Abstract
Purpose
The clinical studies carried out in the last few decades unequivocally introduced activated androgen receptor (AR) as a pathogenic feature of human malignancies which not only endows cancer cells with survival advantage, but also may be exploited for anticancer interventions.
Patients and Methods
In this study, we have investigated the expression profile of AR and EMT-related genes in fresh gastric cancer (GC), adjacent nontumor and normal gastric tissues, as well as the effect and molecular mechanisms of AR inhibition in GC cell lines.
Results
Amongst 60 GC patients, 66.7% overexpressed AR that was remarkably correlated with the overexpression of Snail, β-catenin, Twist1, and STAT3. AR overexpression was also remarkably associated with unfavorable outcome (HR=3.478, P=0.001); however, multivariate Cox regression analysis indicated that it was not an independent prognostic factor (HR=2.089, P=0.056). This study has investigated simultaneous assessment of AR and EMT-related genes expression and indicated that concurrent overexpression of AR and Snail is an independent unfavorable factor for GC overall survival after adjustment with other variables (HR=2.382, P=0.021). Interestingly, the inhibition of AR signaling by potent AR antagonist enzalutamide suppressed cell growth, migration and invasion of GC cells via regulation of apoptosis-, cell cycle-, and EMT-related gene expressions.
Conclusion
Our findings have clinical importance proposing AR as an important prognostic factor involved in GC progression and metastasis, and submit AR inhibition as an appealing therapeutic approach for GC patients, either as a single agent or in a combined-modal strategy.
Keywords: androgen receptor, gastric cancer, epithelial-mesenchymal transition, EMT, prognosis, targeted therapy, enzalutamide
Introduction
Gastric cancer (GC) currently ranks as the fourth most common cancer and the second most common cause of cancer-related deaths worldwide.1 In spite of endorsed and exponential efforts to improve diagnostic and therapeutic strategies, clinical studies have not yet translated into better prospects in GC and many patients are diagnosed at advanced stages with poor prognosis. Based on the fact that the median survival of patients receiving standard chemotherapies is less than 13 months,2 there is a crucial need for novel prognostic markers and more effective treatment strategies against advanced GC. Evidence indicated that the incidence of GC is remarkably higher in males over females with a ratio of 2:1,3 putting this tumor as well as hepatocellular carcinoma (HCC), bladder cancer and pancreatic cancers into a family of malignancies defined as male-predominant cancers.4,5 Given this, clarifying the factors which cause sex-related disparity of GC may result in unrevealing pivotal pathways involved in gastric carcinogenesis. Of great interest, the involvement of sex hormone receptors (ERα, ERβ, PgR and AR) in GC pathogenesis has been examined in a fair number of studies;1,6–11 however, the results are controversial and inconclusive.
Androgen receptor (AR), is a member of the evolutionarily conserved nuclear receptor superfamily, which acts as a transcription factor and regulates the expression of several genes in both androgen-dependent and independent manners.12–14 Mounting studies have demonstrated that AR, rather than androgen, functions as an oncoprotein by modulating proliferation and metastasis especially in male-predominant tumors.12–16 Several previous studies on HCC revealed that AR-positive patients had a higher rate of tumor recurrence with lower overall survival (OS) when compared to AR-negative patients.17,18 Moreover, Okitsu et al showed that phosphorylation of STAT3 and MAPK induced by IL6 leads to the activation of AR in pancreatic cancer cells, which in turn promotes cancer cell migration.19 Although there is limited evidence asserting the prognostic significance of AR in GC,1,8,10,11,20,21 these results are conflicting. While a previous report indicated that AR expression is associated with early TNM stage of GC,7 other studies reported AR as an unfavorable factor for GC outcome.1,8,11
During recent years efforts to suppress the AR pathway have led to the identification of several inhibitors whose anticancer effects were studied thoroughly in various types of tumors such as HCC, prostate cancer, pancreatic cancer, bladder cancer and triple-negative breast cancer.22–26 Enzalutamide (ENZ), also known as MDV-3100, is a novel FDA-approved AR antagonist which affects androgen signaling more effectively than other inhibitors.27 Kawahara et al showed that inhibition of AR using ENZ could effectively reduce AR-positive bladder cancer cell growth, migration, and invasion.22 They also assessed multiple anti-AR drugs in UMUC3 xenograft-bearing mice and demonstrated that only ENZ could significantly suppress the tumor growth. En masse, the present study aims at investigating the expression and prognostic role of AR in GC patients, along with the assessment of the plausible correlation between its expression profile and overall survival of the patients. Moreover, this study examines the molecular mechanisms underlying the effects of AR inhibition using ENZ, either as a single agent or in combination with 5-FU, to propose a possible complex network in which AR signaling pathway could promote progression and metastasis of GC.
Patients and Methods
Patients and Clinicopathological Data
During June 2016 to June 2017, 75 newly diagnosed GC patients who referred to Kasra, Madaen, or Imam Khomeini hospitals, Tehran, Iran and experienced gastrectomy were entered in the study. Notably, patients without sufficient clinicopathological data and patients who were lost to follow-up, suffered from double primary tumors or received radiotherapy and/or chemotherapy before surgery were not included in this study. Amongst all patients, 60 fresh tumor tissues and adjacent nontumor tissue samples were used for further investigations. In addition, 50 fresh normal gastric samples were obtained from cases who had undergone endoscopy procedure at the Digestive Oncology Research Center, Digestive Diseases Research Institute, Shariati hospital, Tehran, Iran. For reliable gene expression analysis, all fresh samples were stabilized in RNA later solution (RNAlater RNA Stabilization Reagent, QIAGEN, Germany) within 15 min of excision. We regularly observed GC patients from the date of surgery until the end of our study period (May, 2020) or earlier in case of a patient’s death due to cancer. This period of time was defined as overall survival (OS). Informed consents were signed by all patients. The present study committed to the principles of the Declaration of Helsinki 1964 as well as the Hematology, Oncology and Stem Cell Transplantation Research Institute, Shariati hospital, and approved by the Clinical Research Ethics Committee of Tehran University of Medical School with the approval code: ir.TUMS.horcsct.rec.1394.103.10.
Human Gastric Cancer Cell Lines
Three human GC cell lines (KATO III, AGS, and MKN45), and one prostate cancer cell line (LNCaP) were obtained from the National Cell Bank of Iran (NCBI; Tehran, Iran). CRL-5822 (NCI-N87), a human GC cell line was a generous gift from Avicenna Research Institute, ACECR, Tehran, Iran. KATO III, MKN45, and CRL-5822 were obtained from metastatic sites; in contrast, AGS is an adenocarcinoma cell line from the stomach. All the cell lines received from NCBI and also the gifted cells (CRL-5822) were authenticated by STR profiling (Cell ID™ system, Promega) and were routinely checked for mycoplasma infection using PCR and direct culture methods. GC cell lines were cultured according to ATCC recommendations and maintained at 37°C under humidified atmosphere with 5% CO2.
Chemicals and Antibodies
Enzalutamide (MDV3100) and dihydrotestosterone (DHT) were purchased from Selleckchem (Houston, TX, USA) and were dissolved in DMSO. In all treatments, final concentrations of DMSO did not exceed 0.1% (v/v). Monoclonal anti-caspase-3 and β-actin were obtained from Abcam, Mediqip; USA and Santa Cruz Biotechnology, respectively.
Total RNA Preparation
RiboEx reagent (GeneAll Biotechnology Co., South Korea) was used to extract total RNA from cell line lysates or the RNAlater-stabilized tissues. PrimeScriptTM RT reagent Kit (Takara, Japan) and an ABI Veriti Thermocycler (Applied Biosystems) were applied to synthesize complementary DNAs for 15 min at 37°C, and five seconds at 85°C.
