Abstract
White light endoscopy has proven to be a very powerful tool in oncology. There is still, however, a need for better endoscopic techniques to overcome the current limitations of white light optics. New technologies that allow higher sensitivity, improved microanatomy and molecular characterization have been available for in vitro microscopy and are now being translated into in vivo endoscopy. Endoscopic molecular imaging is still in its infancy but holds the promise for enhancing sensitivity for early lesions, thus allowing earlier diagnosis and enabling early image-guided endoscopic intervention. A key feature of endoscopic molecular imaging is its increased sensitivity and specificity, which will be illustrated in this article, as well as describing perspectives on its future use in oncologic surgery.
Keywords: cancer, fluorescence, endoscopy, imaging probe, molecular imaging
Endoscopy has been a powerful medical diagnostic and therapeutic tool for over 50 years. Improvements in fiberoptics, camera sensitivity, and light-emitting diodes and lasers have resulted in more versatile endoscopes that can now access virtually every body cavity, crossing multiple medical disciplines [1]. Advantages of conventional endoscopy are that it is cost effective, portable, accurate and provides a means to ‘see and treat’ early cancers. However, optical endoscopy, which relies on a visible range light, has inherent limitations [2]. Optical endoscopy relies on changes in morphological and color features on the surface and these architectural changes are sometimes late or nonspecific indicators of disease. Furthermore, it is challenging to detect smaller, flat and depressed lesions with subtle color or morphological changes. Hence, there is a need for better endoscopic techniques to improve the detection of cancer.
Developments in endoscopy have improved not only morphological detection but also tissue characterization. For instance, image-enhanced endoscopy (IEE) is a technique that offers in vivo histopathology by actual staining of the lesions or by virtually enhancing the image using magnified and/or narrow band imaging (NBI) to elicit subtle morphological features not visible with conventional white light endoscopy (WLE). WLE can be combined with endoscopic ultrasound (EUS) to provide deeper cross-sectional images. Confocal laser endomicroscopy (CLE) provides in situ optical biopsies hence, guiding decisions regarding the best place to sample for a conventional needle biopsy. However, IEE, EUS and CLE have not been widely adopted because they require specialized training both to operate the equipment and interpret the results. Moreover, the high magnification of these methods means that they can only cover a small portion of the surface, requiring these methods to be combined with WLE. Hence, a potential issue is having a sufficient number of well-trained physicians to screen a large number of patients with these methods. It should be noted that it is not feasible to have an on-site pathologist provide real-time histopathologic analysis during every procedure. Thus, endoscopists themselves will have to become trained in ‘microendoscopic’ histopathology, if this method is to advance further. Alternatively, automated real-time image processing methods may need to be developed to assist endoscopists in identifying regions of pathology. Even under the best conditions histopathologic evaluation takes a lot training and patience, and it is difficult to combine these attributes with an active procedure, such as endoscopy where there is a premium on minimizing patient time (e.g., to reduce sedation and costs). Thus, there is much room for further improvement in endoscopic techniques, which are easy to use yet clinically superior to WLE.
To further illustrate the challenges of current endoscopic methods, it is instructive to consider endoscopy of the GI tract (GIT). Standard screening and detection of cancer in the GIT relies on gross morphological changes with WLE rather than earlier molecular events [1,3]. In the upper GIT, Barrett’s esophagus, a precursor to adenocarcinoma, and its transition phase, high-grade dysplasia, which is associated with progression to adenocarcinoma, are both ideally suited for early detection using endoscopic surveillance. Even though earlier diagnosis has been associated with improved patient outcome [4,5], routine surveillance is still controversial [6] owing to its cost, invasiveness, sampling error and low specificity with the currently recommended sampling guidelines [7]. In the case of screening for colon cancer, limitations of conventional WLE include missing nonpolypoid lesions, right-sided (proximal) disease and flat lesions [8,9]. Clinical studies have shown a significant miss rate of more than 20% for adenomas [10] and more than 25% of dysplastic lesions may be flat and, therefore, difficult to detect [11]. Even advanced endoscopic techniques, such as IEE, that continue to rely on structural and morphological changes in pathologic tissue have yielded disappointing results with a significant miss rate for GIT neoplasia [10]. Thus, there is a need for a so-called ‘red-flag’ method that identifies potential lesions that require more detailed evaluation.
There have been profound advances in endoscopic instrumentation. With the miniaturization of endoscopes, virtually every body cavity is amenable to endoscopy, including very small structures such as breast ducts, vessels and lymphatics. It is well established that transformed cells and tissues express molecular changes well in advance of morphologic alterations, holding out the promise of harnessing advanced optics with more specific imaging agents for the early detection and treatment of cancer. Optical fluorescence molecular imaging takes advantage of this concept, enabling the assessment of cancer molecular biology. Therefore, molecular imaging technology, adapted to endoscopy, may play a valuable role in diagnostics. In comparison with other imaging modalities (e.g., nuclear imaging and MRI), optical imaging is cost effective, widely available, portable, does not involve ionizing radiation and offers the possibility of real-time imaging to guide biopsy and therapy.
In this perspective review, we will focus on specific advances in endoscopy that have the potential to be easily translated to the clinical environment. These emerging technologies are still in their infancy and are not commercially available yet. This article will describe advances in image processing, magnification and fluorescence that may result in molecular endoscopy becoming a commonplace clinical reality (Table 1).
Table 1.
Advantages and disadvantages of the various endoscopic techniques.
| Technique | Applicability | Advantages | Disadvantages |
|---|---|---|---|
| Chromoendoscopy | Image enhancement Improves detection of pathologic lesions |
Recognition of subtle morphological changes Safe, simple and inexpensive Can be carried out using standard endoscopic equipment |
Time consuming Requires the use of exogenous stains |
| Virtual chromoendoscopy (excluding narrow band imaging) | Distinguishes between normal and malignant tissues | Easy to use by the press of a button and can be used as a ‘red-flag’ technique No need for exogenous stains Safe, simple and inexpensive Can be carried out using standard endoscopic equipment |
Learning curve – standardized training to become competent |
| Endomicroscopy (excluding confocal laser endomicroscopy) | In vivo optical biopsies Possibility of molecular imaging | Highly magnified (~1000-fold) Noninvasive in vivo virtual biopsies without the need for tissue fixation and sampling Easily integrated at the tip of standard endoscopic equipment Miniaturizing allows its use in any orifice of the body |
Needs to be combined with a red-flag technique Needs to use a fluorescent dye (e.g., fluorescein) Demands an experienced endoscopist/pathologist on site due to learning curve |
| Autofluorescence imaging | Distinguishes normal from malignant lesions via endogenous fluorophores | No need for an exogenous agent hence decreased complexity, regulatory issues and cost | Low specificity and high false-positive rate Autofluorescence imaging image difficult to interpret – depends on a complex mix of endogenous fluorophores |
| Molecular imaging | Distinguishes normal from malignant lesions Red flag for screening Allows for patient stratification for target therapy Allows for image-guided surgery |
Identifies lesions before morphologic changes become evident hence earlier diagnosis and more accurate staging Increased sensitivity and specificity Noninvasive, inexpensive and potentially widely available |
Limited depth of penetration Limited number of targeted surface biomarkers |
Selected optical endoscopic technologies
Image-ehanced endoscopy
Using WLE successfully depends on the ability to distinguish abnormal morphologies (e.g., a polyp) and colors from normal background mucosa or epithelium. The term IEE encompasses various means of enhancing the image contrast during endoscopy. One means of enhancing the image is to apply a dye to the tissue to accentuate the contrast between normal and abnormal tissue, a process known as chromoendoscopy (Figure 1) [12].
