Abstract
Background
Non-small cell lung cancer (NSCLC) remains the most commonly diagnosed malignancy and the leading cause of cancer death worldwide. Circular RNAs (circRNAs) have been demonstrated to play critical roles in human carcinogenesis, including NSCLC. However, it is still unclear whether circRNA nuclear factor I X (circNFIX) is implicated in the molecular pathogenesis of NSCLC.
Methods
The expression levels of circNFIX, miR-212-3p and a disintegrin and metalloproteinases 10 (ADAM10) were detected by quantitative real-time polymerase chain reaction (qRT-PCR) or Western blot. Cell viability was gauged by the Cell Counting Kit-8 (CCK-8) assay, and cell migration and invasion were determined by transwell assays. Glucose uptake and lactate product were determined using the assay kits. Targeted relationships among circNFIX, miR-212-3p and ADAM10 were verified by dual-luciferase reporter and RNA pulldown assays. Additionally, the xenograft model assays were carried out to analyze the role of circNFIX in tumor growth in vivo.
Results
Our data revealed that circNFIX was overexpressed in NSCLC and predicted poor prognosis of NSCLC patients. CircNFIX knockdown suppressed NSCLC cell viability, migration, invasion and glycolysis in vitro and hampered tumor growth in vivo. Mechanistically, CircNFIX acted as a molecular sponge of miR-212-3p, and the repressive effect of circNFIX knockdown on NSCLC cell malignant progression was mediated by miR-212-3p. Moreover, ADAM10 was a direct target of miR-212-3p, and circNFIX influenced ADAM10 expression by sponging miR-212-3p in NSCLC cells. Furthermore, the silencing of ADAM10 hindered NSCLC cell viability, migration, invasion and glycolysis in vitro.
Conclusion
Our findings first identified that the knockdown of circNFIX, a highly expressed circRNA in NSCLC, exerted a repressive role in NSCLC malignant progression at least in part through targeting the miR-212-3p/ADAM10 axis, illuminating a novel understanding of circRNA regulation in NSCLC.
Keywords: NSCLC, circNFIX, miR-212-3p, ADAM10, malignant progression
Introduction
Lung cancer remains the most commonly diagnosed malignancy and the leading cause of cancer death worldwide in 2018.1 Accounting for approximately 85% of all diagnosed cases, non-small cell lung cancer (NSCLC) is the most prevalent type of lung cancer.2 Significant advances in the treatment of NSCLC have been achieved in the past 20 years; however, the overall survival rates of NSCLC are still very low, especially in metastatic disease.3 Targeted therapy has recently greatly improved the clinical outcomes of NSCLC patients.4,5 Therefore, understanding the underlying mechanism of NSCLC progression will provide the basis for molecularly targeted interventions.
Circular RNAs (circRNAs) are covalently closed RNAs that exert diverse cellular functions from normal development to disease.6 Growing studies have confirmed that circRNAs regulate gene expression through functioning as “sponges” of microRNAs (miRNAs) in many human cancers, including NSCLC.7–9 For instance, Chen et al demonstrated that hsa_circ_100146 was overexpressed in NSCLC, and its knockdown restrained tumor cell malignant behaviors via directly interplaying with miR-615-5p and miR-361-3p.10 Zhang et al highlighted that has_circ_0072083 exerted a potential oncogenic activity in NSCLC tumorigenesis via reducing cullin 4B inhibition by sponging miR-101-3p.11 Moreover, Cui et al uncovered that hsa_circ_0043278 served as a sponge of miR-520f to contribute to the carcinogenesis of NSCLC.12 Recent work in several laboratories illuminated that circRNA nuclear factor I X (circNFIX, hsa_circ_0049658) accelerated the malignant progression and chemoresistance in glioma.13–15 The precise mechanisms underlying the regulation of circNFIX in glioma via sequestrating a certain miRNA were also included in these researches. However, whether circNFIX is involved in the molecular pathogenesis of NSCLC remains unknown.
MiRNAs are attractive candidates as potential oncogenes or tumor suppressors in the tumorigenesis and malignant progression of NSCLC.16,17 For example, Chen et al reported that miR-148a exerted an anti-tumor activity in NSCLC by targeting Wnt1.18 Huang et al uncovered that miR-34c-3p down-regulation contributed to NSCLC metastasis via up-regulating integrin α2β1.19 MiR-212-3p, a tumor-related miRNA, was reported to be underexpressed in NSCLC and its up-regulation exhibited a repressive impact on NSCLC cell growth and metastasis.20 Of interest, when we used the starBase database to help identify the mechanisms of circNFIX in NSCLC progression, we found two complementary regions among circNFIX, miR-212-3p and a disintegrin and metalloproteinases 10 (ADAM10). Therefore, we undertook to examine whether the miR-212-3p/ADAM10 axis could serve as a molecular mediator of circNFIX in NSCLC pathogenesis. For the first time, we identified circNFIX, an up-regulated circRNA in NSCLC, regulated NSCLC malignant progression by targeting the miR-212-3p/ADAM10 axis.
Materials and Methods
Clinical Samples and Cells
The Ethics Committee of SSL Central Hospital of Dongguan City approved all protocols. In the present project, we analyzed human specimens, including 55 NSCLC tissues and 55 matched non-tumor lung tissues, which were collected from patients enrolled at SSL Central Hospital of Dongguan City, after obtaining written informed consent. The clinicopathologic features of all patients are displayed in Table 1. Other tumors, cardiovascular diseases, metabolic diseases, severe trauma, and recent infections were excluded in this study. Patients with incomplete information were also excluded. The follow-up information was obtained by telephone visits every three months from March 2011 to August 2017. These specimens were stored in liquid nitrogen until RNA extraction.
