Abstract
Eco-immunology seeks evolutionary explanations for the tremendous variation in immune defense observed in nature. Assays to quantify immune phenotypes often are crucial to this endeavor. To this end, we suggest that more use could (and arguably should) be made of the veterinary and clinical serological toolbox. For example, measuring the magnitude and half-life of parasite-specific antibodies across a range of host taxa may provide new ways of testing theories in eco-immunology. Here, we suggest that antibody assays developed in veterinary and clinical immunology and epidemiology provide excellent tools—or at least excellent starting points for development of tools—for tests of such hypotheses. We review how such assays work and how they may be optimized for new questions and new systems in eco-immunology. We provide examples of the application of such tools to eco-immunological studies of seabirds and mammals, and suggest a decision-tree to aid development of assays. We expect that addition of such tools to the eco-immunological toolbox will promote progress in the field and help elucidate how immune systems function and why they vary in nature.
Introduction
Understanding how natural variation in immune defense relates to evolutionary fitness and to variation in life history at the individual, population, and species levels is the purpose of ecological immunology, or eco-immunology (Sheldon and Verhulst 1996; Demas and Nelson 2011; Schmid-Hempel 2011). Eco-immunology is thus rooted in the broader context of evolutionary ecology and focuses on immunity as a cohort of phenotypic mechanisms that provide benefits to the host (e.g., survival despite infection) but also carry costs (e.g., autoimmunity or depletion of resources that might otherwise be devoted to reproduction) (Lochmiller and Deerenberg 2000; Graham et al. 2005; Hawley and Altizer 2011). Such costs may help to explain the maintenance of heterogeneity in immune defense in nature (Boots et al. 2009; Buehler et al. 2010). For example, the life-history traits of hosts and parasites are expected to shape the optimal magnitude and specificity of immune responses: long-lived host species are expected to invest more in costly, highly specific, and persistent immunity, particularly against virulent parasites, compared with short-lived host species (Miller et al. 2007; Garnier et al. 2012a; Boots et al. 2013). Testing these theoretical predictions in natural systems has, however, proven challenging (Sheldon and Verhulst 1996; Demas and Nelson 2011; Pedersen and Babayan 2011; Schmid-Hempel 2011).
Eco-immunological studies are empirically challenging for a variety of reasons. These include the difficulty of obtaining the right type and quantity of sample at the right time to capture the activity of ever-dynamic immune systems, and of obtaining accurate and relevant phenotypic measures of immunity for the particular question and system under study. Here, we argue that the development of the serological toolbox in recent years has put measurement of parasite-specific antibodies in wild animals within the reach of evolutionary ecologists. Development of these serological tools often has been inspired by concerns for wildlife populations affected by particular infectious diseases, including those of zoonotic potential (e.g., avian influenza). Epidemiological insights have been gained by the use of these new reagents and by careful interpretation, given limited knowledge of the timing of exposure and the persistence of antibodies in wild animals (Gilbert et al. 2013). Some of these tools already have been applied to generate eco-immunological insights, as outlined below, and we argue that this bodes well for tailoring assays to other questions and systems.
Eco-immunological studies already use a wide array of laboratory techniques when quantifying immune phenotypes (reviewed by Boughton et al. 2011). Most of these assays can be applied to a broad range of host taxa and have been chosen for their ease of use, especially because sample volumes for blood or plasma can be extremely limited when collected non-invasively in the wild. The downside is that many of these assays provide limited insight into the specific acquired immune response, so they do not provide appropriate data for tests of all eco-immunological hypotheses. An example is the measure of the wing-web swelling following injection of phytohemagglutinin (PHA) in birds, a technique that has been widely used since the early days of eco-immunology. PHA is a mitogen that non-specifically stimulates several components of the innate and acquired cellular response that are integrated in the resulting measure of the thickness of the wing-web 24 h after injection (Salaberria et al. 2013). Another common measure that lacks specificity is the measure of total antibodies, such as total IgG in mammals or IgY in birds. Although variations in total immunoglobulin levels may be informative about varied efficacy of defense in some cases, they are also likely to vary substantially due to a suite of other factors such as recency of exposure to infection. The use of such measures is especially problematic when specific acquired immunity is the focus of the eco-immunological predictions being tested. For instance, parasite-specific measures are essential to test the hypothesis that longer lived species should exhibit stronger specific (particularly antibody-mediated) immune defenses in order to reduce costs of re-infection (termed the “pace-of-life” hypothesis) (Lee 2006). To complement measures of innate markers and of primary immune response following injection of a novel antigen (e.g., Martin et al. 2006), one should therefore aim to measure the specific immune markers that could protect against re-infection through immunological memory.
Our primary focus is on antibody responses of vertebrate species, particularly birds and mammals. Tools to dissect genetic polymorphisms and changes in gene expression in wild animals are treated elsewhere (e.g., Turner and Paterson 2013), and have been empowered by modern genomics. Measurements of immune phenotype at the protein level are less well discussed, yet are crucial to understanding variation in specific immunological memory; Major Histocompatibility Complex (MHC) and cytokine promoter polymorphisms will affect the acquisition of immunity, but only by measuring the induced effector phenotypes (e.g., concentrations and half-lives of specific antibodies or T cells) can eco-immunologists begin to assess the costs and benefits of such specific and persistent defense. Furthermore, antibodies present various logistical advantages for studies in the wild (Graham et al. 2011), including their robustness to variations in post-sampling treatment under field conditions (Hoye 2011). In addition, once basic assays are established, a wide array of specificities and functional types often are available for measurement.
Different approaches to quantifying specific acquired immunity may be required in avian and mammalian systems as their immune systems are anatomically and mechanistically different. The most striking differences between the immune systems of mammals and birds can be found at the anatomical level. B lymphocytes mature in the bursa of Fabricius in birds versus in the bone marrow of mammals; birds also lack lymph nodes like those of mammals and instead develop diffuse lymphoid tissue at the sites of antigenic stimulation (Schat et al. 2013). Differences also are evident at the molecular level as immunoglobulin Y (IgY, the main immunoglobulin of birds) is structurally different from immunoglobulin G (IgG, the main immunoglobulin of mammals); the heavy chain of IgG contains three constant fragments, whereas the one of IgY contains four constant regions (Kovacs-Nolan and Mine 2012). These differences are likely to affect both function and detection of immunity mediated by B cells and antibodies in the two taxa. Further differences in function and detection arise within taxa, depending upon which parasites hosts harbor (e.g., helminths inducing IgE or bacteria inducing opsonizing IgG isotypes in mammals) (Murphy et al. 2008). Devising measures of immunity that account for such differences is key to empirical study of natural systems.
Below, we first outline how human and veterinary serological assays work and illustrate why these broadly adaptable tools are of potential interest for eco-immunologists. We then discuss examples of the deployment of this toolbox to study eco-immunology in wild seabirds and mammals. Finally, we consider what further insights might emerge from application of these tools. There is increasing appreciation that evolutionary causes of variation in immune defense are of central interest to biomedical immunology (Maizels and Nussey 2013), and putting serological assays into the hands of evolutionary ecologists will aid discovery in this exciting area.