Reverse Transcription (RT) PCR
Complementary DNAs (cDNAs) were amplified using specific primers. B2M (beta-2-microglobulin) was used as a control gene. RT-PCR was performed using Taq DNA polymerase master mix red (Ampliqon, Copenhagen, Denmark) with ABI Veriti Thermocycler (Applied Biosystems). One percent agarose gel electrophoresis was applied to visualize the PCR products.
Real-time Quantitative RT-PCR
The quantitative RT-PCR (qRT-PCR) analysis was performed by LightCycler 96 instrument (Roche Molecular Diagnostics) using SYBRGreen RealQ-PCR Master Mix kit (Ampliqon, Copenhagen, Denmark) as instructed by the manufacturer. Water was used as negative control in the PCR reaction. Although three different housekeeping genes (B2M, HPRT, and GAPDH) were utilized for normalization, B2M proved to be the most constant among the assessed genes with no variation between tissues. Therefore, ΔCt values were calculated to quantify mRNA expression levels by comparing it with the mean Ct values of B2M. The formula 2−(ΔΔCT) was used for calculations as described earlier.28 The sequences of all primers are listed in Supplemental Table 1.
Cytotoxicity Assays
KATO III, MKN45, CRL-5822, AGS and LNCaP cells in logarithmic growth phase were plated at a density of 2500 cells per well. Cell proliferation was assessed by MTT assay as previously described.29 IC50 values were calculated from full dose–response curves to show cytotoxicity. Moreover, to determine the synergism of combinational treatment, MTT assay was applied after 72 h of treatment with ENZ plus fluorouracil (5-FU) or cisplatin in GC cells at the indicated concentrations. Combination index (CIx) and dose reduction index (DRI) were computed using Chou–Talalay method by CalcuSyn software (Biosoft, Cambridge, UK). CIx <1, CIx=1, and CIx >1 represent synergistic effects, additive effects, and antagonism of drugs, respectively.30
Crystal Violet Staining
Crystal violet staining assays were carried out as described earlier.31
Wound Healing Assays
GC cells were cultured in six-well plates to reach about 100% confluency. After 24 h, the cell monolayer was scratched with a pipette tip. After washing out the cells with PBS, they were treated with different concentrations of ENZ. Migration of the cells was assessed 48 h posttreatment under an inverted phase contrast microscope (Olympus).
Zymography
Briefly, equal amounts of the supernatants from the media of ENZ-treated and vehicle-treated cells were run on polyacrylamide gels copolymerized with gelatin A or B (Sigma). Gels were then rinsed in Triton X-100 to remove SDS, followed by incubation in reactivation buffer at 37°C overnight. Next, the gels were stained with Coomassie Brilliant Blue. Clear bands over the blue background showed the areas of enzymatic activity.31
Cell Cycle Analysis
The DNA contents of different steps of cell cycle were assessed with flow cytometry using propidium iodide (PI; 50 μg/mL; Sigma) staining as explained previously.29 The samples were analyzed with a FACSCalibur (BD Bioscience) flow cytometer equipped with CellQuest Pro software.
Flow Cytometric Analysis of Apoptosis
To evaluate the apoptotic effect of ENZ on GC cells, PI and FITC-conjugated Annexin V Staining Kit (Roche Applied Science, Germany) was used for detection by flow cytometry. The results were analyzed using a Partec PAS III flow cytometer and TM FloMax software (Partec).
Western Blot Analysis
GC Cell lysates were assayed for protein concentration using the BCA Protein Assay Reagent kit (Thermo Scientific, USA). Proteins were resolved on SDS-PAGE and transferred to a PVDF membrane (Roche, Germany). After blocking the nonspecific binding sites with 5% skim milk, the membrane was blotted with the human reactive monoclonal anti-caspase-3 (Abcam, USA) followed by incubation with the horseradish peroxidase conjugated secondary antibody (1:1000). Bound antibodies were detected with the ECL Western Blotting Detection System (GE Healthcare, UK). Hybridization with the β-actin was used as the loading control.
Statistical Analysis
Independent samples Mann–Whitney U-test was used to compare the difference in expression of AR and EMT-related genes between gastric tumors and adjacent uninvolved tissues or normal tissues. Correlations between the genes were assessed by the Spearman rank test. Chi-squared or Fisher’s exact tests were applied to investigate the associations between expression of AR and EMT-related genes and clinicopathological data. The expectation of survival was estimated by Kaplan–Meier (log rank test) method. For identification of the prognostic value of AR expression and other variables, univariate and multivariate Cox proportional hazards model was performed. All significant variables in the univariate analysis as well as clinical characteristics considered correlated with prognosis including age and tumor grade were entered to multivariate analysis. Stepwise backward elimination as a reduced model was performed until only significant factors remained in multivariate analysis. Two-tailed Student’s t-test was used to analyze the data of functional experiments. The presented results are the means ±SD of three independent experiments. The IBM SPSS® statistics 22 software and GraphPad Prism Software 8 were employed to perform statistical analysis. Two-tailed P<0.05 was considered statistically significant.
Results
Clinicopathological Characteristics
To assess the expression level of AR and to explore its plausible contributory role in GC, both the fresh tumor and the adjacent nontumor tissues of 60 GC patients were included in the study. Clinicopathological characteristics of patients with GC, including sex, age, tumor size, tumor grade, tumor type, Lauren’s classification, lymphovascular and nerve invasion, tumor shape, tumor location, TNM classification and disease stages, were enrolled as listed in Table 1. Fifty normal cases (25 female, 25 male) with median age of 51 ranging from 19 to 83 were also included for comparison. Among GC patients, 66.7% (40/60) overexpressed AR relative to normal samples. Possible relationship between clinicopathological characteristics and AR expression was analyzed and notably, significant statistical correlation was detected between AR overexpression and lymphovascular invasion, T classification, N classification, and M classification. Importantly, overexpression of AR was more frequently found in patients with advanced TNM stages (P=0.008). When age and gender is concerned, no remarkable association was found.
Table 1.
