Abstract
Plant roots are inhabited by an enormous variety of microorganisms, including fungi, which can control the growth as well as regulate the health of the host plants. The mycobiome composition of the roots of wheat plants, especially spelt, under drought stress has been rarely investigated. Therefore, the aim of the present study was to examine the composition of fungal communities in the root endosphere and rhizosphere of three Triticum aestivum ssp. spelta L. cultivars and one Triticum aestivum ssp. vulgare L. cultivar, grown under drought and controlled conditions in different soil preparations. Culture-dependent fungal community profiling was performed to examine the impact of rhizocompartments (endosphere, rhizosphere), host genotype, watering status and different soil preparation on roots mycobiome structure. A total of 117 fungal strains, belonging to 22 genera, were found to colonize the internal and external parts of roots in T. aestivum ssp. spelta L. and T. aestivum ssp. vulgare L. cultivars. The results showed that the part of root and soil preparation type significantly determined the mycobiome composition of wheat roots.
Introduction
Plant roots are inhabited by different types of microorganisms, including fungi, which can control the growth as well as regulate the health of the host plants. Fungi present in the soil ecosystem not only act as substantial decomposers of biomass and plant symbionts but also pose threats as serious pathogens. These microorganisms are capable of colonizing both the internal and external parts of the plant organs. Fungi that colonize the internal tissue of a plant (endosphere) throughout or at least a part of their life cycle without manifesting any disease in the host are called endophytes [1]. Some of these fungi are known to improve the host’s defense against abiotic and biotic stresses, promote its growth, and reduce/inhibit the expansion of pathogens [2, 3]. The rhizosphere is an external territory, which closely surrounds the plant roots. Plants attract selected fungi inhabiting the soil by producing the exudates, and as a result, the structure of fungal communities in the rhizosphere differs from that in the adjacent soil area [4, 5]. Among the fungal species known to colonize the rhizosphere, the arbuscular mycorrhizal fungi (AMF) are observed in more than 80% of the terrestrial plants [6]. The AMF establish a conjunction between their host and the soil by creating an external hyphal network, which enhances the fitness of the host plant, increases its nutrient uptake, and improves its productivity under drought stress [7]. Apart from the beneficial fungi, some pathogenic species also colonize the rhizosphere. The most detrimental fungal pathogens that inhabit wheat roots are Gaeumannomyces graminis and those belonging to the Fusarium genus (F. avenaceum, F. culmorum, F. graminearum, F. oxysporum, and F. poae) [8–10]. Wheat (Triticum aestivum ssp. vulgare L.) and spelt wheat (Triticum aestivum ssp. spelta L.) are considered as an important source of nourishment for humans and livestock. Therefore, wheat cultivation is extensively performed and is of substantial economic importance [11, 12]. Furthermore, spelt wheat grain is identified as a great source of fiber, vitamins (A, E, D), and microelements (selenium, zinc, copper), and thus, its consumption is known to have a positive impact on human health [13]. It is one of the oldest cereals cultivated during the Roman period, and has regained popularity over the last 30 years. The growing nutritional requirements caused by increasing human population indicate the need for improving the global wheat production. Nonetheless, wheat plants are facing enormous challenges from abiotic and biotic stresses, especially during unpredictable weather conditions arising from climate changes. Drought, salinity, increase or decrease in temperature (hot or cold environment), flooding, ultraviolet radiation, and metal toxicity are the most deleterious abiotic stresses, which reduce crop productivity by up to 50% [14]. Wheat is sensitive to drought stress, especially until heading or germination stage and during the grain filling period [15]. Numerous phenological, physiological, and biochemical changes that are detrimental to wheat plants occur following the period of water deficiency. Drought leads to around 50% increase in the root/shoot ratio, due to a higher level of abscisic acid, which together with auxin, cytokine, and gibberellic acid stimulates the growth of roots but represses the development of shoot [14, 16].
The purpose of this study was to examine the composition of fungal communities in the root endosphere and rhizosphere of three T. aestivum ssp. spelta L. cultivars and one T. aestivum ssp. vulgare L. cultivar, grown under drought and well-watered conditions, in different soil preparations. The study focused on finding answers for two main questions: 1) Does the fungal mycobiome structure vary depending on the area of wheat roots (external and internal), host genotype, watering status, and different soil preparation? 2) Does a specific pattern of root mycobiome occur during drought in wheat plants/do some fungal species or genera specifically colonize the roots of these plants under conditions of water deficiency? Knowledge about the structure and diversity of root mycobiome in wheat is not only important from the ecological point of view but also promising for application in agricultural studies. This study on the native endosphere- and rhizosphere-associated fungal communities of wheat plants, conducted using cultivation-based approaches, might help in the identification of beneficial plant fungi for use in modern agronomy to create new-generation natural growth stimulators or factors capable of increasing plant adaptability to environmental changes.
Materials and methods
Plant material and experimental design
The experimental analyses were performed on the roots of T. aestivum ssp. spelta L. and T. aestivum ssp. vulgare L. plants. Initially, the plants were grown on the experimental field in Złotniki Research Station (52°48′ N, 16°82′ E, Poland), belonging to the Research and Education Center of Gorzyń, Poznań University of Life Sciences. When the wheat seedlings grew up to 8 cm long, they were collected, rinsed in running water, and disinfected using potassium manganate (0.05% KMnO₄) for 1 min. Then, two seedlings found showing the best growth performance were transplanted to polyethylene plastic pots (volume: 7 L). The plants were grown in the greenhouse for 4 months (from April to July) at a temperature between 18°C and 30°C, under a photoperiod of 16:8 h and relative humidity of 60–70%. All the plants were regularly fertilized using Florovit (5 mL/2 L H2O) and ammonium nitrate (1 g/pot) 1 month after the beginning of the experiment.
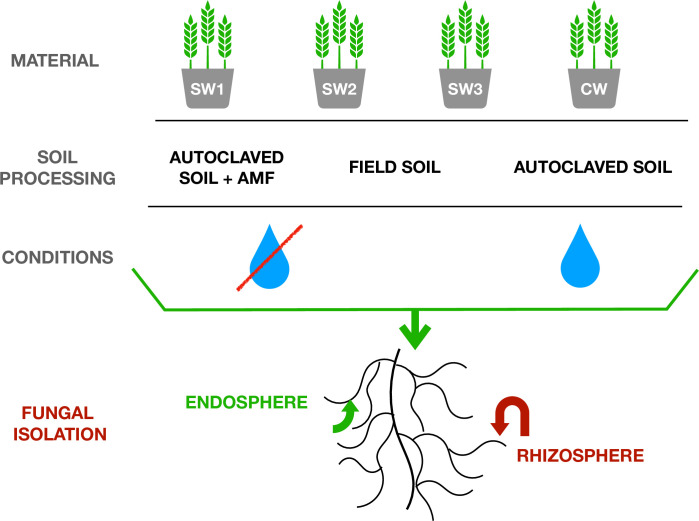
The experimental design was completely randomized in a 2×4×3 factorial scheme with four replications (Fig 1): four wheat cultivars (common wheat: ‘Dakotana’ (KWS Saat, Einbeck, Germany); spelt wheat: ‘Badenstern’, ‘Badenkrone’ (both ZG Raiffeisen eG, Karlsruhe, Germany), and ‘Zollernspelz’ (Saaten Union, Isernhagen, Germany)), two levels of drought stress (control—pots were irrigated with deionized water; drought—pots were not irrigated in the flowering phase), three types of soil preparations (control—sterile, autoclaved soil; nonsterile soil—collected from Złotniki Research Station, with a natural microbiological component; autoclaved soil with the addition of AMF (DAOM 197198 strain of Rhizophagus irregularis, syn. Glomus irregulare; Connectis, Agronutrition, France)), and two areas of roots (endosphere and rhizosphere).
