Abstract
Background
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN) is the most Serious Cutaneous Adverse Reaction (SCAR) often with a fatal outcome. Coronavirus Disease (COVID-19) is caused by Severe Acute Respiratory Syndrome–Coronavirus—2 (SARS-COV2) and is an emergent pandemic for which no cure exist at the moment. Several drugs have been tried often with scant clinical evidence and safety.
Case presentation
Here we report the case of 78-years-old woman with cardiometabolic syndrome and COVID-19. A multidrug regimen including others hydroxychloroquine, antibiotics, dexamethasone and paracetamol, low-molecular-weight-heparin and potassium canrenoate was started. After almost 3 weeks, the patient started to display a violaceous rash initially involving the flexural folds atypical targetoid lesions and showing a very fast extension, blister formation and skin detachments of approximately 70% of the total body surface area and mucous membranes involvement consistent with toxic epidermal necrolysis (TEN). The ALDEN algorithm was calculated inserting all drugs given to the patient in the 28 days preceding the onset of the skin manifestations. The highest score retrieved was for hydroxychloroquine. Other less suspicious drugs were piperacillin/tazobactam, ceftriaxone and levofloxacin.
Conclusions
To our knowledge, this is the first case of TEN in a patient suffering from COVID-19 probably associated with hydroxychloroquine. Given the activation of the immune system syndrome induced by the virus and the widespread off-label use of this drug, we suggest a careful monitoring of skin and mucous membranes in all COVID-19 positive patients treated with hydroxychloroquine in order to early detect early signs of toxicities.
Keywords: COVID 19, SARS-CoV- 2, Toxic Epidermal Necrolysis (TEN), Hydroxychloroquine, Severe Cutaneous Adverse Reactions (SCAR), Adverse Drug Reaction (ADR), Stevens–Johnson Syndrome (SJS), ALDEN Algorithm, SCORTEN Score, Pharmacovigilance
Background
Toxic Epidermal Necrolysis (TEN) is a rare Serious Cutaneous Adverse Reaction (SCAR). It displays an acute onset and is characterized by erythematous or violaceous patches, atypical targetoid lesions, bullae, erosions and skin detachment. It differs from Stevens-Johnson Syndrome (SJS) only in the percentage of skin involvement, which in TEN is greater than 30% of the body surface [1]. Etiopathogenetically, it results from the combination of drug and host genetic factors (such as drug metabolism and T cell clonotypes) resulting in a delayed-type hypersensitivity reaction, where drug or drug-peptide complexes are recognized by T cell receptors [2].
Coronavirus Disease (COVID-19) is caused by Severe Acute Respiratory Syndrome–Coronavirus—2 (SARS-CoV-2) and can be potentially fatal disease [3]. The most common symptoms at onset of COVID-19 illness are fever, cough, and fatigue, followed by dyspnea and diarrhea [4]. Lymphopenia, elevation of creatinine kinase (CK), lactate dehydrogenase (LDH) are also frequently observed. The lung involvement is frequently that of an interstitial pneumonia, COVID-19 later shows an over exuberant inflammatory response with a no correlation between viral load and the worsening of symptoms [5].
At the moment there is no effective cure for COVID-19, several drugs often in combination have been used, often with scant clinical evidence or conflicting results, with the aim of targeting the virus replication and/or the inflammatory process, such as anti-retrovirals, macrolides, hydroxychloroquine and monoclonal antibodies targeted on inflammatory cytokines [6–9]. There is limited data establishing their safety profile during SARS-CoV-2 infection.
Case presentation
Here we report the case of 78-year old woman with several comorbidities (hypertension, obesity, unstable angina, type 2 diabetes) admitted to our hospital for respiratory insufficiency with fever requiring non-invasive ventilation and a diagnosis of COVID-19 lung infection with bacterial superinfection was formulated given the radiological evidence of a bilateral interstitial pneumonia, the positivity of nasopharyngeal swab for SARS-CoV-2 and (Fig. 1). Lymphopenia (0.55 103/µL [1.00–4.00]), elevation of CK (252 U/L [24–170]) and LDH (407 U/L [30–250]) were also observed.