Association Between AR Expression and Clinicopathological Characteristics of Patients with Gastric Cancer
| Clinical Variables | Total Patients: N (%) | Evaluable Patients: N (%) | ||
|---|---|---|---|---|
| 60 (100) | Overexpressed N (%) | Underexpressed N (%) | P | |
| Age (years) median, range | 63, 33–83 | |||
| N <63 | 29 (48.3) | 18 (30) | 11 (18.3) | 0.465 |
| N ≥63 | 31 (51.7) | 22 (36.7) | 9 (15) | |
| Sex | ||||
| Male | 39 (65) | 26 (43.3) | 13 (21.7) | 1.000 |
| Female | 21 (35) | 14 (23.3) | 7 (11.7) | |
| Tumor size (cm) | ||||
| N <5 | 17 (28.3) | 9 (15) | 8 (13.3) | 0.156 |
| N ≥ 5 | 43 (71.7) | 31 (51.7) | 12 (20) | |
| Lauren’s classification | ||||
| Intestinal | 55 (91.7) | 36 (60) | 19 (31.7) | 0.656 |
| Diffuse | 5 (8.3) | 4 (6.7) | 1 (1.7) | |
| Tumor grade | ||||
| Poorly differentiated | 33 (55) | 21 (35) | 12 (20) | |
| Moderately differentiated | 21 (35) | 15 (25) | 6 (10) | 0.919 |
| Well differentiated | 6 (10) | 4 (6.7) | 2 (3.3) | |
| Tumor type | ||||
| Adenocarcinoma | 46 (76.7) | 29 (48.3) | 17 (28.3) | 0.347 |
| Signet ring cell carcinoma | 14 (23.3) | 11 (18.3) | 3 (5) | |
| Lymphovascular invasion | ||||
| Yes | 43 (71.7) | 33 (55) | 10 (16.7) | 0.008* |
| No | 17 (28.3) | 7 (11.7) | 10 (16.7) | |
| Perineural invasion | ||||
| Yes | 50 (83.3) | 33 (55) | 17 (28.3) | 1.000 |
| No | 10 (16.7) | 7 (11.7) | 3 (5) | |
| Tumor shape | ||||
| Ulcerated flat | 48 (80) | 31 (51.7) | 17 (28.3) | |
| Linitis plastica | 5 (8.3) | 4 (6.7) | 1 (1.7) | 0.887 |
| Polypoid | 7 (11.7) | 5 (8.3) | 2 (3.3) | |
| Tumor location | ||||
| Proximal | 28 (46.7) | 17 (28.3) | 11 (18.3) | |
| Middle | 22 (36.7) | 15 (25) | 7 (11.7) | 0.589 |
| Distal | 6 (10) | 4 (6.7) | 2 (3.3) | |
| Diffuse | 4 (6.7) | 4 (6.7) | 0 (0) | |
| T classificationa | ||||
| pT1 | 0 (0) | 0 (0) | 0 (0) | |
| pT2 | 13 (18.3) | 7 (11.7) | 6 (10) | 0.001* |
| pT3 | 19 (31.7) | 8 (13.3) | 11 (18.3) | |
| pT4 | 28 (46.7) | 25 (41.7) | 3 (5) | |
| N classificationa | ||||
| N0 | 19 (31.7) | 8 (13.3) | 11 (18.3) | |
| N1 | 11 (18.3) | 7 (11.7) | 4 (6.7) | 0.002* |
| N2 | 17 (28.3) | 15 (25) | 2 (3.3) | |
| N3 | 13 (21.7) | 10 (16.7) | 3 (5) | |
| M classificationa | ||||
| M0 | 42 (70) | 24 (40) | 18 (30) | 0.019* |
| M1 | 18 (30) | 16 (26.7) | 2 (3.3) | |
| TNM stagea | ||||
| I+II | 22 (36.7) | 10 (16.7) | 12 (20) | |
| III+IV | 38 (63.3) | 30 (50) | 8 (13.3) | 0.008* |
Notes: aThe 8th TNM Classification of Malignant Tumors proposed by the AJCC/UICC. *P<0.05 values are statistically significant.
Abbreviation: AR, androgen receptor.
Overexpression of AR is Correlated with the Expression of EMT-related Genes in Gastric Cancer
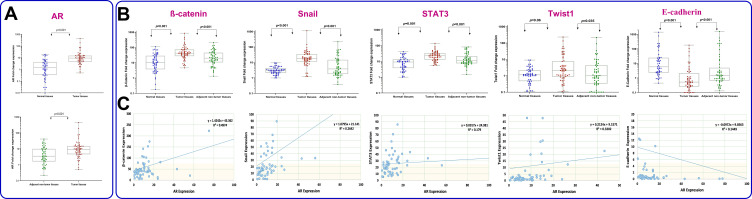
Quantitative real-time PCR was applied to detect the mRNA expression levels of AR, β-catenin, E-cadherin, Snail, Twist1 and STAT3 in gastric tumor, adjacent uninvolved tissues, and normal cases.
In the present study, one of the normal samples with the highest Ct value was considered as a calibrator for each specific gene. To calculate the fold change of gene expression all other samples were compared with the specific calibrator. Thereafter, we used ROC curve to determine the cutoff value of the genes. The values which were higher than cutoff point were defined as overexpression. As shown in Figure 1A and B, the relative mRNA expression values of AR, β-catenin, Snail, Twist1 and STAT3 in GC tissues were significantly higher than those in adjacent nontumor tissues (median expression of AR, 9.39 vs 3.44, P<0.001; β-catenin, 41.59 vs 19.91, P<0.000; Snail, 17.95 vs 3.81, P<0.000; Twist1, 2.1 vs 1.02, P<0.035; STAT3, 23.1 vs 11.3, P<0.001). In contrast, the expression of E-cadherin in GC tissues was significantly lower than adjacent nontumor tissues (0.52 vs 1.44, P<0.001). A similar pattern was also detected when tumor tissues were compared to normal cases (Figure 1); however, the relative mRNA expression difference of Twist1 was not statistically significant. Spearman rank test was used to assess correlation between mRNA expression of AR and EMT-related genes selected in this study (Figure 1C). While there was a strong positive correlation between AR and β-catenin (r=0.705, P=0.004), a negative correlation was detected between AR and E-cadherin (r=−0.385, P=0.003). Notably, correlation coefficients showed that AR expression moderately correlates with Snail, Twist1 and STAT3 (r=0.512, P<0.001; r=0.560, P<0.001 and r=0.424, P=0.001 respectively).
Figure 1.
Graphical box-plot expression profile of (A) AR, (B) β-catenin, Snail, STAT3, Twist1 and E-cadherin at transcriptome level in gastric tumor, adjacent nontumor (N=60) and normal tissues (N=50). Results are the mean of three independent experiments ±SD (P<0.05). (C) Correlation between AR and EMT-related gene expression analyzed by Spearman rank test.
AR, Snail, Twist1 and E-cadherin Expressions Correlate with an Unfavorable Outcome in GC Patients
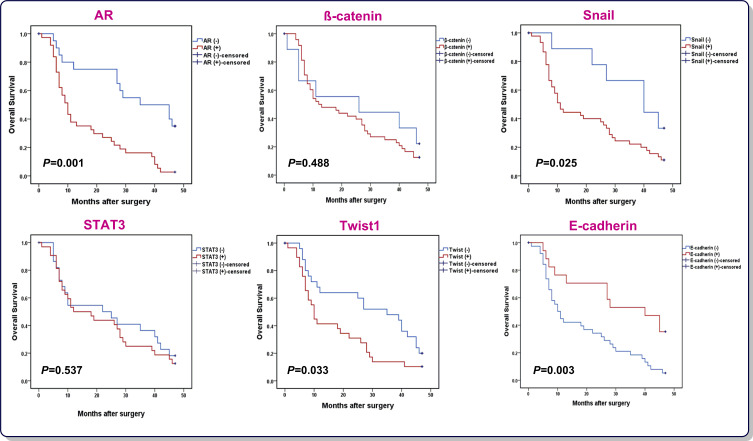
Having established that AR, β-catenin, Snail, Twist1, and STAT3 were upregulated in GC patients, it was tempting to investigate if there is any correlation between the expressions of the aforementioned genes and GC patients OS. All 60 patients were followed-up and then prognostic values of genes were analyzed using Kaplan–Meier method (Figure 2). The rate of OS for patients overexpressing AR was 10% (4/40) in comparison with 35% (7/20) for patients underexpressing AR (P=0.001). Moreover, while both the overexpression of Snail and Twist1 and the underexpression of E-cadherin were found to be unfavorable prognostic factors, other mentioned genes had no remarkable correlation with OS. The univariate and multivariate analysis data from Cox proportional hazards model is reported in Table 2. Regarding the univariate analysis, the tumor size, lymphovascular invasion, perineural invasion, T classification, N classification, M classification, TNM stage, AR expression, Snail expression, Twist1 expression and E-cadherin expression were found to be significant prognostic factors; however, only T, N, and M classifications maintained in the multivariate Cox regression model, indicating that the expression of AR, Snail, E-cadherinand Twist1 were not independent unfavorable factors for OS in GC patients. Of particular interest, Kaplan–Meier analysis indicated higher mortality rate among GC patients who simultaneously overexpressed AR and Snail (HR=3.236, 95%CI=1.688–6.205, P=0.001). Therefore, concurrent overexpression of AR and Snail genes, as a single variable, disclosed to be an independent unfavorable factor for OS adjusted for other variables using multivariate Cox regression model (HR=2.382, 95%CI=1.141–4.971, P=0.021).