Breadth and flexibility of the serological toolbox
Human and veterinary medicine have led to the development of countless assays that quantify precisely many of the effectors involved in the inflammatory and immune responses. Enzyme-linked immunosorbent assays (ELISA) are of particular interest for eco-immunology and disease ecology as they allow the detection and quantification of specific antibodies produced after exposure to pathogens. Briefly, an antigen is coated onto the bottom of wells. When plasma or serum (most commonly, although other types of samples also may be used: e.g., fecal extract) is added to a well, any antibodies specific to the coated antigen will bind, and antigen–antibody complexes are formed. In most cases, a secondary color-marked antibody is used, via enzymatic reaction, to quantify the complexes (“sandwich ELISA”) and the absorbance measured on a spectrophotometer provides a direct estimate of the concentration of a specific antibody. In some cases, the secondary antibody can instead be directed toward the remaining binding sites at the bottom of the well (“competitive ELISA”) and the concentration of the molecule of interest is inversely related to absorbance (Crowther 2009; Wild 2013).
Many such tests have been developed, particularly for diseases of economic importance in domestic animals and diseases of conservation or zoonotic concern in wild animals (as illustrated in Table 1). Although they were not developed for use in wild animals, assays for domestic animals are commercially available and represent valuable starting points for studying the immunology of wild species. Competitive ELISAs, for instance, can readily be applied to samples of wild species as the secondary antibody is antigen-specific and not specific for the host species. In the case of sandwich ELISA, only the secondary antibody needs to be modified to accommodate the host species. These are available for an ever-increasing range of species, as pointed out by Boughton et al. (2011) and as suggested by the taxonomic coverage of the antibody reagents deployed in wild animals in the past year (Table 1). In some cases, specific secondary antibodies have been developed de novo for a given species, for example, Nile crocodile (Ludovisi et al. 2013), and this could be achieved for any host species although the process can be expensive and time-consuming.
Table 1.
Publications in 2013 that reported measurements of concentrations of parasite-specific antibodies in wild animals
| Pathogenic agent | Host Species | Assay method | References |
|---|---|---|---|
| Avian influenza viruses | American black duck (Anas rubripes), Mallard duck (Anas platyrhynchos), Northern pintail (Anas acuta), Northern rockhopper penguin (Eudyptes moseleyi), Southern rockhopper penguin (Eudyptes chrysocome), Macaroni penguin (Eudyptes chrysolophus), Chinstrap penguin (Pygoscelis antarctica), Gentoo penguin (Pygoscelis papua), King penguin (Aptenodytes patagonicus), Tundra swan (Cygnus columbianus), Pacific black brant (Branta bernicla nigricans), Greater white-fronted goose (Anser albifrons), Emperor goose (Chen canagica), Common eider (Somateria mollissima), Cackling goose (Branta hutchinsii), Spectacle eider (Somateria fischeri), Steller’s eider (Polysticta stelleri), Long-tailed duck (Clangula hyemalis), Black scoter (Melanitta nigra) | Commercial anti-influenza nucleoprotein competitive ELISA | Huang et al. (2013), Wilson et al. (2013), Abad et al. (2013) |
| Influenza viruses | Raccoon (Nyctereutes procyonoides) | Commercial anti-influenza nucleoprotein competitive ELISA | Cha et al. (2013) |
| Besnoitia besnoiti | Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Chamois (Rupicapra rupicapra), Mouflon (Ovis musimon) | In-house ELISA using tachyzoite antigen extract and anti-deer IgG secondary antibody | Gutierrez-Exposito et al. (2013) |
| Blue tongue virus | Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Chamois (Rupicapra rupicapra), Alpine ibex (Capra ibex) | Competitive ELISA developed for cattle | Casaubon et al. (2013) |
| Borrelia burgdorferi sensu lato | Small Japanese field mouse (Apodemus argenteus), Large Japanese field mouse (Apodemus speciosus), Grey red-backed vole (Myodes rufocanus), Northern red-backed vole (Myodes rutilus) | Commercial indirect ELISA modified using an anti-mouse (Mus musculus) IgG secondary antibody | Taylor et al. (2013) |
| Bovine viral diarrhea virus | Common eland (Tragelaphus/Taurotragus oryx), Greater kudu (Tragelaphus strepsiceros) | In-house competitve ELISA | Scott et al. (2013) |
| Brucella sp. | Hooded seal (Cystophora cristata), Minke whale (Balaenoptera acutorostrata), Sei whale (Balaenoptera borealis), Fin whale (Balaenoptera physalus), Polar bear (Ursus maritimus), Caribou (Rangifer tarandus groenlandicus), Reindeer (Rangifer tarandus tarandus) | In-house indirect ELISA using peroxidase-conjugated protein A, protein G or protein A/G as a secondary “antibody” | Nymo et al. (2013) |
| Crimean-Congo hemorrhagic fever virus | European brown hare (Lepus europeus) | In-house indirect ELISA using goat anti-rabbit IgG secondary antibody | Nemeth et al. (2013) |
| Foot and mouth disease virus | Buffalo (Syncerus caffer) | Liquid phase blocking ELISA (similar to a competitive ELISA) | Miguel et al. (2013) |
| Francisella tularensis | Wild boar (Sus scrofa), Racoon (Nyctereutes procyonoides), Red fox (Vulpes vulpes) | In-house indirect ELISA using peroxydase-conjugated protein G as secondary “antibody” | Kuehn et al. (2013) |
| Leishmania mexicana | Mantled howler monkey (Alouatta palliata), Guatemalan black howler monkey (Alouatta pigra) | In-house indirect ELISA using crude parasite extracts and an anti-monkey IgG secondary antibody | Rovirosa-Hernandez et al. (2013) |
| Trypanosoma cruzi | |||
| Orthopoxvirus | Roof rat (Rattus rattus) | In-house indirect ELISA using crude Vaccinia virus antigen and goat anti-rat IgG secondary antibody | Salzer et al. (2013) |
| Peste des petits ruminants virus | Buffalo (Syncerus caffer), Thomson’s gazelle (Eudorcas thomsonii), Grant’s gazelle (Nanger granti) | Competitive ELISA developed for sheep and goats | Lembo et al. (2013) |
| Phocine herpesvirus 1 | Harbor seal (Phoca vitulina) | In-house indirect ELISA using peroxidase-conjugated protein A as a secondary “antibody” | Roth et al. (2013) |
| Rabies virus | Raccoon (Nyctereutes procyonoides), Red fox (Vulpes vulpes) | Commercial competitive ELISA; in-house indirect ELISA using an anti-dog IgG secondary antibody | Wasniewski et al. (2013) |
| Rift valley fever virus | Buffalo (Syncerus caffer), Common eland (Tragelaphus/Taurotragus oryx), Giraffe (Giraffa camelopardalis), Waterbuck (Kobus ellipsiprymus), Kongoni/Hartebeest (Alcelaphus buselaphus), Gerenuk (Litocranius walleri), Warthog (Phacochoerus africanus), Impala (Apyceros melampus), Lesser Kudu (Tragelaphus imberbis) | In-house competitve ELISA | Britch et al. (2013) |
| Swine vesicular disease, hepatitis E virus, swine influenza virus, Aujesky’s disease virus, African swine fever virus, classical swine fever virus, porcine parvovirus, porcine rotavirus, porcine respiratory coronavirus, transmissible gastroentiritis virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus | Wild boar (Sus scrofa) | Direct use of kits designed for use in pig farms | Albayrak et al. (2013); Rodriguez-Prieto (2013) |
| Trichinella zimbabwensis | Nile crocodile (Crocodylus niloticus) | In-house indirect ELISA with a specifically produced rabbit anti-crocodile IgG used as secondary antibody | Ludovisi et al. (2013) |
| West Nile virus | White stork (Ciconia ciconia), Goshawk (Accipiter gentilis), Rock dove (Columba livia), Black kite (Milvus migrans), Blackbird (Turdus merula) | Competitive ELISA developed for wild birds screening | Bakonyi et al. (2013) |
The table illustrates the tools that are available across a wide range of wildlife species, especially birds and mammals. The existence of these ELISA kits and host species-compatible secondary reagents suggests that raw materials are available for development of custom assays tailored for application to many eco-immunological systems and questions.