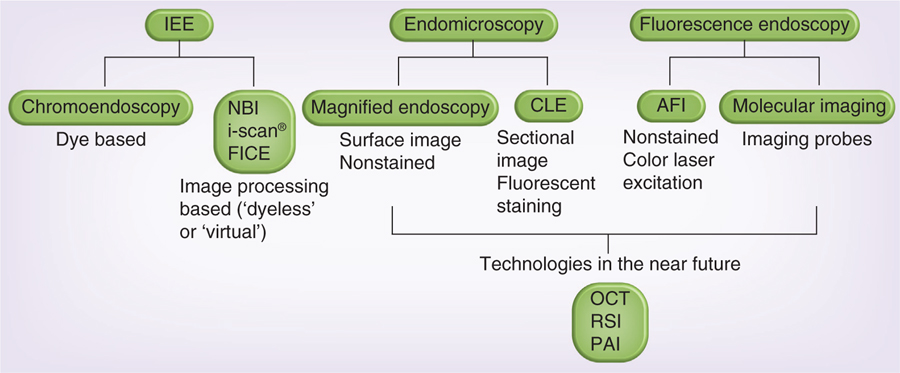
Figure 1. Currently available optical endoscopy methods and new optical technologies, which will be applied in endoscopic methods in the near future.
AFI: Autofluorescence imaging; CLE: Confocal laser endomicroscopy; FICE: Fuji intelligent chromoendoscopy; IEE: Image-enhanced endoscopy; NBI: Narrow band imaging; OCT: Optical coherent tomography; PAI: Photoacoustic imaging; RSI: Raman spectral imaging.
Chromoendoscopy involves the topical application of tissue stains to improve tissue localization, characterization and/or diagnosis during endoscopy [13]. In essence, it improves the recognition of subtle changes in the surface pattern by enhancing the contrast of raised and deepened areas [14]. The stains can be classified as absorptive, nonabsorptive and reactive. Trivedi et al. nicely reviewed the various stains, their indications and the methodologies of various chromoendoscopic techniques [14]. In contrast with chromoendoscopy, digital or ‘virtual chromoendoscopy’ does not apply an actual topical stain but rather generates virtual contrast by accentuating the vasculature and mucosal patterns of the epithelium using rotating filters (NBI) or more recently, postimage acquisition algorithms (iscan® [Illumina, CA, USA] or Fuji intelligent chromo endoscopy [Fujifilm, Tokyo, Japan]), which generates pseudocolorized videoendoscopic images. NBI enhances vessel structures, while iscan or Fuji intelligent chromoendoscopy enhances surface features and vessels [15]. Switching back and forth between WLE and NBI is relatively easy. NBI utilizes narrow band filters: light (essentially blue) with a short wavelength, which penetrates the mucosa superficially and is absorbed by hemoglobin, which highlights mucosal surface patterns and microvascular detail [16]. Owing to the density and shape of microvessels that change in neoplasia, NBI may potentially aid in the diagnosis of neoplasia simply by recognizing tumor-associated microvasculature [17]. IEE, including chromoendoscopy and virtual chromoendoscopy, serve as wide-field or ‘red-flag’ techniques and regions of interest that are detected with these methods can then be subsequently biopsied for histologic evaluation.
In 2008, the American Gastroenterological Association Institute released their guidelines to assist physicians with the appropriate use of novel endoscopic methods [12]. This guideline was developed based on a comprehensive review of the medical literature at that time and is an excellent reference. Briefly, IEE was not universally endorsed in these guidelines for screening colonoscopy owing to the absence of a definitive benefit. However, IEE was found to be useful in the surveillance of ulcerative colitis patients [18]. In 2009, Van den Broek et al. published two systematic reviews of NBI for the detection and differentiation of neoplastic lesions in the GIT [19,20]. They concluded that NBI failed to demonstrate an improved detection rate of neoplasia in the colon but the method seemed more promising for tumor detection in the upper GIT.
While IEE has the advantage of being simple, safe and inexpensive, it falls short in several aspects. First, it can be time consuming, especially in the colon. Second, IEE has a steep learning curve and findings can be difficult to interpret, which can lead to poor reproducibility and an overall lack of standardization. A number of recent studies, however, have shown that with standardized training, the method can be learned relatively quickly [21,22].
Endomicroscopy
Endomicroscopy borrows methods from in vitro microscopy and merges it with endoscopy. Endomicroscopy can be divided into confocal and two-photon endomicroscopy, both of which perform virtual optical sectioning to produce tomographic images within tissue, which, in turn, can be used to generate 3D volumetric images [1]. While confocal microscopy uses single photons in the visible near-infrared (NIR) spectrum to generate fluorescence, two-photon endomicroscopy requires two low-energy photons in the NIR spectrum that together produces enough energy to excite the tissue.
CLE enables in vivo microscopy during an ongoing endoscopic exam. Confocal optics, usually integrated into a microscope, is instead integrated into the tip of the endoscope [23]. While biopsy remains the gold standard for histology, the insertion of the biopsy needle also poses more risk and expense, and CLE potentially could achieve comparable results without resorting to an invasive procedure. CLE requires the use of fluorescent dyes, which are excited by a low-power laser and the resulting tissue fluorescence is reflected back and refocused through a pinhole confocal aperture. The area being examined is scanned systematically in horizontal and vertical planes and a 2D image is generated by stitching together each pixel into a single image. The fluorescent dyes can be topical or systemic. Intravenous fluoresecin, for instance, demonstrates the vasculature, lamina propria and intracellular spaces, while topical acriflavine predominantly stains cellular nuclei. This topic is reviewed in Goetz et al., who provides an excellent overview of different staining protocols [24]. Similarly to CLE, endocytoscopy (ECS) also provides real-time microscopic imaging requiring the use of a contrast agent. However, since ECS is a contact microscopy technique and there is no confocal sectioning, only the very superficial layer of the mucosa can be imaged. A complete review of CLE and ECS can be found in [25].
