Abstract
Background
Significant numbers of chronic obstructive respiratory disease patients are readmitted for Acute Exacerbation (AE) within 30 days of discharge. And these early readmissions have serious clinical and socioeconomic consequences. The objective of our study was to determine the rate of readmission within 30 days of discharge and it’s predictors among patients treated for acute exacerbations of asthma and chronic obstructive pulmonary disease (COPD).
Methods
A prospective cohort study involving 130 patients (asthma = 59, COPD = 71) was conducted from April-September, 2019, in Jimma Medical Center (JMC), South-West Ethiopia. Socio-demographic, clinical, laboratory, and drug-related data were recorded at admission and during hospital stay. Cox regression analysis was performed to identify risk factors for readmissions following an AE of asthma and COPD.
Results
During the study period, 130 (male, 78(60%)) patients were admitted with AE of asthma and COPD. The median age was 59(IQR, 50–70) years. Of 130 patients, 21(18.10%) had a new AE of asthma and COPD that required hospitalization in the 30 days after discharge. The overall median survival time to 30-day readmission was 20 days (IQR, 16–29). Multivariate analysis revealed prolonged use of oxygen therapy (AHR = 4.972, 95% CI [1.041–23.736] and frequent hospital admissions (AHR = 11.482 [1.308–100.793]) to be independent risk factors for early readmissions.
Conclusion
Early hospital readmission rates for AE of asthma and COPD were alarmingly high. Frequent hospital admission and long-term oxygen therapy during hospital stay were independent predictors of 30-day readmission.
Background
Asthma and Chronic Obstructive Pulmonary Disease (COPD), commonly called chronic respiratory diseases, are common diseases with a heterogeneous distribution worldwide. The clinical course is punctuated by intermittent episodes of acute worsening of symptoms, termed exacerbations [1]. Exacerbations of asthma and COPD are defined as an acute onset and worsening of respiratory symptoms beyond the baseline level that require a change in medication for mild cases and emergency department (ED) visits or hospitalization in more severe cases [2]. Exacerbations due to lack of early prevention of the risk factor and co-morbidity are the main cause of hospital admission [1, 3–5].
According to the Global Burden ofDisease (GBD) report in 2015, asthma and COPD affected an estimated 358.2 and 174.5 million people, respectively [1, 3–5]. In the same year, COPD was responsible for 3.2 million deaths and 0.4 million deaths from asthma. COPD alone is accounted for 2.6% disability adjusted life years (DALYs), while 1.1% of the DALYs belonged to asthma [1]. Similarly, COPD is attributed to nearly 800,000 hospitalizations and estimated $50 billion in healthcare expenditures, while the healthcare expenditure due to asthma was $56 billion [3, 5].
In Africa, a spirometry based study showed the prevalence of COPD to be 13.4%. But, it was 4% by non-spirometry study [6], and that of asthma was 12.8% [7]. In Ethiopia, the prevalence of asthma was 29.6% [7], while COPD was 17.8% [6]. Asthma is responsible for 0.6% of death, while death attributed to COPD ranges from 3% to 5.2% [8].
Acute exacerbations of chronic obstructive respiratory diseases like asthma may result in excess medication use, emergency department visits, hospitalization, and even death. It also reduces quality of life and leads to time off work and school with the associated emotional and financial stress which accounts for almost 50% of total costs [9]. Similarly, acute exacerbations of COPD (AECOPD) may result in morbidity, and mortality as well as loss of lung function and impaired health status [10–12]. Prevention or amelioration of exacerbations has become a major therapeutic outcome as it is a common event with a median of 1.2 to 2.4 exacerbations per patient per year [13], and the frequency of exacerbations increases with increased severity of the disease [14–16].
Furthermore, unplanned 30 days hospital readmissions are a major health care burden accounting for as much as 58% of total costs in Medicare insured populations [17]. Nearly 20% of all Medicare discharges are readmitted within 30 days and among all discharge diagnoses, asthma and COPD are major causes [18]. Although studies are scarce in resource limited settings, risk-prediction model was developed focusing on which co-morbidities and risk factors affect 30-day readmission rates for asthma and COPD. Factors like tobacco use, diabetes mellitus, infections, and obesity, frequent hospital visits, ageare some of them [19, 20]. Readmissions in asthma and COPD patients have serious clinical and socioeconomic consequences [21]. A recent study showed that up to 20% of COPD patients readmitted due to AECOPD within 30days after discharge [22].
To date, there is no study evaluating the rate of readmission within 30 days of discharge among asthma and COPD patients in Ethiopia. The aim of this study was therefore to determine the rate of readmission within 30 days of discharge and it’s predictors among asthma and COPD patients presenting with acute exacerbations.
Methods
Study design and setting
This prospective cohort study was conducted at a University hospital in Ethiopia (Jimma Medical Center (JMC)) over a period of 6 months starting from April-September, 2019. JMC is located in Jimma town, 355 km from Addis Ababa, capital city. It is one of the oldest public hospitals found in the South -Western part of the country that runs under Jimma University. It is currently the only medical center in this part of the country. This medical center gives a service for a catchment’s population of 15 million and has more than 800 beds, 1600 staff members. It has several specialty clinics. As one of the specialty clinics, chest clinic has outpatient department (OPD) and pulmonology unit, which was the major source of patients for this study.
Study population and variables
We included all adult (age>18 years) patients with acute exacerbations of asthma and COPD admitted to medical wards, ED, and ICU of JMC during the study period. Cardiac asthma, Asthma-COPD overlap, asthma or COPD misdiagnosed patients and patients unwilling to participate in the study were excluded.
Data were collected on patient-related factors (socio-demographic, body mass index (BMI)), social drug use (smoking status), medication adherence, living with pet animals), disease-related factors (laboratory findings, clinical presentation, severity ofasthma and COPD at admission, duration of AE of asthma and COPD, and co morbidities), and drug related factors (in-hospital medications, past medication history, medications at discharge). The lung function test (Forced expiratory volume (FEV1), Forced vital capacity (FVC), Peak expiratory flow rate (PEFR), and FEV1/FVC) was performed by spirometryV1.34:CAR36ML3500SR4 (brand: carefusion-microlab3500 spirometry). Laboratory results of the first blood test in index admission (hemoglobin, white blood cell count (WBC), creatinine, blood urea nitrogen (BUN)) were collected. Complete blood counts (CBC) were measured using hematologic analyzers: XT-1800i (Sysmex, Japan), KX-21 N™ (Sysmex, Japan), and Cell-Dyn 18001(Abbot, USA). Blood tests including serum creatinine (Cr), blood urea nitrogen (BUN) were analyzed using chemistry analyzers: ABX Pentra 400(Horiba, USA), Dirui DR-7000D (DIRUI, Changchun, China) and HumaLyzer 3000 (HUMAN, Wiesbaden, Germany). The severity of asthma or COPD disease was evaluated using the global initiative for asthma (GINA 2019) and global obstructive lung disease (GOLD 2019) classification, respectively [23, 24]. Adherence to asthma and COPD medications inhalation techniques were assessed by passive observation of patient’s ability to demonstrate the10 steps of inhalation technique, the validated questionnaire widely used in chronic obstructive diseases.