Fig 1. Research design of the conducted experiment.
SW: spelt wheat, 1: ‘Badenstern’, 2: ‘Badenkrone’, 3: ‘Zollernspelz’; CW: common wheat ‘Dakotana’. AUTOCLAVED SOIL: control—sterile soil; FIELD SOIL: nonsterile soil, collected from Złotniki Research Station with a natural microbiological component; SOIL + AMF: autoclaved soil with the addition of arbuscular mycorrhizal fungi: DAOM 197198 strain of Rhizophagus irregularis.
Soil preparation
The soil (0–20 cm layer depth) used for the greenhouse experiment was collected at the Złotniki Research Station, from the field where spelt wheat and common wheat were grown. According to the FAO/WRB classification [17], the soil used in the study was a luvisol of light clay sand grade, shallowly deposited on a light clay belonging to a good rye complex. The pH was 5.7, and it had an average phosphorus content of 120.6 mg P2O5 kg-1 (very high) and an average potassium content of 122.5 mg K2O kg-1 (high) with 0.59% organic matter. A part of the collected soil was first sieved in 4-mm mesh, autoclaved at 120°C for 1 h, and subsequently stored for 2 days before it was used in the greenhouse. Half of the pots filled with this autoclaved soil medium were inoculated by watering 2 mL per two plants of spore suspension of R. irregularis DAOM 197198 strain (2×103 spores mL−1 sterile water), while the remaining pots filled with the autoclaved soil medium were treated with 2 mL of sterile water.
Drought stress conditions
Drought stress was initiated in the flowering phase (BBCH 65–69) and was maintained for 8 days. The drought symptoms were evaluated in tested plants by observing the leaves vigor and measuring the soil humidity using a probe (ThetaProbe, Eijkelkamp Penetrologger SN, Giesbeek, The Netherlands). Wheat plants showing rolled-up leaf blades, grown in different soil variants with a low moisture level (5–8%), were selected for further analyses.
Sampling and fungi isolation
To evaluate the fungal communities, the roots were separated from wheat plants at late milk maturity stage in BBCH 73–77 phase, after 8 days of drought. The collected roots were placed in separate paper bags, transported to the laboratory, and stored for 24 h at 4°C until processing.
Fungal isolates were obtained from endosphere and rhizosphere of both wheat and spelt wheat roots. To isolate the endophytic fungi, the plant roots were sectioned into 4- to 5-cm-long pieces and were surface sterilized by treating with 70% ethanol and 0.5% active chlorine and rinsing five times in sterile distilled water. Then, the root samples were cut with a sterile scalpel to obtain 1-cm-long sections. Both sterilized and nonsterilized parts of roots were placed on Potato Dextrose Agar (PDA; Oxoid™, Thermo Fisher Scientific, Waltham, Massachusetts, United States) supplemented with ampicillin (50 mg mL-1). The samples were incubated at 22°C for 1–2 weeks or until the emergence of mycelia. Putative fungal colonies were isolated by performing three (or more if needed) rounds of subculture on PDA, until a visually homogeneous culture (confirmed by observing under a light microscope (Zeiss)) was obtained. Pure cultures were either subsequently used for DNA isolation or were transferred to tubes containing SNA (synthetic nutrient-poor agar) and preserved in sterile mineral oil at 4°C for further analyses.
DNA isolation, PCR amplification, and sequencing
For DNA isolation, 40 mg of mycelium obtained from homogeneous cultures was used. DNA was extracted from the mycelium using Wizard® Genomic DNA Purification Kit (Promega, Madison, Wisconsin, United States), according to the manufacturer’s protocol. For fungal identification, DNA sequencing of the internal transcribed spacer (ITS) region, small-subunit (SSU) or large-subunit (LSU) nrRNA, and protein-coding markers (beta-tubulin (tub2) and translation elongation factor 1-alpha (tef1)) was performed. The primers and PCR conditions used in the study are described in Table 1. DNA amplification was performed on a T-1000 Thermal Cycler (BioRad, Hercules, California, United States). Each PCR mix contained approximately 50 ng of DNA, 1.0 μM of each primer, 0.2 mM of each dNTP (Sigma-Aldrich, Saint Louis, Missouri, United States), and 1.25 unit of DreamTaq Green DNA Polymerase (Thermo Fisher Scientific, Waltham, Massachusetts, United States). After amplification, the PCR products were separated and examined on a 1.5% agarose gel added with Simply Safe dye (EURx, Poland) and 100-bp DNA Ladder Plus (Thermo Fisher Scientific, Waltham, Massachusetts, United States). Then, 2.5 μL of each amplicon was purified by incubating for 30 min at 37°C with 2 units of Exonuclease I (Thermo Fisher Scientific, Waltham, Massachusetts, United States) and 0.4 unit of FastAP Thermosensitive Alkaline Phosphatase I (Thermo Fisher Scientific, Waltham, Massachusetts, United States). Subsequently, the enzymes were inactivated at 80°C for 15 min. DNA sequencing was performed using the BigDye3.1® Kit under the following conditions: 96°C for 2 min; 25 cycles of 30 s at 96°C, 30 s at 50°C, and 4 min at 60°C. The sequencing product was cleaned using 0.4 g of Sephadex® G-50 beads (Sigma-Aldrich, Saint Louis, Missouri, United States), with a diameter of 20–50 μm, on MultiScreen Filter Plates HTS (Merck Millipore, Burlington, Massachusetts, United States). Capillary electrophoresis procedure was performed using ABI3730 Genetic Analyzer (Applied Biosystems, Foster City, California, United States) in the Institute of Biochemistry and Biophysics of the Polish Academy of Sciences (Warsaw).
Table 1. PCR primers and conditions applied for molecular fungal identification.