Figure 2.
Kaplan–Meier curves of OS for GC patients (N=57) according to AR, β-catenin, Snail, STAT3, Twist1 and E-cadherin expression (log rank test).
Table 2.
Univariate and Multivariate Cox Regression Analysis of Overall Survival in Patients with Gastric Cancer
| Variables | Univariate Cox | Multivariate Cox | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Sex | ||||||
| Male | 1.000 | |||||
| Female | 0.785 | 0.388–1.591 | 0.636 | |||
| Age (years) median, range | ||||||
| n <63 | 1.000 | 1.000 | ||||
| n ≥63 | 1.364 | 0.693–2.133 | 0.346 | 1.000 | 0.249–1.967 | 0.499 |
| Tumor size (cm) | ||||||
| n <5 | 1.000 | 1.000 | ||||
| n ≥5 | 2.670 | 1.309–5.447 | 0.007* | 1.625 | 0.841–3.702 | 0.254 |
| Lauren’s classification | ||||||
| Intestinal | 1.000 | |||||
| Diffuse | 2.452 | 0.741–8.112 | 0. 142 | |||
| Tumor grade | ||||||
| Well differentiated | 1.000 | |||||
| Moderately differentiated | 1.269 | 0.272–5.150 | 0.822 | |||
| Poorly differentiated | 1.337 | 0.296–6.040 | 0.705 | |||
| Lymphovascular invasion | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 2.619 | 1.280–6.759 | 0.008* | 2.096 | 0.387–2.312 | 0.763 |
| Perineural invasion | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 2.540 | 1.006–6.460 | 0.050* | 1.346 | 0.214–3.345 | 0.866 |
| T classificationa | ||||||
| pT1 | ||||||
| pT2 | 1.000 | 1.000 | ||||
| pT3 | 2.523 | 1.000–6.480 | 0.050* | 3. 252 | 0.934–11.822 | 0.064 |
| pT4 | 4.955 | 1.971–12.374 | 0.001* | 3.724 | 1.155–12.005 | 0.028* |
| N classificationa | ||||||
| N0 | 1.000 | 1.000 | ||||
| N1 | 2.479 | 1.268–5.886 | 0.041* | 2.846 | 0.980–8.341 | 0.055 |
| N2 | 6.306 | 2.680–15.573 | 0.001* | 4.958 | 1.319–14.832 | 0.003* |
| N3 | 8.801 | 3.361–22.104 | 0.001* | 11.614 | 3.419–38.832 | 0.001* |
| M classificationa | ||||||
| M0 | 1.000 | 1.000 | ||||
| M1 | 3.353 | 1.709–6.471 | 0.002* | 3.947 | 1.733–9.389 | 0.001* |
| TNM stagea | ||||||
| I+II | 1.000 | |||||
| III+IV | 19.259 | 5.579–66.411 | 0.001* | |||
| AR | ||||||
| Underexpressed | 1.000 | 1.000 | ||||
| Overexpressed | 3.478 | 1.580–8.768 | 0.001* | 2.089 | 0.998–4.643 | 0.056 |
| β-catenin | ||||||
| Underexpressed | 1.000 | |||||
| Overexpressed | 1.321 | 0.437–3.564 | 0.499 | |||
| E-cadherin | ||||||
| Underexpressed | 1.000 | 1.000 | ||||
| Overexpressed | 0.372 | 0.139–0.982 | 0.005* | 0.665 | 0.280–1.158 | 0.355 |
| Snail | ||||||
| Underexpressed | 1.000 | 1.000 | ||||
| Overexpressed | 3.618 | 1.028–10.406 | 0.046* | 2.152 | 0.974–6.100 | 0.084 |
| Twist | ||||||
| Underexpressed | 1.000 | 1.000 | ||||
| Overexpressed | 1.865 | 1.005–4.629 | 0.040* | 1.001 | 0.310–1.444 | 0.279 |
| Stat3 | ||||||
| Underexpressed | 1.000 | |||||
| Overexpressed | 1.259 | 0.504–2.446 | 0.564 | |||
Notes: aThe 8th TNM Classification of Malignant Tumors proposed by the AJCC/UICC. *P<0.05 values are statistically significant.
Abbreviations: HR, hazard ratio; CI, confidence interval; AR, androgen receptor.
Inhibition of AR Signaling Suppresses the Proliferation of GC Cell Lines
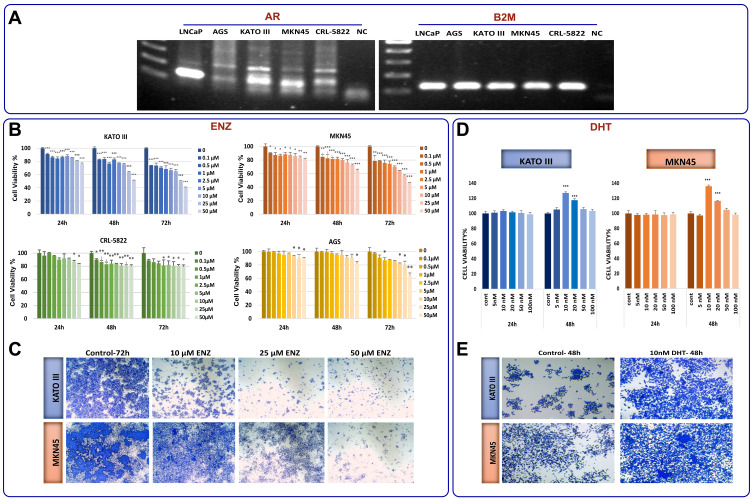
Based on increased expression of AR as well as significant correlation of this receptor with an unfavorable outcome in GC patients, it was reasonable to investigate the effect of AR inhibition on GC cell lines. First we used conventional PCR to examine the basal mRNA expression level of AR in four gastric cancer cell lines and LNCaP cells, as an AR-positive cell line.32 While all the tested GC cell lines expressed AR, we found that AR expression levels in MKN45 and KATO III cells were higher than AGS and CRL-5822 (Figure 3A). Accordingly, when GC cell lines were exposed to the increasing concentrations (0.1–50 µM) of a novel AR antagonist enzalutamide (ENZ),27 the survival of all tested cells were decreased in concentration- and time-dependent manners (Figure 3B). It is worth mentioning that KATO III and MKN45 cells were more sensitive to the inhibitor compared to AGS and CRL-5822 cells harboring lower amount of the receptor.
Figure 3.
The anticancer effects of ENZ on GC cell lines. (A) Basal level of AR mRNA expression in four gastric cancer cell lines and LNCaP cells detected by RT-PCR. Digital images of the gels were captured using a Bio-Rad gel documentation system using Image Lab Software. The samples derived from the same Gel. Our desired AR band is at 105 bp. Nonspecific bands disappeared after optimizing PCR condition and increasing the annealing temperature in conventional PCR. (B) The effect of ENZ on proliferation of GC cells and LNCaP cells was determined by MTT assay 24, 48, and 72 h posttreatment. Data are given as mean ±SD. (C) The effects of ENZ on GC cell viability were demonstrated by crystal violet staining 72 h posttreatment. The cultures were stained with crystal violet and imaged by an inverted microscope (images acquired at 4× magnification). (D) The effect of DHT (nM) on proliferation of GC cells was determined by MTT assay 24, 48, and 72 h posttreatment. Data are given as mean ±SD. (E) The effects of DHT on GC cell viability were demonstrated by crystal violet staining 48 h posttreatment. Statistically significant values of *P<0.05, **P<0.01, and ***P<0.001 were determined compared with the control.
