Abstract
During the past 85 years of antibiotic use, we have learned a great deal about how these ‘miracle’ drugs work. We know the molecular structures and interactions of these drugs and their targets and the effects on the structure, physiology and replication of bacteria. Collectively, we know a great deal about these proximate mechanisms of action for virtually all antibiotics in current use. What we do not know is the ultimate mechanism of action; that is, how these drugs irreversibly terminate the ‘individuality’ of bacterial cells by removing barriers to the external world (cell envelopes) or by destroying their genetic identity (DNA). Antibiotics have many different ‘mechanisms of action’ that converge to irreversible lethal effects. In this Perspective, we consider what our knowledge of the proximate mechanisms of action of antibiotics and the pharmacodynamics of their interaction with bacteria tell us about the ultimate mechanisms by which these antibiotics kill bacteria.
Subject terms: Antibiotics, Bacterial physiology, Cellular microbiology
We know a lot about antibiotics and their targets; however, how antibiotics actually kill bacteria is not entirely clear and is up for debate. In this Perspective, Baquero and Levin reflect on this ultimate action of antibiotics and consider different mechanisms and modulating factors.
Introduction
Antibiotics have been used to treat bacterial infections for nearly 85 years, or more than a century if Paul Ehrlich’s arsenate, also called ‘compound 606’ or ‘Salvarsan’, is included1. During this time, we have learned a great deal about these drugs. We know their molecular structure and that of their targets in the bacteria, how they bind to those targets and the immediate consequences of that binding on the physiology and structure of the exposed bacteria. Curiously, for the vast majority of antibiotics, what is not known, and is subject of some controversy, is how these drugs actually kill bacteria2 and/or prevent their replication3. For example, why do some ribosome-targeting drugs, such as most but not all aminoglycosides, rapidly kill bacteria, whereas others, such as the macrolides, prevent replication and kill at low rates if at all? Why is inhibition of protein synthesis lethal in some cases and only static in others? It is useful to put this question into the context of what Ernst Mayr described as cause and effect in evolutionary biology4–6. Mayr used ‘proximate causation’ to refer to immediate factors (for example, physiology or mutation) of processes and ‘ultimate causation’ to refer to the ‘final reasons’ for the outcome (for example, natural selection or evolution). We use these terms similarly to respectively refer to the primary biochemical mechanisms by which antibiotics exert their action (the traditional ‘mechanisms of action’) and the final result of the process (bactericidal action).We know a great deal about the proximate causes of antibiotic action, how antibiotics interact with the cell, but we know vastly less about the ultimate causes, why, when confronted with antibiotics, bacteria die, and why they do so at different rates.
In this Perspective, we consider what the knowledge of the proximate causes of antibiotic action tells us about these ultimate causes, and what we know and need to know to truly understand how antibiotics kill bacterial cells. We discuss these processes in general and for antibiotics of specific classes.
When is a bacterium dead?
Central to understanding how antibiotics kill or prevent the replication of their target bacteria is knowing when a bacterium is dead and when it is no longer capable of dividing. Whereas the former is irreversible, the latter is expected to be transient, but if cell division is prevented for a long time, the bacterium is effectively dead by convention. In an essay written many years ago, Peter Medawar defined death as the non-reversible loss of individuality, a state that requires physical destruction of the biological “structure of the self” (ref.7), dissipation of the internal content of the cell by irreversible membrane damage or irreversible breaks and disorganization of its individual genetic content produces death. Other changes in the structural and physiological status quo may not result in death, but from the limited perspective of bench scientists conveniently working with bacteria whose viable cell densities can be estimated from data on colony-forming units, if a bacterium cannot form a colony, it is officially dead.
Deadly events are those for which the antimicrobial agents or procedures immediately destroy the integrity of the cell (similar to a crash or explosion), whereas deadly processes resemble a mortal illness (acute or chronic), finally leading to the collapse of physical or genetic individuality. Probably, most of the deadly processes result from the antimicrobial-induced starvation or destruction of a key cellular components needed to maintain the cellular envelope or genetic integrity. In this way, an antimicrobial, including chemical disinfectants, could produce virtually immediate death of a bacterium at higher concentrations and at lower concentrations could produce morbidity and a slow approach to death. Chlorine and its effects on the membranes of bacteria is an excellent example of this8,9.
One antibiotic, many actions
Traditionally, in vitro studies of bacteria and antibiotics ignore the inconvenient reality of the physical and temporal heterogeneity of bacterial populations and their interactions with these drugs. However, it is clear that for a comprehensive understanding of how antibiotics do their bactericidal and bacteriostatic actions, this complexity must be considered, but rarely is. For convenience and the parametric reductionism manifest in so much of biology, the study of antibiotic action is treated as a static process´, and the use of minimum inhibitory concentrations as the unique pharmacodynamic parameter is reflection of this approach10. The interaction between antibiotics and their target bacteria is a dynamic process. Bacteria are in a continuous state of flux: their populations are heterogeneous and composed of cells of a diversity of ages (corresponding to the time since they were produced by cell division) and physiological states. Antibiotics act at variable concentrations, and their effects on bacteria might differ with the number of molecules effectively interacting with each cell.
The concept of ‘hormesis’ applies here. The term ‘hormesis’ was coined by Southam and Ehrlich in 1943 to describe biphasic dose responses of the same compound acting on a biological substrate11. This term was resurrected by Julian Davies in reference to different types of action at different antibiotic concentrations, ranging from promoting death to serving as signals in interbacterial communication12,13. Hormesis is considered a fundamental concept in medicine and biology14,15. Hormesis and, more generally, multiphasic processes are certainly a reality for the antibiotic treatment of bacterial infections. In treated patients, the concentrations of antibiotics vary in time and space (intracellular, tissues or organic fluids); pharmacokinetics is a highly dynamic process. The individual cells of the target bacteria are confronted with variable concentrations of the treating drug, which has different but overlapping effects on their physiology. Antibiotic susceptibility tests based on agar diffusion or the response of bacteria to antibiotics in continuous culture16 offer a more realistic view of the antibiotic action than the gold standard of estimating the minimum concentration of antibiotics necessary to prevent replication (the minimum inhibitory concentration) by exposing well-mixed bacterial populations at relatively low densities growing exponentially to fixed antibiotic concentrations.
We have considered hormesis for individual cells, as it should be. However, the consequences of the ‘one antibiotic, many actions’ paradigm are particularly relevant in assays to determine killing curves, which are normally performed on relatively dense bacterial populations (on the order of 107 cells per millilitre). Such high numbers ensure substantial heterogeneity in the physiological conditions of each bacterial cell, so different antibiotic actions are expected to apply in a population of cells with different ages and physiological states.
What pharmacodynamics tells us
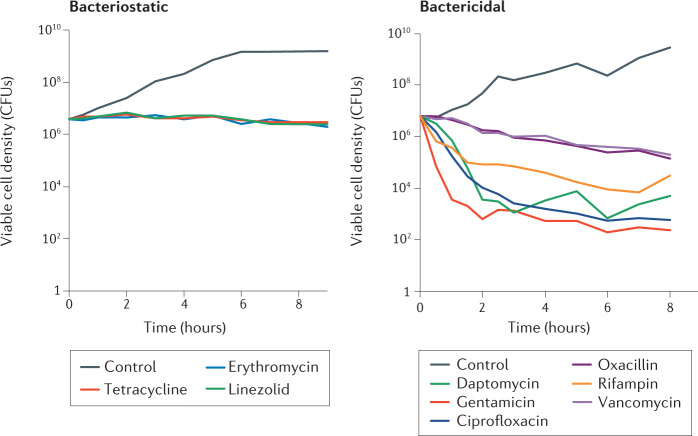
What does the relationship between the concentration of antibiotics and the rate of growth and death of bacteria (that is, pharmacodynamics) tell us about how antibiotics do their bacteriostatic and bactericidal actions? As can be seen in Fig. 1, antibiotics differ considerably in the rates at which they kill bacteria. Although the drug dose, relative to the minimum inhibitory concentration, to which these growing populations of Staphylococcus aureus are exposed (10 times the minimum inhibitory concentration) is the same for the nine drugs, the rates at which the viable cell densities decline (that is, the raters at which the bacteria are killed) differ considerably among these drugs. Antibiotics that are deemed bacteriostatic kill at low rates, whereas those deemed bactericidal kill at higher but very different rates. The rates at which oxacillin and vancomycin kill during the 8 hours of the experiment are not much greater than those of the bacteriostatic antibiotics. The rates of decline in the density of S. aureus exposed to gentamicin, daptomycin, ciprofloxacin and rifampin are not monotonic. In the case of rifampin, resistant mutants emerged. For the other three drugs, there is a levelling off in the kill rate, which can be attributed to persistence17,18. The bacteria recovered from these time–kill experiments were as susceptible to these three drugs as their ancestors used to start the experiment.
