SGZ neurogenesis (Brezun and Daszuta 1999). Restoring innervation by recuperation or by directly implanting serotonergic neurons into the dentate gyrus reverses the decline caused by raphe lesions (Brezun and Daszuta 2000a, 2000b). Similarly, selective lesions to the locus ceruleus damage the noradrenergic inputs into the dentate gyrus and also result in a decrease in the proliferation of SGZ neuronal precursors (Kulkarni et al 2002). The impact of noradrenergic and serotonergic lesions on SGZ neurogenesis are quantitatively similar, suggesting a comparable role for both. Furthermore, unlike SRIs, tricyclic inhibitors of norepinephrine reuptake (TCAs) continue to increase neurogenesis in 5-HT1A receptor knock out mice (Santarelli et al 2003). This suggests that although both serotonin and norepinephrine comparably regulate neurogenesis, they may be doing so by different mechanisms.
Neurogenesis also occurs in the subventricular zone, but SRIs and TCAs have no effect on SVZ neurogenesis (Malberg et al 2000; Santarelli et al 2003); however, there appears to be a role for dopamine in regulating SVZ neurogenesis (Hoglinger et al 2004; Kippin et al 2005). Antipsychotic medications that have no affinity for serotonin receptors and no clinical antidepressant efficacy affect subventricular, but not SGZ, neurogenesis (Kippin et al 2005). Antipsychotic medications that have affinity for serotonin receptors and clinical efficacy for treating depression also increase cellular proliferation in the SGZ (Kodama et al 2004). Regulation of subventricular neurogenesis by dopamine and potential specificity of dopamine D2 receptor blockers for the subventricular and not subgranular neurogenesis may prove important in understanding the pathophysiology of schizophrenia and Parkinson’s disease and is beyond the scope of this review. It is important to note here, however, that serotonin- and norepinephrine-modulating antidepressants appear to regulate neurogenesis specifically in the SGZ, whereas dopamine-specific antipsychotics may have SVZ-specific effects. Similarly, exercise and enriched environment have antidepressant-like activity in animals and stimulate neurogenesis in the hippocampus without affecting SVZ neurogenesis (Brown et al 2003).
Although antidepressants affect hippocampal neurogenesis and dopamine specific antipsychotic medications affect the SVZ, the role of dopamine and serotonin receptors in regulating neurogenesis seems to be more anatomically promiscuous. Detailed pharmacologic analyses of the role of serotonin receptors in regulating SGZ neurogenesis have been informative to this end. The 5-HT1A receptors are necessary for SRI-induced increase in neurogenesis (Santarelli et al 2003). Consistently, 5-HT1A agonists stimulate neurogenesis in the SGZ (Banasr et al 2004; Santarelli et al 2003) and SVZ (Banasr et al 2004), whereas 5-HT1A antagonists decrease SGZ neurogenesis (Radley and Jacobs 2002). It is not yet clear whether 5-HT1A is directly involved in proliferation of progenitor cells, but transfecting fibroblasts with the gene encoding the receptor was sufficient to increase mitotic index (Varrault et al 1992). The presence of the 5-HT1A receptors on neuronal progenitors remains to be demonstrated. Thus, 5-HT1A receptors appear to have a positive regulatory role in hippocampal and subventricular neurogenesis and may directly increase cell division. The role of 5-HT2 receptors in regulating neurogenesis is less clear. One study provides evidence for a negative regulatory role of 5-HT2C specifically in neurogenesis in the ventral hippocampus of rats (Banasr et al, in press). The last finding is consistent with segregation of hippocampal function along its dorsoventral axis (Bannerman et al 2004), and the observation that stimulation of ventral, but not dorsal 5-HT2C is anxiogenic in rats (Alves et al 2004). Inhibition of 5-HT2C in humans has shown promise as an antidepressant strategy. These data suggest that serotonin can regulate neurogenesis in different ways through its numerous receptors in the SGZ and SVZ; however, compounds with demonstrated antidepressant efficacy appear to increase only hippocampal neurogenesis.