| Locus | Amplicon length | Primer | Primer sequence 5’-3’ | PCR conditions | Reference |
|---|---|---|---|---|---|
| Internal Transcribed Spacer (ITS) region of the rRNA | ~450-800bp | ITS1F | CTT GGT CAT TTA GAG GAA GTA A | 1. 95˚C– 5 min 2. 95˚C– 30 s 3. 52˚C– 30 s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 8 min |
[18] |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | ||||
| Translation elongation factor 1-alpha (tef1) | ~600 bp | EF1-1018F | GAY TTC ATC AAG AAC ATG AT | 1. 95˚C– 2 min 2. 66˚C-56˚C touchdown (9 cycles) 3. 95˚C– 30 s 4. 56˚C– 1 min 5. 72˚C– 1 min Steps 3–5 x 36 cycles 6. 72˚C– 10 min |
[19] |
| EF1-1620R | GAC GTT GAA DCC RAC RTT GTC | ||||
| beta-tubulin (tub2) | ~ 500 bp | Bt2a | GGT AAC CAA ATC GGT GCT GCT TTC | 1. 95˚C– 3 min 2. 95˚C– 30 s 3. 58˚C– 30 s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 10 min |
[20] |
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | ||||
| Translation elongation factor 1-alpha (tef1) | ~700 bp | ef1 | ATG GGT AAG GA(A/G) GAC AAG AC | 1. 95˚C– 3 min 2. 95˚C– 30 s 3. 58˚C– 30s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 10 min |
[21] |
| ef2 | GGA (G/A)GT ACC AGT (G/C)AT CAT GTT | ||||
| Translation elongation factor 1-alpha (tef1) | ~700 bp | Ef728M | CATCGAGAAGTTCGAGAAGG | 1. 95˚C– 3 min 2. 95˚C– 30 s 3. 58˚C– 30 s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 10 min |
[22, 23] |
| Tef1R | GCCATCCTTGGAGATACCAGC | ||||
| Small Subunit (SSU, 18S) of the rRNA | ~1200 bp | NS1 | GTA GTC ATA TGC TTG TCT C | 1. 95˚C– 5 min 2. 95˚C– 30 s 3. 52˚C– 30 s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 8 min |
[18] |
| NS4 | CTT CCG TCA ATT CCT TTA AG | ||||
| Large Subunit (LSU, 28S) of the rRNA | ~1200 bp | LROR | ACC CGC TGA ACT TAA GC | 1. 95˚C– 5 min 2. 95˚C– 30 s 3. 52˚C– 30 s 4. 72˚C– 1 min Steps 2–4 x 35 cycles 5. 72˚C– 8 min |
[24, 25] |
Bioinformatics and statistical analysis
The DNA sequences were analyzed using DNA Star Software (Madison, Wisconsin, United States) and BLASTn (Basic Local Alignment Search Tool) algorithm (National Centre for Biotechnology Information (NCBI), http://www.ncbi.nlm.nih.gov/BLAST/). All the sequences contained fragments of ITS, SSU and LSU regions, and tub2 and tef1 genes. These were deposited in NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/), and their accession numbers are provided in S1 Table. Charts illustrating the composition of fungal communities identified in the tested conditions were prepared using ggplot2 and VennDiagram packages for R software [26, 27]. Evolutionary analyses were conducted in MEGA X [28], by applying the Maximum Likelihood method and the Tamura–Nei model [29]. The bootstrap consensus tree inferred from 500 replicates was taken to represent the evolutionary history of the sequences analysed [30]. The initial trees for the heuristic search were obtained automatically by applying the Neighbor-Joining and BioNJ algorithm to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach, and then selecting the topology that had the superior log-likelihood value. A discrete Gamma distribution was applied to model the evolutionary rate differences among sites (5 categories (+G, parameter = 0.8233)). Furthermore, the rate variation model was used to allow some sites to be evolutionarily invariable ([+I], 14.86% sites). The phylogeny tree was generated using the iTOL software, v. 5.5.1 [31].
To examine the differences in the structure of fungal communities among the studied groups, we performed principal coordinate analysis (PCoA), based on the unweighted and weighted UniFrac distances, using phyloseq package in R [32]. To determine the significance of differences observed in the calculated distances, permutational multivariate analysis of variance (PERMANOVA) with a permutational number of 999 was applied, using the adonis function of the vegan package in R [33].
Results
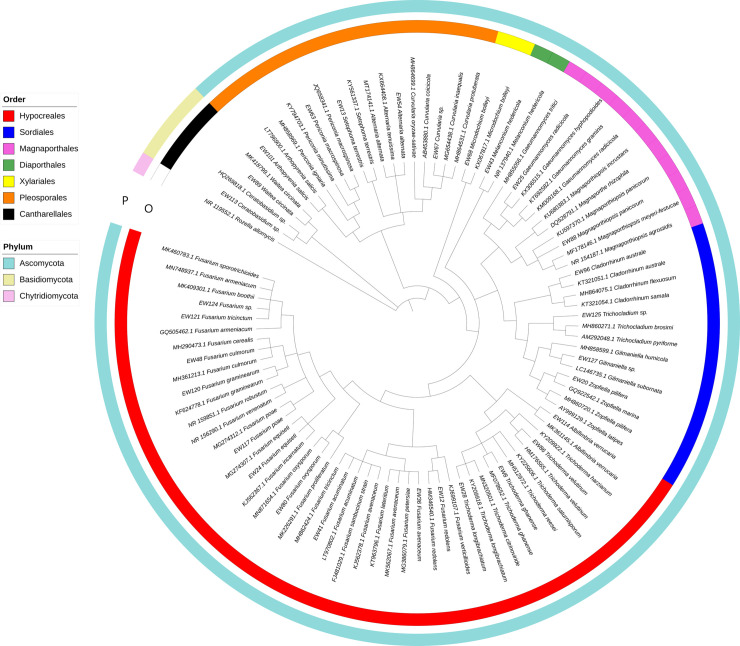
A total of 117 fungal strains were isolated from the endosphere and rhizosphere of the common wheat cultivar ‘Dakotana’ and the spelt wheat cultivars ‘Badenstern’, ‘Badenkrone, and ‘Zollernspelz’, grown in three types of soil preparations (control—autoclaved, sterile soil; nonsterile soil—collected from Złotniki Research Station, with a natural microbiological component; autoclaved soil with AMF (DAOM 197198 strain of R. irregularis) added), under two different growth conditions (watered and not watered during the flowering phase). The majority of the identified fungi belonged to phylum Ascomycota, and among them, the orders Hypocreales and Pleosporales were found to be predominant (Fig 2). Waitea circinata, Ceratobasidium sp., Marasmius sp., and Rhizoctonia solani were the only Basidiomycota observed in the studied wheat roots. A higher number of fungal strains were observed in the rhizosphere than in the endosphere (63 and 54 strains, respectively); however, the endophytic fungal communities demonstrated a similar level of diversity (25 and 27 strains were observed, respectively). Of the identified fungi, 33% were present in both the internal and external parts of the roots. Nonetheless, Albifimbria verrucaria, Alternaria alternata, Curvularia sp., Fusarium acuminatum, F. culmorum, Fusarium equiseti, F. poae, Gaeumannomyces radicicola, Melanconium hedericola, Mucor circinelloides, Marasmius sp., Rhizoctonia sp., Trichoderma velutinum, and Trichoderma ghanense were observed only in the rhizosphere and not in the endosphere, whereas Arthopyrenia salicis, Gilmaniella sp., F. graminearum, Magnaporthiopsis panicorum, Magnaporthiopsis sp., Penicillium crustosum, Periconia macrospinosa, Trichocladium sp., Trichoderma sp., Setophoma sp., Zopfiella pilifera, and Zopfiella sp. were observed only in the inner part of the plant roots.
Fig 2. Evolutionary analysis of the ITS region of root-associated fungi identified in common and spelt wheat, using the Maximum Likelihood method.
The analysis involved 95 nucleotide sequences and contained a total of 695 positions in the final dataset. Branches corresponding to partitions reproduced in less than 50% of bootstrap replicates (500 replicates) are collapsed.