Based on the prominent cytotoxicity of ENZ on KATO III and MKN45, further experiments were expanded on these cell lines. As presented in Figure 3C, the results of crystal violet staining assay showed that the inhibition of AR could decrease the proliferation of KATO III and MKN45 cells both in time- and concentration-dependent manners. To validate our results, we assessed the effect of AR activation on GC cells proliferation upon treatment of the cells using a potent AR agonist dihydrotestosterone (DHT). Consistent with the results achieved upon treatment of the cells with ENZ, we found that not only had the activation of AR significantly increased cell viability after 48 h, but it also promoted cancer cell proliferation both in KATO III and MKN45 cells (Figure 3D and E).
Antiproliferative, Apoptotic, and Antimetastatic Effects of AR Inhibition on KATO III and MKN45 GC Cells
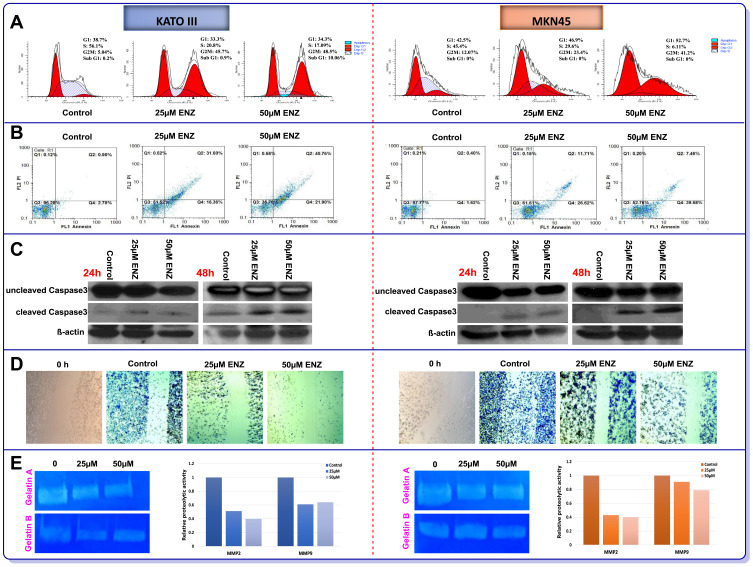
In the light of the antiproliferative effect of AR inhibition on GC cell lines and to explore the potential mechanism of action of the inhibitor, we assessed both the cell cycle pattern and apoptosis induction using flow cytometry. As shown in Figure 4A, while untreated KATO III and MKN45 cells were mainly in S phase, 48 h treatment with ENZ resulted in a remarkable decrease in the fraction of S phase as well as the induction of G2/M cell cycle arrest in both cell lines. Our data also revealed that the percentage of apoptotic dead cells was robustly increased in the cultures of the cells treated with the inhibitor in a concentration-dependent manner (Figure 4B). We next examined the level of a key enzyme involved in apoptosis, caspase-3, in GC cells, 24 and 48 h posttreatment with ENZ (Figure 4C). ENZ significantly induced cleaved caspase-3 in a time- and dose-dependent manner in both GC cell lines. Evidences indicated that AR could contribute in the invasion of tumor cells through the induction of MMP-2 and MMP-9 enzymatic levels.1,33 As depicted in Figure 4D, ENZ at the concentrations of 25 μM and 50 μM significantly inhibited cell migration in both KATO III and MKN45 cell lines, compared with the vehicle-treated cells. In agreement with wound healing assay, we found that ENZ clearly reduced both MMP-2 and MMP-9 activities in GC cell lines 48 h posttreatment (Figure 4E). Intensities of clear bands demonstrated the diminishing gelatinolytic activities of MMP2 and MMP9 against the blue background of the stained gelatin.
Figure 4.
The effects of ENZ on induction of (A) Cell cycle arrest and (B) Apoptosis in GC cell lines 48 h posttreatment. The flow cytometry graphs of cell cycle and apoptosis are representative of three independent experiments with similar outcomes. The effects of ENZ (25 and 50 μM) on activation of caspase-3 (cleaved form) in GC cells were determined by Western blot analysis, 24 and 48 h posttreatment (C). The blots are representative of three independent experiments with similar outcomes. β-actin was used as loading control. The effects of ENZ on migration and metastasis of GC cell lines (D) Representative pictures of wound healing scratch assays of vehicle and ENZ treated cells (images acquired at 10× magnification). (E) Representative gelatin zymogram showing MMP9 and MMP2 activities in ENZ treated cells after 48 h. Gelatinolytic activities are visualized as clear bands against the blue background of stained gelatin and the intensity of each band was quantified by GelQuant.net.
AR Signaling Targets the EMT-related Genes Transcription in GC Cells
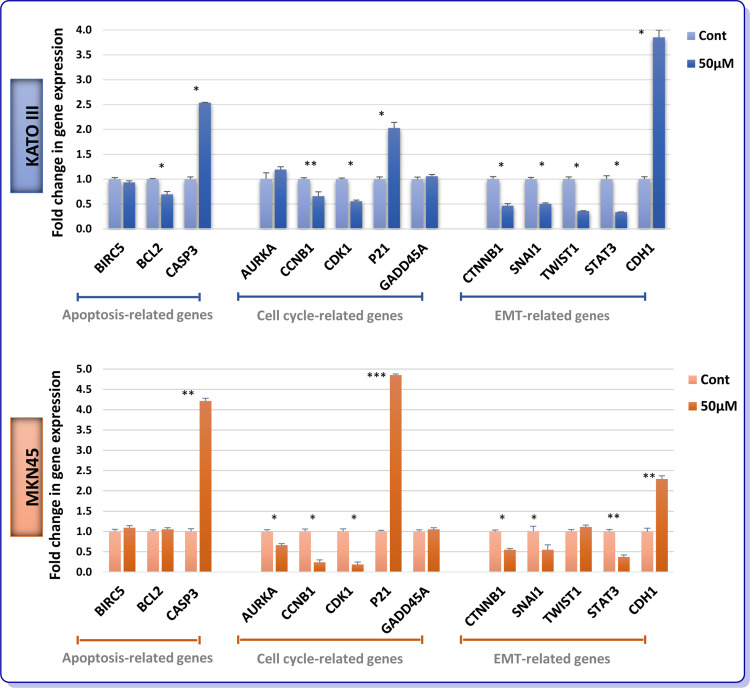
To delve into the molecular mechanisms by which AR inhibition could either induce apoptosis or suppress cell cycle progression in GC cells, we aimed to analyze the mRNA expression levels of apoptosis- and cell cycle-related genes using real-time PCR. In addition, molecular analysis of genes involved in epithelial-mesenchymal transition (EMT) was also examined to shed more light on the suppressive effect of the inhibitor on the invasive ability of KATO III and MKN45. As presented in Figure 5, not only could ENZ significantly alter pro- and anti-apoptotic genes, but it also induce its growth suppressive effect through a p21-mediated G2/M arrest in both GC cell lines. Intriguingly, our data showed that the mRNA expression of β-catenin, Snail, Twist1, and STAT3 were significantly diminished, while the mRNA level E-cadherin was upregulated upon treatment with ENZ. A similar pattern was also observed in MKN45 cells, except for the expression of Twist1 which did not remarkably vary in this cell line (Figure 5).
Figure 5.
Effect of ENZ on apoptosis, cell cycle and EMT-related genes expression. After 48 h treatment with ENZ (50 µM), the GC cells were harvested for quantitative real-time PCR test. Gene expression levels were normalized to B2M. Data are given as mean ±SD. Statistically significant values of *P<0.05, **P<0.01, and ***P<0.001 were determined compared with the control.
Abbreviations: BIRC5, survivin; CASP3, caspase-3; AURKA, aurora kinase A; CCNB1, cyclin B1; CDK1, cyclin-dependent kinase 1; GADD45A, growth arrest and DNA damage inducible alpha.