The experiments discussed here provide strong support for a regulatory role of hippocampal neurogenesis by monoamines. Together these data are compelling, yet they remain only correlative with the ability of antidepressants to modulate behavior. One recent experiment suggests that SGZ neurogenesis is necessary for monoamine modulators to affect behavior. Santarelli and colleagues demonstrated that disrupting neurogenesis by x-ray irradiation abrogated the effect of chronic antidepressant treatment in stress- and conflict-based experimental paradigms (Santarelli et al 2003). In the same set of experiments, mice devoid of 5-HT1A receptors failed to increase neurogenesis or demonstrate behavioral responses to SRIs while responding normally to TCAs. Disruption of SGZ, but not SVZ neurogenesis resulted in the loss of response to both types of medications. Moreover, similar results were recently obtained for the ability of stimulators of the cannabinoid system to modulate behavior in rats (Jiang et al 2005). Activators of the CB1 cannabinoid receptors increased neurogenesis and decreased anxiety-like and helpless-like behavior in two paradigms. The drugs failed to produce a neurogenic or behavioral response when hippocampal neurogenesis was ablated by irradiation. Together these experiments demonstrate that in some animals, SGZ neurogenesis is necessary for antidepressant action in certain stress- and conflict-based models of depression and anxiety; whether stimulation of SGZ neurogenesis is sufficient for eliciting a behavioral effect remains to be determined.
Together, the animal studies indicate that
neurogenesis in the SGZ and SVZ is differentially responsive to treatment with antidepressant and antipsychotic medications,
SGZ neurogenesis is highly sensitive to stress and treatment with antidepressants and enriched environment,
antidepressant treatment reverses the effects of stress on neurogenesis and behavior, and
neurogenesis is necessary for antidepressants to exert their behavioral effect in novelty-suppressed feeding and chronic unpredictable stress.
The potential therapeutic value of regulating hippocampal neurogenesis remains to be determined. Currently chronic inhibition of the serotonin transporter seems to yield reliable increase in neurogenesis, behavioral improvement in animal models of depression and anxiety and anxiolytic and antidepressant efficacy.
Animal Models of Depression
All of the experimental data on the role of neurogenesis in stress and antidepressant treatment come from analysis of brains from animals exposed to behavioral paradigms designed to model psychosocial stress and antidepressant response. Of course the subjective feeling of depressed mood is not possible to model in rodents. Therefore, different animal models of depression rely on reproducing either some aspect of behavioral manifestations of the depressive syndrome or a behavioral response to antidepressants. As previously mentioned, the therapeutic response to antidepressant treatment suffers from considerable latency. Cellular and structural plasticity are thought to be the biological underpinnings of this therapeutic latency; however, antidepressants cause rapid changes in synaptic serotonin and norepinephrine (David et al 2003). Consistently, patients report onset of nausea, jitteriness, heightened anxiety, and sexual dysfunction acutely after initiating antidepressant medications (Figure 2). Changes in synaptic serotonin and norepinephrine may underlie the acute changes experienced by patients. Similarly, some animal models reproduce an acute response to antidepressant treatment, and others demonstrate a chronic response. Because changes in synaptic monoamines precede plastic adaptations in response to antidepressant treatment, response to acute treatment in animal models may potentially serve as an indicator for antidepressant efficacy without modeling plastic adaptations. Unfortunately, evidence of acute antidepressant response predicting therapeutic efficacy in patients is lacking. Animal models characterized by acute response thus may fall short in recapitulating the pathophysiology of the psychiatric syndrome (construct validity) despite their excellent pharmacologic (predictive) validity. Therefore, although such models may be useful in developing drugs that work by modulating monoamines, they have not been useful in identifying truly novel cellular and molecular pathways for therapeutic intervention (Nestler et al 2002).
Figure 2.