Outcome measurement and method of validations
Both Spirometry measurements and inhalation techniques were assessed after patients became clinically stable without respiratory distress. Before spirometry measurements, patients were asked to omit short-acting inhaled bronchodilators for 4–6 hours, and long-acting oral and inhaled agents for 12 hours. Spirometry was done initially then Salbutamol 400mcg inhaler administered in four separate doses and spirometry was repeated after 15minutes [24].
Patients were diagnosed as AECOPD, if the post bronchodilator (400mcg of SABA in our setting) peak flow measurement after 15 minutes was increased by less than 12% or 200ml from baseline of PEF or FEV1 (pre-bronchodilator) or evaluated FEV1 was less than or equal to 80% predicted from age, height, and sex, and FEV1/FVC ratio was less than 0.7, plus clinical feature of dyspnea, chronic cough and sputum productions [24].
Patients were diagnosed as AE of asthma if the post bronchodilator (400mcg of SABA) peak flow measurement after 15 minutes was increased by 12% or 200ml from baseline of PEF or FEV1 (pre-bronchodilator) and FEV1/FVC ratio was less than 0.7 and oxygen saturation≥90, plus clinical feature of cough, wheezing shortness of breath and presence of risk factors [25]. Finally, 30-day readmission information was obtained when readmitted to the hospital or through telephone from the family or the patient itself.
Sample size determination and sampling technique
Since the number of patients encountered during the study period was very limited, we included all patients who fulfilled the eligibility criteria and there is no special technique employed.
Data collection tool and procedure
The data collection tool was developed based on previous similar studies [11, 14, 16, 26–30] and active patient follow-up chart. Data were collected both from active patient follow-up chart and patient interview.
Data quality assurance
A carefully designed data collection tool was used to collect important data required to meet the stated objectives. Prior to the actual data collection process, pre-test was conducted on 10 randomly selected eligible patients and the data collection tool was modified accordingly. Four data collectors (two pharmacists with Bachelor of Pharmacy degree and two clinical nurses with Bachelor of Science degree) and four supervisors (general practitioners) were hired and two-day training on the data collection tool and general procedures was provided by the principal investigator. The supervisors coordinate data collectors and facilitate the daily activities. All filled checklists were reviewed for completeness and consistency on a daily basis by supervisors and principal investigator.
Statistical analysis
Data were entered into Epi-Data 4.02.01 for cleaning and exported to STATA 14 for analysis. Descriptive analysis was performed and results were presented by text, tables and figures. Survival estimates for 30-day readmission was checked by Kaplan-Meier (log-rank test). Multicolinearity test was performed to check for co-linearity between independent variables.
X2 test was performed to check adequacy of cells before performing Cox regression. Cox regression model assumption of proportional hazards was checked by testing an interaction of covariates with time. Bivariate Cox regression was performed to identify candidate variables for multivariable Cox regressions. Variables with p-value < 0.25 in bivariate regression were considered as candidates for multivariable Cox regression. Multivariable Cox regression was performed to identify independent predictors of 30-day readmission. Hazard ratio was used as a measure of strength of associationand p-value < 0.05 was considered todeclare statistical significance.
Ethical approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of Jimma University. It has designated with an IRB number of IHRPGD/549/109. After informing the overall concern of the study and confidentiality of their personal information, participants were requested for verbal consent; for those patients unable to speak, consent was requested from their relatives. For those who could write and read, written consent was taken.
Operational definition of terms
Active smokers: refers to patients smoking a cigarette in the past 3 months before the current hospital admission [31].
Adherence (inhalation techniques): is the degree to which known asthma and COPD patients’ medication-taking behavior (inhalation techniques steps) corresponds with agreed recommendations from a healthcare provider. Good adherence was defined as an average adherence to study medications of >80% over the whole period the subject was in the study except critical steps. Poor adherence (not adhere to inhalation techniques) was defined as average adherence to inhaled medication ≤80% including critical steps or missed at least one critical steps [32].
Critical step: procedure if it is missed which leads to poor asthma control. These are place mouthpiece between teeth and lips, simultaneously press canister and breathing in slowly and inhaler out of mouth and hold breathe for 5–10 sec [33].
Efficient: Refers when patients can perform all critical steps regardless of non-critical steps [34].
Inefficient: Refers when patients miss any one or more critical steps regardless of performance of non-criticalsteps [34].
Index admission: The first occurrence of the patient’s hospital encounter for asthma or
COPD within the study period [35] and inpatient stay discharged alive with no missing length of stay (LOS) [36].
Readmission is an inpatient stay that begins within 30 days of the discharge date of an index admission.
The readmission rate is the percentage of index admissions that are readmitted within 30 days [36].
30-day readmission timing: any unplanned consecutive admission within 30 days (1–30) after being discharged alive from a hospital stay where asthma or COPD was a principal diagnosis during the study period [35].
Spirometer: the apparatus commonly used to measure the volume of air exchanged during breathing and the respiratory rate.
Forced vital capacity (FVC): the maximum volume of air in the lung after a forceful exhalation following forceful inhalation.
Forced expiratory volume (FEV1)-the volume of air expired forcefully in one second.
Peak expiratory flow rate (PEFR)-a maximum expiratory air flow beyond which the flow cannot be increased any more even with greatly increased additional force.
FEV1/FVC (FEV1%): the percentage of the FVC expired in the first second of maximal forced expiration following full inspiration. Predicted values greater than 80% is usually considering as normal.
Obstructive disorder: FEV1/FVC ratio <0.7 and percent predicted FEV1<80% predicted normal, FVC value reduced or normal.
Chronic obstructive respiratory diseases: are obstructive lung diseases that include either asthma or COPD (ICD-10, 2019).
Long-term oxygen therapy: use of oxygen for greater or equal to 16hrs per days and for multiple days (GOLD-2019).