Fig. 1.

The X-ray shows bilateral and extensive interstitial infiltrates
A multidrug regimen with hydroxychloroquine 200 mg twice a day, sodium enoxaparin and dexamethasone were started. Besides, an antibiotic treatment with ceftriaxone for 1 day, followed by piperacillin/tazobactam for 4 days was given. The patient was initially treated in a sub-intensive care unit and after the favorable clinical evolution was transferred to our general medicine division, 18 days after the admission. At the arrival in our unit, the patient started to display a violaceous erythematous rash mainly involving the flexural folds. The patient was still taking hydroxychloroquine, along with levofloxacin (started the day before); other drugs were reported in Table 1.
Table 1.
Calculation of the ALDEN score for each drug administrated during the hospitalization (chronic treatments were excluded) [10]
| DRUG | ALDEN score | Causal link |
|---|---|---|
| Enoxaparin | −2 | Very unlikelya |
| Oseltamivir | −2 | Very unlikely |
| Ceftriaxone | +1 | Unlikely |
| Potassium Canrenoate | −3 | Very unlikely |
| Pantoprazole | −2 | Very unlikelya |
| Hydroxychloroquine | +4 | Possible |
| Piperacillin/Tazobactam | +1 | Unlikely |
| Dexamethasone | 0 | Unlikely |
| Levofloxacin | +1 | Unlikely |
| Paracetamol | −1 | Very unlikelya |
< 0: very unlikely
0–1: unlikely
2–3: possible
4–5: probable
≥ 6: very probable
are-challenge without any adverse reactions. Score (−12; +10)
The rash presented in the course of 3 days a rapid extension with the involvement of the whole trunk and buttocks, reaching approximately 70% of the total body surface area, with the appearance of atypical targetoid lesions and the formation of blisters, with subsequent skin detachment (Figs. 2, 3 and 4). Nikolsky’s sign was present. A severe desquamation of the buccal and nasal mucosa was also observed. The patient referred severe skin pain requiring morphine. Blood tests did not show eosinophilia or alterations of liver and renal function tests.
Fig. 2.
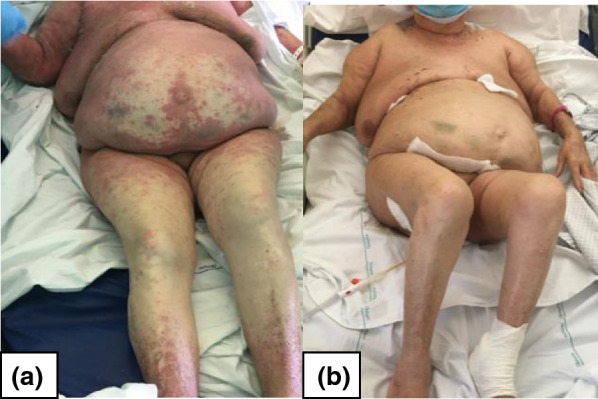
The violaceous rash extension with atypical targetoid elements (at day 3 from clinical onset) is depicted in a. b shows the complete resolution (after 6 weeks)
Fig. 3.
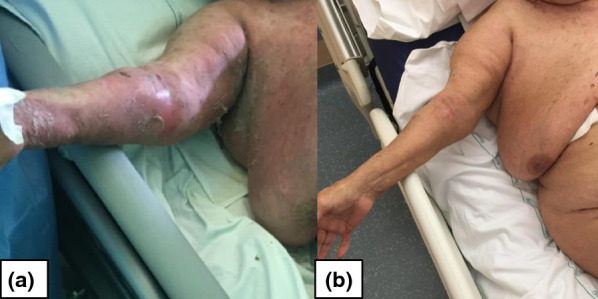
The extensive skin detachment is showed in a (at day 5) and its favorable evolution at 6 weeks in (b)
Fig. 4.