Obviously species-specific secondary antibodies are mostly available for species of economic importance or for laboratory model species. However, this encompasses wild species which have a domestic counterpart,for example, rabbits (Pathak et al. 2012), wolves (Nelson et al. 2012), horses (Franson et al. 2011), sheep (see below), wild boar (Table 1) or are commonly used as laboratory models in biomedicine, for example, mice (Abolins et al. 2011), rats (Devalapalli et al. 2006), baboons (Gicheru et al. 2009), and chimpanzees (Nerrienet et al. 2005). It is interesting to note the relative paucity of eco-immunological studies of specific immune defense in wild populations of model species such as primates or small rodents, although there is probably the widest library of immunological reagents and techniques available for these species due to their place in human medical research. For instance, none of these species is present in the review of wildlife serological studies published in 2013 we compiled in Table 1, although these species might have been studied via alternative approaches in the wild (Archie 2013; Turner and Paterson 2013). For a relatively wide range of species, reagents would be available in phylogenetically close species and one can then take advantage of the cross-reactivity of antibodies developed for a related species. Although defining a threshold for positivity when cross-reactive antibodies are used can be problematic (Peel et al. 2013), carefully chosen positive and negative controls allow for good power of detection. These can include individuals from geographically distant areas as negative controls (when the pathogen is endemic) or culture-positive or PCR-positive individuals as positive controls. In addition, in species with domestic or laboratory counterparts, samples from experimentally infected individuals are excellent controls. Another option that has been successfully used in the wild (Table 1) is to replace the secondary antibody with a protein with non-specific binding properties,for example, protein G, protein A or protein M (e.g., Nymo et al. 2013; Grover et al. 2014).
Another consideration in development of assays is the availability of antigens of relevant parasites. Although refined antigens can be used when available, crude extract can be a source of antigen that can be obtained easily for some parasites such as some bacteria and macroparasites (in particular helminths). For example, parasites obtained from the blood or feces of wild animals can be washed, freeze-thawed and ground to generate suitable extracts of antigens for use in ELISA, as is common practice in studies of immunology in the laboratory. The same secondary antibodies that would be used to modify commercial kits can be used to complete the assay. The development of such an assay does take time, for example, to optimize the concentrations of the coating antigen, the sample itself, and the secondary-detection antibody. This is particularly true when detection-reagents from several different species need to be compared for sensitivity and specificity.
Such approaches have allowed study of a wide range of species; for studies published during the calendar year of 2013, parasite-specific antibodies have been measured in at least 71 different wild species (Table 1). It should be noted, however, that most of these studies are strongly rooted in epidemiology (see Gilbert et al. 2013), rather than in the evolutionary framework that characterizes eco-immunological approaches. Below, we provide examples of how such assays can be applied to provide insights into the eco-immunology of wild animals.
Applying the toolbox to avian systems: eco-immunological insights from seabirds
Seabirds represent interesting models for eco-immunology, in particular because they display a wide range of life-history traits (e.g., representing several orders of magnitude of variation in lifespan and reproductive schedules and thus have potential to test the pace-of-life hypotheses). In addition, they are usually relatively faithful to their breeding sites, thus allowing longitudinal monitoring of individuals within breeding colonies. Seabirds, however, are phylogenetically distant from commercially available species such as chickens, and thus have restricted potential for direct application of laboratory techniques of use in the poultry industry. Other complications of seabirds’ systems include limited access to the individuals as this usually is possible only during the reproductive season and, for some species, the limited amount of blood that can be obtained safely. The latter complication can be overcome to some extent when measuring antibodies by taking advantage of the transfer of antibodies in the eggs (Gasparini et al. 2001; Grindstaff et al. 2003; Boulinier and Staszewski 2008). Eggs can be collected easily and level of antibodies in the yolk directly reflects circulating antibodies in the female parent (Gasparini et al. 2002; Grindstaff 2010). In addition, extraction of antibodies from egg yolks is easy in the laboratory and produces large sample volumes (Kuiken et al. 1998; Pearce-Duvet et al. 2009). Sampling eggs may be justified in the wild in species for which controls of reproduction are being implemented or when several eggs are produced but only a limited number of them produce fledglings.
A first approach successfully used in seabirds has been to take advantage of commercially available competitive ELISA kits to measure specific antibodies. So far, these kits mostly have been applied to understanding epidemiology; however when prevalence is sufficiently high and when a specific antibody reflects resistance as well as exposure, infectious diseases may represent models of interest for eco-immunological studies. For instance, avian influenza viruses are infectious agents that are of both eco-immunological and epidemiological interest and that have been repeatedly found in seabirds (Munster et al. 2007). Using antibodies extracted from egg yolks and plasma of adult female yellow-legged gulls (Larus michahellis; Fig. 1A), Hammouda et al. (2012) showed that levels of antibodies in the females were correlated with the levels in the eggs but that the latter levels decreased within clutches over the course of the laying sequence. This pattern suggests variation among mothers in the extent of maternal transfer of immunity, as well as differential benefits to offspring, depending upon laying order. Each of these findings is of tremendous interest in eco-immunology (Grindstaff et al. 2003; Boulinier and Staszewski 2008) and more broadly in the evolutionary ecology of maternal effects (Mousseau and Fox 1998). In addition, immunological data obtained by ELISA can be used to parameterize mathematical models. For instance, Hoye et al. (2011) estimated persistence of anti-influenza antibody over the annual cycle of a migrating species, the pink-footed goose (Anser brachyrhynchus). The half-life they reported is shorter than would be expected for such a long-lived species under the pace-of-life hypothesis (Hoye et al. 2011). This suggests a role for the ecology of the pathogen in governing the immune dynamics of the host. This result also emphasizes that the ecology and evolution of parasites likely play an important role in shaping the evolution of immunological memory in their hosts and that this can only be studied by measuring specific markers of the immune response.
Fig. 1.
Examples of wild species to which the veterinary immunology toolbox has been applied successfully. (A) Yellow-legged gull (Larus michahellis) in the Mediterranean region. (B) Black-legged kittiwake (Rissa tridactyla) in Norway. (C) Soay sheep (Ovis aries) on the island of Hirta in the St. Kilda Archipelago, Scotland. See text for more details on the measures of immunity performed in each case. All photographs © R. Garnier.