Results
Characteristics of study cohort
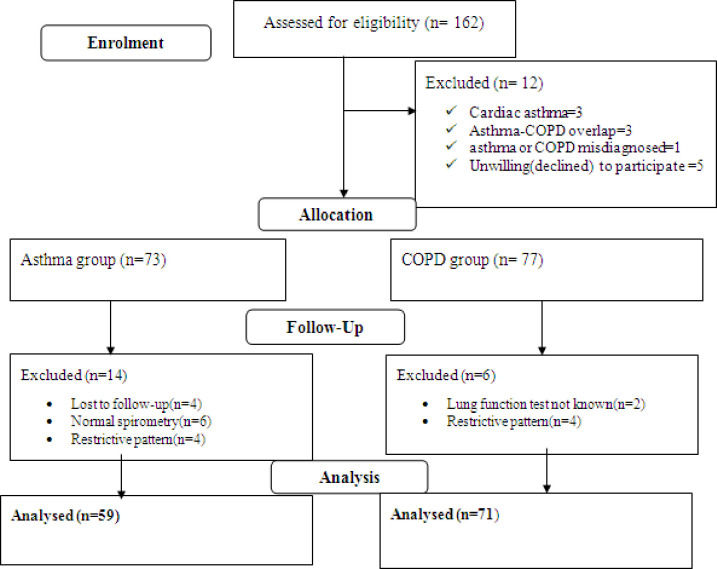
In total, 162 asthma/COPD adult patients were admitted to hospital for acute exacerbations. Twelve patients were excluded from enrolment because of not meeting inclusion criteria and not willing to participate. During the follow-up period, 20 patients were excluded due to loss to follow-up, 4 patients, 14 patients not obstructive, and two patients lung function test unknown. One hundred thirty patients met the eligibility criteria (asthma = 59, COPD = 71) and included into the final analysis. Eligible participants were followed for a median period of 5 days (IQR, 3–14 days). (Fig 1).
Fig 1. Flow diagram of study participants (asthma/COPD patients) at JMC from April-September, 2019.
Description of socio-demographic characteristics
Of 130 eligible patients with chronic obstructive respiratory disease, 78(60%) were male. The median age of patients was 59(IQR, 50–70) years. Sixty-eight (52.31%) patients were from rural areas. Majority, 73(56.15%) of patients had no formal education (could not read and write). Seventy 70 (53.85%) patients had history of living with pet animals (cats, dogs). Twenty-eight (21.54%) were active smokers. Of these, 25(35.21%) were COPD patients, and 3(5.08%) were asthmatic. Twenty three (82.14%) of the smokers consume1-10 cigarettes per day. At baseline, 49 (37.69%) of patients had low body mass index (BMI<18.5kg/m2). (Table 1).
Table 1. Baseline socio-demographic characteristics of study cohort at JMC, April-September, 2019.
| Variables | Asthma (N = 59) n (%) | COPD (N = 71) n (%) | Overall(N = 130) n (%) | χ2p-value | |
|---|---|---|---|---|---|
| Gender | Male | 29 (49.15) | 49 (69.01) | 78(60.00) | 0.021 |
| Age | Median(in years) | 53(IQR,35–61) | 60(IQR,53–70) | 59(IQR,50–70 | 0.145 |
| BMI | <18.5kg/m2 | 14 (23.73) | 35(49.30) | 49(37.69) | 0.003 |
| ≥18.5kg/m2 | 45(76.27) | 36(50.70) | 81(62.31) | ||
| Marital status | Single | 5 (8.47) | 3(4.23) | 8(6.15) | 0.604 |
| Married | 50 (84.75) | 63(88.73) | 113(86.92) | ||
| Widowed | 4 (6.78) | 5(7.04) | 9(6.92) | ||
| Residence | Urban | 32 (54.24) | 30(42.25) | 62(47.69) | 0.173 |
| Rural | 27(45.76) | 41(57.75) | 68(52.31) | ||
| Educational level | Can’t read & write | 26(44.07) | 47(66.20) | 73(56.15) | 0.040 |
| Primary | 17(28.81) | 13(18.31) | 27(20.77) | ||
| Post-primary | 16(27.12) | 11(15.49) | 30(23.08) | ||
| Occupation | Employed | 25(42.37) | 26(36.62) | 51(39.23) | 0.658 |
| Farmer | 18(30.51) | 27(38.03) | 45(34.62) | ||
| Housewife | 16(27.12) | 18(25.35) | 34(26.15) | ||
| Living status | With family | 56 (94.92) | 67(94.37) | 123(94.62) | 0.890 |
| Alone | 3(5.08) | 4(5.63) | 7(5.38) | ||
| Live with pet animals | Yes | 35(59.32) | 35(49.30) | 70(53.85) | 0.254 |
| Smoking status | Active smokers | 3(5.08) | 25(35.21) | 28(21.54) | <0.001 |
| Non-smokers | 46(77.97) | 32(45.07) | 78 (60.00) | ||
| Ex-smokers | 10(16.95) | 14(19.72) | 24(18.46) | ||
| Active smokers cig/day | 1–10 | 3(100.00) | 20 (80.00) | 23(82.14) | 0.393 |
| 11–20 | 0(0) | 5(20.00) | 5(17.86) | ||
*BMI = Body mass index, IQR = inter quartile range
Clinical characteristics
Of total patients,111(85.38%) had history of previous chronic obstructive respiratory disease with the median duration of 3 years (IQR, 2–6 years) for COPD, and 10 years (IQR, 4.5–19 years) for asthma. The overall median length of hospital stay was 5 days (IQR, 3–14 days). The median length of hospital stay for acute exacerbation of asthma was 3 days (IQR, 3–6 days); while for acute exacerbation of COPD was 8 days (IQR, 4–19 days). Seventy-nine (60.77%) patients had history of hospital admission and nearly half, 38(48.10%) of them had two admissions per annum. Eight (6.15%) patients had history of ICU admission (asthma = 4, COPD = 4).
Fifty-one (39.23%) patients had history of oxygen therapy prior to the current study, of which 15(29.41%) were on oxygen for ≥16hrs. Majority, 117(90%) of patients had history of difficulty in falling asleep during night, to which asthma accounted for 57(48.72%) patients and 60 (51.28%) were accounted for COPD patients. According to GINA guideline, 34(29.06%) patients encountered severe night attacks, 7*/week. Sixty (46.15%) patients had history of limitation to perform daily activities (asthma = 26, COPD = 34) (Table 2).