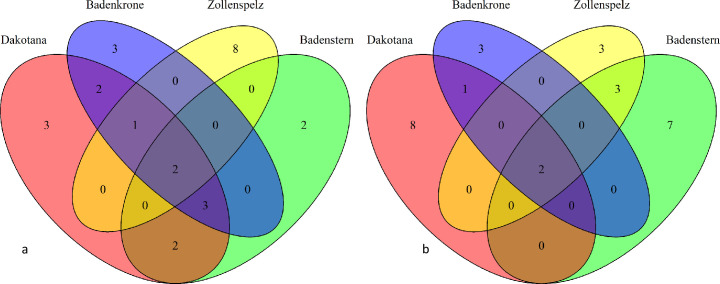
The composition of fungal communities was also found to vary depending on the host genotype (Fig 3). In the endosphere, only Microdochium bolleyi and Setophoma terrestris were identified in all the studied cultivars. The ‘Zollernspelz’ cultivar demonstrated the most unique pattern of fungal composition in the root endosphere. By contrast, 65% of the fungi observed in the rhizosphere of this cultivar were similar to those observed in the ‘Badenstern’ cultivar. Fusarium avenaceum and Fusarium redolens were the only fungal species observed in the rhizosphere of all the wheat genotypes analyzed in the study. A majority of the fungi identified in the rhizosphere of ‘Dakotana’ were found to be exclusive to this cultivar, whereas 75% of the fungal endophytes observed in this cultivar were also detected in the remaining analysed wheat genotypes.
Fig 3.
Comparison of fungi identified in ‘Badenstern’, ‘Badenkrone, ‘Zollernspelz’, and ‘Dakotana’ wheat in the root (a) endosphere and (b) rhizosphere.
In general, the composition and abundance of the wheat root-associated fungi were not found to be significantly influenced by drought stress. Fungi identified in wheat roots under drought and control conditions, were listed in Table 2. Fusarium avenaceum, M. bolleyi, Cladorrhinum australe, F. redolens, W. circinata, F. oxysporum, Ceratobasidium sp., R. solani, T. ghanense, Fusarium tricinctum, Periconia sp., and S. terrestris were observed in both plants grown under drought and those grown in well-watered conditions. However, Trichoderma longibrachiatum and T. velutinum were observed only in the plants grown under drought stress, while Zopfiella sp., M. hedericola, A. verrucaria, G. radicicola, and A. salicis were observed exclusively in the irrigated plant groups.
Table 2. Comparison of fungi identified in roots endosphere and rhizosphere of wheat plants under drought and controlled conditions.
| ENDOSPHERE | RHIZOSPHERE | |||
|---|---|---|---|---|
| DROUGHT | CONTROL | DROUGHT | CONTROL | |
| 1. | Cladorrhinum australe | Arthopyrenia salicis | Alternaria alternata | Albifimbria verrucaria |
| 2. | Fusarium avenaceum | Ceratobasidium sp. | Ceratobasidium sp. | Cladorrhinum australe |
| 3. | Fusarium oxysporum | Cladorrhinum australe | Cladorrhinum australe | Culvularia sp. |
| 4. | Fusarium redolens | Fusarium avenaceum | Fusarium avenaceum | Fusarium acuminatum |
| 5. | Fusarium sp. | Fusarium graminearum | Fusarium culmorum | Fusarium avenaceum |
| 6. | Gilmaniella sp. | Fusarium oxysporum | Fusarium redolens | Fusarium equiseti |
| 7. | Magnaporthiopsis panicorum | Fusarium redolens | Fusarium sp. | Fusarium poae |
| 8. | Microdochium bolleyi | Fusarium sp. | Fusarium tricinctum | Fusarium redolens |
| 9. | Penicillium crustosum | Fusarium tricinctum | Microdochium bolleyi | Gaeumannomyces radicicola |
| 10. | Periconia macrospinosa | Magnaporthiopsis sp. | Periconia sp. | Marasmius sp. |
| 11. | Rhizoctonia solani | Microdochium bolleyi | Rhizoctonia sp. | Melanconium hedericola |
| 12. | Setophoma terrestris | Periconia sp. | Setophoma terrestris | Microdochium bolleyi |
| 13. | Trichocladium sp. | Setophoma sp. | Trichoderma ghanense | Mucor circinelloides |
| 14. | Trichoderma longibrachiatum | Setophoma terrestris | Trichoderma longibrachiatum | Rhizoctonia solani |
| 15. | Waitea circinata | Trichoderma sp. | Trichoderma velutinum | Setophoma terrestris |
| 16. | - | Waitea circinata | Waitea circinata | Trichoderma ghanense |
| 17. | - | Zopfiella pilifera | - | Waitea circinata |
| 18. | - | Zopfiella sp. | - | - |
Soil preparation strongly affected the mycobiome structure of wheat roots. Table 3 shows fungi identified in wheat roots grew in soils after three different types of processing. The roots collected from the plants grown in nonautoclaved field soil demonstrated the most diverse mycobiome composition (21 taxons). Moreover, more than 70% of the identified fungi (A. salicis, Curvularia sp., Periconia sp., P. macrospinosa, Zopfiella sp., Z. pilifera, F. equiseti, Marasmius sp., Setophoma sp., S. terrestris, G. radicicola, M. circinelloides, Magnaporthiopsis sp., M. panicorum, and T. velutinum) were observed exclusively in the nonautoclaved soil group. Fungi belonging to genera Setophoma, Periconia, Magnaporthiopsis, Fusarium, Michrodochium, and Waitea were most frequently isolated from the plants grown in nonautoclaved soil. However, wheat plants grown in the autoclaved soil also demonstrated equally high species richness (19 taxons), with almost 50% of the species identified were noticed only in this group (A. alternata, F. culmorum, F. graminearum, F. tricinctum, Trichocladium sp., Trichoderma sp., T. longibrachiatum, Gilmaniella sp., Rhizoctonia sp.). Only three of the observed species, namely F. avenaceum, M. bolleyi, and W. circinata, were commonly observed in all the analyzed soil preparations. Of the soil groups analyzed, the autoclaved and AMF-added groups showed high similarity in the structure of fungal communities (A. verrucaria, Ceratobasidium sp., C. australe, F. poae, R. solani, T. ghanense). By contrast, the field soil having a natural microorganism composition presented a diverse fungal reservoir; thus, only two and one species, belonging to genus Fusarium, were shared between the field soil and AMF-added soil and between the field soil and autoclaved soil, respectively.