The first clinical study of in vivo endomicroscopy was completed in 2004 and demonstrated that CLE was predictive of colonic pathology when compared with gold-standard ex vivo histology [23]. These results were confirmed in a follow-up trial by the same group, resulting in more widespread acceptance of the method [26]. Hsiung et al. were the first to use this CLE to target specific molecular targets instead of using the nonspecific dyes that provide anatomical and not functional data [27]. In this study, a specific heptapeptide was conjugated with fluorescein and tested in patients undergoing colonoscopy, and this agent showed more selective binding to dysplastic tissue compared with normal mucosa. Some limitations of the study included the use of a peptide that was discovered by phage display, which leaves the molecular target unknown, and, furthermore, the peptide did not improve on the reported performance of endoscopic CLE using the nonspecific intravenously administered fluorescein. Goetz et al. employed a similar strategy with fluorescent-labeled antibodies against EGFR and VEGF in rodent models, human specimens and, more recently, in patients using CLE [28–31]. These studies demonstrated that molecularly targeted optical probes were successful in identifying and characterizing pathology.
Since endomicroscopy is highly magnified (~1000-fold) it is impractical to analyze the entire gastrointestinal mucosa in this manner. Thus, this technique must be combined with a red-flag technique, such as IEE, that identifies a specific site for higher resolution analysis. A number of clinical studies, both in gastric and colonic cancer, have demonstrated improved overall accuracy of IEE combined with endomicroscopy for detection and characterization [32,33]. CLE has also been used in the detection of inflammatory bowel disease, celiac sprue and microscopic colitis [34].
Advances in flexible fiberoptic imaging devices have paved the way for the miniaturization of confocal microscopy [35]. It has even become possible to perform CLE with the same needles used for EUS-guided fine-needle aspiration [36]. Currently, there are two commercially available US FDA-approved systems: a tip-integrated confocal laser endomicroscope and a flexible fiber-based confocal miniprobe (pCLE), which have allowed examination of the biliary and pancreatic ducts [37,38] and may also have a role in the evaluation of organs outside the GIT. For example, pCLE has been used for the assessment of cervical intraepithelial neoplasia during colposcopy [39,40] and the detection of bladder cancer [41]. These and other applications of pCLE can be found in an excellent review by Wallace et al. [34].
CLE imaging has the potential to replace actual biopsies and could certainly help guide biopsies so as to maximize the yield. However, CLE also has a number of limitations including that it may be contraindicated in patients with a dye-specific allergy, and some topical stains can cause DNA damage and, thus, are potentially mutagenic. In addition, given that CLE has a steep learning curve and is highly dependent on the knowledge and skills of the observer, it will involve significant commitments to training in pathology and/or the presence of a pathologist in the endoscopy suite [42,43].
Autofluorescence imaging
Video autofluorescence imaging (AFI) depends on changes in the fluorescence profile of endogenous fluorophores (collagen, nicotinamide, adenine dinucleotide, flavin, porphyrins and hemoglobin) to distinguish normal from malignant lesions [44]. As cancerous transformations lead to morphologic and biochemical alterations in the stroma, which may affect the composition of the tissue, the autofluorescence spectrum is also affected and, thus, could serve as a useful diagnostic marker [45]. This is a very attractive strategy since it does not require an exogenous agent to be administered, greatly reducing the complexity, regulatory issues and cost. Abnormal autoflorescence patterns in neoplastic tissues have been attributed to an increased nuclear:cytoplasmic ratio, loss of collagen and neovascularization [46]. Video AFI has been used to investigate the detection of cancer in the lung, stomach, esophagus and colon [1,47,48]. Even though AFI demonstrates high sensitivity, a major drawback is its low specificity and high false-positive rate when compared with WLE. Its low specificity stems from the fact that autofluorescence alterations may not be specific for neoplasia and may also be seen in inflammation. Moreover, the AFI image is difficult to interpret as it depends on a complex mix of endogenous fluorophores. An endoscopy system (endoscopic trimodal imaging) is commercially available that incorporates high-resolution WLE, AFI and NBI (Lucera®; Olympus, Tokyo, Japan), and a number of studies suggest that AFI may be more useful when combined with another imaging technique, such as NBI. However, studies have not consistently demonstrated the superiority of this method when compared with conventional WLE for detecting GIT neoplasia [49,50]. Technically, the setting of AFI is easily adaptable to fluorescence molecular imaging, which employs target-specific fluorescent imaging probes.
Molecular imaging
Optical fluorescence molecular imaging is an emerging imaging approach based on unique molecular changes that occur in diseased cells but not in normal tissue [51]. Fluorescence, light emitted when an excited molecule transitions from its lowest singlet state to its ground state, is the source of contrast for most in vivo optical imaging. Fluorescence intensity imaging (FII) occurs when an injected or applied fluorophore is excited by a light source and subsequently emits a photon of characteristic wavelength, typically longer than the excitation wavelength, a phenomenon known as the Stokes shift. FII techniques are most applicable to oncologic targets near tissue surfaces (e.g., breast cancer, polyps and bladder cancers), directly visible with an endoscope or during open surgery (e.g., peritoneal tumors, colon cancers and lymph nodes).
Identifying the unique molecular signature of specific cancer cells could permit earlier detection of neoplasia. Advantages of optical molecular imaging includes the ability to repeatedly and nondestructively assess tissue in vivo without ionizing radiation, the possibility of multiplexing molecular markers simultaneously increasing sensitivity and specificity, and the potential for quantitating these findings [52]. Thus, molecular imaging may improve sensitivity and specificity of endoscopic cancer screening leading to earlier diagnosis, more accurate staging and image-guided surgical intervention for the complete removal of neoplasia with tumor-free margins. Moreover, molecular imaging may assist in selecting patients for the most appropriate systemic therapy.
Imaging probes
Specific molecular probes are designed to target disease-specific biomarkers hence allowing targeted molecular imaging. Targeted molecular imaging probes consist of three basic parts: a targeting moiety for specificity, a carrier to optimize pharmacokinetics and a fluorophore for signaling [53]. Targeting moieties can include antibodies, antibody fragments, peptides and drugs. Carrier molecules can be nanoparticles, proteins and polyethylene glycol. Nonactivatable fluorophores are ‘always on’ whether they are bound or unbound to the target but activatable fluorophores fluoresce only after certain conditions are met, for instance, binding to the target cell. In this way, the target:background ratios remain very high and sensitivity is improved. This topic has been reviewed extensively by Kobayashi et al. [53,54].
Cell-specific molecular receptors (whether intracellular or on the cell surface) must be present in sufficient quantity to be detectable. FII is sensitive, offering the ability to detect as little as 10−9–10−12 M of a probe. FII also has high temporal (multiple frame/s) and spatial resolution (2–3 mm). Furthermore, a broad range of fluorophores are available in the research setting, with emissions ranging from visible spectrum (390–650 nm) to the NIR spectrum (650–900 nm), the latter in general being preferred owing to the deeper tissue penetration. Most optical imaging probes are still in the pre-clinical stages of development, however, several nonspecific optical probes such as fluorescein and indocyanine green (ICG) have been employed clinically in conjugates to target moieties.