Table 2. Baseline clinical characteristics of study cohort at JMC, April-September, 2019.
| Variables | Overall(N = 130) N (%) | Asthma (N = 59) N (%) | COPD(N = 71) N (%) | p -value | |
|---|---|---|---|---|---|
| Duration of chronic respiratory disease(year) | Known | 111(85.38) | 10 (IQR,4.5–19) | 3 (IQR,2–6) | 0.756 |
| New | 19(14.62) | Newly admitted | Newly-admitted | ||
| History of hospital admission | Yes | 79(60.77) | 34(57.63) | 45(63.38) | 0.504 |
| Number of hospital admission per year | 1*/year | 24(30.38) | 9(26.47) | 15(33.33) | 0.017 |
| 2*/year | 38(48.10) | 22(64.71) | 16(35.56) | ||
| ≥ 3*/year | 17(21.52) | 3(8.82) | 14(31.11) | ||
| History of ICU admission | Yes | 8(6.15) | 4(6.78) | 4(5.63) | 0.787 |
| History of oxygen therapy(prior to the current study) | Yes | 51(39.23) | 23(38.98) | 28(39.44) | 0.958 |
| duration of oxygen therapy /day | <16hrs | 36(70.59) | 15(65.22) | 21(75.00) | 0.046 |
| ≥16hrs | 15(29.41) | 8 (34.78) | 7(25.00) | ||
| Difficulty in falling asleep | Yes | 117(90.00) | 57(96.61) | 60(84.51) | 0.022 |
| Frequency of night attacks | 3–4*/month | 83(70.94) | 39(68.42) | 44(73.33) | 0.164 |
| 7*/week | 34(29.06) | 18(31.58) | 16(26.67) | ||
| Limit performing daily activities | Yes | 60(46.15) | 26(44.07) | 34(47.89) | 0.133 |
| Last hospitalizations | ≥12 month | 34(26.15) | 15(25.42) | 19(26.76) | 0.970 |
| <12 month | 45(34.62) | 19(32.20) | 26(36.62) | ||
| Not-admitted | 51(39.23) | 25(42.37) | 26(36.62) | ||
| Chest clinic physician visit | ≥12 month | 10(7.69) | 4(6.78) | 6(8.45) | 0.229 |
| <12 month | 77(59.23) | 34(57.63) | 43(60.56) | ||
| Never visit | 43(33.08) | 21(35.59) | 22(30.99) | ||
ICU-intensive care unit
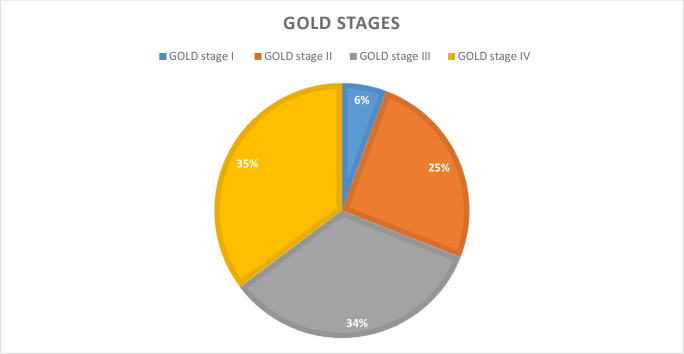
Based on GOLD severity staging, 71COPD patients, 25(35.21%) were GOLD stage IV and 24(33.80%) were GOLD stage III (Fig 2).
Fig 2. Clinical staging for COPD using GOLD at JMC, April -September, 2019.
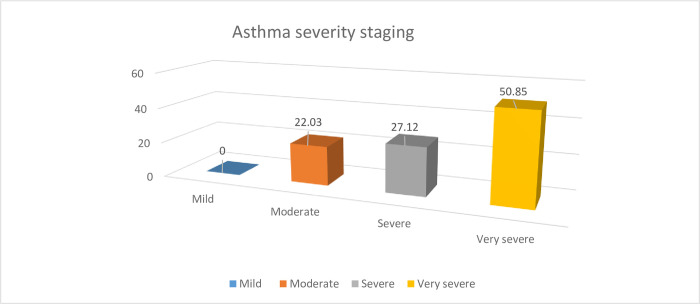
According to GINA staging severity, 30(50.85%) had severe asthma and 16(27.12%) had very severe asthma (Fig 3).
Fig 3. Clinical staging for asthma using GINA guideline at JMC, April -September, 2019.
Sixty-one (46.92%) patients (asthma = 20, COPD = 41) had co-morbidities. Of these, pulmonary hypertension, 34(47.89%), severe community-acquired pneumonia (SCAP), 30(42.25%), and heart failure (IHD), 23(32.39%) were the most common in COPD patients, while SCAP 32(54.24%), hypertension, 6(10.17%), and heart failure, 6(10.17%) were mostly seen in asthma patients (Table 3).
Table 3. Comorbid conditions among study cohort at JMC, April-September, 2019.
| Variables | Overall(N = 130) | Asthma (N = 59) | COPD(N = 71) | p -value | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Common co-morbidity (n = 61) | yes | 61(46.92) | 20(33.90) | 41(57.75) | 0.007 |
| Heart failure(IHD) | yes | 29(22.31) | 6(10.17) | 23(32.39) | 0.002 |
| Hypertensions | yes | 17(13.08) | 6(10.17) | 11(15.49) | 0.370 |
| Diabetes mellitus | yes | 4(3.08) | 1(1.69) | 3(4.23) | 0.406 |
| Cardiac arrhythmia | yes | 7(5.38) | 1(1.69) | 6 (8.45) | 0.089 |
| Depression | yes | 1(0.77) | 0(0) | 1(1.41) | 0.360 |
| Pulmonary HTN | yes | 39(30.00) | 5(8.47) | 34(47.89) | 0.000 |
| Renal failure | yes | 8(6.15) | 0(0) | 8(11.27) | 0.008 |
| Cancer | yes | 4(3.08) | 2(3.39) | 2(2.82) | 0.851 |
| DVT | yes | 2(1.54) | 1(1.69) | 1(1.41) | 0.895 |
| Pneumonia(SCAP) | yes | 62(47.69) | 32(54.24) | 30(42.25) | 0.173 |
SCAP: severe community acquired pneumonia, IHD: ischemic heart disease, DVT: deep vein thrombosis, HTN: hypertension
Drug-related and investigation findings
Of the total admitted patients, 100 (76.92%) patients had been using salbutamol inhaler since diagnosis. Eighty-three (63.85%) patients had history of antibiotics usage and 51 (39.23%) patients had history of long-term oxygen therapy≥ 16hrs per day (asthma = 22, COPD = 29). The most frequently used drugs during hospital stay were salbutamol inhaler, 128(98.46%), oxygen therapy, 94(72.31%), and prednisolone, 81(62.31%). The most frequently used antibiotics were ceftriaxone injection, 97(74.62%) and azithromycin, 91(70%).
With regard to laboratory findings, twenty (15.38%) patients had elevated haemoglobin (asthma = 2, COPD = 18). Fifty-nine (45.38%) patients presented with raised white blood cells. Creatinine was also increased in 21(16.5%) patients and blood urea nitrogen also elevated in 16 (12.31%) patients (asthma = 5, COPD = 11) (Table 4).