Stimulatory Effect of Enzalutamide on 5-Fluorouracil Cytotoxicity on GC Cell Lines
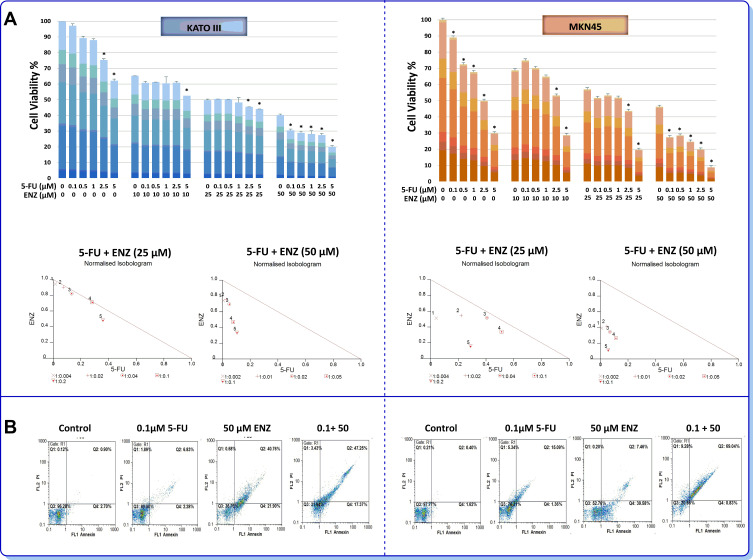
Given the frequent acquisition of chemoresistant phenotype in GC34 as well as the proven effect of ENZ on metastatic castration-resistant prostate cancer (mCRPC),35 we wondered if the inhibition of AR signaling may achieve higher cytotoxicity with 5-fluorouracil (5-FU) as one of the most effective chemotherapy drugs used in GC patients. Notably, combination of ENZ with 5-FU clearly displayed a synergistic effect on cell survival in both GC cell lines. As presented in Figure 6A, normalized isobolograms of combination of AR inhibitor (25 and 50 µM) and 5-FU (0.1, 0.5, 1, 2.5, and 5 µM) revealed that all the points are located below the line of additive effect. Combination index (CIx) and dose reduction index (DRI) of ENZ and 5-FU in GC cells are presented in Table 3. Stimulatory effect of AR inhibition on the cytotoxic effect of 5-FU against GC cell lines was further validated using flow cytometric analysis of annexin-V/PI assay. As presented in Figure 6B, we found that 5-FU in combinational treatment induced apoptosis more than single agent therapy in GC cells, as the percentage of apoptotic dead cells was remarkably higher in the cultures of the cells co-treated with ENZ and 5-FU.
Figure 6.
Synergistic activity of 5-fluorouracil with ENZ-targeted therapy (A) AR inhibiting enhances sensitivity to 5-FU in GC cells. The effects of ENZ in combination with 5-FU on cell proliferation were investigated by MTT assay 72 h posttreatment. In normalized isobolograms of combination of ENZ (10, 25, 50 μM) and 5-FU (0.1, 0.5, 1, 2.5, and 5 μM), the connecting line represents additivity. Data points located below the line indicate a synergistic drug-drug interaction and data points above the line indicate an antagonistic drug-drug interaction. Statistically significant values of *P<0.05 were determined compared with the control. (B) The combinatory effects of ENZ and 5-FU to induce apoptosis in GC cells assessing by flow cytometry. The apoptosis graphs are representative of three independent experiments with similar outcomes.
Table 3.
Combination Index (CIx) and Dose Reduction Index (DRI) of ENZ and 5-FU Combination in MKN45 and KATO III Cells.
| Concentrations (μM) | fa | CIx | DRI | ||
|---|---|---|---|---|---|
| ENZ | 5-FU | ENZ | 5-FU | ||
| KATO III | |||||
| 10 | 0.1 | 0.241 | 0.947 | 1.107 | 23.149 |
| 10 | 0.5 | 0.248 | 1.07 | 1.156 | 4.87 |
| 10 | 1 | 0.325 | 0.808 | 1.785 | 4.034 |
| 10 | 2.5 | 0.341 | 1.079 | 1.939 | 1.777 |
| 10 | 5 | 0.413 | 1.11 | 2.758 | 1.339 |
| 25 | 0.1 | 0.405 | 0.957 | 1.062 | 64.034 |
| 25 | 0.5 | 0.414 | 0.976 | 1.108 | 13.459 |
| 25 | 1 | 0.434 | 0.954 | 1.218 | 7.507 |
| 25 | 2.5 | 0.465 | 0.993 | 1.406 | 3.549 |
| 25 | 5 | 0.549 | 0.843 | 2.069 | 2.782 |
| 50 | 0.1 | 0.605 | 0.748 | 1.347 | 188.963 |
| 50 | 0.5 | 0.601 | 0.784 | 1.321 | 36.959 |
| 50 | 1 | 0.618 | 0.747 | 1.434 | 20.328 |
| 50 | 2.5 | 0.695 | 0.549 | 2.125 | 12.842 |
| 50 | 5 | 0.754 | 0.44 | 2.985 | 9.537 |
| MKN45 | |||||
| 10 | 0.1 | 0.127 | 1.785 | 0.878 | 1.549 |
| 10 | 0.5 | 0.299 | 1.07 | 2.365 | 1.546 |
| 10 | 1 | 0.351 | 1.247 | 2.943 | 1.103 |
| 10 | 2.5 | 0.524 | 0.96 | 5.665 | 1.276 |
| 10 | 5 | 0.697 | 0.61 | 11.172 | 1.92 |
| 25 | 0.1 | 0.483 | 0.553 | 1.948 | 24.961 |
| 25 | 0.5 | 0.467 | 0.765 | 1.836 | 4.536 |
| 25 | 1 | 0.481 | 0.923 | 1.934 | 2.466 |
| 25 | 2.5 | 0.594 | 0.852 | 2.945 | 1.952 |
| 25 | 5 | 0.774 | 0.442 | 6.449 | 3.48 |
| 50 | 0.1 | 0.725 | 0.403 | 2.534 | 117.691 |
| 50 | 0.5 | 0.715 | 0.459 | 2.42 | 21.856 |
| 50 | 1 | 0.753 | 0.414 | 2.897 | 14.624 |
| 50 | 2.5 | 0.802 | 0.377 | 3.764 | 8.945 |
| 50 | 5 | 0.911 | 0.169 | 8.843 | 17.882 |
Notes: fFa denotes fraction affected. DRI shows the order of magnitude of dose reduction that is allowed in combination for a given degree of effect as compared with the dose of each drug alone.
Discussion
Men develop GC more than women.3 Clarifying the factors that cause gender disparity of GC may help to disclose underlying molecular pathways in gastric carcinogenesis. There are several studies indicating AR as an oncoprotein involved in tumor growth, progression and metastasis in male-predominant cancers.12,14,15 Although GC belongs to this family, few studies have investigated the role of AR in GC prognosis leading to conflicting results. The results obtained in the present study demonstrate that 66.7% of GC patients overexpressed AR relative to normal cases. We also found that the higher expression rate of AR is positively associated with higher lymphovascular involvement rate, increased risk of lymph node metastasis, larger tumor mass, more distant metastasis, and subsequently late TNM stages; shedding light on the fact that AR probably plays an important role in the progression and late-stage carcinogenesis of GC. Not only have many studies revealed that dysregulation of EMT is a prevalent phenomenon in GC,36–39 but also it has been frequently reported that AR could interact with EMT-related genes in other tumors.40–43 Accordingly, our results showed significant alteration in the expression of well-known EMT-related genes in GC samples as compared to normal tissues. While Snail, Twist1, β-catenin and STAT3 were overexpressed in GC tissues, E-cadherin showed notable downregulation. Of particular interest was a strong positive relation found between AR and β-catenin expression in tumor tissues, which was in agreement with several studies demonstrating the direct interaction between the aforementioned genes especially in prostate cancer.41,42,44 In a thought-provoking study on HCC, it was also indicated that AR can promote uncontrolled cell proliferation through regulation of cell cycle related kinase to activate β-catenin/TCF signaling.16 Taken together and as the most straightforward interpretation of our results, we presumed that AR and β-catenin may have an entwined connection for the progression of EMT and metastasis in GC.