Table 1.
Correlation Between CircNFIX Expression and Clinicopathological Parameters of NSCLC Patients
| Characteristics | Number | circZFR Expression | P | |
|---|---|---|---|---|
| High | Low | |||
| 35 | 35 | |||
| Age (years) | 0.473 | |||
| <60 | 33 | 15 | 18 | |
| ≥60 | 37 | 20 | 17 | |
| Distant metastasis | 0.016* | |||
| Present | 32 | 11 | 21 | |
| Absent | 38 | 24 | 14 | |
| Tumor size (cm) | 0.629 | |||
| ≤2 | 30 | 16 | 14 | |
| >2 | 40 | 19 | 21 | |
| TNM stage | 0.002** | |||
| I, II | 37 | 25 | 12 | |
| III, IV | 33 | 10 | 23 | |
| HER-2 status | 0.339 | |||
| Positive | 34 | 15 | 19 | |
| Negative | 36 | 20 | 16 | |
| PR status | 0.229 | |||
| Positive | 31 | 13 | 18 | |
| Negative | 39 | 22 | 17 | |
| ER status | 0.473 | |||
| Positive | 37 | 17 | 20 | |
| Negative | 33 | 18 | 15 | |
Notes: *P < 0.05 or **P < 0.01.
NSCLC cell lines (A549, H1299, H1975 and H1650 cells, ATCC, Manassas, VA, USA) and human bronchial HBE1 cells (Bnbio, Beijing, China) were propagated in RPMI-1640 medium (ATCC) under standard conditions as previously reported.9 All cell culture reagents were provided by Gibco (Life Technologies, Lucerne, Switzerland).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
RNA isolated from clinical specimens and cells using the TRIzol reagent (Life Technologies) averaged 46 μg/mL (OD260/OD280 = 1.84–1.96) when quantified by a spectrometer (Roche, Basel, Switzerland). To quantify circNFIX and ADAM10, reverse transcription (RT) was conducted on 100 ng of total RNA using an ABI cDNA RT Kit (Applied Biosystems, Darmstadt, Germany), and then cDNA was amplified by qRT-PCR in a 25 μL of reaction containing Taq DNA Polymerase (1 μL) as per the suggestion of manufacturers (Applied Biosystems), with β-actin as an internal control. The expression of miR-212-3p was tested by qRT-PCR with TaqMan MicroRNA Assay (Applied Biosystems)21 using total RNA (100 ng), normalizing the results to U6 expression. The details of primers (Sangon Biotech, Shanghai, China) are shown in Table 2.
Table 2.
Primers Sequences Used for PCR
| Primers for PCR (5ʹ-3ʹ) | ||
|---|---|---|
| CircNFIX | Forward | AGGAGATGCGGACATCAAAC |
| Reverse | GTGAAATACGGGCTCGACTG | |
| ADAM10 | Forward | GGAGTGTACGTGTGCCAGTTCTG |
| Reverse | GGTTCGACCACTGAAGTGCCTAC | |
| β-actin | Forward | CATGTACGTTGCTATCCAGGC |
| Reverse | CGCTCGGTGAGGATCTTCATG | |
| miR-212-3p | Forward | CGGCGGTAACAGTCTCCAGTC |
| Reverse | GTGCAGGGTCCGAGGT | |
| U6 | Forward | CTCGCTTCGGCAGCACA |
| Reverse | AACGCTTCACGAATTTGCGT | |
Generation of Gene and miR-212-3p Knockdown or Overexpressing Cells
For the knockdown of circNFIX studies in vitro and in vivo, short hairpin RNA (shRNA) lentiviruses targeting circFNIX (sh-circ#1 or sh-circ#2) and negative control (sh-NC) were obtained from Hanbio (Shanghai, China) and then were used to infect A549 and H1299 cells as per the manufacturer’s directions. Vector-transduced cells were selected in the presence of 1 μg/mL of puromycin. The miR-212-3p mimic and inhibitor (anti-miR-212-3p, GenePharma, Shanghai, China) were chemical oligonucleotides designed to up-regulate and down-regulate the miR-212-3p level, respectively, and scrambled oligonucleotide sequences (miR-NC mimic and anti-NC) were used as the negative control. The sequences of the commercial siRNA against ADAM10 (si-ADAM10, GenePharma) and nontarget siRNA (si-NC) were si-ADAM10, 5ʹ-CAGUGUGCAUUCAAGUCAA-3ʹ and si-NC, 5ʹ-CUCCGAACGUGUCACGUT-3ʹ. Cells were transiently transfected with 30 nM of the indicated oligonucleotides using Lipofectamine 3000 (Life Technologies) as recommended by the manufacturers.
Cell Viability Assay
sh-NC or sh-circ#1-transducing A549 and H1299 cells were transfected with or without anti-miR-212-3p or anti-NC, and A549 and H1299 cells were introduced with si-ADAM10 or si-NC, followed by the culture for 24 h in a cell incubator. The colorimetric Cell Counting Kit-8 (CCK-8, Yesen, Shanghai, China) assay was used to gauge cell viability as per the manufacturer’s recommendations.
Transwell Migration and Invasion Assays
Cells were carried out various transfections in these assays as described above. The treated cells were plated at 20,000 cells each well into the top chamber of 24-well transwells with non-coated membrane (8 μm pore, BD Biosciences, Heidelberg, Germany) for migration assays or at 40,000 cells each well into Matrigel-coated chamber (BD Biosciences) for invasion assays. In both assays, RPMI-1640 medium containing 10% fetal bovine serum (Gibco) was added into the lower chamber as a chemoattractant. Twenty-four hours later, the penetrated cells through the pores of the membrane were stained and visualized under a microscope (Nikon, Tokyo, Japan) at 100× magnification.