Table 3. Fungi identified in roots endosphere and rhizosphere of wheat plants grew in soil after different preparation: Autoclaved (CONTROL), autoclaved with the addition of arbuscular mycorrhizal fungi: DAOM 197198 strain of Rhizophagus irregularis (AMF) and nonsterile collected on field (FIELD SOIL).
| CONTROL | AMF | FIELD SOIL |
|---|---|---|
| Albifimbria verrucaria | Albifimbria verrucaria | Arthopyrenia salicis |
| Alternaria alternata | Ceratobasidium sp. | Fusarium oxysporum |
| Ceratobasidium sp. | Cladorrhinum australe | Microdochium bolleyi |
| Cladorrhinum australe | Fusarium acuminatum | Curvularia sp. |
| Fusarium avenaceum | Fusarium avenaceum | Fusarium avenaceum |
| Fusarium culmorum | Fusarium oxysporum | Fusarium equiseti |
| Fusarium graminearum | Fusarium poae | Fusarium oxysporum |
| Fusarium poae | Fusarium redolens | Fusarium redolens |
| Fusarium sp. | Melanconium hedericola | Fusarium sp. |
| Fusarium tricinctum | Microdochium bolleyi | Gaeumannomyces radicicola |
| Gilmaniella sp. | Penicillium crustosum | Magnaporthiopsis panicorum |
| Microdochium bolleyi | Rhizoctonia solani | Magnaporthiopsis sp. |
| Rhizoctonia solani | Trichoderma ghanense | Microdochium bolleyi |
| Rhizoctonia sp. | Waitea circinata | Mucor circinelloides |
| Trichocladium sp. | Periconia macrospinosa | |
| Trichoderma ghanense | Periconia sp. | |
| Trichoderma longibrachiatum | Setophoma terrestris | |
| Trichoderma sp. | Trichoderma velutinum | |
| Waitea circinata | Waitea circinata | |
| Zopfiella pilifera | ||
| Zopfiella sp. |
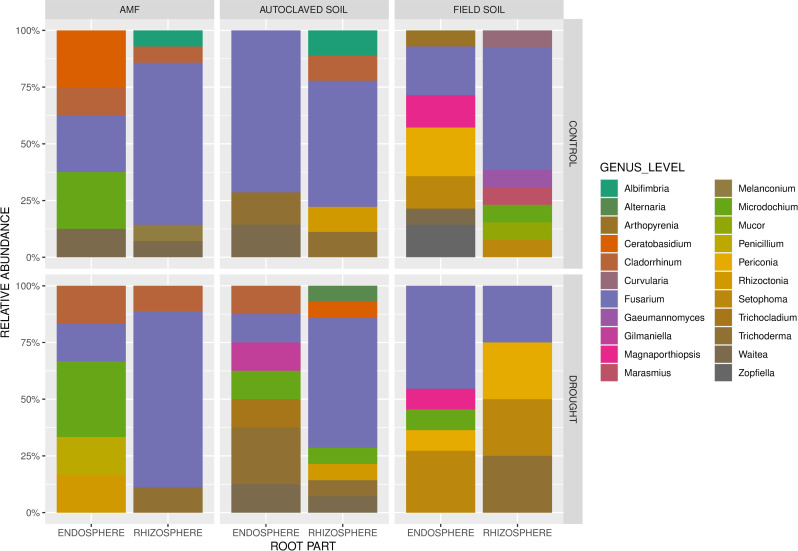
The compiled results of fungal identification at the genus level across soil with different microbiological components, root parts, and watering status are shown in Fig 4. A distinct endophytic structure was observed in three studied soil preparation types depending on the drought existence. Greater number of different genera were identified in roots from field soil and from AMF soil under controlled condition, than in the same groups under drought stress. On the other hand, the opposite pattern was observed in the autoclaved soil, in which the fungal communities were found to be much more diverse in wheat roots under the water-deficient condition. The amount of Fusarium sp. was lower in the endosphere of plants grown in the AMF and autoclaved soil under drought, in contrast to the field soil plants in which the amount of these species was higher in the endosphere than in the rhizosphere. Moreover, Trichoderma sp. were found more often under drought, compared to the roots of wheat plants grown in controlled conditions.
Fig 4. Genus-level relative abundance (%) of the identified fungi across different soil treatment, root part, and watering status.
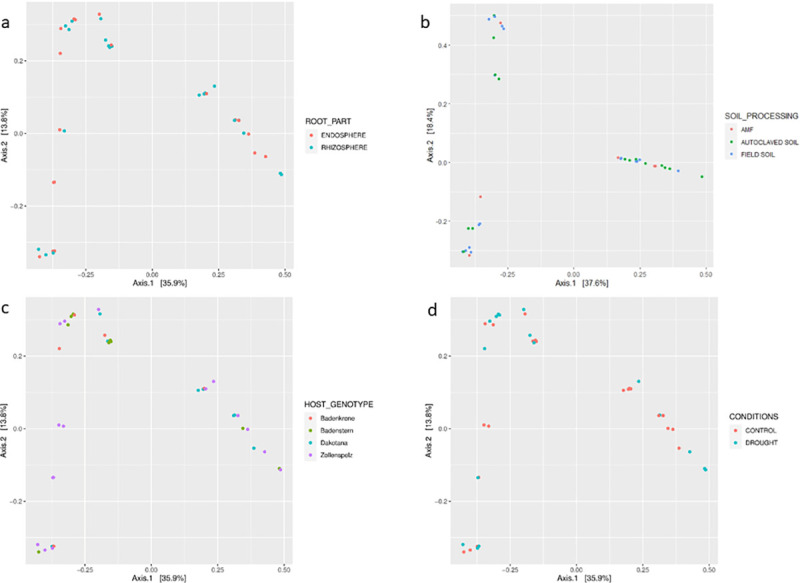
PCoA, performed based on unweighted (Fig 5) and weighted (data not shown) UniFrac distances, showed the differences in biological communities across parts of roots, watering conditions, host varieties, and different soil preparation. Unweighted UniFrac considers only the sequence distances, whereas weighted UniFrac also includes abundance information. PERMANOVA performed based on unweighted UniFrac distances revealed that the part of the roots and different soil treatment had a significant impact on the composition of fungal communities in wheat roots (p<0.05 and p<0.01, respectively). The influence of different soil preparation was also confirmed by PERMANOVA conducted based on weighted UniFrac (p<0.01).
Fig 5. Principal coordinate analysis of the fungal community structure across different root parts, watering conditions, cultivars, and soil preparation with various microbiological components, based on unweighted UniFrac distances.
Discussion
Here, wheat plant have been used as a model to investigate the impact of rhizocompartments (endosphere, rhizosphere), host genotype, watering status and different soil preparation on roots mycobiome structure by detailed characterization of the root fungal communities. In the present study, we have demonstrated the existence of 20 species (C. australe, F. avenaceum, F. equiseti, F. oxysporum, F. redolens, F. tricinctum, Fusarium sp., G. radicicola, Magnaporthiopsis sp., Marasmius sp., M. bolleyi, M. circinelloides, P. crustosum, Periconia sp., Setophoma sp., S. terrestris, T. ghanense, T. longibrachiatum, W. circinata, Z. pilifera) in the internal parts and on the surface of the roots in common wheat and 31 species (A. verrucaria, A. alternata, A. salicis, Ceratobasidium sp., C. australe, Curvularia sp., F. acuminatum, F. avenaceum, F. culmorum, F. graminearum, F. oxysporum, F. poae, F. redolens, F. tricinctum, Fusarium sp., Gilmaniella sp., M. panicorum, Magnaporthiopsis sp., M. hedericola, M. bolleyi, P. macrospinosa, Periconia sp., R. solani, Rhizoctonia sp., S. terrestris, Trichocladium sp., T. longibrachiatum, Trichoderma sp., T. velutinum, W. circinata, Zopfiella sp.) in the case of spelt wheat. Most of the species identified in this study as inhabiting the roots of common wheat have already been described as wheat root-associated fungi in the previous studies [34–38]. Nevertheless, in the case of spelt wheat, knowledge on root-inhabiting fungal species remains limited [39].