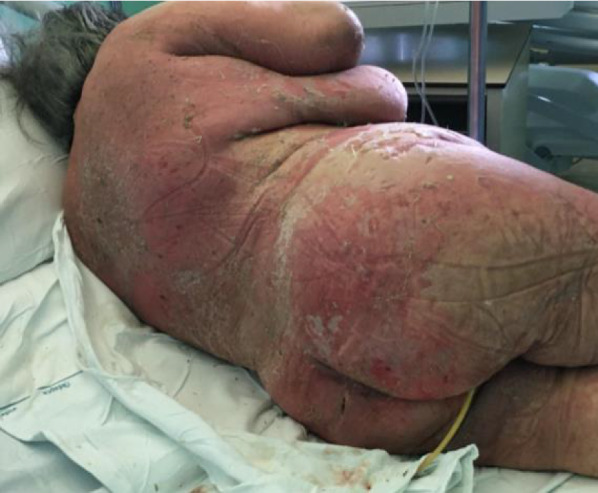
The figure shows the extensive disepithelialization with subcutaneous oozing and bleeding
A clinical diagnosis of toxic epidermal necrolysis (TEN) also confirmed by the dermatologist was formulated. Methylprednisone 1 mg/kg, as an intravenous bolus regiment was administred along with intravenous immunoglobulin (IVIG) 1 mg/kg for 3 days. Then oral prednisone 1 mg/kg daily was given and subsequently tapered in 1 month.
To identify the culprit drug, the ALDEN algorithm was calculated (Table 1) [10] (Additional file 1). The highest score retrieved was for hydroxychloroquine, with a possible correlation (+4 score). Other less suspicious drugs were piperacillin/tazobactam, ceftriaxone and levofloxacin. All suspected drugs according to the ALDEN algorithm were promptly stopped (Additional file 2).
The patient was cared for by the wound care service of the hospital with topical therapy, a marked improvement in the skin conditions was observed progressively over a period of 6 weeks, until the complete resolution (Figs. 2 and 3). Paracetamol, pantoprazole, enoxaparin were tolerated after the reaction, ruling out their correlation with the skin reaction.
Due to the COVID-19 epidemic, no skin biopsies were technically feasible for logistical problems. However, the clinical manifestations were highly compelling for SJS/TEN. Other differential diagnosis included Drug-Reaction with Eosinophilia and Systemic Symptoms (DRESS), which was excluded given the absence of eosinophilia and internal organ involvement, Staphylococcal Scalded Skin Syndrome (SSSS), which is a pediatric form, and bullous dermatoses that were ruled out in our case given the minor mucous involvement and intense skin inflammation.
Discussion
SJS/TEN is the most severe SCAR being associated with a high mortality rate [1]. It is a delayed reaction occurring after 4–28 days from drug exposure, therefore it is of paramount importance to acquire precise retrospective pharmacological information for a long period of time preceding the onset of skin manifestations.
STS/TEN has an approximate incidence of 1 or 2 cases/1,000,000 annually. It results from the clonal expansion of CD8 + cytotoxic T lymphocytes (CTLs) and Natural Killer cell (NK),which are major histo-compatibility complex (MHC)-restricted and induce epidermal apoptosis [1, 11, 12]. Recently, it has been shown that low molecular weight drugs can directly bind the T cell receptor (TCR) of T cells or the Human Leukocyte Antigen (HLA) of antigen-presenting cells [13–16].
Usually implicated drugs are allopurinol, anticonvulsant drugs nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics such as cephalosporins, aminopenicillins and rarely macrolides, as shown in the EuroSCAR study, together with the REACT project and the regiSCAR project [17–21].
The ALDEN algorithm represents the gold standard tool in SJS/TEN to identify the culprit drug and to discriminate it from the “innocent” drugs which can be safely administered. It gives to each drug taken by patient a score, ranging from −12 to +10, which corresponds to the probability of having caused the reaction, ranging from very unlikely to very probable [10]. Besides, from a recent analysis a significant statistic for agreement (kappa 0.571) was found between ALDEN score for related-drugs (ALDEN ≥ 4) and lymphocyte transformation test (LTT) results performed after recovery [22].
According to the ALDEN algorithm results, the most likely implicated drug in our case is hydroxychloroquine.