Measurement of Glucose Uptake and Lactate Product
Glucose uptake and lactate product in transfected cells were tested using a colorimetric Glucose Uptake Assay Kit and Lactate Assay Kit (Abcam, Cambridge, UK) based on the guidance of manufacturers.
Western Blot
Clinical specimens and cells were homogenized in RIPA buffer (Life Technologies) as per the protocols of manufacturers. Fifty micrograms of protein was subjected to a standard Western blot analysis9 using SDS-polyacrylamide gels and nitrocellulose membranes (Millipore, Molsheim, France). The membranes binding sample protein were incubated with primary antibodies against matrix metalloprotease 9 (MMP9, 1:1000, Sigma-Aldrich, Tokyo, Japan), MMP2 (1:2000, Abcam), hexokinase 2 (HK2, 1:1000, Sigma-Aldrich), ADAM10 (1:1000, Abcam), GAPDH (1:2000, Sigma-Aldrich), and anti-rabbit or anti-mouse IgG secondary antibody (1:5000, Abcam). The immune complexes were visualized by chemiluminescence (GE Healthcare, Waukesha, WI, USA).
Animal Studies
All procedures were performed according to the Academia Sinica IACUC and Council of Agriculture Guidebook for the Care and Use of Laboratory Animal, and this study was approved by the Ethics Committee on Use and Care of Animal at SSL Central Hospital of Dongguan City. Twelve 6-week-old male BALB/c nude mice (Guangdong Research Center of Laboratory Animal, Foshan, China) were used for the xenograft model assays. sh-NC or sh-circ#1-transducing A549 cells were subcutaneously inoculated into the left flank of nude mice at 5 × 106 cells per mouse in 100 μL PBS (n = 6 per group). Tumor volume was measured every week after 7 days implantation and calculated by using the formula: volume = length × width2 × 0.5. At the end of the animal experiments, all mice were sacrificed and xenograft tumors were excised for weight.
Dual-Luciferase Reporter Assay
The directly targeted miRNAs of circNFIX and miR-212-3p molecular targets were predicted by starBase v.2 bioinformatic tool (http://starbase.sysu.edu.cn/). circNFIX wild-type luciferase reporter (circNFIX-WT) and ADAM10 3ʹUTR reporter (ADAM10-3ʹUTR-WT) containing the miR-212-3p-binding sequence, and the mutant reporters with miss-matched miR-212-3p-binding sites (circNFIX-MUT and ADAM10-3ʹUTR-MUT) were constructed by Uptbio (Changsha, China). Cells of 60% confluence were cotransfected with 100 ng of circNFIX-WT, circNFIX-MUT, ADAM10-3ʹUTR-WT or ADAM10-3ʹUTR-MUT and either 30 nM of miR-212-3p mimic or miR-NC mimic. Cell lysates were prepared at 24 h post-transfection, and the ratio of firefly to Renilla luciferase was detected with Dual-luciferase Assay System as recommended by the manufacturers (Promega, Madison, WI, USA).
RNA Pulldown Assays
Cell extracts were prepared using RIPA buffer and then incubated with the biotin-labeled miR-212-3p mimic (Bio-miR-212-3p, Hanbio) or control oligonucleotide (Bio-NC, Hanbio) for 2 h at 4°C, before adding to the streptavidin beads (Sigma-Aldrich) for 2 h. RNA complexes were harvested, and total RNA was isolated, followed by the determination of circNFIX level.
Statistical Analysis
Statistical analyses for all data were performed by a two-sided Student’s t-test, Mann–Whitney U-test or analysis of variance (ANOVA). The correlation between miR-212-3p and circNFIX expression levels in tumor tissues was analyzed using Spearman rank correlation coefficients. The Kaplan–Meier survival curve and Log rank test were used to analyze the overall survival of NSCLC patients. Error bars represented standard deviation (SD), except where indicated otherwise. The P values were *P < 0.05, **P <0.01 and ***P < 0.001.
Results
Up-Regulation of CircNFIX Predicted Poor Prognosis of Patients with NSCLC
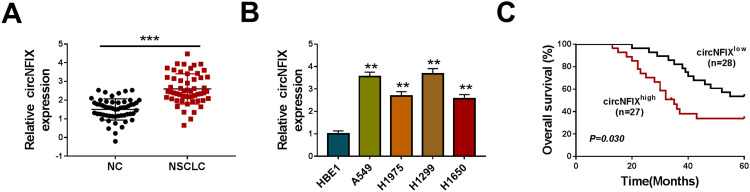
For preliminary observation for the involvement of circNFIX in NSCLC progression, we first determined its expression level in NSCLC tissues and cell lines. As demonstrated by qRT-PCR, in comparison to their counterparts, circNFIX was prominently overexpressed in NSCLC tissues and cells (Figure 1A and B). To examine whether circNFIX was associated with NSCLC prognosis, these patients were divided into high circNFIX level group and low circNFIX level group according to the median of circNFIX expression. Kaplan–Meier survival curves revealed that the patients in low circNFIX level group had a longer survival time that those in high circNFIX level group (Figure 1C).
Figure 1.