Table 4. Drug-related and laboratory variables recorded at JMC, April-September, 2019.
| Variables | Over all (n, (%) | Asthma (n, (%) | COPD (n, %) | P-value | ||
|---|---|---|---|---|---|---|
| Past Antibiotics uses history | yes | 83(63.85) | 33(55.93) | 50(70.42) | 0.087 | |
| Past Long term O2 therapy(≥16hrs) | yes | 51(39.23) | 22(37.29) | 29 (40.85) | 0.679 | |
| Past Salbutamol inhaleruse history | yes | 100(76.92) | 48(81.36) | 52 (73.24) | 0.274 | |
| Past selmaterol inhaler | No | 129(99.23) | 58(98.31) | 71(100.00) | 0.271 | |
| Past Bechlomethasone inhaler | yes | 33(25.38) | 13(22.03) | 20(28.17) | 0.424 | |
| Prednisolone tablet | yes | 35(26.92) | 14(23.73) | 21(29.58) | 0.454 | |
| Baseline oxygen saturations | <90% | 83(63.85) | 32(54.24) | 51(72.86) | 0.038 | |
| ≥90% | 47(36.15) | 27(45.76) | 20(28.17) | |||
| Current medications(on admission) | ||||||
| Oxygen therapy | yes | 94(72.31) | 40(67.80) | 54(76.06) | 0.295 | |
| Duration of oxygen therapy | <16hrs | 77(81.91) | 35(87.50) | 42(77.78) | 0.226 | |
| ≥16hrs | 17(18.09) | 5(12.50) | 12(22.22) | |||
| Salbutamol inhaler | yes | 128(98.46) | 58(98.31) | 70 (98.59) | 0.895 | |
| Salmeterol inhaler | yes | 5(3.85) | 2(3.39) | 3 (4.23) | 0.805 | |
| Bechlomethasone inhaler(ICS) | yes | 57(43.85) | 22(37.29) | 35(49.30) | 0.170 | |
| Prednisolone tablet | yes | 81(62.31) | 35(59.32) | 46(64.79) | 0.522 | |
| Hydrocortisone injections | yes | 43(33.08) | 21(35.59) | 22(30.99) | 0.286 | |
| Budesonide+formoterol inhalations | No | 125(96.15) | 59(100.00) | 66(92.96) | 0.038 | |
| Drugs for comorbidities | ||||||
| Ceftriaxone | yes | 97(74.62) | 42(71.19) | 55(77.46) | 0.413 | |
| Azithromycin | yes | 91(70.00) | 40(67.80) | 51(71.83) | 0.617 | |
| Vancomycin | yes | 5(3.85) | 1(1.69) | 4(5.63) | 0.245 | |
| Doxycycline | yes | 3(2.31) | 0(0) | 3(4.23) | 0.110 | |
| Laboratory parameter | ||||||
| Hgb g/dl | High | 20(15.38) | 2(3.39) | 18(25.35) | 0.001 | |
| Normal | 103(79.23) | 56(94.92) | 47(66.20) | |||
| Low | 7(5.38) | 1(1.69) | 6(8.45) | |||
| WBC | High | 59(45.38) | 28(47.46) | 31(43.66) | 0.851 | |
| normal | 68(52.31) | 30(50.85) | 38(53.52) | |||
| low | 3(2.31) | 1(1.69) | 2 (2.82) | |||
| Serum creatinine | High | 21(16.15) | 6(10.17) | 15(21.13) | 0.237 | |
| normal | 92(70.77) | 45(76.27) | 47(66.20) | |||
| low | 17(13.08) | 8(13.56) | 9(12.68) | |||
| BUN | High | 16(12.31) | 5(8.47) | 11(15.49) | 0.079 | |
| normal | 55(42.31) | 21(35.59) | 34(47.89) | |||
| Not measured | 59(45.38 | 33(55.93) | 26 (36.62) | |||
Hgb-hemoglobin, WBC- white blood cell, BUN-blood urea nitrogen
Spirometer results for pre and post bronchodilator
On admission, lung function test was performed for all candidate patients. The FEV1/FVC was <0.7 in 130 patients showing obstructive pattern. The relationship between pre-bronchodilator values and bronchodilator response was estimated and declared as acute exacerbation of asthma or COPD (severity).
Measurements of inhalation techniques adherence
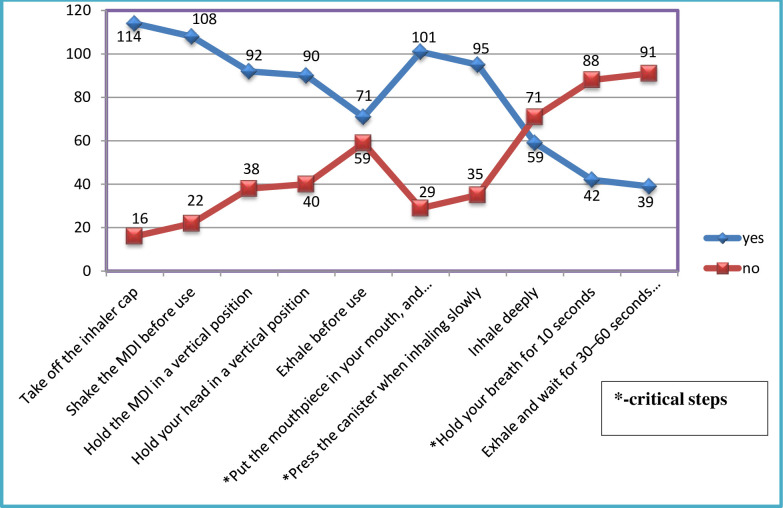
For all eligible patients, 10 step-wise inhalation techniques were provided to check the adherence of effective inhalations. Fourteen (10.78%) patients totally could not perform inhalation techniques correctly including the three critical steps and declared as “inefficient”. The remaining 116(89.23%) patients missed at least one steps, except the three critical inhalation techniques and declared as “efficient”. As depicted in Fig 4, 114 patients performed step one correctly (blue color), 16 patients cannot perform correctly (red color), and so on. As steps goes up, the ability to remember and perform effectively was decreased i.e. 91(70%) could not perform correctly the last steps (exhale and wait for 30–60 seconds before the other inhaler) (red color). (Fig 4).
Fig 4. Instructions measurements for MDI (step-wise correct use of the inhalation techniques) at JMC, April-September, 2019.
*MDI-metered dose inhalations.
Rate of 30-days readmission
Of 130 admitted patients, 21(18.10%) had a new AE of asthma and COPD that required hospitalization in the 30-days after discharge. Fourteen (12.07%) of them were asthmatic and 7(6.03%) were COPD patients. The total analysis time at risk was 3,705 days, making an overall incidence rate of 30-day readmission 55.8 per100, 000 person-years, (95% CI, [36.4–85.6]). The incidence rate of 30-day readmission for acute exacerbation of COPD was 59.9 per 100,000 person-years, 95% CI [35.9–158.3), whereas that of asthma was 189.9 per100, 000 person-years, 95% CI [112.5–320.8].