Hydroxychloroquine is an 4-aminoquinoline with a low molecular weight (333,9 Da) [23–26]. It has been used for decades to treat rheumatologic conditions (with a dose of 6 mg/kg) with a general good safety, even though in last years cases of skin reactions, also severe, have increasingly been reported [27].
We searched the Eudravigilance database and found 30 cases of TENs related to the use of hydroxychloroquine [28].
Hydroxychloroquine is being profusely used to treat SARS-CoV-2 infection with an unconventionally high dosage (from 200 mg twice a day to 200 mg three times a day, which is independent of body weight sometimes with a loading dose, due to its high volume of distribution).
Interestingly, some new severe skin manifestation to hydroxychloroquine have also been reported during the treatment of SARS-CoV-2 positive patient [29–31].
In this instance, it is not to be discounted that a rare side effect, such as TEN, occurring with a not commonly associated drug, hydroxychloroquine, could have been favored by the particular immune stimulation induced by the virus SARS-CoV-2 [3]. SJS/TEN, and SCAR more generally, have been classically demonstrated to be associated with viral replication [11, 32–34].
To our knowledge, this is the first case of TEN in a COVID-19 positive patient.
Another salient aspect of the case is the favorable evolution of the patient given that this type of SCAR is typically associated with a bad prognosis [35–37], even more so because the patient displayed all the negative typical prognostic factors also for COVID-19 [38–41], indeed the calculation of the severity-of-illness score for toxic epidermal necrolysis (SCORTEN) in our patient led to an estimated mortality rate of 58.3% (CI 36,6 –77,59) (Table 2) [42, 43].
Table 2.
Calculation of the Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) in our patient
| Risk factor | Score | Patient score | |
|---|---|---|---|
| 0 | 1 | ||
| Age | < 40 years | > 40 years | 1 |
| Associated malignancy* | No | Yes | 0 |
| Heart rate (beats/min) | < 120 | ≥ 120 | 1 |
| Serum urea (mg/dL) [mmol/L] | ≤ 28 [10] | > 28 [10] | 1 |
| Detached or compromised body surface | < 10% | ≥ 10% | 1 |
| Serum bicarbonate (mEq/L) | ≥ 20 | < 20 | 0 |
| Serum glucose (mg/dL) [mmol/L] | ≤ 250 [14] | > 250 [14] | 0 |
| Total | 4 | ||
More risk factors result in a higher score and a higher mortality rate (%) as follows:
0–1 = 3.2% (CI: 0.1-16.7)
2 = 12.1% (CI: 5.4-22.5)
3 = 35.5% (CI: 19.8-53.5)
4 = 58.3% (CI: 36.6-77.9)
≥5 = 90% (CI: 55.5-99.8)
CI: Confidence Interval [42, 43]
aMalignancy: evolving cancer and hematological malignancies
It is possible that the prompt diagnosis of TEN, the suspension of all the suspected drugs (in particularly hydroxychloroquine) and the administration of steroid and IVIG, both targeting the inflammatory syndrome initially triggered by the virus, may have had contributed to a better prognosis, [1, 5].
Further studies are needed to assess the safety profile of hydroxychloroquine in patients with COVID-19.
Conclusion
To our knowledge, this is the first case of TEN associated with hydroxychloroquine in patient suffering from COVID-19. Given the widespread off-label use of this drug, the high degree of immune activation induced by the virus and the recent increase of hydroxychloroquine-related SCARs, we believe that it is necessary to adequately monitor the skin in all COVID-19 positive patients treated with hydroxychloroquine in order to early detect early signs of toxicities.
Supplementary information
Additional file 1: Details regarding the calculation of the ALDEN score [10].
Additional file 2: Timing of the drugs administered to the patients regarding the calculation of the ALDEN score [10].
Acknowledgements
Not applicable.