CircNFIX was overexpressed in NSCLC and predicted poor prognosis of NSCLC patients. CircNFIX expression by qRT-PCR in 55 pairs of NSCLC tissues and adjacent non-tumor tissues (A), HBE1, A549, H1299, H1975 and H1650 cells (B). (C) Analysis for the overall survival of NSCLC patients in high (n = 27) or low (n = 28) circNFIX level group using Kaplan–Meier survival analysis and Log rank test.
Notes: **P < 0.01 or ***P < 0.001
Knockdown of CircNFIX Repressed NSCLC Cell Viability, Migration, Invasion and Glycolysis in vitro and Attenuated Tumor Growth in vivo
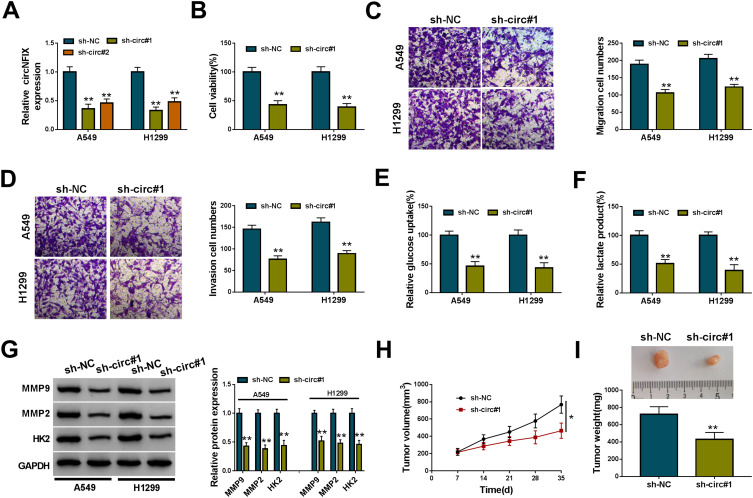
To identify the function of circNFIX in NSCLC malignant progression, we then performed in vitro loss-of-function analyses by silencing circNFIX with shRNA targeting circNFIX (sh-circ#1 and sh-circ#2). Transduction of sh-circ#1/#2, but not the negative sh-NC control, significantly down-regulated the expression of circNFIX in both A549 and H1299 cells (Figure 2A). Because sh-circ#1 transduction caused the more significant reduction, we selected it for further investigations. In both cell lines, the data of CCK-8 and transwell assays showed that in contrast to the control group, circNFIX knockdown strikingly suppressed cell viability (Figure 2B), migration (Figure 2C) and invasion (Figure 2D). Moreover, the depletion of circNFIX reduced glucose uptake (Figure 2E) and lactate product (Figure 2F), indicating the inhibition of circNFIX knockdown on cell glycolysis. Additionally, Western blot data revealed that circNFIX depletion triggered a prominent reduction in MMP9, MMP2 and HK2 levels in the two NSCLC cells (Figure 2G).
Figure 2.
CircNFIX knockdown repressed NSCLC malignant progression in vitro and in vivo. (A) CircNFIX expression by qRT-PCR in A549 and H1299 cells transduced with sh-NC, sh-circ#1 or sh-circ#2. Cell viability by CCK-8 assay (B), cell migration and invasion by transwell assay (C and D), glucose uptake and lactate product using the assay kits (E and F), the levels of MMP9, MMP2 and HK2 by Western blot (G) in sh-NC- or sh-circ#1-transduced A549 and H1299 cells. (H and I) sh-circ#1-transduced or sh-NC-infected A549 cells were subcutaneously implanted into nude mice (n = 6 per group), and tumor volume was measured every week after 7 days implantation, tumor image was photographed and tumor weight was gauged at the end of the animal experiments.
Notes: *P < 0.05 or **P < 0.01
We also asked whether circNFIX could influence tumor growth in vivo. To address this possibility, we implanted the sh-circ#1-transduced or sh-NC-infected A549 cells into the nude mice. In comparison to the negative control, the knockdown of circNFIX significantly weakened tumor growth in vivo, as presented by the decreased tumor volume and weight (Figure 2H and I).
CircNFIX Acted as a Molecular Sponge of miR-212-3p
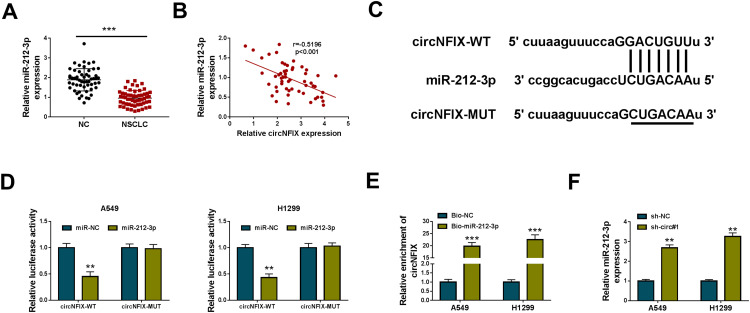
In NSCLC tissues, miR-212-3p expression was significantly reduced when comparing to the normal tissues (Figure 3A), and it was inversely correlated with circNFIX level (Figure 3B). Of interest, using starBase v.2 software, the predicted data revealed that circNFIX harbored seven nucleotides (GACUGUU) that are complementary to miR-212-3p (Figure 3C). When we performed dual-luciferase reporter assays, the cotransfection of circNFIX wild-type luciferase reporter (circNFIX-WT) and miR-212-3p mimic into the two NSCLC cells generated a lower luciferase activity than in cells cotransfected with miR-NC mimic (Figure 3D). However, the mutant of the seed region (circNFIX-MUT) remarkably abolished the repressive effect of miR-212-3p (Figure 3D). RNA pulldown analyses showed that in contrast to the Bio-NC control, circNFIX enrichment level was significantly elevated by Bio-miR-212-3p (Figure 3E). Furthermore, miR-212-3p expression was prominently increased by circNFIX silencing in the two NSCLC cells (Figure 3F).