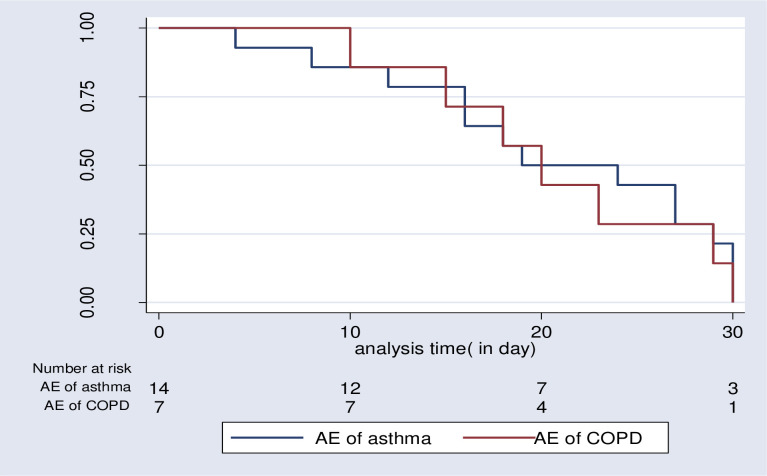
The overall median time to 30-day readmission was 20 days (IQR, 16–29). The median time to 30-day readmission for AECOPD was 20 days (IQR, 15–29), while 21.5days (IQR.16-29) for acute exacerbation of asthma, (p = 0.773). (Fig 5).
Fig 5. Survival estimates for 30-day readmission among patients admitted with acute exacerbations of COPD or asthma at JMC, April-September, 2019.
Predictors of 30-day readmission
Several variables such as history of hospital admission, use of high dose salbutamol inhaler, chest clinic physician visit, occupational status, residence, and other four variables (age, BMI, smoking status, and educational status) were omitted from analysis due to violation of cell adequacy test and two variables (comorbidity and living status) removed due to co-linearity effect. Therefore, nine variables were candidates for bivariate Cox-regression analysis (p<0.25) depicted in Table 5. Further multivariate Cox-regression identified prolonged use of oxygen therapy and frequent hospitalization per yearto be independent predictors of 30-day readmission.
Table 5. Crude and adjusted Cox-proportional hazard regression for predictors of 30-days readmission of the cohort at JMC, April- September, 2019.
| Variables | 30-day readmission | CHR[CI 95%] | p-value | AHR[CI 95%] | P-value | |
|---|---|---|---|---|---|---|
| category | ||||||
| Bechlomethasone inhalation | No | 1 | 1 | |||
| yes | 2.42[1.021–5.756] | 0.045 | 2.426[0 .680–8.651] | 0.172 | ||
| Number of hospitalization/year | 1*/year | 1 | 1 | |||
| 2*/year | 7.69[0.99–59.57] | 0.051 | 6.467[0.768–54.446] | 0.086 | ||
| ≥3*/year | 11.86[1.458–96.53] | 0.021 | 11.482[1.308–100.793] | 0.028* | ||
| antibiotics | No | 1 | 1 | |||
| yes | 2.529[0.851–7.516] | 0.095 | 0.418[0.093–1.879] | 0.256 | ||
| LTOT | <16hrs | 1 | 1 | |||
| ≥16hrs | 5.818[2.129–15.892] | 0.001 | 4.972 [1.041–23.736] | 0.044* | ||
| daily activities | No | 1 | 1 | |||
| yes | 3.175[0.703–14.336] | 0.133 | 1.140[0.377–3.444] | 0.816 | ||
| Frequency of Night attacks | 3–4*/mnth | 1 | 1 | |||
| 7*/week | 0.235[0.031–1.808] | 0.164 | 1.151[0.338–3.921] | 0.822 | ||
| Baseline O2 saturation | ≥90% | 1 | 1 | |||
| <90% | 1.897[0.695–5.179] | 0.211 | 0.780[0.176–3.458] | 0.744 | ||
| Prednisolone tablet | No | 1 | 1 | |||
| yes | 1.781[0.738–4.298] | 0.199 | 0.623[0.193–2.011] | 0.274 | ||
| FEV1 | 0.987 [0.962–1.012] | 0.131 | 1.0156[0.988–1.044] | 0.274 | ||
LTOT = long term oxygen therapy, Mnth-month, FEV1: Forced expiratory volume in 1 sec, AHR—Adjusted Hazard Ratio, COR-Crude Odd Ratio, CI- Confidence interval
Accordingly, patients who were on oxygen therapy for ≥16hrs per 24hrs had 5 times increased hazard of 30-day readmission (AHR = 4.972, 95% CI [1.041–23.736]. Similarly, patients with three times or greater admissions per year had 11 times increased hazard of 30-day readmission (AHR = 11.482[1.308–100.793]) (Table 5).
Discussion
This prospective cohort study summarized the rate of 30-day readmission and it’s predictors among patient treated for acute exacerbation of chronic obstructive respiratory disease, asthma, and COPD. During the study period, 21(18.01%) patients were readmitted, making an overall incidence rate of 55.8 per100, 000 person-years (COPD = 7(6.03%), asthma = 14(12.07%). It was 59.9 per 100,000 person-year, 95% CI, [35.9–158.3), and 189.9 per100, 000 person-year, 95% CI [112.5–320.8] for AE of COPD and Asthma, respectively. The overall median survival time to 30-day readmission was 20 days (IQR, 16–29). The median survival time to 30-day readmission for AECOPD was 20 days (IQR, 15–29), and that of AE of asthma was 21.5 days (IQR.16-29). Prolonged use of oxygen therapy and frequent hospital admissions were independent predictors for 30-day readmission.
Regarding an overall rate of 30-days readmission, our finding (21(18.01%)) was almost similar with studies conducted in Chicago [37], Ohio [38], Spain [26], and USA [39] which revealed an overall 30-days readmission rate of 18.5%, 18.1%, 17.98%, and 19.4% respectively. However, it’s inconsistent with studies conducted by Buyantseva et al [40], and Meservey et al [41] that reported a higher 30-days readmission rate of 39%, and 23% respectively. Similarly, a lower 30-days readmission rate was reported in USA (9.2%) and UK (4.7%) [42]. This variation might be due to difference in socio-economic status, sample size, and duration of study period (6 months vs. greater than one year).
In this study, one hundred sixteen (89.23%) patients were adherent (efficient) to inhalation technique. This adherence rate is relatively better than the study conducted by Kebede et al, 87(62.14%) [28]. In a study conducted in Turkey, nearly 23% of the patients had suboptimal adherence to inhalation technique [43]. Difference in the methodology used to measure adherence level might contribute to the variation. Furthermore, the direct involvement of clinical pharmacists in the patient care process and regular provision of counseling on medication use in our set up may contributed for better adherence.