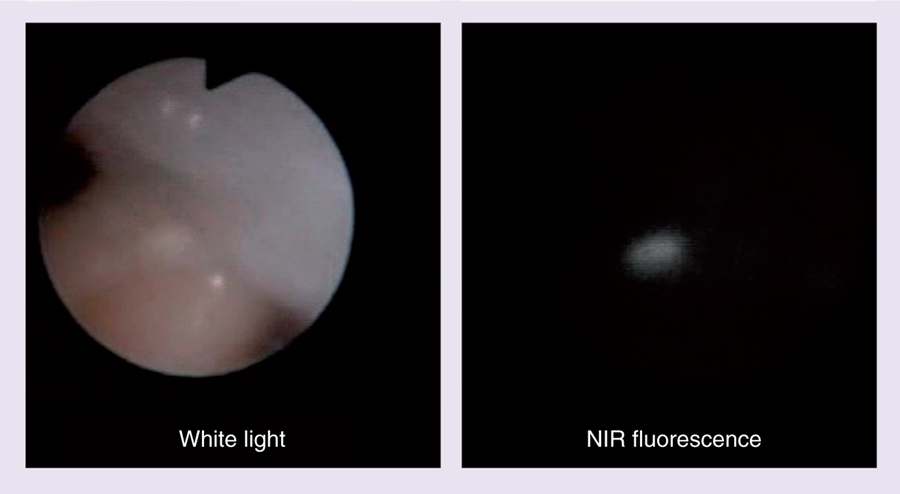
Exogenous fluorescent dyes fall into two categories: the organic small molecule (e.g., a fluorophore dye) and nanosized particles (e.g., quantum dots). Both types of dye can be conjugated to a high-affinity ligand (e.g., antibody, antibody fragment and peptides), to provide molecular specificity. However, the pharmacokinetics of nanosized particles will be very different from small molecules. There are several characteristics of a successful optical molecular probe for medical imaging, including proper wavelength (typically in the NIR), brightness, bio- and photo-stability, and pharmacokinetics including absence of nonspecific tissue accumulation, which has been previously reviewed by Kobayashi et al. [54]. Among the NIR fluorophores, the cyanine-based dyes have been used most extensively for in vivo FII owing to their improved tissue penetration, lower autofluorescence and large Stokes shift, allowing better rejection of excitation light [55,56]. However, there are other types of NIR dyes that merit attention. NIR dyes can be synthesized based on many different platforms including phthalocyanines, cyanines, boron-dipyrromethenes (BODIPYs) and squaraines. Since most NIR dyes can be excited with a broad visible light range, WLE is not compromised and interventional endoscopic procedures can be easily performed (Figure 2). Indeed, such dual real-time imaging of white light and NIR fluorescence has been previously described [57,58]. Furthermore, since the optical imaging system requires only a minor modification of WLE, it is simple and inexpensive compared with other imaging methods, it can be easily translated into the clinic.
Figure 2. Near-infrared fluorescence endoscope images of a tiny peritoneal nodule of ovarian cancer in a mouse abdomen.
Since visible range white light was used as the excitation light, a full color white light image and a near-infrared fluorescence image specific for cancer could be concurrently obtained by simply separating light using a dichroic mirror. NIR: Near infrared.
An important consideration when using exogenous probes is to determine the desired route of administration in order to gain maximal access to the target. The two routes most commonly used to deliver optical agents are the systemic, intravenous route and the topical route. Molecular changes in neoplastic cells can be present inside the cell or at the cell surface, hence topical agents may be relevant, for instance, enhancing the GIT during endoscopy. Advantages of systemic delivery include a more even distribution of the probe throughout the body and the lesion, and the ability to penetrate more deeply into tumors. However, potential side effects may be greater than with topical application, since the probe comes into contact with more off-target organs. Since a much lower amount of probe is required for topical application, there are potentially fewer safety concerns and a lower chance of developing an immunogenic reaction. Multiple topical probes may also be serially used since they are easily washed off the mucosa.
Nonspecific imaging probes
Before considering highly targeted probes, we will discuss nonspecific probes that are simply the fluorophore itself. Fluorophores, by themselves, have been used to image cancer cells in vivo because there is preferential accumulation of the probe within the tumor depending on its vascularity and/or impaired tumor capillary permeability, a phenomenon referred to as the enhanced permeability and retention effect. The only fluorescent contrast agents approved by the FDA for clinical applications are fluorescein and ICG, a NIR agent. Even though fluorescein is a nonspecific dye, it can be conjugated with targeting probes, providing specific labeling of cancer cells and this will be discussed later in the article. By contrast, because ICG conjugation chemistry is difficult, it has remained a nonspecific contrast agent until recently.
ICG has a long clinical history and has been used to measure hepatic clearance [59], cardiovascular function and is used during retinal angiography. ICG has many desirable, clinical properties including its safety profile [56,60], which has prompted its use in many other applications, including image-guided surgery. For example, it has been shown to be useful in a number of nononcologic surgical procedures, such as intraoperative angiography during neurosurgery, coronary bypass surgery, liver surgery, reconstructive microsurgery after oncologic surgery and laparoscopic cholecystectomy [61]. ICG has also been studied for image-guided oncologic surgery. A complete review of this subject can be found in [62]. Additionally, ICG has been used in a number of studies as a lymphatic tracer in sentinel node mapping procedures in breast, skin, gastrointestinal, non-small-cell lung, oropharyngeal and gynecological cancers [62]. This makes use of the proclivity of ICG to bind albumin in the body, thus becoming a macromolecular agent. The use of NIR imaging instead of conventional radioscintigraphy for sentinel node imaging is much more convenient, as well as safer for the surgeon. ICG has also been evaluated for the intraoperative identification of tumors such as hepatobiliary cancer [63,64], colorectal–liver metastases [65,66] and disseminated peritoneal ovarian cancer [67]. As mentioned earlier, current intraoperative assessment of tumors and their margins relies on the naked eye and palpations, which are notoriously misleading. Thus, ICG could potentially aid surgeons in detecting tumors that might otherwise be missed and in delineating negative tumor margins after complete resection of cancer.
Since ICG is already a FDA-approved agent, it can be readily translated for human use, requiring only institutional review board approval for its off-label use. Its nonspecificity and lack of need for conjugation make it very easy to use and generally applicable for most cancers. ICG also has several disadvantages. It is considered an ‘always on’ probe and thus, the difference between the target and background is reduced. Since ICG is bound to albumin in vivo, circulation times are prolonged resulting in higher background signals. Targeting ICG with a conjugating ligand could improve this by making ICG activatable (see the ‘Antibody/protein ligand-based activatable probes’ section). Another problem with ICG is that it relies on the enhanced permeability and retention effect, and this effect can be very small for partially albumin-bound ICG macromolecules. Thus, a wide timeframe is required for successful imaging, usually approximately 24 h after injection, hence translation for routine clinical application may be impractical.
Molecular-specific imaging probes
Location of the target is an important consideration: for example, the target could be a secreted protein, or extracellular matrix protein or enzyme, or it could be attached to the cell surface, such as a receptor or transporter. For example, in esophageal cancer, Wong et al. established that periostin was present, by examining the tumor invasion gene signature with gene expression data (Shanxi cohort) in 53 patients [68]. They developed a polyclonal antiperiostin antibody conjugated with a NIR dye (Cy5.5). The optical probe was able to achieve a tumor:background ratio (TBR) of approximately 1.5, which is typical for ‘always on’ fluorescent imaging probes. One must remember that cellular targets may be overexpressed in non-neoplastic conditions, such as wound injury, inflammation and fibrosis that may lower the specificity of the optical probe. This is of significance in gastrointestinal neoplasia where inflammation may be common in the background: for example, in the upper GIT, inflammation may be due to gastroesophageal reflux disease, infection, smoking and certain foods, while in the lower GIT inflammation may be the result of infection and inflammatory bowel disease.