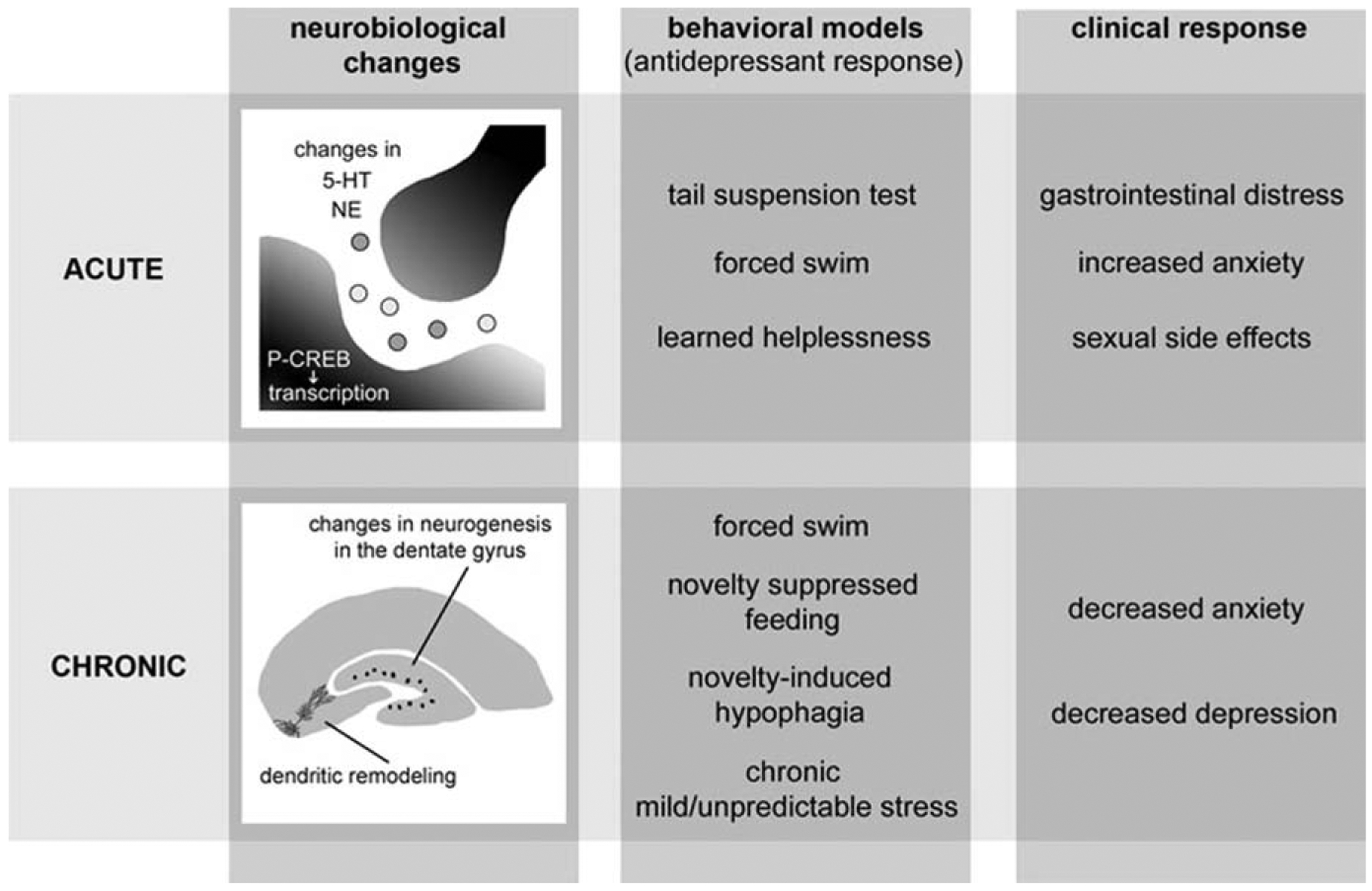
Acute and chronic responses to antidepressant treatment: treatment with monoamine modulators has acute effects on hippocampal neurochemistry, behavioral responses in animal models, and clinical responses. Chronic treatment results in hippocampal plasticity with increased neurogenesis and separate effects in animal models and clinical use. See text for references.