Fig. 1. Killing rates of different antibiotics.
The findings of time–kill experiments measured as changes in the viable cell density of Staphylococcus aureus Newman exposed to 10 times the minimum inhibitory concentration of nine different antibiotics in Mueller–Hinton II medium at 37 °C with aeration are shown. Bacteriostatic antibiotics (left) stop growth and colony-forming units (CFUs) remain stable. By contrast, bactericidal antibiotics (right) kill bacteria and thus the CFUs drop. Of note, a resistant mutant emerged in the culture treated with rifampin, leading to resumed growth at the end of the experiment.
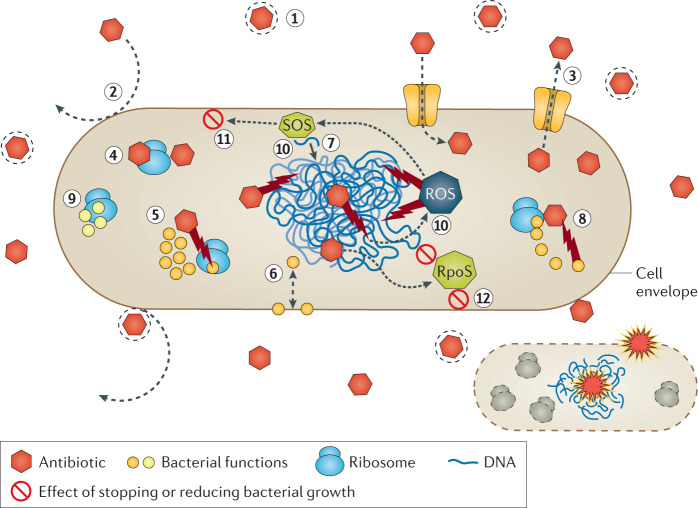
There are a number of possible, but not mutually exclusive, explanations for these differences in killing rates, which we illustrate in Fig. 2. We believe the following explanations to be particularly relevant: (1) only free drug is active against the target bacteria, and protein binding of the drug decreases the rate of killing19; (2) there are structures (such as porins) and mechanisms facilitating drug uptake, but also barriers that prevent the drug from entering the cells20; (3) the drug can be pumped out, so the concentration needed for killing takes longer to achieve21,22; (4) the antibiotics with weak target-binding affinity will take longer to achieve the doses necessary for killing than those with greater affinity23; (5) the targeted function might increase in the presence of the drug, thereby compensating for the inhibition by the drug24; (6) the target function corresponds to the build-up of a cellular structure with slow turnover, which increases the amount of time for the antibiotic to kill25,26; (7) the cells repair the damage produced by the antibiotics at rates that differ between drugs27; (8) the damaged bacteria have inducible antibiotic-deactivating mechanisms28; (9) the bacteria use alternative metabolic pathways that, to some extent, bypass those inhibited by the antibiotic29,30; (10) antibiotics differ in the extent to which they induce reactive oxygen species (ROS; deleterious) or SOS (potentially protective) responses and thereby the rate at which they kill the exposed bacteria31–34; (11) members of the antibiotic-exposed populations are either not replicating or are replicating slowly, and as such are killed at lower rates than the more active members of the population or their death is delayed; (12) the antibiotics produce a kind of ‘stationary phase’ by activating the general RpoS-mediated stringent response35. Notably, under very effective ‘death-delaying conditions’, before the point of no return is reached, it would be difficult to determine whether a cell is in the process of dying. However, when this point of no return is surpassed, the last resources are invested in programmed cell death, and the bacterium induces its own lysis and DNA degradation (apoptosis)36.
Fig. 2. Factors influencing the bactericidal activity of antibiotics in a susceptible bacterial cell.
Only free drug is active (1) and protein binding, for example to albumin or other plasma proteins, can reduce the level of available and thus active drug, as commonly observed for β-lactams. Uptake systems (such as porins) and barriers can prevent the drug from entering cells (2) or the drug is pumped out (3). Greater or weaker target-binding affinity also influences activity (4). The targeted function can also increase in the presence of the drug, thereby compensating for inhibition (5; for example, upregulation of RpoB by rifampin in mycobacteria). The target function involves the build-up of a cellular structure with slow turnover (for example, peptidoglycan), which increases the amount of time for the antibiotic to kill (6). The cells repair the damage produced by the drug (7), involving the SOS system. The bacteria have inducible antibiotic-deactivating mechanisms (8; for example, β-lactamases). The bacteria use alternative functions, bypassing those that are inhibited (9). Antibiotics differ in the extent to which they induce reactive oxygen species (ROS; deleterious) or SOS (potentially protective) responses (10). Low replication rates (involving the SOS system) reduce the killing activity (11). Activation of the RpoS-mediated stringent response produces a kind of ‘stationary phase’, reducing bactericidal potency (12). The two ultimate causes (bottom right) of a bactericidal effect are loss of spatial individuality by rupture of the limits with the environment (broken green line indicating disruption of the cell envelope) and loss of genetic individuality (broken blue line indicating disruption of the genome).
Although it is convenient for investigators to separately consider the pharmacodynamics of the interaction of antibiotics and bacteria and the changes in antibiotic concentration with different therapeutic schedules (that is, the pharmacokinetics of the drug), bacteria do not have that luxury — the pharmacodynamics of antibiotic action is highly dependent on pharmacokinetics. On first consideration, it would seem the higher the concentration of the drug to which the bacteria are exposed, the higher the rate at which they are killed. This is the case for many antibiotics, but not for all. Commonly, but not universally, the rate at which bacteria are killed by antibiotics is proportional to the maximum rates of growth of their populations37. One interpretation of this association is that death occurs when the demand for resources is great, and the amount of resources is severely limited by drug action. If only a fraction of the population of bacteria is in a ‘susceptible mode’ (replicating) at any time, the full bactericidal activity will be achieved only after prolonged periods of exposure (time-dependent killing, as is the case of β-lactams)38. On the pharmacodynamics side, it may well be that the rate at which bacteria are killed depends on the multiplicity of targets simultaneously affected by antibiotic action, which provokes a chaotic and difficult-to-compensate chemical destructuring of the cell. This sort of mechanism has been invoked to explain the rapid killing (minutes) by most biocides, such as disinfectants or antiseptics39,40. This rapid death by ‘multiple targets’ is also consistent with the frequent increase in the bactericidal effect measured in time–kill curves of synergistic antibiotic combinations41 or in phage–antibiotic combinations42,43.
Towards a bactericidal coefficient?
Considering the plethora of factors contributing to the rates at which antibiotics kill bacteria, we can appreciate the difficulty of maintaining the tradition of classifying antibiotics as bactericidal and bacteriostatic. As noted in Fig. 1, at the concentration used, as measured by decline in colony-forming unit estimates of densities, all nine antibiotics examined killed S. aureus, although those deemed bacteriostatic killed at a lower rate than those considered bactericidal. Moreover, the rate at which a bacteriostatic antibiotic kills one species of bacteria, for example, Escherichia coli, can be markedly less than the rate at which it kills another species, for example, Campylobacter jejuni3. A possibility for dealing with this conceptual challenge is to attribute a bactericidal coefficient to each pair of antibiotic and bacterial species, considering the amount of killing at a given antibiotic concentration and time in established standardized conditions. This view originated in the field of disinfectants (phenol coefficient, a measure of the bactericidal activity of a chemical compound in relation to phenol), and the calculation of ‘specific bactericidal activities’ (SBA method) is supported by the National Committee for Clinical Laboratory Standards44–46 but we are urging for the updating of such an approach47.