A complete recounting of all probes for optical molecular imaging that have been developed is beyond the scope of this review; however, a few additional examples will be illustrative. For instance, in order to develop another agent for esophageal cancer, gene expression profiling from 75 specimens revealed that glycans might be a good target for cancer and dysplastic lesions, the latter often being missed on conventional endoscopy [69]. A topical probe was created by conjugating a glycan-binding lectin to an Alexa Fluor® 680 (Invitrogen, CA, USA). The choice of a NIR imaging fluorophore permitted a deeper assessment of the disease while the lectin added specificity. Within 10 min of application, lesions became visible with a TBR of approximately five. Dye that was not bound to the target tissue readily washed off the normal mucosa. Importantly, regions of inflammation failed to take up the probe. Topical application can improve the TBR by many fold compared with systemic application.
Smart activatable probes
A unique aspect of optical imaging is that fluorescence can be switched off or on. A switched off probe is referred to as ‘quenched’ while a switched on probe is ‘dequenched’ or ‘activated’. Molecular imaging probes can be designed so that they activate at the target but are otherwise quenched. This leads to remarkable gains in TBR. When using antibodies, which are usually slow to clear from the vasculature, huge gains in TBR can be realized if a tumor-specific activatable design is employed. Therefore, in an antibody-based targeting probe, highly specific delivery is achieved when the signaling molecule is only activated at the sites of the targeted cancer cells thus, reducing nonspecific background signals. Therefore, the advantage of activatable probes includes very high TBR with greater sensitivity and specificity.
There are a variety of activating mechanisms for optical probes, including self-quenching, photon-induced electron transfer, intermolecular interactions (hetero- or homo-dimer formations) and fluorescence resonance energy transfer. In some cases the activation may occur within seconds but in other cases the time required may be measured in hours or days, which impacts the practicality of each mechanism [53].
Antibody/protein ligand-based activatable probes
Antibodies are a commonly used targeting moiety in vitro and in vivo due to their high specificity. Many antibodies have been developed against a wide range of targets and they generally have very high affinities with slow off rates for their cell-surface antigens. Moreover, the antibody is designed so that conjugations can occur in constant regions of the molecule using a similar chemical reaction without interfering with the binding affinity at the Fv portion. Antibodies can be used as a vector to deliver a quenched optical probe to a specific target, whereupon it is activated. For instance, a pH-activatable fluorophore, based on the BODIPY was conjugated to a cancer-targeting monoclonal antibody (mAb), anti-HER2 [70]. Ex vivo and in vivo imaging of HER2-expressing lung cancer cells in mice demonstrated that the probe was highly specific for tumors with minimal background signal. Furthermore, because the acidic pH in lysosomes is maintained by an energy-consuming proton pump, only viable cancer cells were successfully visualized. This same mechanism could be used with any internalizing antibody demonstrating a very flexible approach to probe design. To demonstrate the versatility of the method, a mouse model of peritoneal metastasis of ovarian cancer was targeted with galactosamine-conjugated serum albumin (GSA) conjugated to the pH-activatable BODIPY. With in vivo fluorescence microendoscopy, tiny tumor sites, invisible to the naked eye, became clearly visible, demonstrating that this method might be useful as an aid to laparoscopic surgery by helping the surgeon identify resectable lesions that would otherwise be invisible. Another advantage of this probe is its reversibility. Since the probes lose signal when they leak out of the cell into a nonacidic environment, cellular viability can be assessed since this is an energy-consuming process. Thus, viable cancer cells can be distinguished from dead or apoptotic cells permitting a better delineation of the relevant target population of cells.
In a related strategy, GSA could be used as the carrier molecule and self-quenching could be used as the activatable mechanism. For instance, by attaching multiple copies of carboxy-X-rhodamine (ROX) to the tumor-targeting protein, GSA, an activatable probe for ovarian cancer was created [71]. GSA binds to the d-galactose receptor, which is overexpressed on ovarian cancer cells. It is subsequently internalized and then is unfolded and degraded into small fragments within the lysosome. In an in vivo investigation, 20 ROX molecules were attached to each GSA molecule (GSA-20ROX), resulting in a highly quenched molecule. However, after intraperitoneal injection of the compound, the peritoneal ovarian cancer metastases became clearly visible. GSA-20ROX was able to achieve higher sensitivity and specificity than GSA-1ROX, which was an ‘always on’ version of the probe.
As mentioned previously, ICG is a highly desirable fluorophore owing to its extensive history of use in humans. However, ICG is well known to lose its fluorescence when conjugated to other molecules. This prevented its targeted use until it was realized that ICG was simply being quenched in the conjugated state. Ogawa et al., recognizing this, developed an activatable NIR probe composed of monoclonal antibodies and ICG [60]. When bound to the antibody the ICG is quenched; however, upon binding and internalization to the target cell, the antibody–ICG conjugate is internalized, whereupon the ICG is liberated from the conjugate, leading to activation in a cell-specific manner. This strategy employs two already FDA-approved components (ICG and a mAb), which improves the likelihood of clinical translation. It should be noted to those unfamiliar with the FDA or similar regulatory approval agencies, that such agencies generally consider the conjugation of two approved compounds to nonetheless represent a new compound subject to retesting. However, because each component has well-understood toxicities, the level of proof needed for initial toxicity testing is lower than for completely novel compounds. With the antibody–ICG combination, fluorescence activation only occurred after internalization within targeted cells with minimal fluorescence outside the cell. ICG was conjugated to daclizumab (Dac), panitumumab (Pan) and trastuzumab (Tra; all three are US FDA approved monoclonal antibodies) at 1:1 or 1:5 antibody:ICG ratios. Surprisingly, even the 1:1 conjugate showed quenching. During in vivo studies, target tumors were specifically visualized with ICG-conjugated Dac with a high TBR. The fluorescence intensity of the tumor increased only in the targeted ATAC4 (IL2–Rα+) tumors, and it was higher for Dac–ICG (1:5) than Dac–ICG (1:1). The background and the nontarget tumor fluorescence was low for both Dac–ICG (1:1) and Dac–ICG (1:5). When the EGFR1-targeting antibody, Pan, and the HER2 targeting antibody, Tra, were conjugated with ICG (Pan–ICG [1:5] and Tra–ICG [1:5]) and were injected into mice bearing both EGFR1+ (MDA-MB468 and A431) and HER2+ tumors (3T3/HER2+), only EGFR1+ tumors were visualized with Pan–ICG (1:5) and only HER2+ tumors were visualized with Tra–ICG (1:5). Thus, mAb–ICG is a generally targetable activatable probe for in vivo molecular imaging that can be conjugated with a highly specific mAb and makes possible the detection and characterization of tumors in vivo.