Figure 3.
CircNFIX acted as a sponge of miR-212-3p. (A) MiR-212-3p expression by qRT-PCR in 55 pairs of NSCLC tissues and adjacent non-tumor tissues. (B) The correlation between miR-212-3p expression and circNFIX level in NSCLC tissues using the Spearman test. (C) Schematic of the complementary sequence for miR-212-3p within circNFIX identified by starBase v.2 software and the mutation of the seed region. (D) Relative luciferase activity in A549 and H1299 cells cotransfected with circNFIX-WT or circNFIX-MUT and miR-212-3p mimic or miR-NC mimic. (E) The enrichment level of circNFIX by Bio-miR-212-3p or Bio-NC in both A549 and H1299 cells. (F) MiR-212-3p expression by qRT-PCR in A549 and H1299 cells transduced with sh-NC, sh-circ#1.
Notes: **P < 0.01 or ***P < 0.001
CircNFIX Knockdown Repressed NSCLC Cell Viability, Migration, Invasion and Glycolysis in vitro by miR-212-3p
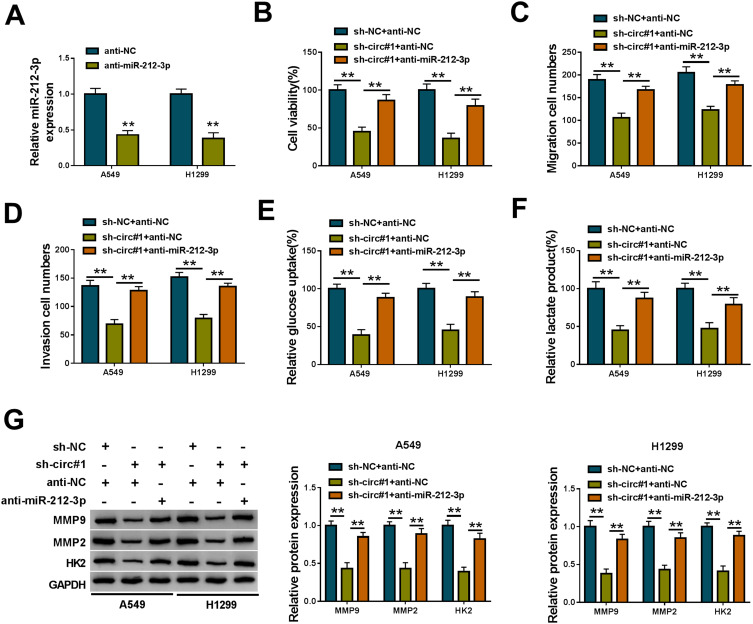
To examine whether miR-212-3p was involved in the regulation of circNFIX on NSCLC progression, we transfected anti-miR-212-3p into the sh-circ#1-transduced NSCLC cells. In comparison to the control group, anti-miR-212-3p introduction led to a significant decrease in miR-212-3p expression (Figure 4A). Functional analyses revealed that the down-regulation of miR-212-3p dramatically abolished the repression of circNFIX knockdown on cell viability (Figure 4B), migration (Figure 4C), invasion (Figure 4D) and glycolysis (Figure 4E and F). Moreover, the decreased level of miR-212-3p significantly abrogated the reduction of circNFIX knockdown on MMP9, MMP2 and HK2 levels (Figure 4G).
Figure 4.
CircNFIX knockdown repressed NSCLC cell malignant progression in vitro by miR-212-3p. (A) MiR-212-3p expression by qRT-PCR in A549 and H1299 cells transfected with anti-NC or anti-miR-212-3p. sh-NC- or sh-circ#1-transduced A549 and H1299 cells were transfected with anti-NC or anti-miR-212-3p, followed by the assessment of cell viability by CCK-8 assay (B), cell migration and invasion by transwell assay (C and D), glucose uptake and lactate product using the assay kits (E and F), MMP9, MMP2 and HK2 levels by Western blot (G).
Note: **P < 0.01
CircNFIX Regulated ADAM10 Expression by Acting as a Sponge of miR-212-3p
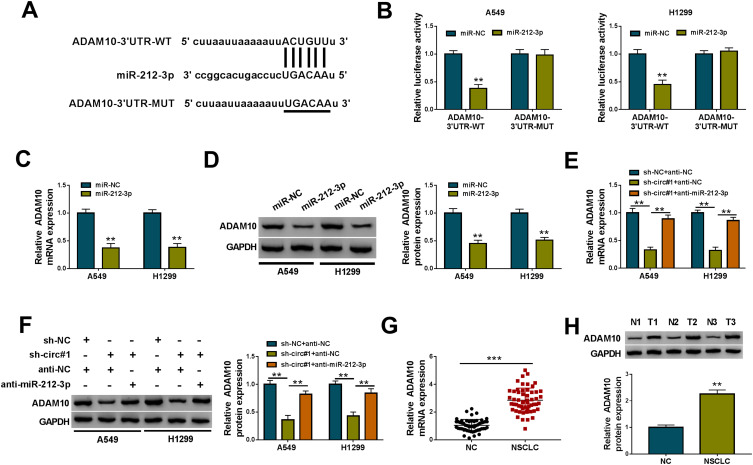
Using starBase v.2 software, a putative target sequence for miR-212-3p was found within the 3ʹUTR of ADAM10 mRNA (Figure 5A). Transfection of ADAM10 3ʹUTR reporter construct (ADAM10-3ʹUTR-WT) in the presence of miR-212-3p mimic led to a remarkable reduction in luciferase activity, and this effect was completely abolished by the mutation of the target sequence (ADAM10-3ʹUTR-MUT, Figure 5B). Moreover, ADAM10 mRNA and protein levels were notably down-regulated by the elevated expression of miR-212-3p in the two cells (Figure 5C and D).