Another interesting approach to the eco-immunology of seabirds is to control the exposure of the hosts and then use competitive ELISAs to measure their response to a known challenge. One simple way to take advantage of the veterinary toolbox is to use vaccines that have been developed for poultry to manipulate the exposure of birds in the wild (Staszewski and Boulinier 2004). Not all vaccines are suitable for this approach. Some vaccines indeed use live attenuated bacteria or viruses, and introducing these into the wild may have devastating consequences. Only vaccines relying on heat-killed or chemically killed parasites or recombinant antigens should be used to manipulate exposure in natural populations. One such vaccine in birds is available for Newcastle Disease Virus (NDV), and several competitive ELISAs are available, thanks to the poultry industry, to quantify individual variation in immune response to vaccination. In seabirds, this vaccine has been used to successfully manipulate the antigen exposure of black-legged kittiwakes, Rissa tridactyla (Staszewski et al. 2007; Fig. 1B) so as to study the functional role of maternal antibodies depending on the timing of exposure, and of Cory’s shearwater, Calonectris diomedea (Garnier et al. 2012b). The latter study then measured specific anti-NDV IgY throughout the particularly long rearing period of the chicks in this species. As antibodies are not transferred after hatching in most bird species, this approach allowed the monitoring of the decay of maternal antibodies in the chicks, and highlighted the very long half-life of IgY in this species of seabirds with a very slow pace of life. In Cory’s shearwater, IgY decayed over three times slower than the rate usually observed in chickens and most of the chicks followed during this study still had detectable antibodies 70 days after hatching (Garnier et al. 2012b). The relative roles of phylogeny and pace of life and the detailed mechanism underlying this very long persistence, however, remain to be determined. This would require measuring antibody half-life in a variety of species, probably using the widely applicable commercial competitive ELISAs.
When only sandwich ELISAs are available, using anti-chicken IgY secondary antibody has proven successful against a wide array of avian species. In seabirds, this has been successfully used to document the marine cycle of the Lyme disease bacteria belonging to the Borrelia burgdorferi sensu lato complex (Olsén et al. 1993; Gasparini et al. 2001). As Lyme disease is an important zoonosis in humans, ELISA kits are commercially available and some of them include the subtypes of bacteria that are commonly found in the marine cycle (Olsen et al. 1995). Replacing the secondary antibody in these kits by an anti-chicken IgY antibody allowed for the detection of anti-Borrelia antibodies in four out of 10 species of seabirds both in the North Atlantic and the North Pacific (Staszewski et al. 2008; Lobato et al. 2011). It is important to note that these ELISAs use the same secondary antibodies for very different species. Affinity of this antibody is likely to vary among species and this would result in different thresholds of positivity among species. One solution is to perform western blots (which, with antigen bound to a blot probed with serum or plasma, tend to be more sensitive than ELISAs) to confirm the positive status of individuals. As this would be the course of action of clinicians in the face of a positive ELISA in a human patient, all the reagents to perform these assays are commercially available and, again, only the secondary antibody needs to be modified. The thresholds are then set by using the mean optical density measure of all the individuals confirmed as positives by the western blot. The resulting cut-off fits the bimodal distribution of optical densities observed when using this ELISA in kittiwakes reasonably well (Chambert et al. 2012), although further refinements can include directly modeling a mixture of two distributions, one for positive and one for negative individuals, respectively, to determine the seropositivity (Choquet et al. 2013). Overall, these approaches show that this parasite is widespread in seabirds in both the North Atlantic and the North Pacific but that difference in prevalence exist between species (Staszewski et al. 2008; Lobato et al. 2011). These results suggest that adaption in the tick vector and differences in the immune response between host species may be interacting to explain such complex dynamics. In addition, antibody levels can be used in models of population dynamics to estimate the effects of parasites on the fitness of their hosts. In the case of Lyme disease, antibody levels can be used as markers of exposure to the bacteria and included as covariates in multi-event capture-mark-recapture models. Chambert et al. (2012) showed that there was no statistical support for an effect of seropositivity on the survival of kittiwakes and thus concluded that exposure to Lyme disease did not affect survival over winter in this system. This illustrates the need to analyze serological data in the light of the fitness of the host to test eco-immunological predictions (Telfer et al. 2002; Raberg et al. 2003).
Overall, these examples illustrate that eco-immunological insights can be gained in non-model bird species when measuring immune responses elicited by parasite-specific antigens. When no parasite can be readily identified, vaccination can be chosen so that a convenient immunological technique can be used. In any case, measuring antigen-specific markers can help shed light on the evolutionary ecology of immune responses at the individual, population, and species levels.
Applying the toolbox to mammals: the case study of the Soay sheep
Eco-immunological studies of wild-mammal systems may likewise benefit from techniques developed for domestic animals and wildlife species (Table 1), or even for humans. The longitudinally monitored Soay sheep (Fig. 1C) population of St Kilda archipelago (Scotland) represent a case study of this approach. The sheep were introduced onto the island of Hirta in the 1930s from a stock of sheep left unmanaged for thousands of years on the neighboring island of Soay (Clutton-Brock and Pemberton 2004). The Soay sheep belong to the same species as the domestic sheep, Ovis aries, and thus can benefit from all the reagents developed for domestic sheep, including some commercially available detection-reagents (secondary anti-sheep immunoglobulins). Gastrointestinal nematodes, and in particular a pathogenic strongyle species infecting the Soay sheep, Teladorsagia circumcincta, contribute to high mortality during certain winters (Grenfell et al. 1992; Coulson et al. 2001; Hayward et al. 2011).
Defined strongyle antigens associated with protective immunity in sheep (e.g., Nisbet et al. 2013; Shaw et al. 2013) are increasingly available to enable quantification of specific antibody-mediated defense against these nematodes. However, crude preparations of antigen are still routinely used by veterinary immunologists. For example, whole-parasite lysate can be used to coat ELISA wells. In the Soay sheep, higher titers of antibodies measured in this way proved to be associated with lower counts of nematode eggs in the feces (Hayward et al., submitted for publication). However, the assays also used a commercially available secondary reagent that measured the aggregate all-isotype antibody response, despite the fact that IgA, IgE, and IgG participate differentially in clearance of nematodes from domestic sheep (Stear et al. 1995; Strain et al. 2002; Williams et al. 2010). Furthermore, the assay used crude antigens from adult nematodes and veterinary research suggests these may be less important for resistance than is the immune response to larval antigens (Stear et al. 1999).
Veterinary immunologists on the collaborative team therefore prepared a crude lysate of L3 larval parasites and developed, through an admittedly arduous, although standard (Wild 2013) process, secondary antibodies to permit detection of the different functional variants of immunoglobulin (IgA, IgE, IgG, and IgM) of sheep. These refinements allowed quantification of different functional types of antibody specific to the larval nematode parasite of interest. Interestingly, different isotypes and specificities of antibodies proved to relate differently to fitness of Soay ewes (Nussey et al. 2014). For instance, L3-specific IgG was an independent predictor of overwinter survival, suggesting that a specific immune response to gastrointestinal worms may improve fitness. These results illustrate the utility of drawing upon veterinary immunology to measure specific immunity, and thereby to understand the fitness-consequences of variations in immunity in nature.
In a separate eco-immunological study, human ELISAs were adapted for use in the Soay sheep (in an approach that parallels the Lyme disease example in seabirds). By replacing the anti-human secondary antibody in the kit with the commercially available sheep-specific secondary antibody, autoreactive antibodies (“anti-nuclear antibodies”, ANA) were measured in the Soay sheep (Graham et al. 2010). The antigens used in these assays are nucleic acids and proteins derived from the nucleus of human cell lines. Although not from sheep, these molecules are strongly conserved within mammals. This marker of autoimmunity proved to be a predictor of overwinter survival in adult females (Graham et al. 2010) and the positive effect of this aspect of the immune response on survival was independent of the positive effect of the anti-nematode IgG outlined above (Nussey et al. 2014).