Since the prognosis of advanced GC is dramatically poor, identification of proper markers that precisely predict aggressiveness and progression of the disease could improve the outcome of GC patients. In this study, not only did the results of the Kaplan–Meier analysis reveal that AR, Snail and Twist1 overexpression were significantly correlated with the overall survival of GC patients, but also assessing HR using univariate Cox regression model showed that they were remarkable risk factor for the disease prognosis; notably, after adjustment with other variables, we found that AR, Snail and Twist1 overexpression cannot be used as independent prognostic factors. Although these results are consistent with a previous report,8 Kominea et al claimed a controversial issue suggesting that AR is an independent prognostic factor for GC patients11 which may be explained, at least partly, by different cutoff points, experimental methods, sample size, or different populations that were assessed. To the best of our knowledge, no study has addressed simultaneous assessment of AR and EMT-related gene expressions and this study represents for the first time that concurrent overexpression of AR and Snail can be used as a precise independent unfavorable factor for GC OS (HR=2.382, P=0.021).
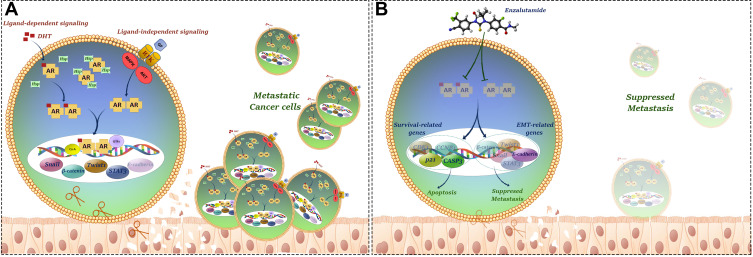
Given our results showing AR overexpression in GC tissues on the one hand, and controversial findings concerning hormonal therapy using sex hormone receptors antagonists on the other hand this encouraged us to investigate the effect of a novel AR antagonist ENZ in GC cell lines. Our results showed that ENZ, as an FDA-approved drug for metastatic castration-resistant prostate cancer patients,27 could significantly inhibit GC cell growth and proliferation in time- and concentration-dependent manners. Our supplementary examinations further support our results that activating AR using an AR agonist DHT could effectively induce GC cell proliferation which is consistent with a study showing that AR activation promoted HCC growth by the suppression of DNA damage repairing system.12 Anti-tumor effect of AR inhibition using ENZ was further confirmed using flow cytometric and molecular investigations of cell cycle and apoptosis, where we found that ENZ could regulate G2/M transition and provoke apoptosis via downregulation of cyclin-B1 and Cdk1 as well as upregulation of p21 and caspase-3 in GC cell lines. We also authenticated activation of caspase-3 following ENZ treatment using Western blot analysis. In a study on triple-negative breast cancer, Zhu et al showed that AR inhibition using bicalutamide induced apoptosis and exerted an inhibitory effect on cell growth by regulating p21, p73, and cyclin D1 expressions.45 Apart from ENZ cytotoxicity, our results showed that AR suppression could overwhelm the ability of GC cell lines to migrate and invade possibly through modulation of EMT-related genes expression and suppression of MMPs. Taken altogether and to provide a better overview with respect to our results, we provided a schematic model of the possible complex network in which AR crosstalks with EMT- and survival-related pathways to promote progression and metastasis of gastric cancer (Figure 7).
Figure 7.
Schematic model of the possible complex network in which AR crosstalks with apoptosis, cell cycle and EMT-related pathways to promote growth, proliferation and metastasis of gastric cancer. (A) Metastatic gastric cancer cells. (B) Inhibition of invasion by ENZ in gastric cancer cells. AR inhibition using ENZ suppressed metastatic properties of the cells through modulation of EMT and survival related-gene expressions.
Despite the achievement of magnificent breakthroughs to improve GC survival, the most important factor causing failure in the treatment strategy of the disease is the acquisition of chemoresistance. Bearing this in mind, many studies have been lately dedicated to find innovative targeted therapy, in particular in the context of combined-modal strategy with traditional chemotherapy. Since the most well-known chemotherapy regime admitted for GC is 5-FU monotherapy or as part of a combination therapy,46 we wondered if ENZ could augment its cytotoxic effects. Our findings suggest that combination of ENZ with even low concentration of 5-FU (0.1 µM) could attain synergistic effects; highlighting the fact that AR inhibition using ENZ, either as a single agent or in combination with chemotherapy, could provide a better therapeutic outcome in GC patients. However, further researches especially in vivo studies are required to illustrate the accurate impact of AR in GC carcinogenesis along with assessment of anti-AR therapy in GC patients.
Acknowledgments
This study was supported by a grant from Hematology/Oncology and Stem Cell Transplantation Research Centre, Shariati hospital, Tehran University of Medical Sciences, Tehran, Iran. (Grant numbers: 94-03-36-30292, 94-03-36-30293).
Funding Statement
This study was supported by a grant from Hematology, Oncology and Stem Cell Transplantation Research Institute, Shariati hospital, Tehran University of Medical Sciences, Tehran, Iran. (Grant numbers: 94-03-36-30292, 94-03-36-30293).
Abbreviations
AR, androgen receptor; GC, gastric cancer; EMT, epithelial-mesenchymal transition; ENZ, enzalutamide; DHT, dihydrotestosterone; 5-FU, 5-fluorouracil.
Data Sharing Statement
All data generated or analyzed during this study and its supplementary information files are included in this published article.
Ethics Approval
Our research was approved by the Clinical Research Ethics Committee of Tehran University of Medical School (TUMS) and complied with the ethical principles of the HORC-SCT, Shariati hospital and the Helsinki Declaration of 1964 and later versions (Ethics Committee approval code: ir.TUMS.horcsct.rec.1394.103.10).