Two rodent models that have established predictive validity and acute antidepressant response are the tail suspension test and the forced swim test (Lucki 2001). Of these two tests, the forced swim test was demonstrated to have a specifically chronic response to antidepressant treatment with lack of response after an intermediate “subchronic” period (Dulawa et al 2004). Chronic treatment of rats by running (Bjornebekk et al 2005) or antidepressants (Drigues et al 2003) increased neurogenesis and improved performance on the forced swim test. In the former study, a strain that did not upregulate neurogenesis in response to running also failed to improve its forced swim test performance (Bjornebekk et al 2005). Dulawa and colleagues also observed behavioral response to chronic treatment only in one out of the four strains of mice tested (Dulawa et al 2004). The affected strain is widely considered to exhibit baseline behavior consistent with high trait anxiety. Within the animal strains that respond behaviorally to pharmacologic treatment on the forced swim test, neurogenesis was necessary in one rat strain (Jiang et al 2005) and not necessary in one mouse strain (Holick, personal communication). Strain differences in response to chronic anti-depressant treatment are consistent with the selective treatment response by depressed and anxious individuals. Chronic treatment in animals temporally parallels the clinical antidepressant response, emphasizes importance of an appropriate experimental treatment course in a responsive strain, and highlights the potential relevance of plasticity to depression and antidepressant treatment.
Approach–avoidance tests such as open field, novelty-suppressed feeding, novelty-induced hypophagia, and elevated plus maze all exploit the animal’s conflict to explore and feed with its innate fear of exposure to open spaces and predators. These tests are reviewed in detail elsewhere (Dulawa and Hen 2005; Gordon and Hen 2004). Out of these conflict-based tests, open field, novelty-induced hypophagia, and novelty-suppressed feeding were all shown to have a response specific to chronic antidepressant treatment (Bodnoff et al 1988; Dulawa et al 2004; Merali et al 2003; Santarelli et al 2003). The novelty-suppressed feeding study demonstrated no acute effect. The requirement of neurogenesis for the behavioral effect of chronic antidepressant treatment was strain-specific in novelty-suppressed feeding and present in one strain of mice (Santarelli et al 2003) and one strain of rats (Jiang et al 2005). Antidepressant response was not dependent on neurogenesis in novelty-induced hypophagia in a different mouse strain (Holick, personal communication). This strain–species difference is consistent with the results from the forced swim test reviewed earlier. The data also illustrate that the behavioral effects of drug depend on neurogenesis in some but not other drug-responsive strains. This mechanistic difference highlights the adaptive plasticity that enables the brain to recruit different cellular processes toward directing the same behavior in the context of genetic variability.
Although demonstrating response to chronic treatment, approach–avoidance tests have better face validity for anxiety disorders than depressive disorders. Accordingly, animals undergoing approach-avoidance tests respond acutely to purely anxiolytic benzodiazepines, which act as agonists at the GABA receptors (reviewed in Dulawa and Hen 2005; Gordon and Hen 2004). In one study, the behavioral effects seen in novelty-suppressed feeding after chronic treatment with antidepressants were not reversible by acute treatment with a GABA antagonist (Bodnoff et al 1988). This finding adds further support to the notion that acute neurochemical changes and chronic plastic adaptations affect behavior by distinct mechanisms. The anxiety-like face validity of the conflict-based paradigms underscores the relationship of anxiety and depressive disorders in humans. There are few nonanxiolytic antidepressants, and these two types of disorders share a common genetic burden and a high comorbidity (Kendler et al 1992; Roy et al 1995). Nevertheless, the disorders are distinct entities (American Psychiatric Association, 2000) and the conflict-based paradigms may best serve to model the subset of the disorders that are highly comorbid or be specific for anxiety disorders.
Social stress paradigms are numerous and have been used extensively to study the effects of stress on the brain. As discussed earlier, several stress paradigms were used to demonstrate stress-induced changes in behavior and plasticity and reverse some of these changes with antidepressant treatment. Overmier and colleagues noted that some animals after exposure to inescapable shock became reluctant to seek escape even when it became available (Overmier and Seligman 1967). The increased escape latency following inescapable shock exposure was termed “learned helplessness,” has excellent face validity, and has been used extensively to model depression. Early studies of learned helplessness demonstrated changes in the HPA axis consistent with ones seen in some MDD patients and response to acute treatment with antidepressants (reviewed in Cryan and Mombereau 2004). Unfortunately, results varied significantly between investigators, and reliability of the test was problematic (Porsolt 2000; Vollmayr and Henn 2001). Recent modifications in the learned helplessness paradigm may improve reliability, but pharmacologic predictive validity of the modified test remains to be assessed. Malberg and colleagues were able to block both decrease in cellular proliferation as well as increased escape latency by administering fluoxetine during a 9-day exposure to inescapable shock (Malberg and Duman 2003). Notably, rats that develop learned helplessness in response to inescapable shock do not differ in baseline neurogenesis from rats that do not (Vollmayr et al 2003). This is consistent with no decreases in baseline neurogenesis in 5-HT1A knockout mice (Santarelli et al 2003) and may indicate that mounting a neurogenic response, but not baseline cellular proliferation, is important for mood and anxiety disorders. Thus, learned helplessness has excellent face validity and in modified form may prove reliable, but predictive validity of the modified paradigm, and hence its utility for pharmacologic studies, need to be further determined. It is important to note that treatment studies discussed in this review all focus on brain’s ability to mount a neurogenic or neurogenotoxic response to interventions and not on baseline neurogenesis per se.