In closing, we note that the benefits of having a well-studied domestic counterpart can go well beyond access to reagents for antibody ELISAs. As monoclonal antibodies specific to surface markers of ovine T-cells (CD4, CD8, and γδ T-cell receptor) are available, flow cytometry can be used to differentiate and quantify different subpopulations of T-lymphocytes. These tools have been used to study patterns of lymphocyte phenotype with age in the Soay sheep (Nussey et al. 2012). Nussey et al. (2012) also quantified the expression of other markers such as FoxP3 for which no ovine-specific secondary antibody was available; they thus used anti-rat FoxP3 secondary antibody, which had previously been shown to cross-react with ovine FoxP3 (Rocchi et al. 2011). This example illustrates that non-specific secondary antibodies developed for one species may be of use in others. There may thus be scope for wider application of such tools in the wild.
These examples from the Soay sheep system illustrate the benefits of crosstalk between veterinary immunologists and eco-immunologists. Access to state-of-the-art serological reagents relatively specific to a wild system enables measurement of parasite-specific antibodies. This can in turn reveal how variations in immune phenotype between individuals determine life-history characteristics such as winter survival, and ultimately fitness.
Perspectives
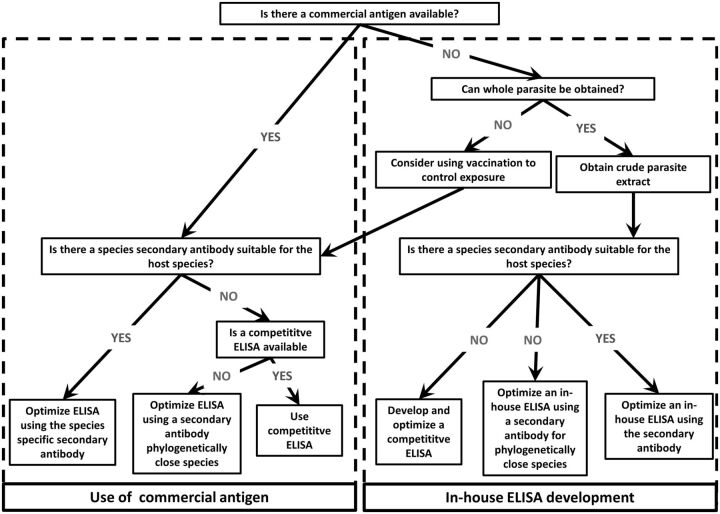
These examples show that specific immunological measures can represent achievable goals in wild animal species. Some tools of veterinary immunology are immediately applicable, whereas others must be tailored. We suggest that the process of choosing which approach is best suited to a particular situation can be summarized in a few simple steps (Fig. 2). Briefly, this includes first choosing between the use of commercial antigens or the in-house production of crude parasite extracts. If a commercial product exists or if a vaccination has been performed, a sandwich ELISA might be modified, preferably using a species-specific secondary antibody or, if that is not available, a secondary antibody developed for a phylogenetically related species. Alternatively, a competitive ELISA might be available. Similar steps can be followed when using parasite extracts but the development of a competitive ELISA would probably prove more challenging as it requires (polyclonal) antibodies directed against the parasite extract. In any case, the process presented in Fig. 2 illustrates that all the steps are possible in wild systems.
Fig. 2.
Flow-chart for selection of ELISA. When no assay has been developed for a given host species and its parasites, one can choose whether to develop an in-house ELISA or to adapt an existing commercial kit by using this flow-chart.
In the current review, we have put the focus on ELISA techniques that represent accessible ways to measure parasite-specific antibodies, even when one has to start from scratch. Serology has indeed been widely used and discussed in an epidemiological context and some that are problem-specific to its application in the wild, such as the determination of cut-off values between positive and negative individuals, have been discussed (Gilbert et al. 2013). Solutions are emerging (e.g., Cizauskas et al. 2014) that will likely only increase the potential for eco-immunological studies based on parasite-specific serological approaches.
The measure of parasite-specific antibodies is not accessible only through the use of ELISA and in some cases other laboratory techniques are available. These include, for instance, viral neutralization or western blots, which can provide additional information unavailable by the use of ELISAs. It is also important to note that antibodies are not always protective, although coupling measures of immune phenotype with data on parasite burden and fitness of the host can clarify the contribution of antibody to defense (Graham et al. 2011). More generally, eco-immunological studies may benefit from measuring a combination of these specific and general markers of immunity—e.g., natural and parasite-specific antibodies in the same animals—or paired measures of specific immunity against several parasites (Viney et al. 2005). Such dual approaches are required to detect effects such as the facilitation of susceptibility to tuberculosis, a disease that in individuals infected by nematodes is almost exclusively controlled by a cellular immune response (Ezenwa et al. 2010).
Whether assays focus on antibodies or not, we argue that building upon advances in veterinary and human immunology can only help eco-immunology grow. Conversely, understanding which factors affect the ecology and evolution of the immune systems in wild contexts will provide important insights into immunology (Maizels and Nussey 2013) and epidemiology, especially when considering that wildlife is the main provider of emerging infectious diseases in human populations (Jones et al. 2008). Like Hawley and Altizer (2011), we believe that cross-fertilization between disease ecology, epidemiology, and eco-immunology would facilitate the advancement of all these fields.
Acknowledgments
We thank the organizers of the Methods and Mechanisms in Eco-Immunology Symposium at SICB 2014 for the invitation to participate. We also thank our many collaborators for their efforts to adapt veterinary and human immunoassays for use in wild seabirds and sheep. We also thank Thierry Boulinier, two anonymous reviewers, and the editor for their insightful comments.