Enzymatically activatable probes
A major class of activatable probes are enzymatically smart probes, which were first introduced in 1999 when Weissleder et al. designed a conjugate, such that tumor-associated lysosomal protease activity (cathepsin B and D) could activate a NIR probe [72,73]. The NIR fluorophore (Cy5.5) was self-quenched as it was anchored to a peptide backbone that was cleaved in the presence of cathepsin B or D. After cleavage, the Cy5.5 becomes activated at the tumor site. Having demonstrated this proof of principle, this same group went on to develop a variety of probes each activated by a different enzyme. For instance, a probe was designed around MMP-2, an enzyme commonly found during tumor growth in the extracellular matrix. The development of enzymatically activated probes stimulated much interest in the concept of characterizing tumors using molecular imaging. Optical imaging was appealing owing to its high sensitivity and relatively low expense. One drawback to these agents, however, was the relatively long incubation period needed to convert the probe to its activated state. By changing the dye to ProSense® 750 (Perkin Elmer Inc., MA, USA), this incubation time could be reduced with better fluorescence output [74,75].
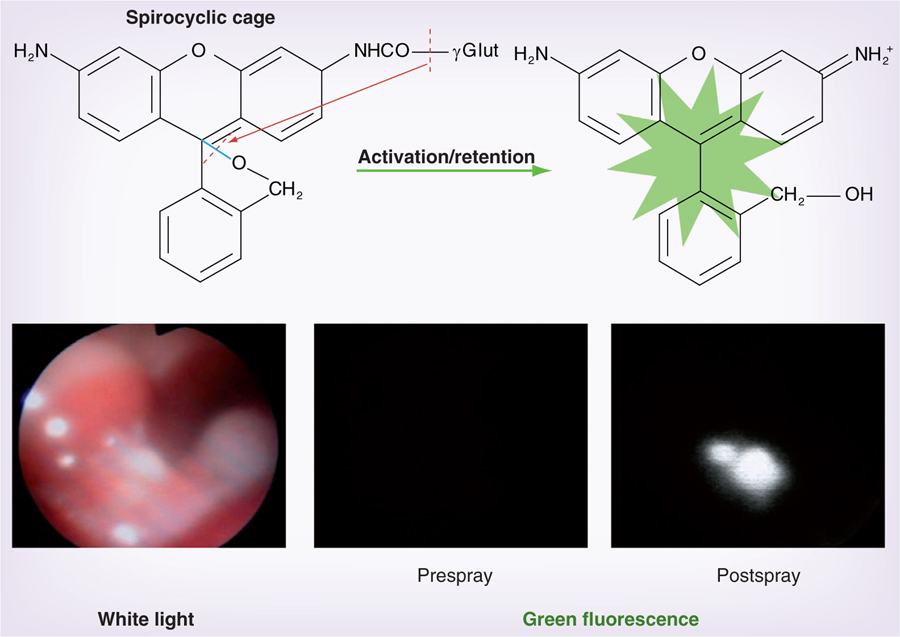
One disadvantage of enzyme-mediated dequenching based on peptide scaffolds is that this process is relatively slow, requiring multiple cleavages on a single molecule to be fully activated, a process that takes many hours to be achieved. An alternative is a directly activated probe. For instance, GGT, which is a cell-surface-associated enzyme related to glutathione metabolism, has been shown to be overexpressed in various types of human cancers [76], including cervical and ovarian cancers [77]. The probe, γ-glutamyl hydroxymethyl rhodamine green (γGlu-HMRG) is rapidly activated by a one-step cleavage reaction to produce green light with high efficiency (Figure 3). Thus, GGT can be considered a potential biomarker for early cancer detection. Its role in tumorigenesis is not completely understood but it is implicated in tumor progression, invasion and drug resistance. γGlu-HMRG is completely quenched by spirocyclic caging and but is activated rapidly with a one-step enzymatic reaction in the presence of GGT. When γGlu-HMRG encounters GGT on the cancer cell surface, it is hydrolyzed by the enzyme yielding the highly fluorescent and hydrophobic product, HMRG, which can permeate the lipid bilayer of the plasma membrane to enter cancer cells and accumulate mainly in the lysosome. In the mouse model of disseminated human peritoneal ovarian cancer, enhancement of lesions was seen within 1 min of topically spraying the peritoneal surface with γGlu-HMRG. This makes the probe potentially useful for detecting small tumors during debulking procedures. van Dam et al. investigated the potential value of intraoperative tumor-specific fluorescence imaging in the detection of peritoneal ovarian cancer for improvement of cytoreductive surgery. This group targeted FR-α, which is overexpressed in ovarian caners [78]. They administered folate-fluorescein isothiocyanate and observed that during surgery, FR-α-positive tumors were identified within 2–8 h after injection.
Figure 3. Serial fluorescence endoscopy images of a tiny peritoneal ovarian cancer before and after spraying a GGT activatable probe with a white light image.
A cancer nodule does not show fluorescence before spraying the GGT activatable probe, however, bright green fluorescence is shown 5 min after spraying the probe.
In addition to applications for peritoneal tumors, the γGlu-HMRG agent has also been evaluated in a model of colitis-associated colon cancer. Even though colonoscopy is recommended in patients with long-standing colonic inflammation for the detection of early tumors, it can be difficult to detect early tumors against the background of inflammation [79]. It is note-worthy that it is possible to differentiate between inflammation and tumors using γGlu-HMRG fluorescence. The rapidity with which this agent is activated makes it practical to consider for clinical translation, which differentiates this agent from the others discussed where activation times were typically much longer. γGlu-HMRG does not show any detectable fluorescence in the basal state, Notably, this enzymatic probe is internalized after cleavage (in comparison with the other enzymatic probes), thus, labeling cancer cells internally. Furthermore, the fluorescence is not short lived and lasts for at least 1 h. This is important to allow time for interventional procedures without the need to reapply the agent.
Thus, a number of different strategies can be used to create activated molecular imaging probes that can be incorporated into intraoperative procedures in order to guide surgery. All of these techniques can potentially be combined with a simple handheld confocal imaging device to allow optical biopsies for confirmation. The fluorescent endoscopic imaging technique could be readily integrated into the existing endoscopes not requiring any special training owing to its ease of use and minimal cost.
Conclusion & future perspective
Optical molecular imaging techniques are rapidly approaching clinical translation and their future has never been brighter. A number of optical probes are under development for fluorescence-guided surgery, including IEE, confocal laser microendoscopy and molecular imaging [75,80]. Furthermore, new optical technologies, including optical coherent tomography, Raman spectral imaging and photoacoustic imaging have been applied to endoscopy (Figure 1). Among them, optical coherent tomography and photoacoustic imaging will provide high-resolution, real-time, sectional images of deep lesions that might be superior to EUS. The simultaneous-use, multiple target-specific fluorescent agents raise the possibility of non-invasive tissue profiling. Raman spectral imaging might be able to provide tissue-profiling information without using an imaging probe. Therefore, advantages of new types of optical endoscopy are improved characterization of pathology at the point of care. The widespread adoption of NIR probes will enable deeper penetration into tissues. The development of rapidly activatable molecular imaging probes heralds an era of image-guided surgery that promises to improve outcomes and decrease morbidity. A few endoscopic molecular imaging probes or technologies have been used or are ready to use in clinical trials. Therefore, these new endoscopic molecular imaging technologies will be available in patients within 2 or 3 years. With the rapid development of newer optical endoscopic techniques, comparative studies will be crucial to assess the added value of such advances to current existing technologies.