Figure 5.
CircNFIX regulated ADAM10 expression by acting as a sponge of miR-212-3p. (A) Schematic of the putative target sequence for miR-212-3p within the 3ʹUTR of ADAM10 mRNA and the mutation of the target sequence. (B) Luciferase assays in A549 and H1299 cells transfected with ADAM10-3ʹUTR-WT or ADAM10-3ʹUTR-MUT, together with miR-212-3p mimic or miR-NC mimic. ADAM10 mRNA and protein levels by qRT-PCR and Western blot in A549 and H1299 cells transfected with miR-NC mimic or miR-212-3p mimic (C and D), sh-NC- or sh-circ#1-transduced A549 and H1299 cells transfected with anti-NC or anti-miR-212-3p (E and F), NSCLC tissues and adjacent non-tumor tissues (G and H).
Notes: **P < 0.01 or ***P < 0.001
We next determined whether circNFIX influenced ADAM10 expression. As expected, in comparison to their counterparts, ADAM10 mRNA and protein levels were significantly decreased by circNFIX knockdown in the two cells, and these effects were dramatically reversed by anti-miR-212-3p transfection (Figure 5E and F). Additionally, ADAM10 mRNA and protein levels were strikingly up-regulated in NSCLC tissues (Figure 5G and H).
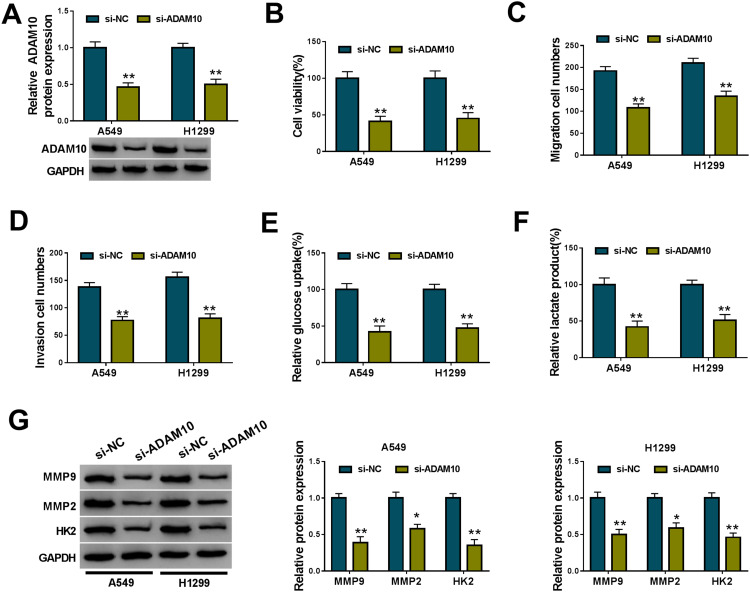
Knockdown of ADAM10 Hindered NSCLC Cell Viability, Migration, Invasion and Glycolysis in vitro
A crucial question was how ADAM10 regulated NSCLC cell malignant progression in vitro. To test this, we reduced the expression of ADAM10 using si-ADAM10. The data of Western blot showed that ADAM10 protein expression was strikingly reduced by si-ADAM10 (Figure 6A). In contrast to the negative group, the silencing of ADAM10 led to a remarkable suppression in cell viability (Figure 6B), migration (Figure 6C) and invasion (Figure 6D), as well as glycolysis (Figure 6E and F) in both A549 and H1299 cells. Furthermore, ADAM10 knockdown resulted in decreased MMP9, MMP2 and HK2 levels in the two cells when comparing to the control group (Figure 6G).
Figure 6.
ADAM10 knockdown hampered NSCLC cell malignant progression in vitro. A549 and H1299 cells were transiently transfected with si-NC or si-ADAM10. (A) ADAM10 expression by Western blot in transfected cells. (B) CCK-8 assay for cell viability. (C and D) Transwell assay for cell migration and invasion. (E and F) Glucose uptake and lactate product using the assay kits. (G) Western blot for MMP9, MMP2 and HK2 levels in transfected cells.
Notes: *P < 0.05 or **P < 0.01
Discussion
Despite decades of researches, effective therapies against NSCLC are still limited.22 Understanding the mechanisms of NSCLC pathogenesis and identifying novel therapeutic targets have been still challenging. Emerging evidence is pointing towards circRNAs as vital regulators in cancer biology.23,24 However, the mechanistic characterization of circRNAs in NSCLC pathogenesis remains rather poor. Here, we sought to identify the precise roles of circNFIX, an oncogene in glioma,13–15 in NSCLC malignant progression.
Here, we first uncovered a prominent up-regulation of circNFIX in NSCLC and the elevated expression of circNFIX predicted poor prognosis of these patients, demonstrating the important involvement of circNFIX in NSCLC pathogenesis. MMP9 and MMP2 are closely associated with tumor cell migration and invasion.25,26 Enhanced glycolysis, known as the “Warburg effect”, is a hallmark of cancer and the suppression of glycolysis is recognized as an anti-tumor approach.27 HK2 is a crucial catalyzing enzyme during glycolytic process in glycolysis.28 Our results first demonstrated that circNFIX knockdown weakened NSCLC cell viability, migration, invasion and glycolysis in vitro and hampered tumor growth in vivo.