References
- Abad FX, Busquets N, Sanchez A, Ryan PG, Majó N, Gonzalez-Solís J. Serological and virological surveys of the influenza A viruses in Antarctic and sub-Antarctic penguins. Antarct Sci. 2013;25:339–44. [Google Scholar]
- Abolins SR, Pocock MJ, Hafalla JC, Riley EM, Viney ME. Measures of immune function of wild mice, Mus musculus. Mol Ecol. 2011;20:881–92. doi: 10.1111/j.1365-294X.2010.04910.x. [DOI] [PubMed] [Google Scholar]
- Albayrak H, Ozan E, Cavunt A. A serological survey of selected pathogens in wild boar (Sus scrofa) in northern Turkey. Eur J Wildl Res. 2013;59:893–97. doi: 10.1007/s10344-013-0743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie EA. Wound healing in the wild: stress, sociality and energetic costs affect wound healing in natural populations. Parasite Immunol. 2013;35:374–85. doi: 10.1111/pim.12048. [DOI] [PubMed] [Google Scholar]
- Bakonyi T, Ferenczi E, Erdélyi K, Kutasi O, Csörgő T, Seidel B, Weissenböck H, Brugger K, Bán E, Nowotny N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in 2008/2009, central Europe. Vet Microbiol. 2013;165:61–70. doi: 10.1016/j.vetmic.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Boots M, Best A, Miller MR, White A. The role of ecological feedbacks in the evolution of host defence: what does theory tell us? Phil T Roy SocB. 2009;364:27–36. doi: 10.1098/rstb.2008.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots M, Donnelly R, White A. Optimal immune defence in the light of variation in lifespan. Parasite Immunol. 2013;35:331–8. doi: 10.1111/pim.12055. [DOI] [PubMed] [Google Scholar]
- Boughton RK, Joop G, Armitage SAO. Outdoor immunology: methodological considerations for ecologists. Funct Ecol. 2011;25:81–100. [Google Scholar]
- Boulinier T, Staszewski V. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol Evol. 2008;23:282–8. doi: 10.1016/j.tree.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Britch S, Binepal Y, Ruder M, Kariithi H, Linthicum K, Anyamba A. Rift Valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS One. 2013;8:e66626. doi: 10.1371/journal.pone.0066626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler DM, Tieleman BI, Piersma T. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr Comp Biol. 2010;50:346–57. doi: 10.1093/icb/icq055. [DOI] [PubMed] [Google Scholar]
- Casaubon J, Chaignat V, Vogt H-R, Michel AO, Thür B, Ryser-Degiorgis M-P. Survey of bluetongue virus infection in free-ranging wild ruminants in Switzerland. BMC Vet Res. 2013;9:1–10. doi: 10.1186/1746-6148-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S-Y, Seo H-S, Kang M, Jang H-K. Serologic survey for antibodies to canine parvovirus and influenza virus in wild raccoon dogs (Nyctereutes procyonoides) in South Korea. J Wildlife Dis. 2013;49:200–2. doi: 10.7589/2012-06-158. [DOI] [PubMed] [Google Scholar]
- Chambert T, Staszewski V, Lobato E, Choquet R, Carrie C, McCoy KD, Tveraa T, Boulinier T. Exposure of black-legged kittiwakes to Lyme disease spirochetes: dynamics of the immune status of adult hosts and effect on their survival. J Anim Ecol. 2012;81:986–95. doi: 10.1111/j.1365-2656.2012.01979.x. [DOI] [PubMed] [Google Scholar]
- Choquet R, Carrie C, Chambert T, Boulinier T. Estimating transitions between states using measurements with imperfect detection: application to serological data. Ecology. 2013;94:2160–5. doi: 10.1890/12-1849.1. [DOI] [PubMed] [Google Scholar]
- Cizauskas CA, Bellan SE, Turner WC, Vance RE, Getz WM. Frequent and seasonally variable sublethal anthrax infections are accompanied by short-lived immunity in an endemic system. J Anim Ecol. 2014 doi: 10.1111/1365-2656.12207. doi: 0.1111/1365-2656.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T, Pemberton J. Soay sheep: dynamics and selection in an island population. Cambridge (UK): 2004. p. Cambridge University Press. [Google Scholar]
- Coulson T, Catchpole EA, Albon SD, Morgan BJT, Pemberton JM, Clutton-Brock TH, Crawley MJ, Grenfell BT. Age, sex, density, winter weather, and population crashes in Soay sheep. Science. 2001;292:1528–31. doi: 10.1126/science.292.5521.1528. [DOI] [PubMed] [Google Scholar]
- Crowther JR. The ELISA guidebook. 2nd ed. New York (NY): Humana Press; 2009. [Google Scholar]
- Demas GE, Nelson RJ. Ecoimmunology. New York (NY): Oxford University Press; 2011. [Google Scholar]
- Devalapalli A, Lesher A, Shieh K, Solow J, Everett M, Edala A, Whitt P, Long R, Newton N, Parker W. Increased levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scand J Immunol. 2006;64:125–36. doi: 10.1111/j.1365-3083.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE. Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. The American Naturalist. 2010;176:613–24. doi: 10.1086/656496. [DOI] [PubMed] [Google Scholar]
- Franson JC, Hofmeister EK, Collins GH, Dusek RJ. Seroprevalence of West Nile Virus in feral horses on Sheldon National Wildlife Refuge, Nevada, United States. Am J Trop Med Hyg. 2011;84:637. doi: 10.4269/ajtmh.2011.10-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier R, Boulinier T, Gandon S. Coevolution between maternal transfer of immunity and other resistance strategies against pathogens. Evolution. 2012a;66:3067–78. doi: 10.1111/j.1558-5646.2012.01665.x. [DOI] [PubMed] [Google Scholar]
- Garnier R, Ramos R, Staszewski V, Militão T, Lobato E, González-Solís J, Boulinier T. Maternal antibody persistence: a neglected life history trait with implications from albatross conservation to comparative immunology. Proc Biol Sci. 2012b;279:2033–41. doi: 10.1098/rspb.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J, McCoy KD, Haussy C, Tveraa T, Boulinier T. Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc Biol Sci. 2001;268:647–50. doi: 10.1098/rspb.2000.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J, McCoy KD, Tveraa T, Boulinier T. Related concentrations of specific immunoglobulins against the Lyme disease agent Borrelia burgdorferi sensu lato in eggs, young and adults of the kittiwake (Rissa tridactyla) Ecol Lett. 2002;5:519–24. [Google Scholar]
- Gicheru M, Jeneby M, Macharia J, Carlsson H-E, Suleman M. Prevalence of antibodies and cell mediated immune response against Leishmania major in feral nonhuman primates from Kenya. Acta Tropica. 2009;109:136–40. doi: 10.1016/j.actatropica.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Gilbert AT, Fooks A, Hayman D, Horton D, Müller T, Plowright R, Peel A, Bowen R, Wood J, Mills J. Deciphering serology to understand the ecology of infectious diseases in wildlife. Ecohealth. 2013;10:298–313. doi: 10.1007/s10393-013-0856-0. [DOI] [PubMed] [Google Scholar]
- Graham AL, Allen JE, Read AF. Evolutionary causes and consequences of immunopathology. Annu Rev Ecol Evol Syst. 2005;36:373–97. [Google Scholar]
- Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–5. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- Graham AL, Shuker DM, Pollitt LC, Auld SKJR, Wilson AJ, Little TJ. Fitness consequences of immune responses: strenghtening the empirical framework for ecoimmunology. Funct Ecol. 2011;25:5–17. [Google Scholar]
- Grenfell BT, Price OF, Albon SD, Clutton-Brock TH. Early development and population fluctuations in Soay sheep. Nature. 1992;355:823–6. doi: 10.1038/355823a0. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Initial levels of maternally derived antibodies predict persistence time in offspring circulation. J Ornithol. 2010;151:423–8. [Google Scholar]