Executive summary.
Limitation of standard endoscopic screening
Standard endoscopic screening and detection of cancers in the GI tract relies on relatively late gross morphological changes rather than earlier molecular events. Limitations of white light endoscopy have long included the detection of nonpolypoid lesions, right-sided (proximal) disease in the colon and flat lesions, all of which account for a significant miss rate.
The problem with current endoscopic enhanced imaging techniques
We have already witnessed the use of enhanced imaging techniques, such as image-enhanced endoscopy, endomicroscopy and autofluorescence imaging, to help distinguish malignant cells from normal tissue. However, these techniques have been slow to be adopted in routine clinical practice due to inconsistent results, the need for specialized training, cost concerns, and intra- and inter-observer variability.
The advantage of endoscopic molecular imaging
Optical molecular imaging may improve the detection of cancer with enhanced sensitivity and specificity that is being realized with the advent of technologic advances in both equipment and design of molecular probes. It holds the promise of being easily disseminated and used for routine clinical care since it does not required specialized training, is highly accessible and inexpensive, and, yet, is highly sensitive.
Consideration of imaging probes
Imaging probes can be designated as nonspecific probes, specific probes and smart activatable probes; all of which have their own unique advantages and disadvantages. Important considerations include the tumor-to-background ratio, time needed for activation, route of administration and clearances, especially in the setting of image-guided interventional procedures.
Molecular imaging may play a vital role in patient care
Optical molecular imaging is still in its infancy with a bright future that may revolutionize the field of oncology and image-guided surgery, and promises to improve outcomes and decrease morbidity.
Acknowledgments
Financial & competing interests disclosure The authors are employees of the NIH. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This work was made possible through the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Leona M and Harry B Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at www.fnih.org/work/programs-development/medical-research-scholars-program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Elahi SF, Wang TD. Future and advances in endoscopy. J. Biophotonics 4(7–8), 471–481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford R, Ogawa M, Choyke PL, Kobayashi H. Molecular probes for the in vivo imaging of cancer. Mol. Biosyst. 5(11), 1279–1291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomini CP, Leung SY, Chen X et al. A gene expression signature of genetic instability in colon cancer. Cancer Res. 65(20), 9200–9205 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. N. Engl. J. Med. 349(23), 2241–2252 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Wang LS, Chow KC, Chi KH et al. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am. J. Gastroenterol. 94(7), 1933–1940 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett’s esophagus. Gastroenterology 138(3), 854–869 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid BJ, Weinstein WM, Lewin KJ et al. Endoscopic biopsy can detect high-grade dysplasia or early adenocarcinoma in Barrett’s esophagus without grossly recognizable neoplastic lesions. Gastroenterology 94(1), 81–90 (1988). [DOI] [PubMed] [Google Scholar]
- 8.Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin. Gastroenterol. Hepatol. 6(10), 1117–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 139(4), 1128–1137 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Heresbach D, Barrioz T, Lapalus MG et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 40(4), 284–290 (2008). [DOI] [PubMed] [Google Scholar]
- 11.O’Brien MJ, Winawer SJ, Zauber AG et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin. Gastroenterol. Hepatol. 2(10), 905–911 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Kaltenbach T, Sano Y, Friedland S, Soetikno R. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology 134(1), 327–340 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Fennerty MB. Tissue staining. Gastrointest. Endosc. Clin. N. Am. 4(2), 297–311 (1994). [PubMed] [Google Scholar]
- 14.Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. Q. J. Med. 106(2), 117–131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz M, Kiesslich R. Advanced imaging of the gastrointestinal tract: research vs. clinical tools? Curr. Opin. Gastroenterol. 25(5), 412–421 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Ng SC, Lau JY. Narrow-band imaging in the colon: limitations and potentials. J. Gastroenterol. Hepatol. 26(11), 1589–1596 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Machida H, Sano Y, Hamamoto Y et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 36(12), 1094–1098 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm. Bowel Dis. 11(3), 314–321 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Curvers WL, van den Broek FJ, Reitsma JB, Dekker E, Bergman JJ. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video). Gastrointest. Endosc. 69(2), 307–317 (2009). [DOI] [PubMed] [Google Scholar]
- 20.van den Broek FJC, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos). Gastrointest. Endosc. 69(1), 124–135 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Patel SG, Rastogi A, Austin G et al. Gastroenterology trainees can easily learn histologic characterization of diminutive colorectal polyps with narrow band imaging. Clin. Gastroenterol. Hepatol. 11(8), 997–1003 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Neumann H, Vieth M, Fry LC et al. Learning curve of virtual chromoendoscopy for the prediction of hyperplastic and adenomatous colorectal lesions: a prospective 2-center study. Gastrointest. Endosc. 78(1), 115–120 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Kiesslich R, Burg J, Vieth M et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127(3), 706–713 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Goetz M, Kiesslich R. Advances of endomicroscopy for gastrointestinal physiology and diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 298(6), G797–G806 (2010).• Review of endomicroscopy in the GI tract.
- 25.Arya AV, Yan BM. Ultra high magnification endoscopy: is seeing really believing? World J. Gastrointest. Endosc. 4(10), 462–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest. Endosc. 62(5), 686–695 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Hsiung PL, Hardy J, Friedland S et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med. 14(4), 454–458 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz M, Ziebart A, Foersch S et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 138(2), 435–446 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Zuo X, Li C et al. In vivo molecular imaging of epidermal growth factor receptor in patients with colorectal neoplasia using confocal laser endomicroscopy. Cancer Lett. 330(2), 200–207 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Foersch S, Kiesslich R, Waldner MJ et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 59(8), 1046–1055 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Hoetker MS, Kiesslich R, Diken M et al. Molecular in vivo imaging of gastric cancer in a human–murine xenograft model: targeting epidermal growth factor receptor. Gastrointest. Endosc. 76(3), 612–620 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Shahid MW, Buchner A, Gomez V et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging in detection of dysplasia in duodenal polyps. J. Clin. Gastroenterol. 46(5), 382–389. [DOI] [PubMed] [Google Scholar]
- 33.Kiesslich R, Goetz M, Lammersdorf K et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 132(3), 874–882 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology 136(5), 1509–1513 (2009).• Review of the fiber-based confocal miniprobe and its applications.