How bacteria die
Death by exogenous disruption of cell envelopes
Cell envelopes are the hallmark of cellular individuality, the limit between the ‘self’ and ‘non-self’. Many antimicrobial agents kill cells by direct disruption of cell envelopes48. Of course, mechanical disruption of these envelopes by grinding, abrasion, high-pressure carbon dioxide or passing them through a narrow valve under high pressure (similar to a French press), ultrasonication and cavitation produces rapid bacterial death49. Other physical destructuring methods include use of dry ice with ethanol, boiling, osmotic or hydrostatic pressure and extreme pH values.
Chemical disruptors of cell envelopes include detergents, biocides, halogens and toxic gases50. More similar to therapeutic antimicrobials are cell wall-destroying enzymes, bacteriocins and antimicrobial peptides, including host defence peptides of insects, reptiles or higher animals (for example, cecropins, magainins and defensins). These agents rapidly kill bacteria by producing holes in their envelopes (particularly the cellular membrane). A number of antibiotics that are used clinically, such as the cyclic peptides (polymyxin B and colistin) and lipopeptides (telavancin and daptomycin), kill by disrupting cell membranes. Similarly to that of detergents, the killing action of these agents may be better described as ‘physical’ rather than biological. At low concentrations of these agents, bacteria can compensate or repair the resulting damage and they can replicate at a rate that exceeds the killing rate.
Death by endogenous disruption of cell envelopes
Bacteria can commit suicide by disrupting their cell envelopes, leading to stiffness, strength loss and osmotic lysis. Autolysins, first studied in Streptococcus pneumoniae, are enzymes that degrade the peptidoglycan (cell wall) substrate and include glycosidases (muramidases, as LytA, lysozymes, glucosaminidases and transglycosylases), amidases and endopeptidases51,52. The cidABC and lrgAB operons of S. aureus have been shown to influence bacterial death by post-translational regulation of peptidoglycan hydrolase activity53. In all these cases, as well as in E. coli, β-lactams trigger autolysin release by disturbing the balance between peptidoglycan synthesis and hydrolysis, which is necessary for growth of the cell wall54. Bacterial growth requires constant synthesis and turnover of the cell wall to insert new molecules, and the latter process relies on peptidoglycan cleavage enzymes, including glycosidases, amidases and endopeptidases55. The same enzymes can produce lethal damage — the physiology of growth can be converted into the physiology of death.
The effect of autolytic enzymes seems to depend on teichoic acids in Gram-positive bacteria (at least in S. pneumoniae) and on lipopolysaccharides (LPS) in Gram-negative bacteria. The mechanism of antibiotic induction of autolysins, as shown in S. pneumoniae, is based on the sequestration of the major autolysin LytA by membrane-bound lipoteichoic acids. In mutant Bacillus subtilis strains, in which teichoic acid–autolysin binding is altered by reduction of positively charged d-alanine esters in teichoic acids, autolysis is increased under β-lactam exposure56. In S. pneumoniae, the availability of precursors and products of teichoic acids regulates the protease FtsH, influencing the balance between lipid membrane-associated teichoic acids and cell wall (peptidoglycan)-associated teichoic acids. Teichoic acid polymers can account for more than 60% of the mass of the Gram-positive cell wall57. Under penicillin (a β-lactam) exposure (or prolonged stationary phase), FtsH degrades the lipid membrane-associated teichoic acid synthase TacL, leading to a short circuit in the normal teichoic acid balancing mechanism, favouring synthesis of cell wall-associated teichoic acids, which stimulates cell wall-destructive LytA activity, ending in cell lysis58.
In Gram-negative bacteria, LPS in the outer membrane is the functional equivalent of the lipid membrane-associated teichoic acids in Gram-positive bacteria. In this case, the protease FtsH alter the turnover of LpxC, an essential enzyme for virtually all Gram-negative bacteria, which is involved in the first step of LPS biosynthesis, formation of lipid A59. Similarly to FtsH in S. pneumoniae, FtsH in Gram-negative bacteria is regulated by the availability of precursors and products of the LPS synthetic pathway, including acyl-acyl carrier protein (acyl-ACP) precursors. Acyl-ACP accumulation probably correlates with a decrease in fatty acid synthesis in S. aureus and also in E. coli, as in vivo data are consistent with acyl-ACP targeting the same two proteins in both species59,60.
In E. coli, the outer membrane LPS and the cell membrane phospholipid synthesis pathways compete for fatty acids61, leading to a destabilization of the outer membrane (less LPS). It should be remembered that the maintenance of the outer membrane integrity in Gram-negative bacteria is probably as important as the maintenance of the integrity of the cell wall, and its failure produces blebbing and killing62. In summary, the viability of the cell depends on a balanced synthesis of membrane phospholipids, fatty acids and cell wall constituents63, and this coordination is altered by β-lactam exposure.
However, autolysis can also occur without specific induction of autolysins but rather as a consequence of disbalance (uncoupling) between cell wall synthesis and degradation, owing to lack of control of peptidoglycan hydrolase turnover, typically involving low molecular weight penicillin-binding proteins that function as peptidoglycan-binding peptidases in E. coli64–66.
How endogenous mechanisms can degrade the cytoplasmic membrane is less clear. Aminoglycosides interact with ribosomes, leading to production of mistranslated proteins. These proteins are misassembled in the membrane and are rapidly degraded, which contributes to bacterial killing67,68. Degradation of misassembled membrane proteins is the result of a proteolytic ‘quality control’ system, which includes the membrane-integrated protease FtsH69. Other ATP-dependent AAA+ proteases, including ClpP and the Lon proteases, which are present in many bacterial species, are also involved in the proteolysis of defective and misfolded proteins. As in the case for the cell wall, a disbalance in physiological proteolytic processes of the cell might result in membrane alteration and cell death70,71.
As described in the following section, antibiotics might promote the formation of superoxides, leading to the oxydation of cysteine and methionine, resulting in protein damage. In other words, we can consider the replacement of senescent or damaged proteins as a requirement for maintaining life72,73. An intriguing possibility that we believe is worthy of further exploration is a lethal threshold, a minimum rate of protein synthesis that bacteria require to repair or compensate structural damage. In the absence of structural repair, bacteria are killed74.
Death by irreversible DNA damage
Similarly to the disruption of cellular membranes, the disruption of DNA integrity is a marker of the loss of individuality. Endogenous cellular mechanisms damage DNA integrity, which can be the result of a stress response that induces the production of ROS (see earlier), reactive nitrogen species, reactive carbonyl species, lipid peroxidation products and endonucleases. Of course, external conditions can also damage cellular DNA, including UV light, ionizing radiation and genotoxic chemicals.
A number of antibiotics directly cause DNA breaks, such as bleomycin. Also, exposure to fluoroquinolones results in DNA breaks75. Extensive DNA damage can induce a special mode of cell death. Single-stranded DNA resulting from damage triggers the protein RecA, which is involved in the inactivation of LexA. LexA is a repressor of SOS response genes, and its inactivation leads to a cascade of events resulting in an alteration of the cellular membrane and DNA fragmentation. LexA is one of the most over-represented transcriptional regulators following fluoroquinolones treatment76. DNA double-strand breaks can also be produced by antibiotic exposure and are potentially more lethal than single-strand breaks. A major inducer of the SOS response are ROS, producing DNA double-breaks. Their (not necessarily entirely successful) repair involves the RecBCD system, comprising a helicase that unwinds DNA strands and a nuclease that makes single-stranded nicks77.