The results from the animal studies discussed here highlight the importance of using a model with an appropriate genetic background and adequate validity. Moreover, good predictive validity may not be informative in identifying novel mechanisms of pathophysiology. Modeling a response specific to chronic treatment may prove helpful to this end. Using multiple tests in a susceptible strain remains the most rigorous methodology in the absence of a gold standard model with construct validity.
Conclusions and Future Directions
This review focused on hippocampal plasticity following stress and reversal of these changes by treatment with modulators of monoamines. Clinical studies indicate that psychosocial stress management and monoamine regulation by pharmacologic agents are both important for improving depressive symptoms. Genetic studies suggest that stress and monoamines interact in depression (Caspi et al 2003) and its treatment (Binder et al 2004). Current pharmacologic treatments for major depression modulate brain monoamines, whereas psychotherapeutic and psychosocial interventions focus on stress reduction, coping techniques, and changing perceptions and attitudes toward events perceived as stressful. Both stress and monoamine modulation induce sustained hippocampal changes that are important for behavioral phenotypes in animal models of depression. In particular, neurogenesis in the hippocampus, but not the SVZ, seems to be highly responsive to both stress and antidepressant treatment. Moreover, at least one study suggests that hippocampal neurogenesis is necessary for antidepressants to change behavior.
Several important questions are raised by the observation that SGZ neurogenesis plays a role in depression and its treatment. First, the cellular targets for stress and antidepressant treatment must be identified. Although it is clear that both affect cellular proliferation, it is not clear whether stress and monoamine modulation act on the hippocampus to promote a neurogenic milieu or if they act on the proliferating cells directly. Evidence for differential expression of receptors for GC and MC on neurons at various stages of differentiation suggests that stress has both direct and indirect control over the birth of new neurons. Definitive studies are lacking, however. Still less is known about the distribution of receptors for serotonin and norepinephrine on the differentiating neurons of the SGZ. Perhaps highly selective genetic manipulation of receptors for stress hormones and monoamines will help to characterize the mechanisms by which these processes regulate neurogenesis.
It is also unclear how and at what stage of differentiation do the new neurons impact mood regulation and hippocampal function. All the studies discussed evaluate S-phase DNA incorporation of a nucleotide analog BrdU to assess neurogenesis. This approach has been productive in assessments of cellular proliferation and single cell lineage studies and has allowed investigators to speculate about neuronal survival. New techniques are needed to perform population-based studies of adult-born neurons, however. Cell population studies will be helpful in establishing which stage of neurogenesis underlies regulation of mood. Cellular proliferation has been linked to depression, but mitotic cells are not yet integrated into neuronal circuits. Differentiating neurons expressing “immature” markers have characteristic physiologic properties (reviewed in Doetsch and Hen 2005). A population of immature neurons could therefore be highly responsive to environmental inputs and determine how environment reinforces existing neuronal circuits by dictating which circuit recruits the new neurons. Some have estimated that immature neurons comprise as much as 5%–10% of the total cellular population of the dentate gyrus in rodents (Snyder et al 2001). Population studies will also help to determine how persistent neurogenesis and its response to environmental stimulation contributes to the cellular composition of the dentate gyrus over time. Although current evidence suggests that as much as 6% of the total dentate granule cell population are added each month in the rat brain (Cameron and McKay 2001), the dentate does not manifest corresponding growth. The net homeostatic maintenance of the granule cell population must therefore be accounted for by death of either young or old neurons. As much as 50%–80% of the new neurons are thought to die within the first month following division (Dayer et al 2003; Santarelli and Saxe, unpublished data), and the life span of adult cells is not clear. It is important to note that the studies on dynamics of neurogenesis reviewed here were all carried out in rodents. The amount and dynamics of neurogenesis in the hippocampi of primates remain an area of much controversy. If adult-born cells represent a cellular population in the hippocampi of primates, reinforcement of undesirable circuitry by the new neurons could then dictate depressive mood and symptoms. A clever approach using changes in atmospheric carbon 14 concentrations following atomic bomb testing in the 1950s was recently used to demonstrate that human cortical cells do not turn over with time (Spalding et al 2005), but the hippocampus was not examined in the study. Population studies on adult-born neurons in animals and humans would serve to delineate the contribution of immature cells to dentate function and determine how much of the dentate gyrus turns over with time and environmental exposure. The role of antidepressants and stress in these phenomena may shed further light on the pathophysiology of depression.