Consent to Participate
All participating patients or their immediate family members signed the informed consents.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang BG, Du T, Zang MD, et al. Androgen receptor promotes gastric cancer cell migration and invasion via AKT-phosphorylation dependent upregulation of matrix metalloproteinase 9. Oncotarget. 2014;5(21):10584–10595. doi: 10.18632/oncotarget.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a Phase III trial. Lancet Oncol. 2008;9(3):215–221. doi: 10.1016/S1470-2045(08)70035-4 [DOI] [PubMed] [Google Scholar]
- 3.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–164. doi: 10.1016/j.critrevonc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, Wan H, Lin Y, Xie X, Li Z, Tan G. Androgen receptor may be responsible for gender disparity in gastric cancer. Med Hypotheses. 2013;80(5):672–674. doi: 10.1016/j.mehy.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 6.Qin J, Liu M, Ding Q, et al. The direct effect of estrogen on cell viability and apoptosis in human gastric cancer cells. Mol Cell Biochem. 2014;395(1–2):99–107. doi: 10.1007/s11010-014-2115-2 [DOI] [PubMed] [Google Scholar]
- 7.Gan L, He J, Zhang X, et al. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12(1):566. doi: 10.1186/1471-2407-12-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W, Liu R, Yan Y, et al. Expression of estrogen receptors and androgen receptor and their clinical significance in gastric cancer. Oncotarget. 2017;8(25):40765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33(2):195–201. doi: 10.1016/j.ejso.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Shimada N, Suzuki T, et al. In situ androgen production in human gastric carcinoma–androgen synthesizing and metabolizing enzymes. Anticancer Res. 2006;26(3A):1935–1939. [PubMed] [Google Scholar]
- 11.Kominea A, Konstantinopoulos PA, Kapranos N, et al. Androgen receptor (AR) expression is an independent unfavorable prognostic factor in gastric cancer. J Cancer Res Clin Oncol. 2004;130(5):253–258. doi: 10.1007/s00432-003-0531-x [DOI] [PubMed] [Google Scholar]
- 12.Ma WL, Hsu CL, Wu MH, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135(3):947–955, 955 e941–945. doi: 10.1053/j.gastro.2008.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol. 2012;42(7):569–577. doi: 10.1093/jjco/hys072 [DOI] [PubMed] [Google Scholar]
- 14.Konduri S, Schwarz MA, Cafasso D, Schwarz RE. Androgen receptor blockade in experimental combination therapy of pancreatic cancer. J Surg Res. 2007;142(2):378–386. doi: 10.1016/j.jss.2006.09.034 [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto H, Yang Z, Chen YT, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99(7):558–568. doi: 10.1093/jnci/djk113 [DOI] [PubMed] [Google Scholar]
- 16.Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2011;121(8):3159–3175. doi: 10.1172/JCI45967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boix L, Castells A, Bruix J, et al. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995;22(6):616–622. doi: 10.1016/0168-8278(95)80217-7 [DOI] [PubMed] [Google Scholar]
- 18.Kanda T, Jiang X, Yokosuka O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J Gastroenterol. 2014;20(28):9229–9236. doi: 10.3748/wjg.v20.i28.9229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okitsu K, Kanda T, Imazeki F, et al. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer. 2010;1(8):859–867. doi: 10.1177/1947601910383417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jukic Z, Radulovic P, Stojkovic R, et al. Gender difference in distribution of estrogen and androgen receptors in intestinal-type gastric cancer. Anticancer Res. 2017;37(1):197–202. doi: 10.21873/anticanres.11306 [DOI] [PubMed] [Google Scholar]
- 21.Fard SS, Saliminejad K, Sotoudeh M, et al. The correlation between EGFR and androgen receptor pathways: a novel potential prognostic marker in gastric cancer. Anticancer Agents Med Chem. 2019;19(17):2097–2107. doi: 10.2174/1871520619666190930142820 [DOI] [PubMed] [Google Scholar]
- 22.Kawahara T, Ide H, Kashiwagi E, et al. Enzalutamide inhibits androgen receptor-positive bladder cancer cell growth. Urol Oncol. 2016;34(10):432e415–423. doi: 10.1016/j.urolonc.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 23.Ao J, Meng J, Zhu L, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6(5):507–515. doi: 10.1016/j.molonc.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda T, Takahashi K, Nakamura M, et al. Androgen receptor could be a potential therapeutic target in patients with advanced hepatocellular carcinoma. Cancers. 2017;9(5). doi: 10.3390/cancers9050043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min A, Jang H, Kim S, et al. Androgen receptor inhibitor enhances the antitumor effect of PARP inhibitor in breast cancer cells by modulating DNA damage response. Mol Cancer Ther. 2018;17(12):2507–2518. doi: 10.1158/1535-7163.MCT-18-0234 [DOI] [PubMed] [Google Scholar]
- 26.Sternberg CN. Enzalutamide, an oral androgen receptor inhibitor for treatment of castration-resistant prostate cancer. Future Oncol. 2019;15(13):1437–1457. doi: 10.2217/fon-2018-0940 [DOI] [PubMed] [Google Scholar]
- 27.Mateo J, Smith A, Ong M, de Bono JS. Novel drugs targeting the androgen receptor pathway in prostate cancer. Cancer Metastasis Rev. 2014;33(2–3):567–579. doi: 10.1007/s10555-013-9472-2 [DOI] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 29.Soleymani Fard S, Jeddi Tehrani M, Ardekani AM. Prostaglandin E2 induces growth inhibition, apoptosis and differentiation in T and B cell-derived acute lymphoblastic leukemia cell lines (CCRF-CEM and Nalm-6). Prostaglandins Leukot Essent Fatty Acids. 2012;87(1):17–24. doi: 10.1016/j.plefa.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 30.Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947 [DOI] [PubMed] [Google Scholar]
- 31.Momeny M, Sabourinejad Z, Zarrinrad G, et al. Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Sci Rep. 2017;7(1):45954. doi: 10.1038/srep45954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malaguarnera R, Sacco A, Morcavallo A, et al. Metformin inhibits androgen-induced IGF-IR up-regulation in prostate cancer cells by disrupting membrane-initiated androgen signaling. Endocrinology. 2014;155(4):1207–1221. doi: 10.1210/en.2013-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Pan T, Zhong X, Cheng C. Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2. Tumour Biol. 2015;36(8):5859–5864. doi: 10.1007/s13277-015-3257-x [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Yashiro M, Qiu H, Nishii T, Matsuzaki T, Hirakawa K. Establishment and characterization of multidrug-resistant gastric cancer cell lines. Anticancer Res. 2010;30(3):915–921. [PubMed] [Google Scholar]
- 35.Drake CG, Sharma P, Gerritsen W. Metastatic castration-resistant prostate cancer: new therapies, novel combination strategies and implications for immunotherapy. Oncogene. 2014;33(43):5053–5064. doi: 10.1038/onc.2013.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawanishi K, Doki Y, Shiozaki H, et al. Correlation between loss of E-cadherin expression and overexpression of autocrine motility factor receptor in association with progression of human gastric cancers. Am J Clin Pathol. 2000;113(2):266–274. doi: 10.1309/JH4Q-25Q5-0TRV-W99U [DOI] [PubMed] [Google Scholar]
- 37.Xing X, Tang YB, Yuan G, et al. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer. 2013;132(11):2589–2596. doi: 10.1002/ijc.27947 [DOI] [PubMed] [Google Scholar]
- 38.Clements WM, Wang J, Sarnaik A, et al. Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62(12):3503–3506. [PubMed] [Google Scholar]
- 39.Ikenoue T, Ijichi H, Kato N, et al. Analysis of the beta-catenin/T cell factor signaling pathway in 36 gastrointestinal and liver cancer cells. Jpn J Cancer Res. 2002;93(11):1213–1220. doi: 10.1111/j.1349-7006.2002.tb01226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YN, Liu Y, Lee HJ, Hsu YH, Chen JH. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol. 2008;28(23):7096–7108. doi: 10.1128/MCB.00449-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64(24):8860–8866. doi: 10.1158/0008-5472.CAN-04-2370 [DOI] [PubMed] [Google Scholar]
- 42.Yang F, Li X, Sharma M, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277(13):11336–11344. doi: 10.1074/jbc.M111962200 [DOI] [PubMed] [Google Scholar]
- 43.Junicho A, Matsuda T, Yamamoto T, et al. Protein inhibitor of activated STAT3 regulates androgen receptor signaling in prostate carcinoma cells. Biochem Biophys Res Commun. 2000;278(1):9–13. doi: 10.1006/bbrc.2000.3753 [DOI] [PubMed] [Google Scholar]
- 44.Lee E, Madar A, David G, Garabedian MJ, Dasgupta R, Logan SK. Inhibition of androgen receptor and beta-catenin activity in prostate cancer. Proc Natl Acad Sci U S A. 2013;110(39):15710–15715. doi: 10.1073/pnas.1218168110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu A, Li Y, Song W, et al. Antiproliferative effect of androgen receptor inhibition in mesenchymal stem-like triple-negative breast cancer. Cell Physiol Biochem. 2016;38(3):1003–1014. doi: 10.1159/000443052 [DOI] [PubMed] [Google Scholar]
- 46.Marin JJ, Al-Abdulla R, Lozano E, et al. Mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16(3):318–334. doi: 10.2174/1871520615666150803125121 [DOI] [PubMed] [Google Scholar]