Probably the main driver leading to double-strand breaks is 8-oxo-2′-deoxyguanosine, which is produced by oxidation of precursor deoxyguanosine triphosphate and causes breaks in conjugation with MutY and MutM, proteins involved in DNA mismatch repair78. As previously stated, it has been proposed that some bactericidal antibiotics ultimately kill bacteria by generating DNA double-strand breaks; chemicals producing breaks are synergistic with these antibiotics79, and thus it can be expected that a shortage of protein synthesis might reduce repair functions, contributing to cell death74. However, this effect is not always apparent in studies using combinations of protein-inhibiting drugs with DNA-damaging antimicrobials or chemical mutagens79,80. Perhaps sequential rather than simultaneous exposure of DNA breaking and protein synthesis-inhibiting drugs would be a more effective way to use combinations of these antibiotics with these different modes of action.
It may well be that antibiotics that drive bacteria into an unspecific stress status mimic other conditions as oligothropy or starvation reduce the viability of bacteria by reducing the availability of nutrients required for essential energy-consuming functions, including DNA repair81. Certainly, E. coli has excess capacity for DNA repair, which compensates DNA damage from nutritional stress82. Perhaps exposure to DNA-breaking antibiotics can surpass this repair capacity.
A paradox that is observed across all fields of biology (including human infections, such as sepsis) is that the very same mechanisms responsible for physiological adaptation, defence and damage repair on crossing a threshold promote death83. In this interpretation, some antibiotics kill by generating an irreversible cascade of events; for example, chromosomal lesions trigger the production of ROS, which damages DNA, which in turn triggers the release of SOS response products intending to rescue the cell from death, but (not fully demonstrated) under high cytotoxicity could lead to destabilization of homeostasis, including iron–sulfur clusters, eventually increasing ROS levels further, resulting in more chromosomal breaks.
Bacteriostatic killing?
Is there a unique mechanism by which antibiotics prevent the replication of bacteria? A few years ago, along with other colleagues, we presented a hypothesis for how ribosome-targeting antibiotics that are deemed bacteriostatic not only prevent the replication of bacteria but might also be lethal3. In accord with our hypothesis, these drugs tie up enough ribosomes for cells not to be able to synthesize enough essential enzymes and other proteins required for replication or to ensure the healthy turnover of envelope components, including the most frequently transcribed proteins in the cell, ribosome proteins and membrane lipoproteins84. As predicted by the model on which this ‘numbers game’ hypothesis was based, as the number of ribosomes is reduced, ribosome-targeting antibiotics will become increasingly bactericidal. Two lines of evidence were presented in support of this hypothesis, both of which were based on the number of ribosomal RNA (rrn) operons. E. coli strains with deletions of five or six of the seven rrn operons85 were killed at a higher rate by azithromycin, chloramphenicol and tetracycline than the ancestral MG1655 strain or strains with more than two rrn operons. In C. jejuni, which has three rrn operons rather than seven as in E. coli, chloramphenicol and azithromycin are bactericidal rather than bacteriostatic as they are for E. coli3.
These results suggest that a low number of functional ribosomes might lead to a kind of lethal protein synthesis threshold. But we restrained ourselves from asserting that crossing this threshold is the ultimate mechanism by which these ribosome-targeting ‘bacteriostatic’ antibiotics kill bacteria. As mentioned already, shortage in key proteins involved in the cellular envelope structure might result in killing, but the association with the quantity of these proteins, or the number of active ribosomes, remains to be demonstrated.
A general killing mechanism?
In 2007, a then graduate student, Michael Kohanski, working with James Collins, presented a general hypothesis for how different bactericidal antibiotics kill both Gram-positive and Gram-negative bacteria and evidence in support of that hypothesis. In accord with that hypothesis, these drugs stimulate the production of highly deleterious hydroxyl radicals, which kill bacteria by oxidative damage, inhibit the tricarboxylic acid cycle, transiently deplete NADH, destabilize iron–sulfur clusters and stimulate the Fenton reaction, resulting in lethal DNA breaks86. In 2013, a series of articles were published pointing out the limitations of the experiments on which Kohanski and colleagues32 based their hypothesis that bactericidal antibiotics work through a common cell death mechanism involving ROS87–89. These authors did not present alternative mechanisms for the bactericidal activity of antibiotics.
This is not a forum to rant on about the details of the experiments performed in these studies and the inferences drawn. It is, however, useful to consider what came out of the ROS ‘debate’ in respect to our understanding of the ultimate mechanism responsible for how antibiotics kill bacteria. To wit, the debate is a compelling argument that there is not a unique mechanism by which antibiotics kill bacteria. In the years since the publication of the article by Kohanski and colleagues32, there have been a number of studies confirming that ROS have an important role in antibiotic-mediated killing of bacteria. These studies present evidence that ROS are frequently synergistic in the killing process with the damage directly caused by the antibiotic in the primary target, and dependent on the background state of cells already stressed by the antibiotic90,91 As suggested by Yang and colleagues47, this killing is not simply a matter of how the drugs act on their targets but is rather the result of an array of downstream consequences of the effects of the drug on that target. As indicated in a recent study by Hong and colleagues34, ROS do indeed have a role in those downstream processes.
Specific antibiotics
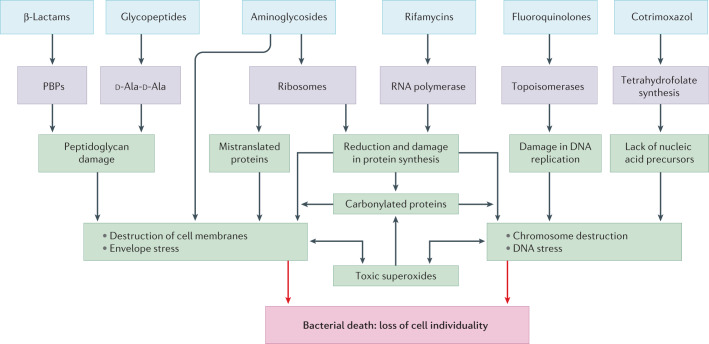
In the following sections, we separately consider antibiotics of six classes, what is known, what has been postulated and what should be known about the ultimate mechanisms by which they kill bacteria. See Fig. 3 for a graphic summary of what follows.
Fig. 3. Successive steps in the process of bacterial killing by antibiotics from six families.
These drugs (blue) directly interact with their targets (purple), which results in structural damage and/or quantitative or qualitative deficiencies of essential cell components. These changes, in turn, lead to envelope stress, DNA damage and/or the production of an excess of reactive oxygen species, which further contribute to the destructuring of cell membranes and nucleic acids. The net effect of these different processes (green) is the ultimate mechanism responsible for the loss of the cell’s individuality, its death (red).
Aminoglycosides
The confidence of clinicians in aminoglycoside therapy is commonly based on the known strong bactericidal effect of these drugs. Although a great deal is known about the proximal mechanism of action of aminoglycosides92,93, no widely accepted or supported hypothesis exists so far for the ultimate mechanism by which these drugs kill bacteria. Three not mutually exclusive hypotheses stand out. A fourth hypothesis, death by superoxides, applies to all bactericidal antibiotics and will be considered separately.
The most commonly offered explanation for the bactericidal action of this class of drugs is that the ribosome–aminoglycoside interactions, mediated by the number and basicity of amino groups in the drug, give rise to toxic mistranslated proteins, which kill by increasing the permeability of the cell membrane94. However, to our knowledge, these toxic proteins have not been isolated, and how they actually kill remains undemonstrated. It is also unclear why these postulated products of mistranslation are not destroyed by the proteases that usually remove mistranslated and misfolded proteins. On the other hand, in opposition of the toxic mistranslated protein hypothesis as the unique mechanism of killing by these drugs is the observation that killing occurs in the absence of aminoglycoside–ribosome binding, as happens with gentamicin in ribosomal 1041A>G mutants that have a single rrn operon95 (B.R.L. and F.B., unpublished observations) and in the presence of the antibiotic resistance gene armA, encoding a 16S ribosomal RNA guanine 1405-N7-methyltransferase96. There is also a pharmacodynamic observation consistent with the toxic mistranslated protein hypothesis. In accord with this hypothesis, the rate of ribosome binding, and thereby the abundance of toxic proteins generated by mistranslation, should be proportional to the growth rate of the target population and the number of ribosomes, which indeed has been observed97. However, some observations question the uniqueness of the toxic mistranslated protein mechanism explaining the bactericidal effect of aminoglycosides. Most importantly, gentamicin can kill E. coli and S. aureus in the stationary phase, when the number of ribosomes is minimal35, and is more bactericidal in E. coli variants with a reduced number of rrn operons3. Collectively, these observations suggest a ribosome-independent (but not alternative) mechanism by which aminoglycosides kill bacteria.