CircRNAs can efficiently inhibit the activity of miRNAs via functioning as a molecular sponge.29 Our data first identified that circNFIX sequestered miR-212-3p by sponging miR-212-3p. Among these predicted miRNAs that potentially pair to circNFIX, miR-212-3p was a strong candidate in the current work owing to its anti-tumor role in a variety of human malignancies, such as ovarian cancer, glioma and osteosarcoma.30–32 Moreover, miR-212-3p restrained the tumorigenesis of gastric cancer and bladder cancer by inhibiting the expression of a target, such as SRY-box transcription factor 6 (Sox6) and nuclear factor IA (NFIA).33,34 A previous document demonstrated the tumor suppressive impact of miR-212-3p in NSCLC malignant progression.20 Here, we were first to uncover that miR-212-3p was a molecular mediator of circNFIX in regulating NSCLC cell malignant progression, arguing for the “sponge” function of circNFIX, as has been reported.13,15
ADAM10, a frequently overexpressed protein in human tumors, contributes to the malignant progression of a plenty of cancers, including pleural mesothelioma, colorectal cancer and breast cancer.35–37 The inhibition of ADAM10 is considered as a potential therapeutic method for cancer.38 Moreover, the overexpression of ADAM10 was discovered in NSCLC, and ADAM10 down-regulation suppressed NSCLC cell metastasis.39 Besides, ADAM10 was reported as a promising biomarker for NSCLC diagnosis and prognosis.40 Our findings first showed that in NSCLC cells, ADAM10 was a direct target of miR-212-3p and circNFIX influenced ADAM10 expression by sponging miR-212-3p. We also unraveled the suppressive impact of ADAM10 knockdown on NSCLC cell malignant progression, as previously reported.39 Meng et al underscored that miR-449a directly targeted ADAM10 to weaken the development of NSCLC.41 However, the direct evidence of the relationship between the circNFIX/miR-212-3p axis and ADAM10 on NSCLC malignant progression was lacked in present, which will be carried out in further work.
In conclusion, our present study identified that the knockdown of circNFIX, a highly expressed circRNA in NSCLC, repressed NSCLC malignant progression through the regulation of the miR-212-3p/ADAM10 axis. To our knowledge, this is the first report of circNFIX on NSCLC pathogenesis, providing a novel understanding of circRNA regulation in NSCLC.
Funding Statement
This work was supported by the Medical Scientific Research Foundation of Guangdong Province, China (No.B2017033) and Scientific Research Project of Traditional Chinese Medicine Bureau of Guangdong Province, China (No.20171273; No.20201368).
Disclosure of Interest
The authors declare that they have no financial or non-financial conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Skřičková J, Kadlec B, Venclíček O. [Non-small cell lung cancer]. Vnitr Lek. 2018;63(11):861–874. Czech. [PubMed] [Google Scholar]
- 3.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Ying S, Hu S, et al. EGFR TKIs impair lysosome-dependent degradation of SQSTM1 to compromise the effectiveness in lung cancer. Signal Transduct Target Ther. 2019;4(1):25. doi: 10.1038/s41392-019-0059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan M, Huang LL, Chen JH, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4(1):61. doi: 10.1038/s41392-019-0099-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer JW, Leung AK. CircRNAs: a regulator of cellular stress. Crit Rev Biochem Mol Biol. 2017;52(2):220–233. doi: 10.1080/10409238.2016.1276882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen LS, Hansen TB, Venø MT, et al. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–565. doi: 10.1038/onc.2017.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan S, Sun D, Pu W, et al. Circular RNA F-circEA-2a derived from EML4-ALK fusion gene promotes cell migration and invasion in non-small cell lung cancer. Mol Cancer. 2018;17(1):138. doi: 10.1186/s12943-018-0887-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang MM, Mai ZT, Wan SZ, et al. Microarray profiles reveal that circular RNA hsa_circ_0007385 functions as an oncogene in non-small cell lung cancer tumorigenesis. J Cancer Res Clin Oncol. 2018;144(4):667–674. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Nan A, Zhang N, et al. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol Cancer. 2019;18(1):13. doi: 10.1186/s12943-019-0943-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Wang X, Hu B, et al. Circular RNA ZFR accelerates non-small cell lung cancer progression by acting as a miR-101-3p sponge to enhance CUL4B expression. Artif Cells Nanomed Biotechnol. 2019;47(1):3410–3416. doi: 10.1080/21691401.2019.1652623 [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Li W, Liu G, et al. A novel circular RNA, hsa_circ_0043278, acts as a potential biomarker and promotes non-small cell lung cancer cell proliferation and migration by regulating miR-520f. Artif Cells Nanomed Biotechnol. 2019;47(1):810–821. doi: 10.1080/21691401.2019.1575847 [DOI] [PubMed] [Google Scholar]
- 13.Ding C, Wu Z, You H, et al. CircNFIX promotes progression of glioma through regulating miR-378e/RPN2 axis. J Exp Clin Cancer Res. 2019;38(1):506. doi: 10.1186/s13046-019-1483-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Zhang Y, Qi L, et al. NFIX circular RNA promotes glioma progression by regulating miR-34a-5p via notch signaling pathway. Front Mol Neurosci. 2018;11(225). doi: 10.3389/fnmol.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding C, Yi X, Wu X, et al. Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett. 2020;479(1):1–12. doi: 10.1016/j.canlet.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Babu N, Advani J, Solanki HS, et al. miRNA and proteomic dysregulation in non-small cell lung cancer in response to cigarette smoke. Microrna. 2018;7(1):38–53. doi: 10.2174/2211536607666180103165343 [DOI] [PubMed] [Google Scholar]