We tracked the changes in roots mycobiome induced by applied conditions, and found out the impact of rhizocompartments and different soil preparation on fungal composition in wheat roots (Fig 5). We observed some compartment specific taxa: A. verrucaria, A. alternata, Curvularia sp., F. acuminatum, F. culmorum, F. equiseti, F. poae, G. radicicola, M. hedericola, M. circinelloides, Marasmius sp., Rhizoctonia sp., T. velutinum, and Trichoderma ghanense exclusively in the rhizosphere and A. salicis, Gilmaniella sp., F. graminearum, M. panicorum, Magnaporthiopsis sp., P. crustosum, P. macrospinosa, Trichocladium sp., Trichoderma sp., Setophoma sp., Z. pilifera, and Zopfiella sp. only in endosphere. A number of previous studies have focused on the endophytes of the common wheat—T. aestivum ssp. vulgare L.; however, they mainly analyzed the differences in structure between plant organs and management strategies or growth stages [35, 40–42]. To the best of our knowledge, no studies published thus far have investigated the fungal structure and dynamics of the endosphere and/or rhizosphere of spelt wheat—T. aestivum ssp. spelta L. However, the endogenous bacterial communities in the endosperm, germ, roots, coleoptiles, and leaves of spelt wheat were described in a recent work [43].
The type of the soil preparation also significantly influenced the mycobiome composition of wheat roots. Mycobiome structure comparison between roots from field soil and autoclaved soil (control) revealed that numerous of identified fungi occurred only in ‘field soil’ samples, this indicates origin from adjacent soil area. We assume that fungi observed in rhizosphere of roots grown in autoclaved soil derived from the inner part of the plant and was able to colonize the sterile niche. Furthermore, the mycobiome structure of roots from autoclaved (control) and AMF soil were similar, so addition of Rhizophagus irregularis didn’t have notable effect on structure of root-associated fungal communities in wheat. The root-associated fungi observed in the plants grown in field soil were the most diverse, whereas the roots isolated from the AMF and autoclaved soil groups presented a similar fungal composition, especially with respect to the abundance of Fusarium ssp. (Fig 4). Other studies on fungal communities colonizing wheat plants have indicated that geographical location, cultivar, growth stage, and leaf position in phyllosphere [42], host maturity and host organ in endosphere [40, 41] and management strategies [41] significantly impacted the fungal reservoir in Triticale.
The ‘Zollernspelz’ spelt wheat cultivar analyzed in this study is a modern, high-yielding variety cultivated in central Europe [44], and our results showed that this cultivar demonstrated the most unique endophytic pattern, as it had the highest number of exclusive endogenous fungi (Fig 3). However, PCoA analysis did not reveal the significant impact of host genotype in roots mycobiome in studied wheat cultivars. By contrast, previous studies indicated that host genotype affects fungal communities structure in wheat phyllosphere on species level (wheat, barley, oat, rye, triticale), as well as cultivar level (6 wheat cultivars) [45]. Presumably, the impact of host genotype on below ground wheat organs mycobiome is limited, or applied by the authors more sensitive method (culture independent approach) influenced obtained results.
Till date, no study has explored how the structures of fungal communities in wheat and spelt wheat roots are affected by drought stress. However, Vujanovic et al. (2019) [46] recently investigated the impact of drought on Triticum durum L. var. durum plants. The authors showed that if the first-generation seeds of T. durum plants were pretreated with an endophytic plant growth promoter (Penicillium sp. SMCD 2318), the second- and third-generation plants exhibited higher drought resistance and positive phenotypic changes under drought stress. We did not observe a significant impact of drought on the composition of fungal communities in wheat roots. Obtained results are in agreement with the recent work, were the abundance of microorganisms residing in rhizosphere of non-irrigated and irrigated wheat plants, were measured using real-time PCR [47]. The authors did not observe significant changes in fungal ITS region abundance in studies samples. Interestingly, the bacterial 16S gene copies were less abundant in non-irrigated soil. Obtained result suggest that fungi are more resistant on water changes in the soil, than bacterial communities. Hawkes et al. (2011) [48] observed greater fungal diversity and abundance in soil with periodically low rainfall and assumed that drought stress moderates competition between fungi. However, we identified similar number of different taxa in rhizosphere under drought and controlled condition (16 and 17, respectively; Table 2) Probably, the composition of fungi in soil closely surrounding roots and from adjacent area presenting different relations under water deficiency conditions. Moreover, we did not notice any drought-specific pattern of fungal composition in the roots of the studied plants, however the M. hedericola, A. verrucaria, G. radicicola, and A. salicis and the species from Zopfiella genus, observed exclusively in the irrigated group, as well as T. longibrachiatum and T. velutinum, found only in the plants grown under drought stress. It is worth mentioning that Trichoderma sp. are known to be associated with plant roots, where these fungi either form a symbiotic relationship or occur as plant endophytes [49–51]. Studies have shown that Trichoderma strains exerted direct effects on host plants by increasing their growth potential and nutrient uptake, efficiency of fertilizer use, percentage and rate of seed germination, and their ability to withstand abiotic stresses, such as drought, salinity, and high temperature, as well as biotic stresses by stimulating their defense [50, 52, 53]. In addition, their natural ability to attack other fungi, and in particular their antagonistic activity toward plant pathogens, such as Botrytis cinerea, Fusarium spp., Pythium spp., R. solani, Verticillium dahilae, and Sclerotinia spp. [49, 54] has contributed to the recognition of Trichoderma spp. as a significant Microbial Biological Control Agent that can help contain the pathogen populations under different agricultural conditions, protect host plants, and enhance vegetative growth, while acting as soil amendments improving the nutrient ability and rate of decomposition and biodegradation [50, 55]. Therefore, the detection of Trichoderma spp. in the roots of wheat plants grown under drought stress conditions reported in the present study may indicate their potential to enhance the resistance of host to abiotic stress, which can contribute to improving wheat cultivation in unfavorable conditions. However, to confirm the prevalence of Trichoderma spp. in wheat roots under water-deficient conditions, the use of high-throughput technologies (next-generation sequencing of the ITS region) on a larger plant group is recommended. Moreover, to prove the beneficial effects of the isolated Trichoderma spp. in terms of increased growth and yield of wheat plants and their enhanced resistance to biotic/abiotic stresses, more comprehensive further research is needed.
Furthermore, our study revealed the coexistence of plant fungal pathogens, symbionts, and commensals in the complex ecosystem of common wheat and spelt wheat roots. Regardless of the growth conditions applied, the genus that was most frequently isolated was Fusarium. Nonetheless, under drought conditions, the abundance of Fusarium sp. in the wheat rhizosphere was lower in the plants grown in field soil. Presumably, the simultaneous occurrence of antagonistic Trichoderma spp. [54] might have caused the observed reduction. Fusarium spp., especially F. graminearum, F. culmorum, F. avenaceum, F. poae, and F. triticum, are mostly known as causal agents of Fusarium head blight, as well as Fusarium foot and root rot, and also cause other detrimental changes in wheat plants [56, 57]. A distinct pattern of Fusarium colonization was noticed between the rhizosphere and endosphere in the studied wheat groups. Among the irrigated plants, the roots growing in the soil with AMF addition and in the nonsterilized field soil demonstrated a lower amount of Fusarium spp. in the endosphere than in the rhizosphere. By contrast, the autoclaved soil group demonstrated a similar abundance of Fusarium spp. in both root parts. On the other hand, the situation was diverse under drought conditions; the roots growing in AMF and autoclaved soils showed a reduction of Fusarium spp. in the endosphere, whereas in field soil the Fusarium colonization was observed to be high in root endosphere. Bokati et al. (2016) [58] observed that Fusarium spp. occurred more frequently in root endosphere from desert soil than in clay soil (42% and 23%, when evaluated using a culture-dependent method; 65% and 17%, when culture-independent methods were applied, respectively).