- Grindstaff JL, Brodie ED, III, Ketterson ED. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc Biol Sci. 2003;270:2309–19. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover RK, Zhu X, Nieusma T, Jones T, Boero I, MacLeod AS, Mark A, Niessen S, Kim HJ, Kong L. A structurally distinct human mycoplasma protein that generically blocks antigen-antibody union. Science. 2014;343:656–61. doi: 10.1126/science.1246135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Expósito D, Ortega-Mora LM, Marco I, Boadella M, Gortázar C, Miguel-Ayanz JMS, García-Lunar P, Lavín S, Álvarez-García G. First serosurvey of Besnoitia spp. infection in wild European ruminants in Spain. Vet Parasitol. 2013;197:557–64. doi: 10.1016/j.vetpar.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Hammouda A, Selmi S, Pearce-Duvet J, Chokri MA, Arnal A, Gauthier-Clerc M, Boulinier T. Maternal antibody transmission in relation to mother fluctuating asymmetry in a long-lived colonial seabird: the yellow-legged gull Larus michahellis. PLoS One. 2012;7:e34966. doi: 10.1371/journal.pone.0034966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DM, Altizer SM. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol. 2011;25:48–60. [Google Scholar]
- Hayward AD, Wilson AJ, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. Natural selection on a measure of parasite resistance varies across ages and environmental conditions in a wild mammal. J Evol Biol. 2011;24:1664–76. doi: 10.1111/j.1420-9101.2011.02300.x. [DOI] [PubMed] [Google Scholar]
- Hoye BJ. Variation in postsampling treatment of avian blood affects ecophysiological interpretations. Methods Ecol Evol. 2011;3:162–7. [Google Scholar]
- Hoye BJ, Munster VJ, Nishiura H, Fouchier RAM, Madsen J, Klaassen M. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos. 2011;120:748–55. [Google Scholar]
- Huang Y, Wille M, Dobbin A, Robertson GJ, Ryan P, Ojkic D, Whitney H, Lang AS. A 4-year study of avian influenza virus prevalence and subtype diversity in ducks of Newfoundland, Canada. Can J Microbiol. 2013;59:701–8. doi: 10.1139/cjm-2013-0507. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–4. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012;3:163–82. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- Kuehn A, Schulze C, Kutzer P, Probst C, Hlinak A, Ochs A, Grunow R. Tularaemia seroprevalence of captured and wild animals in Germany: the fox (Vulpes vulpes) as a biological indicator. Epidemiol Infect. 2013;1:1–8. doi: 10.1017/S0950268812001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Leighton FA, Wobeser G, Danesik KL, Riva J, Heckert RA. An epidemic of newcastle disease in double-crested cormorants from Saskatchewan. J Wildlife Dis. 1998;34:457–71. doi: 10.7589/0090-3558-34.3.457. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–15. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lembo T, Oura C, Parida S, Hoare R, Frost L, Fyumagwa R, Kivaria F, Chubwa C, Kock R, Cleaveland S. Peste des petits ruminants infection among cattle and wildlife in Northern Tanzania. Emerg Infect Dis. 2013;19:2037–40. doi: 10.3201/eid1912.130973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato E, Pearce-Duvet J, Staszewski V, Gomez-Diaz E, Gonzalez-Solis J, Kitaysky A, McCoy KD, Boulinier T. Seabirds and the circulation of Lyme borreliosis bacteria in the North Pacific. Vector Borne Zoonotic Dis. 2011;11:1521–7. doi: 10.1089/vbz.2010.0267. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Ludovisi A, Grange LJL, Morales MAG, Pozio E. Development of an ELISA to detect the humoral immune response to Trichinella zimbabwensis in Nile crocodiles (Crocodylus niloticus) Vet Parasitol. 2013;194:189–92. doi: 10.1016/j.vetpar.2013.01.053. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Nussey DH. Into the wild: digging at immunology’s evolutionary roots. Nat Immunol. 2013;14:879–83. doi: 10.1038/ni.2643. [DOI] [PubMed] [Google Scholar]
- Martin LB, Hasselquist D, Wikelski M. Investment in immune defense is linked to pace of life in house sparrows. Oecologia. 2006;147:565–75. doi: 10.1007/s00442-005-0314-y. [DOI] [PubMed] [Google Scholar]
- Miguel E, Grosbois V, Caron A, Boulinier T, Fritz H, Cornélis D, Foggin C, Makaya PV, Tshabalala PT, de Garine-Wichatitsky M. Contacts and foot and mouth disease transmission from wild to domestic bovines in Africa. Ecosphere. 2013;4:art51. [Google Scholar]
- Miller MR, White A, Boots M. Host life span and the evolution of resistance characteristics. Evolution. 2007;61:2–14. doi: 10.1111/j.1558-5646.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford: Oxford University Press; 1998. [Google Scholar]
- Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WEP, Schutten M, Olsen B, Osterhaus ADME, Fouchier RAM. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:630–8. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. Janeway’s immunobiology. New York (NY): Garland Science; 2008. [Google Scholar]
- Nelson B, Hebblewhite M, Ezenwa V, Shury T, Merrill EH, Paquet PC, Schmiegelow F, Seip D, Skinner G, Webb N. Prevalence of antibodies to canine parvovirus and distemper virus in wolves in the Canadian Rocky Mountains. J Wildlife Dis. 2012;48:68. doi: 10.7589/0090-3558-48.1.68. [DOI] [PubMed] [Google Scholar]
- Németh V, Oldal M, Egyed L, Gyuranecz M, Erdélyi K, Kvell K, Kalvatchev N, Zeller H, Bányai K, Jakab F. Serologic evidence of Crimean-Congo hemorrhagic fever virus infection in Hungary. Vector Borne Zoonotic Dis. 2013;13:270–2. doi: 10.1089/vbz.2012.1011. [DOI] [PubMed] [Google Scholar]
- Nerrienet E, Santiago ML, Foupouapouognigni Y, Bailes E, Mundy NI, Njinku B, Kfutwah A, Muller-Trutwin MC, Barre-Sinoussi F, Shaw GM. Simian immunodeficiency virus infection in wild-caught chimpanzees from Cameroon. J Virol. 2005;79:1312–9. doi: 10.1128/JVI.79.2.1312-1319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet AJ, McNeilly TN, Wildblood LA, Morrison AA, Bartley DJ, Bartley Y, Longhi C, McKendrick IJ, Palarea-Albaladejo J, Matthews JB. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine. 2013;31:4017–23. doi: 10.1016/j.vaccine.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Nussey DH, Watt KA, Clark A, Pilkington JG, Pemberton JM, Graham AL, McNeilly TN. Multivariate immune defences and fitness in a wild mammal: complex but ecologically important associations among different plasma antibodies, host health and survival. Proc Biol Sci. 2014;281:20132931. doi: 10.1098/rspb.2013.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey DH, Watt K, Pilkington JG, Zamoyska R, McNeilly TN. Age-related variation in immunity in a wild mammal population. Aging Cell. 2012;11:178–80. doi: 10.1111/j.1474-9726.2011.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymo IH, Godfroid J, Åsbakk K, Larsen AK, das Neves CG, Rødven R, Tryland M. A protein A/G indirect enzyme-linked immunosorbent assay for the detection of anti-Brucella antibodies in Arctic wildlife. J Vet Diagn Invest. 2013;25:369–75. doi: 10.1177/1040638713485073. [DOI] [PubMed] [Google Scholar]
- Olsen B, Duffy DC, Jaenson TGT, Gylfe Å, Bonnedahl J, Bergström J. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol. 1995;33:3270–4. doi: 10.1128/jcm.33.12.3270-3274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén B, Jaenson TGT, Noppa L, Bunikis J, Bergström S. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature. 1993;362:340–2. doi: 10.1038/362340a0. [DOI] [PubMed] [Google Scholar]