- 17.Leonetti A, Assaraf YG, Veltsista PD, et al. MicroRNAs as a drug resistance mechanism to targeted therapies in EGFR-mutated NSCLC: current implications and future directions. Drug Resist Updat. 2019;42(1):1–11. doi: 10.1016/j.drup.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Min L, Ren C, et al. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS One. 2017;12(2):e0171751. doi: 10.1371/journal.pone.0171751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Yan Y, Liu Y, et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin α2β1. Signal Transduct Target Ther. 2020;5(1):39. doi: 10.1038/s41392-020-0133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu HB, Yang K, Yu HY, et al. Downregulation of long non-coding RNA XIST inhibits cell proliferation, migration, invasion and EMT by regulating miR-212-3p/CBLL1 axis in non-small cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2019;23(19):8391–8402. [DOI] [PubMed] [Google Scholar]
- 21.Rivetti Di Val Cervo P, Lena AM, Nicoloso M, et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109(4):1133–1138. doi: 10.1073/pnas.1112257109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 23.Vo JN, Cieslik M, Zhang Y, et al. The landscape of circular RNA in cancer. Cell. 2019;176(4):869–881.e813. doi: 10.1016/j.cell.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HD, Jiang LH, Sun DW, et al. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JF, Wang P, Yan YJ, et al. IL‑33 enhances glioma cell migration and invasion by upregulation of MMP2 and MMP9 via the ST2-NF-κB pathway. Oncol Rep. 2017;38(4):2033–2042. doi: 10.3892/or.2017.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun XF, Shao YB, Liu MG, et al. High-concentration glucose enhances invasion in invasive ductal breast carcinoma by promoting Glut1/MMP2/MMP9 axis expression. Oncol Lett. 2017;13(5):2989–2995. doi: 10.3892/ol.2017.5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akram M. Mini-review on glycolysis and cancer. J Cancer Educ. 2013;28(3):454–457. doi: 10.1007/s13187-013-0486-9 [DOI] [PubMed] [Google Scholar]
- 28.Jiao L, Zhang HL, Li DD, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2). Autophagy. 2018;14(4):671–684. doi: 10.1080/15548627.2017.1381804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verduci L, Strano S, Yarden Y, et al. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13(4):669–680. doi: 10.1002/1878-0261.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Zhang Y, Wang S, et al. MiR-212-3p suppresses high-grade serous ovarian cancer progression by directly targeting MAP3K3. Am J Transl Res. 2020;12(3):875–888. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Zheng J, Liu X, et al. Knockdown of USF1 inhibits the vasculogenic mimicry of glioma cells via stimulating SNHG16/miR-212-3p and linc00667/miR-429 axis. Mol Ther Nucleic Acids. 2019;14(465):465–482. doi: 10.1016/j.omtn.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Oh JY, Kim EH, Lee YJ. Synergistic autophagy effect of miR-212-3p in zoledronic acid-treated in vitro and orthotopic in vivo models and in patient-derived osteosarcoma cells. Cancers (Basel). 2019;11(11):1812. doi: 10.3390/cancers11111812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang Y, Liu T, Yan J, et al. Gastric cancer proliferation and invasion is reduced by macrocalyxin C via activation of the miR-212-3p/Sox6 pathway. Cell Signal. 2020;66:109430. doi: 10.1016/j.cellsig.2019.109430 [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Chen H, Zhang G, et al. MiR-212-3p inhibits cell proliferation and promotes apoptosis by targeting nuclear factor IA in bladder cancer. J Biosci. 2019;44(4). doi: 10.1007/s12038-019-9903-5. [DOI] [PubMed] [Google Scholar]
- 35.Sépult C, Bellefroid M, Rocks N, et al. ADAM10 mediates malignant pleural mesothelioma invasiveness. Oncogene. 2019;38(18):3521–3534. doi: 10.1038/s41388-018-0669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkiewicz K, Nowakowska-Zajdel E, Strzelczyk J, et al. Serum levels of ADAM10, ADAM12, ADAM17 AND ADAM28 in colorectal cancer patients. J Biol Regul Homeost Agents. 2017;31(4):929–934. [PubMed] [Google Scholar]
- 37.Tsang JYS, Lee MA, Chan TH, et al. Proteolytic cleavage of amyloid precursor protein by ADAM10 mediates proliferation and migration in breast cancer. EBioMedicine. 2018;38(89):89–99. doi: 10.1016/j.ebiom.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith TM, Tharakan A, Martin RK. Targeting ADAM10 in cancer and autoimmunity. Front Immunol. 2020;11(499). doi: 10.3389/fimmu.2020.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, He L, Yuan P, et al. ADAM10 overexpression in human non-small cell lung cancer correlates with cell migration and invasion through the activation of the Notch1 signaling pathway. Oncol Rep. 2012;28(5):1709–1718. [DOI] [PubMed] [Google Scholar]
- 40.Yoneyama T, Gorry M, Sobo-Vujanovic A, et al. ADAM10 sheddase activity is a potential lung-cancer biomarker. J Cancer. 2018;9(14):2559–2570. doi: 10.7150/jca.24601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng H, Huang Q, Zhang X, et al. MiR-449a regulates the cell migration and invasion of human non-small cell lung carcinoma by targeting ADAM10. Onco Targets Ther. 2019;12(3829):3829–3838. doi: 10.2147/OTT.S190282 [DOI] [PMC free article] [PubMed] [Google Scholar]