Although we have evaluated a wide range of risk factors for hospital readmission, only two clinical factors were found to be independent predictors of 30-days readmission in the current study. In our study, patients who were on oxygen therapy for ≥16hrs per day had 5 times increased hazard of 30-day readmission (AHR = 4.972.95% CI [1.041–23.736]. A number of previous similar studies [39, 41], have also reported long term oxygen therapy as a risk factor associated with an increased risk of readmission among patients with chronic obstructive airway diseases. Individuals who stay longer on oxygen therapy likely have severe and advanced disease and are at higher risk for hospital readmission. Furthermore, patients who admitted to the hospital three times or greater in the previous year had 11 times increased hazard of 30-day readmission (AHR = 9.5, 95% CI [1.12–80.16]. This finding is in agreement with several other studies [40, 42, 44, 45]. According to Guerrero M et al., [45], the rate of 30 days readmission was significantly higher in COPD patients with a history of >2 exacerbations in the previous year. Frequent hospital admission is an indicator of disease severity and patients with advanced disease are very prone for readmission.
No association was found between FEV1% predicted and the risk of asthma and COPD readmission. This finding supports the result of a previous study by Bahadori et al., [29] that found no differences between FEV1% predicted and the risk of COPD readmission. However, other studies [27, 30, 46] found FEV1<50% predicted to be predictive of a higher risk of 30-days readmission. The possible justification for the lack of association in the current study might be related to the small sample size, which reduces statistical power to find true association.
The prevalence of COPD/asthma in Ethiopia is comparable with neighboring Sub-Saharan African countries. A prevalence rate of 17.6% was reported for COPD [47] and this is in-line with studies done in Uganda [48] and Tanzania [49]. The prevalence of asthma was reported to be 29.6% [50]. This prevalence was relatively higher compared to the study conducted in Egypt [51], and Uganda [52]. Differences in climate conditions, growing population size, and radical urbanization might contribute to the increment in the prevalence of asthma.
Limitations
The findings of the present study should be interpreted in the context of several possible limitations. First, inclusion of a small number of patients might under power the study. Second, follow-up period of 6 months may be relatively short, but we focused on early 30-days readmission. Third, merging two populations of obstructive diseases may affect the validity of the outcome. Moreover, salbutamol alone was used to test the lung functions in the study due to unavailability of ipratropium, as most of bronchodilator reversibility studies were conducted using the combinations of short-acting beta agonists and anti-muscarinic. Finally, the present study was conducted in a single institution, and so the extrapolation of these findings to other settings must be done with care.
Conclusion
In conclusion, hospital readmission rates for AE of asthma and COPD within 30 days of discharge were alarmingly high. Frequent hospital admission and long-term oxygen therapy during hospital stay were independent predictors of 30-day readmission.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the study participants, data collectors, and pulmonary unit staffs of Jimma Medical Center.
Abbreviations
- AE
Acute Exacerbations
- COPD
Chronic Obstructive Pulmonary Disease
- AHR
Adjusted Hazard Ratio
- AOR
Adjusted odd ratio
- FEV1
Forced Expiratory Volume in One Second
- IQR
Inter quartile range
- SABA
Short Acting Beta Agonist
- LTOT
Long-term oxygen therapy
- JMC
Jimma Medical Center
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 3.Jain VV, Allison R, Beck SJ, Jain R, Mills PK, McCurley JW, et al. Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respir Med. 2014;108(12):1794–800. 10.1016/j.rmed.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM BA. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–73. 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 5.Miller JD, Foster T, Boulanger L, Chace M, Russell MW, Marton JP MJ. Direct costs of COPD in the U.S.: an analysis of Medical Expenditure Panel Survey (MEPS) data. COPD. 2005;2(3):311–8. 10.1080/15412550500218221 [DOI] [PubMed] [Google Scholar]
- 6.Adeloye D, Basquill C, Papana A, Chan KY, Rudan I CH. An estimate of the prevalence of COPD in Africa: a systematic analysis. COPD. COPD. 2015;12(1):71–8. 10.3109/15412555.2014.908834 [DOI] [PubMed] [Google Scholar]
- 7.Adeloye D, Chan KY, Rudan I CH. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–31. 10.3325/cmj.2013.54.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misganaw A, Mariam DH, Ali A AT. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J Heal Popul Nutr. 2014;32(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss KB SS. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol. 2001;107(1):3–8. 10.1067/mai.2001.112262 [DOI] [PubMed] [Google Scholar]
- 10.Donaldson G, Seemungal T, Bhowmik A WJ. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52. 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanner RE, Anthonisen NR CJ. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–64. 10.1164/ajrccm.164.3.2010017 [DOI] [PubMed] [Google Scholar]
- 12.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ WJ. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5):1418–22. [DOI] [PubMed] [Google Scholar]
- 13.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ WJ. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–13. 10.1164/ajrccm.161.5.9908022 [DOI] [PubMed] [Google Scholar]
- 14.Donaldson G, Seemungal T, Patel I, Lloyd-Owen S, Wilkinson T WJ. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir Journal. 2003;22(6):931–6. [DOI] [PubMed] [Google Scholar]
- 15.Decramer M, Rutten-van Mölken M, Dekhuijzen PR, Troosters T, van Herwaarden C, Pellegrino R et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365(9470):1552–60. 10.1016/S0140-6736(05)66456-2 [DOI] [PubMed] [Google Scholar]
- 16.Anthonisen N, Manfreda J, Warren C HE. Antibiotic therapy in exacerbations of chronic obstructive pulmonary. Ann Intern Med. 1987;106(2):196–204. 10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- 17.Fingar K WR. Trends in Hospital Readmissions for Four High-Volume Conditions, 2009–2013. Statistical Brief #196. 2015. [PubMed] [Google Scholar]
- 18.Barrett M, Wier L, Jiang H SC. All-Cause Readmissions by Payer and Age, 2009–2013. Statistical brief# 199. 2006. [Google Scholar]
- 19.Boulet L-P, Boulay M-È, Chanez, Humbert, Bateman, Soriano et al. Asthma-related comorbidities. Expert Rev Respir Med. 2011;5(3):377–93. 10.1586/ers.11.34 [DOI] [PubMed] [Google Scholar]
- 20.Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE CJ. Risk and Protective Factors for Childhood Asthma: What Is the Evidence? Allergy Clin Immunol Pr. 2016;4(6):1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Au DH PJ. Readmissions for Chronic Obstructive Pulmonary Disease, 2008: Statistical Brief #121. 2011 Sep. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65392/ [PubMed]
- 22.Crisafulli E, Torres A, Huerta A, Méndez R, Guerrero M, Martinez R et al. C-reactive protein at discharge, diabetes mellitus and _1 hospitalization during previous year predict early readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. COPD. 2015;12(3):306–14. 10.3109/15412555.2014.933954 [DOI] [PubMed] [Google Scholar]
- 23.Global Initiative for Asthma. 2019 GINA Main Report. www.ginasthma.org/reports (2019).