Ribosome-independent killing by aminoglycosides involves direct killing by ‘surface action’. The polycationic aminoglycoside molecules replace Mg2+ cations and thus destabilize key lipid structures of the outer membrane92,98–101. After aminoglycoside exposure, potassium and intracellular molecules such as nucleotides leak from the bacterial cell immediately, certainly no later than protein synthesis inhibitory effects35,92,102. Besides, aminoglycoside exposure increases alarmone levels, resulting in increased membrane damage103. Recent observations indicate that gentamicin at high concentrations can exert bactericidal activity on ribosomal 1041A>G resistant mutants (B.R.L., I. McCall and F.B., unpublished observations).
As stated before, a direct effect of aminoglycosides on the bacterial cell membrane is not incompatible with the need for ribosomal interaction. Binding to the ribosome triggers a massive secondary, energy-dependent uptake of aminoglycosides104. This uptake will produce a ‘cationic disturbance’ of the membrane integrity and thereby kill without further involvement of ribosomes.
The quinolones
Fluoroquinolones bind to DNA gyrase and topoisomerase IV, leading to the formation of stable drug–enzyme–DNA complexes that block DNA replication and result in DNA double-strand breaks105. Recombination and excision repair is involved in the repair of quinolone-damaged DNA, but continuous induction of these systems in response to exposure to the drug triggers the SOS response31,106. Initially, the quinolone–gyrase–DNA complexes are unstable, and bacteria can recover in the absence of quinolone exposure. However, if exposure is maintained, the complexes become stable, the SOS response continues and when a threshold is crossed, the death process becomes irreversible, even in the absence of the drug34,107.
The activity of ciprofloxacin decreases when bacteria reduce their growth rates108. This effect might contribute to explaining the biphasic dose response of most quinolones, producing a single concentration of maximum kill. The optimal bactericidal concentration probably depends on the SOS response, the formation of superoxides and DNA breaks. Concentrations higher than the optimal bactericidal concentration provoke an immediate SOS-independent inhibition of respiration and growth, with decreased ROS production and less death108. Conversely, at the optimal bactericidal concentration, SOS-derived apoptosis-like death occurs. This pathway depends on RecA and LexA, resulting in cell death associated with membrane depolarization and ROS-induced DNA fragmentation83. In summary, ultimate death by quinolones occurs by the disintegration of DNA mediated by ROS34.
Rifamycins
The target of rifampin (and, in general, rifamycins) is the product of the rpoB gene, the DNA-dependent RNA polymerase. The drug strongly binds to the β-subunit of the core enzyme, thereby inhibiting initiation of transcription; that is, preventing effective protein synthesis109. On first consideration, it may seem that inhibition of protein synthesis is not sufficient to provide rapid killing. However, in practice, rifamycins are considered to have an early bactericidal effect, not only in S. aureus (Fig. 1) and E. coli, but even in slowly growing bacteria such as Mycobacterium tuberculosis. In addition, the killing effect of rifampin is concentration dependent10,110. High rifampin concentrations can even kill bacteria with some types of resistance mutations in the rpoB gene111. By targeting RNA polymerases, rifamycins affect both translation and transcription, which together ensure the coordination of transcriptional activity to the translational needs under various growth rates112,113. An interesting question is whether the inhibition of transcription might produce lethal effects independently from blocking protein synthesis. A classic transcription inhibitor is the toxin MazF, a component of the stress-induced MazF–MazE toxin–antitoxin machinery. In the absence of the antitoxin MazE, MazF inhibits protein synthesis by cleaving mRNA, resulting in later death114. What are the causes of death? It has been suggested that there are a group of mRNAs that are resistant to cleavage by MazF, encoding ‘death proteins’, some of which damage cell envelopes115. A similar mechanism of death can be suggested for rifampin, which also selectively affects the transcription of different genes116. It is possible that rifampin, similarly to ribosome-binding antibiotics, reshapes the cellular proteome rather than just blocking global protein synthesis117,118, but both effects might be synergistic for killing, particularly in species with a low number of rrn operons3. Rifamycins do not stimulate the production of hydroxyl radical production, which could contribute to cell death32.
β-Lactam antibiotics
β-Lactams target penicillin-binding proteins involved in the biogenesis of peptidoglycan119. There is a clear correlation between bacterial growth rate, needs of peptidoglycan biogenesis and cell lysis induced by β-lactams37. Lysis requires functional assembly of the divisome, the cell division machinery120, suggesting that lysis specifically occurs when the cell is ready for division121. Why is the loss of cell wall integrity and lysis the result of reduced peptidoglycan biogenesis? The traditional answer is induction of peptidoglycan autolysins122 or simply that these lysins continue their activity without compensation by biogenesis (see earlier). More recently, it has been proposed that inhibition of penicillin-binding proteins by β-lactams produces a deleterious ‘futile cycle’ of metabolic pathways involved in peptidoglycan synthesis and degradation running simultaneously in opposite directions and promoting deintegration and lysis123. Besides, disruption of cell envelope integrity, leading to bubbling and ‘explosion’ of the cell, particularly in Gram-negative bacteria, is probably triggered by lacking coordination of the multicomponent machinery that links the growth of the different envelope layers, and involves sensing of unassembled outer membrane proteins and LPS in the periplasm123. The possibility of biophysical shearing of different layers, owing to disbalance between cell wall and membrane growth124–126 and resulting in cell lysis, cannot be discarded and is worth further and deeper consideration. Finally, there is evidence that β-lactam antibiotics are bactericidal through DNA damage by ROS34,127.
Vancomycin
Vancomycin is a lipophilic cationic antibiotic that inhibits synthesis of the bacterial cell wall by binding to the dipeptide terminus d-Ala-d-Ala of peptidoglycan pentapeptide precursors, preventing subsequent transpeptidation and transglycosylation and thus peptidoglycan crosslinking128. Deficient crosslinking of the long sugar backbone chains of N-acetylglucosamine/N-acetylmuramic chains results weakens the cell to osmotic damage, leading to cell disintegration. Similarly, this effect explains why vancomycin-exposed cells are much more sensitive to ultrasound25. In S. aureus, the bactericidal effect of vancomycin is generally weaker than that of most β-lactams129. This difference can be explained by the huge size of vancomycin (1,450 Da) compared with oxacillin (401 Da), which impedes diffusion through the cell wall, which is also supported by the finding that thicker peptidoglycan reduces the effect of vancomycin130. Consistently, time–kill curves show that varying the concentration of vancomycin has no effect on the rate or extent of bacterial killing131, probably owing to vancomycin clogging in the cell wall. Independently from the membrane–cell wall shearing effect (see the section “β-Lactam antibiotics”), direct membrane damage can probably be excluded, as vancomycin does not act on protoplasts or Mycoplasma spp., both of which have no cell wall. Superoxide anions might also be involved in the bactericidal activity of vancomycin in Enterococcus spp. and Staphylococcus spp.132.
Sulfonamides and trimethoprim
Sulfonamides and trimethoprim are bacteriostatic, but the combination is synergistic, and has a strongly bactericidal effect133. The two drugs inhibit two sequential steps in tetrahydrofolate synthesis (required for nucleotide synthesis), but this cannot explain the bactericidal synergism. The synergy is more likely due to the disruption of a previously unrecognized metabolic feedback loop by trimethoprim, which results in cyclic mutual potentiation of the effects of the two drugs, leading to amplified depletion of tetrahydrofolate, an essential cofactor in the biosynthesis of thymine127,134. However, the ultimate mechanism of killing by thymine deficiency (the classic ‘thymineless killing’) remains elusive; the deprivation of nutritional requirements normally has a biostatic, but not lethal, effect135,136. Most probably, thymine starvation promotes cell killing by ROS-mediated DNA damage34.