Acknowledgments
This work was supported by grants from NARSAD and NIMH. The authors thank Joshua Gordon, E. David Leonardo, Kerri Hollick, Kristin Klemenhagen, and Elizabeth LeQuesne for critical reading of the manuscript. Figures were designed by Kristin Klemenhagen. Figure 1 will also appear in Dranovsky and Hen’s chapter in Sibley et al, Handbook of Contemporary Neuropharmacology, New York: Wiley (ISBN 0471660531).
References
- Alfonso J, Pollevick GD, Van Der Hart MG, Flugge G, Fuchs E, Frasch AC (2004): Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. Eur J Neurosci 19: 659–666. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD (1965a): Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319– 335. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD (1965b): Post-natal origin of microneurones in the rat brain. Nature 207:953–956. [DOI] [PubMed] [Google Scholar]
- Alves SH, Pinheiro G, Motta V, Landeira-Fernandez J, Cruz AP (2004): Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behav Pharmacol 15:37–43. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual IV-Text Revision. Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Armando I, Tjurmina OA, Li Q, Murphy DL, Saavedra JM (2003): The serotonin transporter is required for stress-evoked increases in adrenal catecholamine synthesis and angiotensin II AT(2) receptor expression. Neuroen- docrinology 78:217–225. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A (2004): Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29:450– 460. [DOI] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A (in press): Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psych. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. (2004): Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28:273–283. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. (2004): Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet 36:1319–1325. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S (2005): The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol 8:357–368. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ (1988): The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 95:298–302. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A (1999): Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 89:999–1002. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A (2000a): Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus 10:37–46. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A (2000b): Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci 12:391–396. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG (2003): Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17: 2042–2046. [DOI] [PubMed] [Google Scholar]
- Burd GD, Nottebohm F (1985): Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J Comp Neurol 240:143–152. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E (1994): Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61:203–209. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD (2001): Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406–417. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E (1998): Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience 82:349–354. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E (1993): Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res 611: 342–346. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. (2003): Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C (2004): In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry 9:326–357. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. (2001): Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A 98:12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, et al. (2002): Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52:1057–1065. [DOI] [PubMed] [Google Scholar]
- David DJ, Bourin M, Jego G, Przybylski C, Jolliet P, Gardier AM (2003): Effects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: A microdialysis study in Swiss mice. Br J Pharmacol 140:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA (2003): Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol 460:563–572. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC (2004): Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42:535–552. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R (2005): Young and excitable: The function of new neurons in the adult mammalian brain. Curr Opin Neurobiol 15:121–128. [DOI] [PubMed] [Google Scholar]
- Drigues N, Poltyrev T, Bejar C, Weinstock M, Youdim MB (2003): cDNA gene expression profile of rat hippocampus after chronic treatment with antidepressant drugs. J Neural Transm 110:1413–1436. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R (2005): Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R (2004): Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharma- cology 29:1321–1330. [DOI] [PubMed] [Google Scholar]
- Duman RS (2004): Depression: A case of neuronal life and death? Biol Psychiatry 56:140–145. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998): Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G (2004): Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell 3:363–371. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F (1983): Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A 80:2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Hen R (2004): Genetic approaches to the study of anxiety. Annu Rev Neurosci 27:193–222. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997): Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998): Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A 95:3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CG (2000): Neurogenesis in the adult brain: Death of a dogma. Nat Rev Neurosci 1:67–73. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC (2004): Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735. [DOI] [PubMed] [Google Scholar]
- Holsboer F (2000): The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X (2005): Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest 115:3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS (1981): Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol 195:323–338. [DOI] [PubMed] [Google Scholar]