Among the endogenous and epiphytic fungi identified in the study, we observed a large group of microorganisms that have been documented with a positive impact on their host. As mentioned earlier, this group includes species from Trichoderma genus (T. ghanense, T. longibrachiatum, T. velutinum, and some unrecognized Trichoderma sp.) which have the ability to antagonize plant-pathogenic fungi and stimulate the growth and defense of host plants [51, 54]. In this study, we also identified A. verrucaria in the rhizosphere of AMF and autoclaved soil. This species was previously isolated from grapes and was also shown to inhibit the growth of B. cinerea causing green mold on this fruit [59]. However, A. verrucaria causes stem necrosis and leaf spot in tomato [60]. Waitea circinata is an orchid myccorhizal fungus and inhibits the growth of Magnaporthe oryzae causing rice blast [61]. Several of the root-associated fungi identified by us belong to the group of latent pathogens. Some of them are major wheat pathogens (e.g. F. poae, F. culmorum, and F. avenaceum), while some identified fungi are known to be pathogens of plants other than wheat; for example, S. terrestris causes pink root rot in squash, canola, and onion [62, 63]. Moreover, plenty of species with unrecognized impact on wheat plants were identified in this study. The relationship between the identified fungi and wheat plants is unknown, and therefore, additional studies, especially focusing on naturally occurring endogenous fungi that are potential sources of biocontrol agents, are needed.
Conclusion
A total of 117 fungal strains, belonging to 22 genera, colonizing the internal and external root parts of T. aestivum ssp. spelta L. and T. aestivum ssp. vulgare L. cultivars were isolated. Here, we found that the type of the root part and soil preparation influence the mycobiome composition of the common and spelt wheat roots. Moreover, Trichoderma longibrachiatum and Trichoderma velutinum, were found exclusively in the plants grown under drought stress. However, water deficiency conditions don’t have significant impact on the roots fungal communities in wheat. In addition, for the first time the fungal reservoir in the root endosphere and rhizosphere of T. aestivum ssp. spelta L. was examined.
Supporting information
(XLSX)
Acknowledgments
We wish to thank Prof. Jerzy Chełkowski for morphological evaluation of molecular identified fungi belongs to Fusarium genus.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the National Science Center in Poland Grant No 2017/27/B/NZ9/01591. Lidia Błaszczyk is PI of mentioned grant. LB contribution in submitted manuscript: Conceptualization, Writing – Review & Editing, Funding Acquisition.
References
- 1.Petrini O. Fungal endophytes of tree leaves Microb Ecol. Springer, New York, NY, 1991; 179–197. [Google Scholar]
- 2.Feng NX, Yu J, Zhao HM, Cheng YT, Mo CH, Cai QY et al. Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci Total Environ. 2017; 352–368. [DOI] [PubMed] [Google Scholar]
- 3.Latz MA, Jensen B, Collinge DB, Jørgensen HJ. Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Divers. 2018; 11: 555–567. [Google Scholar]
- 4.Huang XF, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM. Rhizosphere interactions: root exudates, microbes, and microbial communities. Bot. 2014; 92: 267–275. [Google Scholar]
- 5.Wang Z, Li T, Wen X, Liu Y, Han J, Liao Y, et al. Fungal communities in rhizosphere soil under conservation tillage shift in response to plant growth. Front Microbiol. 2017; 8:1301 10.3389/fmicb.2017.01301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006; 57: 233–266. 10.1146/annurev.arplant.57.032905.105159 [DOI] [PubMed] [Google Scholar]
- 7.Wu QS, Zou YN. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants Arbuscular mycorrhizas and stress tolerance of plants. Singpore: Springer; 2017. pp 21–44. [Google Scholar]
- 8.Juroszek P, von Tiedemann A. Climate change and potential future risks through wheat diseases: a review. Eur J Plant Pathol. 2013; 136.1: 21–33. [Google Scholar]
- 9.Waalwijk C, Kastelein P, De Vries I, Kerényi Z, Van Der Lee T, Hesselink T, et al. Major changes in Fusarium spp. in wheat in the Netherlands. Eur J Plant Pathol. 2003; 109:743–754. [Google Scholar]
- 10.Kwak YS, Weller DM. Take-all of wheat and natural disease suppression: a review. Plant Pathol J. 2013; 29:125 10.5423/PPJ.SI.07.2012.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toreti A, Ottmar C, Matteo Z. Concurrent climate extremes in the key wheat producing regions of the world. Sci Rep. 2019; 9:1–8. 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igrejas G, and Gérard B. The importance of wheat Wheat Quality For Improving Processing And Human Health. Cham: Springer; 2020. pp.1–7. [Google Scholar]
- 13.Andruszczak S, Kwiecinska-Poppe E, Kraska P, Palys E. Yield of winter cultivars of spelt wheat (Triticum aestivum ssp. spelta L.) cultivated under diversified conditions of mineral fertilization and chemical protection. Acta Sci Pol Agric. 2011; 10. [Google Scholar]
- 14.Talaat NB. Abiotic stresses-induced physiological alteration in wheat Wheat Production in Changing Environments. Singapore:Springer; 2019. pp. 1–30. [Google Scholar]
- 15.Ali OA. Wheat Responses and Tolerance to Drought Stress Wheat Production in Changing Environments. Singapore:Springer; 2019. pp. 129–138. [Google Scholar]
- 16.Rauf M, Munir M, ulHassan M, Ahmad M, Afzal M. Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr J Biotechnol. 2007; 6:971–975. [Google Scholar]
- 17.IUSS Working Group WRB. World Reference Base for Soil Resources 2006, first update 2007 World Soil Resources Reports No.103. FAO, Rome: available from: http://www.fao.org/fileadmin/templates/nr/images/resources/pdf_documents/wrb2007_red.pdf [Google Scholar]
- 18.White TJ, Bruns T, Lee SJWT, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18; 1990. pp. 315–322. [Google Scholar]
- 19.Stielow JB, Levesque CA, Seifert KA, Meyer W, Iriny L, Smits, D., et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia: Molecular Phylogeny and Evolution of Fungi. 2015;35:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995; 61:1323–1330. 10.1128/AEM.61.4.1323-1330.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiser DM, del Mar Jiménez-Gasco M, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, et al. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol. 2004; 110: 473–479. [Google Scholar]
- 22.Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999: 91: 553–556. [Google Scholar]
- 23.Kullnig-Gradinger CM, Szakacs G, Kubicek CP. Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycol Res. 2002; 106:757–767. [Google Scholar]
- 24.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990; 172:4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehner SA, Samuels GJ. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Can J Bot. 1995; 73:816–823. [Google Scholar]