Conclusion and final remarks
Surely, we would all love to have a unique, broadly supported hypothesis for how antibiotics kill bacteria; this is particularly so if we were the investigators responsible for providing that hypothesis. This is not the case; there is no a priori reason to expect that the same antibiotics targeting different species of susceptible bacteria would reach these ultimate (killing) effects by the same processes under different growth conditions. On the other, more positive, side, this Perspective suggests that antibiotics with markedly different structures, targets and effects on the structure and physiology of bacteria have proximate mechanisms of action that converge through different processes in the death of bacteria by physical or genetic destructuring, the ultimate effects (Fig. 3). Moreover, we believe that the links between proximate and ultimate mechanisms of the bactericidal and bacteriostatic actions can be elucidated for specific antibiotics and different species of bacteria under different growth conditions.
A multifactorial perspective of bacterial killing will be needed to fully understand how the effects of the primary antibiotic action on targets can be modulated, and eventually amplified, in the context of complex interactions and changes of cell metabolism, and general cellular responses, including ROS production, SOS induction and RpoS regulatory effects. Certainly many of these responses are sensitive to the environment, and therefore the bactericidal effect is expected to differ in various circumstances, bacterial species and lifestyles. A wide field of research is being opened.
But is it worth the effort? We know that bacteria are killed and/or prevented from replicating when exposed to antibiotics, and we know a great deal about the conditions under which these drugs have these bactericidal and bacteriostatic effects. Is this information, which is critical to the clinical applications of these drugs, not entirely sufficient? We suggest it is not. Elucidating how and when different antibiotics prevent the replication of bacteria and kill them is not just an academic exercise. This information will be useful for developing much-needed new antibiotics. It will also be helpful for designing protocols for the administration of existing antibiotics and combinations of antibiotics that are effective clinically, and at the same time minimize the likelihood of emergence and rise of resistance to these drugs in target bacteria and commensals and disturbance of the microbiota.
Acknowledgements
The authors are grateful to their awesome wives, Ros and Adriana, for putting up with them during this period of COVID-19 quarantine and supporting their playing fun games such as reviewing the literature and writing this Perspective. They thank I. McCall for a careful reading of this manuscript, and J. Rodríguez-Beltrán for discussion about some critical concepts. This research was funded by grants from the U.S. National Institutes of General Medical Science (GM091875-17 and 1R35 GM136407-01) to B.R.L and from the Regional Government of Madrid (InGeMICS- B2017/BMD-3691) and the Ramón Areces Foundation to the F.B. laboratory. The authors dedicate this Perspective to the memory of J. Acar137, with whom they would have loved to have discussed the question of how antibiotics kill; from those conversations with Jacques, the authors know they would have had much more to tell you about the answer.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Microbiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fernando Baquero, Email: baquero@bitmailer.net.
Bruce R. Levin, Email: blevin@emory.edu
References
- 1.Ehrlich P. Address in pathology, on chemiotherapy: delivered before the Seventeenth International Congress of Medicine. Br. Med. J. 1913;2:353–359. doi: 10.1136/bmj.2.2746.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright G, Hung D, Helmann J. How antibiotics kill bacteria: new models needed? Nat. Med. 2013;19:544–545. doi: 10.1038/nm.3198. [DOI] [PubMed] [Google Scholar]
- 3.Levin BR, et al. A numbers game: ribosome densities, bacterial growth, and antibiotic-mediated stasis and death. mBio. 2017;8:e02253–16. doi: 10.1128/mBio.02253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr E. Cause and effect in biology. Science. 1961;134:1501–1506. doi: 10.1126/science.134.3489.1501. [DOI] [PubMed] [Google Scholar]
- 5.Laland KN, Sterelny K, Odling-Smee J, Hoppitt W, Uller T. Cause and effect in biology revisited: is Mayr’s proximate-ultimate dichotomy still useful? Science. 2011;334:1512–1516. doi: 10.1126/science.1210879. [DOI] [PubMed] [Google Scholar]
- 6.Ariew A. Ernst Mayr’s ‘ultimate/proximate’ distinction reconsidered and reconstructed. Biol. Philos. 2003;18:553–565. [Google Scholar]
- 7.Medawar, P. The Uniqueness of the Individual (Methuen, 1957).
- 8.Venkobachar CL, Rao A. Mechanism of disinfection: effect of chlorine on cell membrane functions. Water Res. 1997;11:727–729. [Google Scholar]
- 9.Virto R, Manas P, Alvarez I, Condon S, Raso J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Env. Microbiol. 2005;71:5022–5028. doi: 10.1128/AEM.71.9.5022-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regoes RR, et al. Pharmacodynamic functions: a multiparameter approach to the design of antibiotic treatment regimens. Antimicrob. Agents Chemother. 2004;48:3670–3676. doi: 10.1128/AAC.48.10.3670-3676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southam C, Ehrlich J. Effects of extracts of western redcedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 12.Davies J, Spiegelman GB, Yim G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006;9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl Acad. Sci. USA. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabrese EJ. Hormesis: a fundamental concept in biology. Microb. Cell. 2014;1:145–149. doi: 10.15698/mic2014.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese EJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Udekwu KI, Levin BR. Staphylococcus aureus in continuous culture: a tool for the rational design of antibiotic treatment protocols. PLoS ONE. 2012;7:e38866. doi: 10.1371/journal.pone.0038866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigger JW. Treatment of staphylococcal infections with penicillin. Lancet. 1944;244:497–500. [Google Scholar]
- 18.Balaban NQ, et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019;17:460. doi: 10.1038/s41579-019-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey EM, Rybak MJ, Kaatz GW. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob. Agents Chemother. 1991;35:1089–1092. doi: 10.1128/aac.35.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008;6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 23.Clarelli, F. et al. Drug-target binding quantitatively predicts optimal antibiotic dose levels. Preprint at bioRxiv10.1101/369975 (2020). [DOI] [PMC free article] [PubMed]
- 24.Zhu JH, et al. Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription. Nat. Commun. 2018;9:4218. doi: 10.1038/s41467-018-06667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrielsson J, Peletier LA, Hjorth S. In vivo potency revisited - keep the target in sight. Pharmacol. Ther. 2018;184:177–188. doi: 10.1016/j.pharmthera.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Borisova M, Gisin J, Mayer C. Blocking peptidoglycan recycling in pseudomonas aeruginosa attenuates intrinsic resistance to fosfomycin. Microb. Drug Resist. 2014;20:231–237. doi: 10.1089/mdr.2014.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller C, et al. SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science. 2004;305:1629–1631. doi: 10.1126/science.1101630. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby GA. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainardi JL, et al. Novel mechanism of beta-lactam resistance due to bypass of DD-transpeptidation in Enterococcus faecium. J. Biol. Chem. 2000;275:16490–16496. doi: 10.1074/jbc.M909877199. [DOI] [PubMed] [Google Scholar]
- 30.Flensburg J, Skold O. Massive overproduction of dihydrofolate reductase in bacteria as a response to the use of trimethoprim. Eur. J. Biochem. 1987;162:473–476. doi: 10.1111/j.1432-1033.1987.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 31.Piddock LJ, Walters RN. Bactericidal activities of five quinolones for Escherichia coli strains with mutations in genes encoding the SOS response or cell division. Antimicrob. Agents Chemother. 1992;36:819–825. doi: 10.1128/aac.36.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 33.Dorr T, Lewis K, Vulic M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong Y, Zeng J, Wang X, Drlica K, Zhao X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl Acad. Sci. USA. 2019;116:10064–10071. doi: 10.1073/pnas.1901730116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCall IC, Shah N, Govindan A, Baquero F, Levin BR. Antibiotic killing of diversely generated populations of nonreplicating bacteria. Antimicrob. Agents Chemother. 2019;63:e02360–18. doi: 10.1128/AAC.02360-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeters SH, de Jonge MI. For the greater good: programmed cell death in bacterial communities. Microbiol. Res. 2018;207:161–169. doi: 10.1016/j.micres.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 38.Vogelman B, Craig WA. Kinetics of antimicrobial activity. J. Pediatr. 1986;108:835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- 39.Russell AD. Mechanisms of antimicrobial action of antiseptics and disinfectants: an increasingly important area of investigation. J. Antimicrob. Chemother. 2002;49:597–599. doi: 10.1093/jac/49.4.597. [DOI] [PubMed] [Google Scholar]
- 40.Maillard JY. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002;92:16S–27S. [PubMed] [Google Scholar]
- 41.Eliopoulos GM, Eliopoulos CT. Antibiotic combinations: should they be tested? Clin. Microbiol. Rev. 1988;1:139–156. doi: 10.1128/cmr.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhry WN, et al. Synergy and order effects of antibiotics and phages in killing pseudomonas aeruginosa biofilms. PLoS ONE. 2017;12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickey J, Perrot V. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro. PLoS ONE. 2019;14:e0209390. doi: 10.1371/journal.pone.0209390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrissey I. Bactericidal index: a new way to assess quinolone bactericidal activity in vitro. J. Antimicrob. Chemother. 1997;39:713–717. doi: 10.1093/jac/39.6.713. [DOI] [PubMed] [Google Scholar]
- 45.Gottardi W, Klotz S, Nagl M. Superior bactericidal activity of N-bromine compounds compared to their N-chlorine analogues can be reversed under protein load. J. Appl. Microbiol. 2014;116:1427–1437. doi: 10.1111/jam.12474. [DOI] [PubMed] [Google Scholar]
- 46.Barry, A. L. et al. Methods for determining bactericidal activity of antimicrobial agents, approved guideline (CLSI, 1999).