- Kaplan MS (1985): Formation and turnover of neurons in young and senescent animals: an electronmicroscopic and morphometric analysis. Ann N Y Acad Sci 457:173–192. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW (1977): Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 197:1092–1094. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B (2005): The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Arch Gen Psychiatry 62:529–535. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1992): Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry 49:716–722. [DOI] [PubMed] [Google Scholar]
- Kim JB, Ju JY, Kim JH, Kim TY, Yang BH, Lee YS, Son H (2004): Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res 1027:1–10. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D (2005): Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25:5815–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (1996): Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 58:1475–1483. [DOI] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS (2004): Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580. [DOI] [PubMed] [Google Scholar]
- Kole MH, Swan L, Fuchs E (2002): The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci 16:807–816. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA (2002): Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci 16:2008–2012. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. (1996): Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531. [DOI] [PubMed] [Google Scholar]
- Li YF, Zhang YZ, Liu YQ, Wang HL, Yuan L, Luo ZP (2004): Moclobemide up-regulates proliferation of hippocampal progenitor cells in chronically stressed mice. Acta Pharmacol Sin 25:1408–1412. [PubMed] [Google Scholar]
- Lucki I (2001): A prescription to resist proscriptions for murine models of depression. Psychopharmacology (Berl) 153:395–398. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. (2003): Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 100:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS (1999): Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 371:113–122. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS (1995): Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69:89–98. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS (2003): Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000): Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1999): Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2001): Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–277. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS (1968): Selective retention of corticosterone by limbic structures in rat brain. Nature 220:911–912. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H (2003): Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry 54:552– 565. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen BS (2006): The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002): Neurobiology of depression. Neuron 34:13–25. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME (1967): Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol 63:28–33. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN (1984): Neurons generated in the adult brain are recruited into functional circuits. Science 225:1046–1048. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS (2003): Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886. [DOI] [PubMed] [Google Scholar]
- Porsolt RD (2000): Animal models of depression: Utility for transgenic research. Rev Neurosci 11:53–58. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL (2002): 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res 955:264–267. [DOI] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pedersen NL, Mathe AA, Kendler KS (1995): A twin study of generalized anxiety disorder and major depression. Psychol Med 25: 1037–1049. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. (2003): Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999): Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19:5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM (2001): Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85:2423–2431. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J (2005): Retrospective birth dating of cells in humans. Cell 122:133–143. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Li Q, Murphy DL (2004): Life-long serotonin reuptake deficiency results in complex alterations in adrenomedullary responses to stress. Ann N Y Acad Sci 1018:99–104. [DOI] [PubMed] [Google Scholar]
- van der Hart MG, Czeh B, de Biurrun G, Michaelis T, Watanabe T, Natt O, et al. (2002): Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry 7:933– 941. [DOI] [PubMed] [Google Scholar]
- Varrault A, Bockaert J, Waeber C (1992): Activation of 5-HT1A receptors expressed in NIH–3T3 cells induces focus formation and potentiates EGF effect on DNA synthesis. Mol Biol Cell 3:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B (2004): Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- Vinet J, Carra S, Blom JM, Brunello N, Barden N, Tascedda F (2004): Chronic treatment with desipramine and fluoxetine modulate BDNF, CaMKKalpha and CaMKKbeta mRNA levels in the hippocampus of transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuropharmacology 47:1062–1069. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA (2001): Learned helplessness in the rat: Improvements in validity and reliability. Brain Res Brain Res Protoc 8:1–7. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F (2003): Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry 54:1035–1040. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J (2004): The corticoid environment: A determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci 20:2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EY, Herbert J (2005): Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur J Neurosci 22:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EY, Herbert J (2006): Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience 137:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Fairman KR, Meyer JS (1989): Enhanced brain cell proliferation following early adrenalectomy in rats. J Neurochem 53:241–248. [DOI] [PubMed] [Google Scholar]