- Pathak AK, Pelensky C, Boag B, Cattadori IM. Immuno-epidemiology of chronic bacterial and helminth co-infections: Observations from the field and evidence from the laboratory. Int J Parasitol. 2012;42:647–55. doi: 10.1016/j.ijpara.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Pearce-Duvet JMC, Gauthier-Clerc M, Jourdain E, Boulinier T. Maternal antibody transfer in yellow-legged gulls. Emerg Infect Dis. 2009;15:1147–9. doi: 10.3201/eid1507.090036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AB, Babayan SA. Wild immunology. Mol Ecol. 2011;20:872–80. doi: 10.1111/j.1365-294X.2010.04938.x. [DOI] [PubMed] [Google Scholar]
- Peel AJ, McKinley TJ, Baker KS, Barr JA, Crameri G, Hayman DT, Feng Y-R, Broder CC, Wang LF, Cunningham AA. Use of cross-reactive serological assays for detecting novel pathogens in wildlife: Assessing an appropriate cutoff for henipavirus assays in African bats. J Virol Methods. 2013;193:295–303. doi: 10.1016/j.jviromet.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberg L, Stjernman M, Hasselquist D. Immune responsiveness in adult blue tits: heritability and effects of nutritional status during ontogeny. Oecologia. 2003;136:360–4. doi: 10.1007/s00442-003-1287-3. [DOI] [PubMed] [Google Scholar]
- Rocchi MS, Wattegedera SR, Frew D, Entrican G, Huntley JF, McNeilly TN. Identification of CD4(+)CD25(high) Foxp3(+) T cells in ovine peripheral blood. Vet Immunol Immunopathol. 2011;144:172–7. doi: 10.1016/j.vetimm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Prieto V, Kukielka D, Martínez-López B, de las Heras AI, Barasona JÁ, Gortázar C, Sánchez-Vizcaíno JM, Vicente J. Porcine reproductive and respiratory syndrome (PRRS) virus in wild boar and Iberian pigs in south-central Spain. Eur J Wildlife Res. 2013;59:859–67. [Google Scholar]
- Roth SJ, Tischer BK, Kovacs KM, Lydersen C, Osterrieder N, Tryland M. Phocine herpesvirus 1 (PhHV-1) in harbor seals from Svalbard, Norway. Vet Microbiol. 2013;164:286–92. doi: 10.1016/j.vetmic.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Rovirosa-Hernández MDJ, Cortes-Ortíz L, García-Orduña F, Guzmán-Gómez D, López-Monteon A, Caba M, Ramos-Ligonio A. Seroprevalence of Trypanosoma cruzi and Leishmania mexicana in free-ranging howler monkeys in Southeastern Mexico. Am J Primatol. 2013;75:161–9. doi: 10.1002/ajp.22094. [DOI] [PubMed] [Google Scholar]
- Salaberria C, Muriel J, de Luna M, Gil D, Puerta M. The PHA test as an indicator of phagocytic activity in a passerine bird. PLoS One. 2013;8:e84108. doi: 10.1371/journal.pone.0084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JS, Carroll DS, Rwego IB, Li Y, Falendysz EA, Shisler JL, Karem KL, Damon IK, Gillespie TR. Serologic evidence for circulating orthopoxviruses in peridomestic rodents from rural Uganda. J Wildlife Dis. 2013;49:125–31. doi: 10.7589/2012-04-100. [DOI] [PubMed] [Google Scholar]
- Schat KA, Kaspers B, Kaiser P. Avian immunology. 2nd ed. London (UK): Elsevier/Academic Press; 2013. [Google Scholar]
- Schmid-Hempel P. Evolutionary parasitology: the integrated study of infections, immunology, ecology and genetics. Oxford (UK): Oxford University Press; 2011. [Google Scholar]
- Scott TP, Stylianides E, Markotter W, Nel L. Serological survey of bovine viral diarrhoea virus in Namibian and South African kudu (Tragelaphus strepsiceros) and eland (Taurotragus oryx) J South African Vet Assoc. 2013;84:1–3. [Google Scholar]
- Shaw R, Morris C, Wheeler M. Genetic and phenotypic relationships between carbohydrate larval antigen (CarLA) IgA, parasite resistance and productivity in serial samples taken from lambs after weaning. Int J Parasitol. 2013;43:661–7. doi: 10.1016/j.ijpara.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defence and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–21. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Staszewski V, Boulinier T. Vaccination: a way to address questions in behavioral and population ecology? Trends Parasitol. 2004;20:17–22. doi: 10.1016/j.pt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Staszewski V, Gasparini J, McCoy KD, Tveraa T, Boulinier T. Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J Anim Ecol. 2007;76:1215–23. doi: 10.1111/j.1365-2656.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Staszewski V, McCoy KD, Boulinier T. Variable exposure and immunological response to Lyme disease Borrelia among North Atlantic seabird species. Proc Biol Sci. 2008;275:2101–9. doi: 10.1098/rspb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stear M, Bishop S, Doligalska M, Duncan J, Holmes P, Irvine J, McCririe L, McKellar Q, Sinski E, Murray M. Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunol. 1995;17:643–52. doi: 10.1111/j.1365-3024.1995.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Stear MJ, Strain S, Bishop SC. Mechanisms underlying resistance to nematode infection. Int J Parasitol. 1999;29:51–6. doi: 10.1016/s0020-7519(98)00179-9. [DOI] [PubMed] [Google Scholar]
- Strain SAJ, Bishop SC, Henderson NG, Kerr A, McKellar QA, Mitchell S, Stear MJ. The genetic control of IgA activity against Teladorsagia circumcincta and its association with parasite resistance in naturally infected sheep. Parasitology. 2002;124:545–52. doi: 10.1017/s0031182002001531. [DOI] [PubMed] [Google Scholar]
- Taylor K, Takano A, Konnai S, Shimozuru M, Kawabata H, Tsubota T. Differential tick burdens may explain differential borrelia afzelii and borrelia garinii infection rates among four, wild, rodent species in Hokkaido, Japan. J Vet Med Sci. 2013;75:785–90. doi: 10.1292/jvms.12-0439. [DOI] [PubMed] [Google Scholar]
- Telfer S, Bennett M, Bown K, Cavanagh R, Crespin L, Hazel S, Jones T, Begon M. The effects of cowpox virus on survival in natural rodent populations: increases and decreases. J Anim Ecol. 2002;71:558–68. [Google Scholar]
- Turner AK, Paterson S. Wild rodents as a model to discover genes and pathways underlying natural variation in infectious disease susceptibility. Parasite Immunol. 2013;35:386–95. doi: 10.1111/pim.12036. [DOI] [PubMed] [Google Scholar]
- Viney ME, Riley EM, Buchanan KL. Optimal immune responses: immunocompetence revisited. Trends Ecol Evol. 2005;20:665–9. doi: 10.1016/j.tree.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wasniewski M, Guiot A, Schereffer J, Tribout L, Mähar K, Cliquet F. Evaluation of an ELISA to detect rabies antibodies in orally vaccinated foxes and raccoon dogs sampled in the field. J Virol Methods. 2013;187:264–70. doi: 10.1016/j.jviromet.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Wild DG. The immunoassay handbook: theory and applications of ligand binding, ELISA and related techniques. Waltham (MA): Elsevier; 2013. [Google Scholar]
- Williams A, Palmer D, Williams I, Vercoe P, Karlsson L. Faecal dry matter, inflammatory cells and antibodies in parasite-resistant sheep challenged with either Trichostrongylus colubriformis or Teladorsagia circumcincta. Vet Parasitol. 2010;170:230–7. doi: 10.1016/j.vetpar.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Hall JS, Flint PL, Franson JC, Ely CR, Schmutz JA, Samuel MD. High seroprevalence of antibodies to avian influenza viruses among wild waterfowl in Alaska: implications for surveillance. PLoS One. 2013;8:e58308. doi: 10.1371/journal.pone.0058308. [DOI] [PMC free article] [PubMed] [Google Scholar]