- 24.GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019. https://goldcoped.org/wp. Accessed June 25, 2020.
- 25.GA N. The global asthma report. Auckland (New Zealand). 2018.
- 26.Guerrero M, Crisafulli E, Liapikou A, Huerta A, Gabarrús A, Chetta A et al. Readmission for Acute Exacerbation within 30 Days of Discharge Is Associated with a Subsequent Progressive Increase in Mortality Risk in COPD Patients: A Long-Term Observational Study. PLoS One. 2016;11(3):e0150737 10.1371/journal.pone.0150737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao ZHenying OK, Eng P, Tan WC NT. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–95. 10.1111/j.1440-1843.2006.00819.x [DOI] [PubMed] [Google Scholar]
- 28.Kebede B, Mamo G. Determinants of non-adherence to inhaled steroids in adult asthmatic patients on follow up in referral hospital, Ethiopia: cross-sectional study. Asthma Res Pract. 2019;5:1–8. 10.1186/s40733-019-0048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K Bahadori JM FitzGerald RD Levy, T Fera JS. Risk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalization. Can Respir J. 2009;16(4):e43–9. 10.1155/2009/179263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F SJ. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67(5):495–501. [DOI] [PubMed] [Google Scholar]
- 31.Zielińska-Danch W, Wardas W, Sobczak A S-BI. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12(5):484–96. 10.1080/13547500701421341 [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, Anderson JA, Calverley PMA, Celli B, Ferguson GT, Jenkins C et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–43. 10.1136/thx.2009.113662 [DOI] [PubMed] [Google Scholar]
- 33.Hamdan A-J, Ahmed A, Abdullah A-H, Khan M, Baharoon S, Salih SB et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy, Asthma Clin Immunol. 2013;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hämmerlein A, Müller U SM. Pharmacist‐led intervention study to improve inhalation technique in asthma and COPD patients. J Eval Clin Pract. 2011;17(1):61–70. 10.1111/j.1365-2753.2010.01369.x [DOI] [PubMed] [Google Scholar]
- 35.Singh U, Wangia-Anderson V BJ. Chronic Rhinitis Is a High-Risk Comorbidity for 30-Day Hospital Readmission of Patients with Asthma and Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pr. 2019;7(1):279–285.e6. [DOI] [PubMed] [Google Scholar]
- 36.Barrett M, Wier L, Jiang H SC. All-Cause Readmissions by Payer and Age, 2009–2013. HCUP Statistical Brief #199. December 2015. Agency for Healthcare Research and Quality, Rockville, MD. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb199-Readmissions-Payer-Age.pdf [Google Scholar]
- 37.Goto T., Yoshida K., Faridi MK et al. Contribution of social factors to readmissions within 30 days after hospitalization for COPD exacerbation. BMC Pulm Med. 2020;20(107):1136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo AN, Sathiyamoorthy G, Lau C, Saygin D, Han X, Wang X-F et al. Impact of a post-discharge integrated disease management program on COPD hospital readmissions. Respir Care. 2017;62(11):1396–402. 10.4187/respcare.05547 [DOI] [PubMed] [Google Scholar]
- 39.Hijjawi SB, Abu Minshar M SG. Chronic obstructive pulmonary disease exacerbation: a single-center perspective on hospital readmissions. Postgrad Med. 2015;127(4):343–8. 10.1080/00325481.2015.1015394 [DOI] [PubMed] [Google Scholar]
- 40.Buyantseva Larisa V., Joel Brooks, Melissa Rossi EL& TJ. Risk factors associated with 30-day asthma readmissions. J Asthma. 2016;53(7):684–90. 10.3109/02770903.2016.1140773 [DOI] [PubMed] [Google Scholar]
- 41.Meservey AJ, Burton MC, Priest J, Teneback CC DA. Risk of Readmission and Mortality Following Hospitalization with Hypercapnic Respiratory Failure. Lung. 2019;198(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki RY, Daugherty JB, Boudiaf N AF. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. 2017;17(1):74 BMC Pulm Med. 2017;17(1):74. 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capanoglu Murat, Emine Dibek Misirlioglu, Muge Toyran EC& CNK. Evaluation of inhaler technique, adherence to therapy and their effect on disease control among children with asthma using metered dose or dry powder inhalers. J Asthma. 2015;52(8):838–45. 10.3109/02770903.2015.1028075 [DOI] [PubMed] [Google Scholar]
- 44.Almagro P, Barreiro B, de Echagüen AO, Quintana S, Carballeira MR, Heredia JL et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73(3):311–7. 10.1159/000088092 [DOI] [PubMed] [Google Scholar]
- 45.Guerrero M, Crisafulli E, Liapikou A, Huerta A, Gabarrús A, Chetta A, et al. Readmission for Acute Exacerbation within 30 Days of Discharge Is Associated with a Subsequent Progressive Increase in Mortality Risk in COPD Patients: A Long-Term Observational Study. PLoS One. 2016;11(3):e0150737 10.1371/journal.pone.0150737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM AJ. Risk factors of re-admission to hospital for a COPD exacerbation: A prospective study. Thorax. 2003;58(2):100–5. 10.1136/thorax.58.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woldeamanuel G.G., Mingude A.B. & Geta T.G. Prevalence of chronic obstructive pulmonary disease (COPD) and its associated factors among adults in Abeshge District, Ethiopia: a cross sectional study. BMC Pulm Med 19, 181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3:e44–51 10.1016/S2214-109X(14)70337-7 [DOI] [PubMed] [Google Scholar]
- 49.Magitta NF, Walker RW, Apte KK, Shimwela MD, Mwaiselage JD, Sanga AA, et al. Prevalence, risk factors and clinical correlates of COPD in a rural setting in Tanzania. Eur Respir J. 2018;51:1700182 10.1183/13993003.00182-2017 [DOI] [PubMed] [Google Scholar]
- 50.Shine S., Muhamud S. & Demelash A. Prevalence and associated factors of bronchial asthma among adult patients in Debre Berhan Referral Hospital, Ethiopia 2018: a cross-sectional study. BMC Res Notes 12, 608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tageldin MA, Wagih K, Maher O. Study the pattern of bronchial asthmaamong outpatients clinic at Sohag and Akhmeem Chest Hospitals. EgyptSoc Chest Dis Tuberc. 2015;64:313–23. [Google Scholar]
- 52.Kirenga JB, Okot-Nwang M. The proportion of asthma and patterns ofasthma medications prescriptions among adult patients in the chestaccident and emergency units of a tertiary health care facility in Uganda.Afr Health Sci. 2012;12(1):48–53. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.