Abbreviations
- CK
Creatinine Kinase
- COVID-19
COronaVIrus Disease 19
- CTLs
Cytotoxic T Lymphocytes
- DRESS
Drug Reaction with Eosinophilia and Systemic Symptoms
- HLA
Human Leukocyte Antigen
- IVIG
IntraVenous Immunoglobulin
- LDH
Lactate Dehydrogenase
- LTT
Lymphocyte Transformation Test
- MHC
Major Histocompatibility Complex
- NK
Natural Killer
- NSAIDs
NonSteroidal Anti-Inflammatory Drugs
- SARS-CoV-2
Severe Acute Respiratory Syndrome– - Coronavirus—2
- SCAR
Severe Cutaneous Adverse Reaction
- SCORTEN
Severity-of-illness Score for Toxic Epidermal Necrolysis
- SSSS
Staphylococcal Scalded Skin Syndrome
- SJS
Stevens Johnson Syndrome
- TCR
T-Cell Receptor
- TEN
Toxic Epidermal Necrolysis
Authors’ contributions
CMR and FNB collected data. CMR and FNB wrote the draft paper and analyzed the data. CMR, GT and SM followed clinical course of the patient. DZ revised the draft paper. All authors read and approved the final manuscript.
Funding
The authors declare that they have not received any funding.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent to clinical data collection and publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carlo Maria Rossi, Email: carlomariarossi@hotmail.com.
Flavio Niccolò Beretta, Email: flavioniccolo.beretta@gmail.com.
Grazia Traverso, Email: grazia.traverso@asst-nordmilano.it.
Sandro Mancarella, Email: sandro.mancarella@asst-nordmilano.it.
Davide Zenoni, Email: davide.zenoni@asst-nordmilano.it.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12948-020-00133-6.
References
- 1.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010 doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su SC, Chung WH. Cytotoxic proteins and therapeutic targets in severe cutaneous adverse reactions. Toxins. 2013 doi: 10.3390/toxins6010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan HA, Byrareddy SN. The epidemeology and pathogensis of coronavirus (Covid-19) outbreak. J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Cina. Lancet. 2019 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stebbing J, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. 2020 doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo M, De Giglio MAR, Roviello GN. SARS CoV-2: recent Reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) Clin Infect Dis. 2020;2:1–25. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao B, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kraaij T, et al. Tocilizumab in severe COVID-19 pneumonia and concomitant cytokine release syndrome. Eur J Case Reports Intern Med. 2017 doi: 10.12890/2020_001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sassolas B, et al. ALDEN, an algorithm for assessment of drug causality in stevens-johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010 doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 11.Honma M, et al. Toxic epidermal necrolysis with prominent facial pustules: a case with reactivation of human herpesvirus 7. Dermatology. 2010 doi: 10.1159/000319756. [DOI] [PubMed] [Google Scholar]
- 12.Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble fas ligand. Am J Pathol. 2003 doi: 10.1016/S0002-9440(10)64284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy Eur. J. Allergy Clin Immunol. 2019 doi: 10.1111/all.13765. [DOI] [PubMed] [Google Scholar]
- 14.Pichler WJ, Hausmann O. Classification of drug hypersensitivity into allergic, p-i, and pseudo-allergic forms. Int Arch Allergy Immunol. 2017 doi: 10.1159/000453265. [DOI] [PubMed] [Google Scholar]
- 15.Correia O. Cutaneous T-cell recruitment in toxic epidermal necrolysis. Arch Dermatol. 1993 doi: 10.1001/archderm.1993.01680250078010. [DOI] [PubMed] [Google Scholar]
- 16.Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015 doi: 10.1007/s40257-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 17.De Luca F, et al. Tolerated drugs in subjects with severe cutaneous adverse reactions (SCARs) induced by anticonvulsants and review of the literature. Clin Mol Allergy. 2017 doi: 10.1186/s12948-017-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Techasatian L, Panombualert S, Uppala R, Jetsrisuparb C. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children: 20 years study in a tertiary care hospital. World J Pediatr. 2017 doi: 10.1007/s12519-016-0057-3. [DOI] [PubMed] [Google Scholar]
- 19.Frey N, Bodmer M, Bircher A, Jick SS, Meier CR, Spoendlin J. Stevens-johnson syndrome and toxic epidermal necrolysis in association with commonly prescribed drugs in outpatient care other than anti-epileptic drugs and antibiotics: a population-based case-control study. Drug Saf. 2019 doi: 10.1007/s40264-018-0711-x. [DOI] [PubMed] [Google Scholar]
- 20.Diphoorn J, et al. Incidence, causative factors and mortality rates of stevens-johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in Northern Italy: data From the REACT Registry. Pharmacoepidemiol Drug Saf. 2016 doi: 10.1002/pds. [DOI] [PubMed] [Google Scholar]
- 21.Mockenhaupt M. “RegiSCAR project,” Last, 2007. http://www.regiscar.org/ita/Project.html.