- 47.Yang JH, Bening SC, Collins JJ. Antibiotic efficacy-context matters. Curr. Opin. Microbiol. 2017;39:73–80. doi: 10.1016/j.mib.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epand RM, Walker C, Epand RF, Magarvey NA. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta. 2016;1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Harrison ST. Bacterial cell disruption: a key unit operation in the recovery of intracellular products. Biotechnol. Adv. 1991;9:217–240. doi: 10.1016/0734-9750(91)90005-g. [DOI] [PubMed] [Google Scholar]
- 50.Harrison, S. T., Dennis, J. S. & Chase, H. A. in Inhibition and Destruction of the Microbial Cell (ed. Hugo, W. B.) 95–105 (Academic, 1971).
- 51.Mosser JL, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J. Biol. Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 52.Garcia P, Paz Gonzalez M, Garcia E, Garcia JL, Lopez R. The molecular characterization of the first autolytic lysozyme of Streptococcus pneumoniae reveals evolutionary mobile domains. Mol. Microbiol. 1999;33:128–138. doi: 10.1046/j.1365-2958.1999.01455.x. [DOI] [PubMed] [Google Scholar]
- 53.Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitano K, Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob. Agents Chemother. 1979;16:838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin JH, et al. Structural basis of peptidoglycan endopeptidase regulation. Proc. Natl Acad. Sci. USA. 2020;117:11692–11702. doi: 10.1073/pnas.2001661117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wecke J, Perego M, Fischer W. D-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 1996;2:123–129. doi: 10.1089/mdr.1996.2.123. [DOI] [PubMed] [Google Scholar]
- 57.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flores-Kim J, Dobihal GS, Fenton A, Rudner DZ, Bernhardt TG. A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis. eLife. 2019;8:e44912. doi: 10.7554/eLife.44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bittner LM, Arends J, Narberhaus F. When, how and why? Regulated proteolysis by the essential FtsH protease in Escherichia coli. Biol. Chem. 2017;398:625–635. doi: 10.1515/hsz-2016-0302. [DOI] [PubMed] [Google Scholar]
- 60.Parsons JB, Yao J, Jackson P, Frank M, Rock CO. Phosphatidylglycerol homeostasis in glycerol-phosphate auxotrophs of Staphylococcus aureus. BMC Microbiol. 2013;13:260. doi: 10.1186/1471-2180-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.May KL, Silhavy TJ. Erratum for May and Silhavy, “The Escherichia coli phospholipase PldA regulates outer membrane homeostasis via lipid signaling”. mBio. 2018;9:e00718–18. doi: 10.1128/mBio.00379-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao Z, Kahne D, Kishony R. Distinct single-cell morphological dynamics under beta-lactam antibiotics. Mol. Cell. 2012;48:705–712. doi: 10.1016/j.molcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomanek N, et al. Intricate crosstalk between lipopolysaccharide, phospholipid and fatty acid metabolism in Escherichia coli modulates proteolysis of LpxC. Front. Microbiol. 2018;9:3285. doi: 10.3389/fmicb.2018.03285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernadsky G, Beveridge TJ, Clarke AJ. Analysis of the sodium dodecyl sulfate-stable peptidoglycan autolysins of select gram-negative pathogens by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 1994;176:5225–5232. doi: 10.1128/jb.176.17.5225-5232.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Heijenoort J. Peptidoglycan hydrolases of Escherichia coli. Microbiol. Mol. Biol. Rev. 2011;75:636–663. doi: 10.1128/MMBR.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 67.Busse HJ, Wostmann C, Bakker EP. The bactericidal action of streptomycin: membrane permeabilization caused by the insertion of mistranslated proteins into the cytoplasmic membrane of Escherichia coli and subsequent caging of the antibiotic inside the cells due to degradation of these proteins. J. Gen. Microbiol. 1992;138:551–561. doi: 10.1099/00221287-138-3-551. [DOI] [PubMed] [Google Scholar]
- 68.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akiyama Y. Quality control of cytoplasmic membrane proteins in Escherichia coli. J. Biochem. 2009;146:449–454. doi: 10.1093/jb/mvp071. [DOI] [PubMed] [Google Scholar]
- 70.Moreno-Cinos C, et al. ClpP protease, a promising antimicrobial target. Int. J. Mol. Sci. 2019;20:2232. doi: 10.3390/ijms20092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brotz-Oesterhelt H, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 2005;11:1082–1087. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 72.Nystrom T. Not quite dead enough: on bacterial life, culturability, senescence, and death. Arch. Microbiol. 2001;176:159–164. doi: 10.1007/s002030100314. [DOI] [PubMed] [Google Scholar]
- 73.Ezraty B, Gennaris A, Barras F, Collet JF. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017;15:385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 74.Ganesan AK, Smith KC. Requirement for protein synthesis in rec-dependent repair of deoxyribonucleic acid in Escherichia coli after ultraviolet or X irradiation. J. Bacteriol. 1972;111:575–585. doi: 10.1128/jb.111.2.575-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drlica K, Malik M, Kerns RJ, Zhao X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 2008;52:385–392. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singleton MR, Dillingham MS, Gaudier M, Kowalczykowski SC, Wigley DB. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature. 2004;432:187–193. doi: 10.1038/nature02988. [DOI] [PubMed] [Google Scholar]
- 78.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song LY, et al. Exploring synergy between classic mutagens and antibiotics to examine mechanisms of synergy and antibiotic action. Antimicrob. Agents Chemother. 2015;60:1515–1520. doi: 10.1128/AAC.02485-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ocampo PS, et al. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014;58:4573–4582. doi: 10.1128/AAC.02463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kouzminova EA, Kuzminov A. Chromosomal fragmentation in dUTPase-deficient mutants of Escherichia coli and its recombinational repair. Mol. Microbiol. 2004;51:1279–1295. doi: 10.1111/j.1365-2958.2003.03924.x. [DOI] [PubMed] [Google Scholar]
- 82.Le LAT, et al. Nutritional conditions and oxygen concentration affect spontaneous occurrence of homologous recombination events but not spontaneous mutagenesis in Escherichia coli. Genes Genet. Syst. 2020;95:85–93. doi: 10.1266/ggs.19-00008. [DOI] [PubMed] [Google Scholar]
- 83.Bayles KW. Bacterial programmed cell death: making sense of a paradox. Nat. Rev. Microbiol. 2014;12:63–69. doi: 10.1038/nrmicro3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quan S, Skovgaard O, McLaughlin RE, Buurman ET, Squires CL. Markerless Escherichia coli rrn deletion strains for genetic determination of ribosomal binding sites. G3. 2015;5:2555–2557. doi: 10.1534/g3.115.022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imlay JA, Chin SM, Linn S, Toxic DNA. damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 87.Ezraty B, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013;340:1583–1587. doi: 10.1126/science.1238328. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 90.Dwyer DJ, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl Acad. Sci. USA. 2014;111:E2100–E2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. Toxicol. 2015;55:313–332. doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 92.Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura Y, Ikeda M, Nishigaki R, Umemura K. Kinetics of bactericidal activity of aminoglycosides during dynamic dilution. J. Pharmacobiodyn. 1985;8:695–700. doi: 10.1248/bpb1978.8.695. [DOI] [PubMed] [Google Scholar]