- 26.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 27.Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12:35 10.1186/1471-2105-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018; 35:1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993; 10:512–526. 10.1093/oxfordjournals.molbev.a040023 [DOI] [PubMed] [Google Scholar]
- 30.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evol. 1985;39:783–791. [DOI] [PubMed] [Google Scholar]
- 31.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one. 2013; 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, et al. Package ‘vegan’. Community ecology package, version 2; 2013. pp.1–295. [Google Scholar]
- 34.Chen S, Waghmode TR, Sun R, Kuramae EE, Hu C, Liu B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome. 2019; 7:136 10.1186/s40168-019-0750-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenc L, Kwaśna H, Sadowski C, Grabowski A. Microbiota in wheat roots, rhizosphere and soil in crops grown in organic and other production systems. J Phytopathol. 2015; 163: 245–263. [Google Scholar]
- 36.Larran S, Perello´ A, Simo´n MR, Moreno V. Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J Microbiol Biot. 2002;18:683–686 [Google Scholar]
- 37.Larran S, Perello A, Simon MR, Moreno V. The endophytic fungi from wheat (Triticum aestivum L.). World J Microbiol Biot. 2007;23:565–572. [Google Scholar]
- 38.Gqozo MP, Bill M, Siyoum N, Labuschagne N, Korsten L. Fungal diversity and community composition of wheat rhizosphere and non-rhizosphere soils from three different agricultural production regions of South Africa. Appl Soil Ecol. 2020; 151:1035–43. [Google Scholar]
- 39.Kiecana I, Cegielko M, Rachon L, Pastucha A, Wit M, Pojmaj M. The occurrence of fungi on roots and stem bases of Triticum aestivum ssp. spelta L. Thell. grown under two levels of chemical protection and harmfulness of Fusarium graminearum Schwabe to seedlings of selected genotypes. Acta Agrobot. 2016;69. [Google Scholar]
- 40.Comby M, Lacoste S, Baillieul F, Profizi C, Dupont J. Spatial and temporal variation of cultivable communities of co-occurring endophytes and pathogens in wheat. Front Microbiol. 2016;7:403 10.3389/fmicb.2016.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gdanetz K, Trail F. The wheat microbiome under four management strategies, and potential for endophytes in disease protection. Phytobiomes. 2017;1: 158–168. [Google Scholar]
- 42.Sapkota R, Jørgensen LN, Nicolaisen M. Spatiotemporal variation and networks in the mycobiome of the wheat canopy. Front Plant Sci. 2017;8:1357 10.3389/fpls.2017.01357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuźniar A, Włodarczyk K, Grządziel J, Goraj W, Gałązka A, Wolińska A. Culture-independent analysis of an endophytic core microbiome in two species of wheat: Triticum aestivum L.(cv.‘Hondia’) and the first report of microbiota in Triticum spelta L.(cv.‘Rokosz’). Syst Appl Microbiol. 2020; 43:126025 10.1016/j.syapm.2019.126025 [DOI] [PubMed] [Google Scholar]
- 44.Castillo AM, Allue S, Costar A, Alvaro F, Valles MP. Doubled Haploid Production from Spanish and Central European Spelt by Anther Culture. J Agric Sci Technol. 2019;21:1313–1324. [Google Scholar]
- 45.Sapkota R., Knorr K., Jørgensen LN, O’Hanlon KA., Nicolaisen M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015;207:1134–1144. 10.1111/nph.13418 [DOI] [PubMed] [Google Scholar]
- 46.Vujanovic V, Islam MN, Daida P. Transgenerational role of seed mycobiome–an endosymbiotic fungal composition as a prerequisite to stress resilience and adaptive phenotypes in Triticum. Sci Rep.2019; 9:1–13. 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azarbad H, Constant P, Giard-Laliberté C, Bainard LD, Yergeau E. Water stress history and wheat genotype modulate rhizosphere microbial response to drought. Soil Biol Biochem. 2018:126, 228–236. [Google Scholar]
- 48.Hawkes CV, Kivlin SN, Rocca JD, Huguet V, Thomsen MA, Suttle KB. Fungal community responses to precipitation. Glob Chang Biol. 2011;17:1637–1645. [Google Scholar]
- 49.Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, et al. Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol. 2011;16: 749–759. [DOI] [PubMed] [Google Scholar]
- 50.Hermosa R, Viterbo A, Chet I, Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology. 2012;158: 17–25. 10.1099/mic.0.052274-0 [DOI] [PubMed] [Google Scholar]
- 51.Błaszczyk L, Siwulski M, Sobieralski K, Lisiecka J, Jędryczka M. Trichoderma spp.–application and prospects for use in organic farming and industry. J Plant Prot Res. 2014;54:309–317. [Google Scholar]
- 52.Mastouri F, Björkman T, Harman GE. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol Plant Microbe Interact. 2012; 25:1264–1271. 10.1094/MPMI-09-11-0240 [DOI] [PubMed] [Google Scholar]
- 53.Brotman B, Landau A, Cuadros Inostroza A, Takayuki T, Fernie A, Chet I et al. Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PloS Pathog. 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Błaszczyk L, Basinska-Barczak A, Cwiek-Kupczynska H, Gromadzka K, Popiel D, Stepien L. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Pol J Microbiol. 2017;66:85–101. 10.5604/17331331.1234996 [DOI] [PubMed] [Google Scholar]
- 55.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. 10.1038/nrmicro797 [DOI] [PubMed] [Google Scholar]
- 56.Champeil A, Doré T, Fourbet JF. Fusarium head blight: epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004;166:1389–1415. [Google Scholar]
- 57.Balmas V, Scherm B, Marcello A, Beyer M, Hoffmann L, Migheli Q, et al. Fusarium species and chemotypes associated with fusarium head blight and fusarium root rot on wheat in Sardinia. Plant Pathol. 2015; 64:972–979. [Google Scholar]
- 58.Bokati D, Herrera J, Poudel R. Soil influences colonization of root-associated fungal endophyte communities of maize, wheat, and their progenitors. J Mycol. 2016. [Google Scholar]
- 59.Li Z, Chang P, Gao L, Wang X. The Endophytic Fungus Albifimbria verrucaria from Wild Grape as an Antagonist of Botrytis cinerea and Other Grape Pathogens. Phytopathology. 2020;110: 843–850. 10.1094/PHYTO-09-19-0347-R [DOI] [PubMed] [Google Scholar]
- 60.Gilardi G, Matic S, Luongo I, Gullino ML, Garibaldi A. First Report of Stem Necrosis and Leaf Spot of Tomato Caused by Albifimbria verrucaria in Italy. Plant Dis. 2020;12. [Google Scholar]
- 61.Carvalho JC, Sousa KC, Brito DC, Chaibub AA, Luzini AP, Côrtes MV, et al. Biocontrol potential of Waitea circinata, an orchid mycorrhizal fungus, against the rice blast fungus. Trop Plant Pathol. 2015;40:151–159. [Google Scholar]
- 62.Ikeda K, Kuwabara K, Urushibara T, Soyai P, Miki S, Shibata S. Pink root rot of squash caused by Setophoma terrestris in Japan. J Gen Plant Pathol. 2012; 78:372–375. [Google Scholar]
- 63.Yang Y, Zuzak K, Harding M, Neilson E, Feindel D, Feng J. First report of pink root rot caused by Setophoma (Pyrenochaeta) terrestris on canola. Can J Plant Pathol. 2017;39: 354–360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.