- 22.Bellón T, et al. Assessment of drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis: concordance between lymphocyte transformation test and ALDEN. Allergy Eur J Allergy Clin Immunol. 2020 doi: 10.1111/all.14062. [DOI] [PubMed] [Google Scholar]
- 23.Lim HS, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl 1):S11–S15. doi: 10.1177/0961203396005001041. [DOI] [PubMed] [Google Scholar]
- 25.F. D. A. Food and Drug Administration.FDA Approved Drug Products: Hydroxychloroquine Oral Tablets.2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf. Accessed 15 May 2020.
- 26.Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin Immunol. 2018 doi: 10.1016/j.clim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 28.E. M. A. European Medicines Agency. EudraVigilance Oped Data ADR hydroxychloroquine. 2020. https://bi.ema.europa.eu/analyticsSOAP/saw.dll?PortalPages.
- 29.Grandolfo M, et al. Drug reaction with eosinophilia and systemic symptoms syndrome to hydroxychloroquine, an old drug in the spotlight. Dermatol Ther. 2020 doi: 10.1111/dth.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz RA, Janniger CK. Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol Ther. 2020 doi: 10.1111/dth.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitworth AL, Mann NH, Larkum AWD. Cutaneous side-effects of the potential COVID-19 drugs. Ultrasound Obs Gynecol. 2006 doi: 10.1111/1462-2920.12735. [DOI] [Google Scholar]
- 32.Peter J, Choshi P, Lehloenya RJ. Drug hypersensitivity in HIV infection. Curr Opin Allergy Clin Immunol. 2019 doi: 10.1097/ACI.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CW, Cho YT, Hsieh YC, Hsu SH, Chen KL, Chu CY. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020 doi: 10.1111/bjd.18942. [DOI] [PubMed] [Google Scholar]
- 34.Tagajdid MR, Doblali T, Elannaz H, Hammi S, Belfequih B, Mrani S. Reactivation of cytomegalovirus in a patient with Stevens-Johnson syndrome-toxic epidermal necrolysis. Iran J Med Sci. 2013;38(Suppl 2):195–197. [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013 doi: 10.1016/j.jaad.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Papp A, et al. Treatment of toxic epidermal necrolysis by a multidisciplinary team A review of literature and treatment results. Burns. 2018 doi: 10.1016/j.burns.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: an Analysis of Triggers and Implications for Improving Prevention. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Di Stadio A, Ricci G, Greco A, de Vincentis M, Ralli M. Mortality rate and gender differences in COVID-19 patients dying in italy a comparison with other countries. Eur Rewiew Med Pharmacol Sci. 2020 doi: 10.1056/nejmoa2004500. [DOI] [PubMed] [Google Scholar]
- 39.I. S. S. Istituto Superiore di Sanità and I. S. T. A. T. Istituto Nazionale di Statistica. Impatto dell’epidemia covid-19 sulla mortalità totale della popolazione residente primo trimestre 2020. https://www.epicentro.iss.it/coronavirus/pdf/Rapporto_Istat_ISS.pdf. Accessed 15 May 2020.
- 40.D. P. C. DipartimentoProtezione Civile. COVID-19: Monitoraggio della situazione. 2020. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed 15 May 2020.
- 41.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2019 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guégan S, Bastuji-Garin S, Poszepczynska-Guigné E, Roujeau JC, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700068. [DOI] [PubMed] [Google Scholar]
- 43.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. Scorten: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000 doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Details regarding the calculation of the ALDEN score [10].
Additional file 2: Timing of the drugs administered to the patients regarding the calculation of the ALDEN score [10].
Data Availability Statement
Not applicable.