- 35.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, Schnitzer MJ. Fiber-optic fluorescence imaging. Nat. Methods 2(12), 941–950 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker V, Wallace MB, Fockens P et al. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos). Gastrointest. Endosc. 71(7), 1260–1266 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Meining A, Shah RJ, Slivka A et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary structures. Endoscopy 44(3), 251–257 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Meining A, Chen YK, Pleskow D et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest. Endosc. 74(5), 961–968 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Tan J, Quinn MA, Pyman JM, Delaney PM, McLaren WJ. Detection of cervical intraepithelial neoplasia in vivo using confocal endomicroscopy. BJOG 116(12), 1663–1670 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Tan J, Delaney P, McLaren WJ. Confocal endomicroscopy: a novel imaging technique for in vivo histology of cervical intraepithelial neoplasia. Expert Rev. Med. Devices 4(6), 863–871 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Sonn GA, Jones SN, Tarin TV et al. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol. 182(4), 1299–1305 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Dunbar KB, Montgomery EA, Canto MI. The learning curve of in vivo confocal laser endomicroscopy for prediction of Barrett’s esophagus. Gastroenterology 134(4), A62–A63 (2008). [Google Scholar]
- 43.Kiesslich R, Anagnostopoulos GK, Axon A et al. Interobserver variation and standardized training for confocal laser endomicroscopy image interpretation in the upper and lower GI tract. Gastrointest. Endosc. 65(5), AB354 (2007). [Google Scholar]
- 44.Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Ann. Rev. Phys. Chem. 47, 555–606 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Keereweer S, Kerrebijn JD, van Driel PB et al. Optical image-guided surgery – where do we stand? Mol. Imag. Biol. 13(2), 199–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragunath K. Autofluorescence endoscopy – not much gain after all? Endoscopy 39(11), 1021–1022 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Wang TD, van Dam J, Crawford JM, Preisinger EA, Wang Y, Feld MS. Fluorescence endoscopic imaging of human colonic adenomas. Gastroenterology 111(5), 1182–1191 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Cothren RM, Sivak MV, Van Dam J et al. Detection of dysplasia at colonoscopy using laser-induced fluorescence: a blinded study. Gastrointest. Endosc. 44(2), 168–176 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Ohkawa A, Miwa H, Namihisa A et al. Diagnostic performance of light-induced fluorescence endoscopy for gastric neoplasms. Endoscopy 36(6), 515–521 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Curvers WL, van Vilsteren FG, Baak LC et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett’s neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest. Endosc. 73(2), 195–203 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology 138(3), 828–833.e1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi H, Hama Y, Koyama Y et al. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano. Lett. 7(6), 1711–1716 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc. Chem. Res. 44(2), 83–90 (2011).•• Review of the design of activatable fluorescence probes.
- 54.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 110(5), 2620–2640 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma R, Wendt JA, Rasmussen JC, Adams KE, Marshall MV, Sevick-Muraca EM. New horizons for imaging lymphatic function. Ann. NY Acad. Sci. 1131, 13–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weissleder R A clearer vision for in vivo imaging. Nat. Biotechnol. 19(4), 316–317 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi H, Longmire MR, Choyke PL. Polychromatic in vivo imaging of multiple targets using visible and near infrared light. Adv. Drug Deliv. Rev. 65(8), 1112–1119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Funovics MA, Alencar H, Su HS, Khazaie K, Weissleder R, Mahmood U. Miniaturized multichannel near infrared endoscope for mouse imaging. Mol. Imag. 2(4), 350–357 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Alford R, Simpson HM, Duberman J et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol. Imag. 8(6), 341–354 (2009). [PubMed] [Google Scholar]
- 60.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 69(4), 1268–1272 (2009).•• Original work for the activatable indocyanine green and monoclonal antibody conjugates.
- 61.Alander JT, Kaartinen I, Laakso A et al. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imag. 940585 (2012).• Reviews the uses of indocyanine green in intraoperative surgery.
- 62.Schaafsma BE, Mieog JS, Hutteman M et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 104(3), 323–332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoki T, Murakami M, Yasuda D et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J. Hepatobiliary Pancreat. Sci. 17(5), 590–594 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Mizuno S, Isaji S. Indocyanine green (ICG) fluorescence imaging-guided cholangiography for donor hepatectomy in living donor liver transplantation. Am. J. Transplant. 10(12), 2725–2726 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Gotoh K, Yamada T, Ishikawa O et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J. Surg. Oncol. 100(1), 75–79 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery 144(1), 39–48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosaka N, Mitsunaga M, Longmire MR, Choyke PL, Kobayashi H. Near infrared fluorescence-guided real-time endoscopic detection of peritoneal ovarian cancer nodules using intravenously injected indocyanine green. Int. J. Cancer 129(7), 1671–1677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong GS, Habibollahi P, Heidari P et al. Optical imaging of periostin enables early endoscopic detection and characterization of esophageal cancer in mice. Gastroenterology 144(2), 294–297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bird-Lieberman EL, Neves AA, Lao-Sirieix P et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Med. 18(2), 315–321 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Koyama Y, Hama Y, Urano Y, Nguyen DM, Choyke PL, Kobayashi H. Spectral fluorescence molecular imaging of lung metastases targeting HER2/neu. Clin. Cancer Res. 13(10), 2936–2945 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Hama Y, Urano Y, Koyama Y, Gunn AJ, Choyke PL, Kobayashi H. A self-quenched galactosamine–serum albumin–rhodamineX conjugate: a ‘smart’ fluorescent molecular imaging probe synthesized with clinically applicable material for detecting peritoneal ovarian cancer metastases. Clin. Cancer Res. 13(21), 6335–6343 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug. Chem. 10(5), 892–896 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 17(4), 375–378 (1999).• Original work for the protease-activated, near-infrared fluorescent probe.
- 74.Habibollahi P, Figueiredo J-L, Heidari P et al. Optical imaging with a cathepsin b activated probe for the enhanced detection of esophageal adenocarcinoma by dual channel fluorescent upper GI endoscopy. Theranostics 2(2), 227–234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheth RA, Upadhyay R, Stangenberg L, Sheth R, Weissleder R, Mahmood U. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol. Oncol. 112(3), 616–622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitsunaga M, Kosaka N, Choyke PL et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut 62(8), 1179–1186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urano Y, Sakabe M, Kosaka N et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci. Transl. Med. 3(110), 110–119 (2011).•• Original work on topical GGT-activated fluorescent probe.
- 78.van Dam GM, Themelis G, Crane LM et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 17(10), 1315–1319 (2011). [DOI] [PubMed] [Google Scholar]
- 79.The role of colonoscopy in the management of patients with inflammatory bowel disease. American Society for Gastrointestinal Endoscopy. Gastrointest. Endosc. 48(6), 689–690 (1998). [DOI] [PubMed] [Google Scholar]
- 80.Hama Y, Urano Y, Koyama Y, Choyke PL, Kobayashi H. d-galactose receptor-targeted in vivo spectral fluorescence imaging of peritoneal metastasis using galactosamin-conjugated serum albumin-rhodamine green. J. Biomed. Opt. 12(5), 051501 (2007). [DOI] [PubMed] [Google Scholar]