- 94.Nagel R, Chan A. Mistranslation and genetic variability: the effect of streptomycin. Mutat. Res. 2006;601:162–170. doi: 10.1016/j.mrfmmm.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Ying L, Zhu H, Shoji S, Fredrick K. Roles of specific aminoglycoside-ribosome interactions in the inhibition of translation. RNA. 2019;25:247–254. doi: 10.1261/rna.068460.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez-Zorn B, et al. armA and aminoglycoside resistance in Escherichia coli. Emerg. Infect. Dis. 2005;11:954–956. doi: 10.3201/eid1106.040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haugan MS, Lobner-Olesen A, Frimodt-Moller N. Comparative activity of ceftriaxone, ciprofloxacin, and gentamicin as a function of bacterial growth rate probed by escherichia coli chromosome replication in the mouse peritonitis model. Antimicrob. Agents Chemother. 2019;63:e02133–18. doi: 10.1128/AAC.02133-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taber HW, Mueller JP, Miller PF, Arrow AS. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 1987;51:439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peterson AA, Hancock RE, McGroarty EJ. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J. Bacteriol. 1985;164:1256–1261. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kadurugamuwa JL, Clarke AJ, Beveridge TJ. Surface action of gentamicin on Pseudomonas aeruginosa. J. Bacteriol. 1993;175:5798–5805. doi: 10.1128/jb.175.18.5798-5805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dubin DT, Hancock R, Davis BD. The sequence of some effects of streptomycin in Escherichia Coli. Biochim. Biophys. Acta. 1963;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- 103.Ji X, et al. Alarmone Ap4A is elevated by aminoglycoside antibiotics and enhances their bactericidal activity. Proc. Natl Acad. Sci. USA. 2019;116:9578–9585. doi: 10.1073/pnas.1822026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bryan LE, Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob. Agents Chemother. 1983;23:835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin. Infect. Dis. 2001;32:S9–S15. doi: 10.1086/319370. [DOI] [PubMed] [Google Scholar]
- 106.Phillips I, Culebras E, Moreno F, Baquero F. Induction of the SOS response by new 4-quinolones. J. Antimicrob. Chemother. 1987;20:631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- 107.Mustaev A, et al. Fluoroquinolone-gyrase-DNA complexes: two modes of drug binding. J. Biol. Chem. 2014;289:12300–12312. doi: 10.1074/jbc.M113.529164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smirnova, G. V. & Oktyabrsky, O. N. Relationship between Escherichia coli growth rate and bacterial susceptibility to ciprofloxacin. FEMS Microbiol. Lett. 365 (2018). [DOI] [PubMed]
- 109.Nakamura Y, Yura T. Effects of rifampicin on synthesis and functional activity of DNA-dependent RNA polymerase in Escherichia coli. Mol. Gen. Genet. 1976;145:227–237. doi: 10.1007/BF00325817. [DOI] [PubMed] [Google Scholar]
- 110.Gumbo T, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 2007;51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z, et al. Could high-concentration rifampicin kill rifampicin-resistant M. tuberculosis? Rifampicin MIC test in rifampicin-resistant isolates from patients with osteoarticular tuberculosis. J. Orthop. Surg. Res. 2014;9:124. doi: 10.1186/s13018-014-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burmann BM, et al. A NusE:NusG complex links transcription and translation. Science. 2010;328:501–504. doi: 10.1126/science.1184953. [DOI] [PubMed] [Google Scholar]
- 113.Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328:504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Amitai S, Yassin Y, Engelberg-Kulka H. MazF-mediated cell death in Escherichia coli: a point of no return. J. Bacteriol. 2004;186:8295–8300. doi: 10.1128/JB.186.24.8295-8300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amitai S, Kolodkin-Gal I, Hananya-Meltabashi M, Sacher A, Engelberg-Kulka H. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genet. 2009;5:e1000390. doi: 10.1371/journal.pgen.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saito K, et al. Rifamycin action on RNA polymerase in antibiotic-tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc. Natl Acad. Sci. USA. 2017;114:E4832–E4840. doi: 10.1073/pnas.1705385114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kannan K, Vazquez-Laslop N, Mankin AS. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell. 2012;151:508–520. doi: 10.1016/j.cell.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 118.Mosaei H, Zenkin N. Inhibition of RNA polymerase by rifampicin and rifamycin-like molecules. EcoSal Plus. 2020 doi: 10.1128/ecosalplus.ESP-0017-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl Acad. Sci. USA. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chung HS, et al. Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc. Natl Acad. Sci. USA. 2009;106:21872–21877. doi: 10.1073/pnas.0911674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee AJ, et al. Robust, linear correlations between growth rates and beta-lactam-mediated lysis rates. Proc. Natl Acad. Sci. USA. 2018;115:4069–4074. doi: 10.1073/pnas.1719504115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu. Rev. Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- 123.Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159:1300–1311. doi: 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mercier R, Kawai Y, Errington J. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell. 2013;152:997–1007. doi: 10.1016/j.cell.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 125.Koonin EV, Mulkidjanian AY. Evolution of cell division: from shear mechanics to complex molecular machineries. Cell. 2013;152:942–944. doi: 10.1016/j.cell.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Booth S, Lewis RJ. Structural basis for the coordination of cell division with the synthesis of the bacterial cell envelope. Protein Sci. 2019;28:2042–2054. doi: 10.1002/pro.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minato Y, et al. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018;9:1003. doi: 10.1038/s41467-018-03447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs. 2004;64:913–936. doi: 10.2165/00003495-200464090-00001. [DOI] [PubMed] [Google Scholar]
- 129.Joukhadar C, Pillai S, Wennersten C, Moellering RC, Jr, Eliopoulos GM. Lack of bactericidal antagonism or synergism in vitro between oxacillin and vancomycin against methicillin-susceptible strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2010;54:773–777. doi: 10.1128/AAC.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui L, et al. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2006;50:428–438. doi: 10.1128/AAC.50.2.428-438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J. Antimicrob. Chemother. 1996;38:589–597. doi: 10.1093/jac/38.4.589. [DOI] [PubMed] [Google Scholar]
- 132.Ladjouzi R, et al. Analysis of the tolerance of pathogenic enterococci and Staphylococcus aureus to cell wall active antibiotics. J. Antimicrob. Chemother. 2013;68:2083–2091. doi: 10.1093/jac/dkt157. [DOI] [PubMed] [Google Scholar]
- 133.Amyes SG. Bactericidal activity of trimethoprim alone and in combination with sulfamethoxazole on susceptible and resistant Escherichia coli K-12. Antimicrob. Agents Chemother. 1982;21:288–293. doi: 10.1128/aac.21.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Then R, Angehrn P. Nature of the bacterial action of sulfonamides and trimethoprim, alone and in combination. J. Infect. Dis. 1973;128:498–501. doi: 10.1093/infdis/128.supplement_3.s498. [DOI] [PubMed] [Google Scholar]
- 135.Ahmad SI, Kirk SH, Eisenstark A. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu. Rev. Microbiol. 1998;52:591–625. doi: 10.1146/annurev.micro.52.1.591. [DOI] [PubMed] [Google Scholar]
- 136.Khan SR, Kuzminov A. Thymineless death in Escherichia coli is unaffected by chromosomal replication complexity. J. Bacteriol. 2019;201,:e00797–18. doi: 10.1128/JB.00797-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cambau E, et al. Jacques F. Acar (1931–2020) Clin. Microbiol. Infect. 2020;26:1261–1263. [Google Scholar]