Abstract
Background
The teratogenic risks of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are well documented, but prescribing these in younger women in primary care is becoming increasingly frequent.
Aim
To record how frequently women of childbearing age, who are prescribed an ACE inhibitor or ARB, receive preconception advice and/or are prescribed contraception, and how many pregnancies, terminations, and miscarriages occur in this population. Additionally, to ascertain whether patterns in the above differ across age groups.
Design and setting
Cross-sectional study conducted among patients from 141 general practices in East London.
Method
Women aged 15–45 years who were issued a prescription for an ACE inhibitor or ARB between 1 October 2018 and 1 January 2019 inclusive were included. An electronic search strategy was designed to extract pseudonymised data concerning preconception and contraception advice, contraception, and pregnancies from the electronic clinical system; this was applied to the selected cohort on 1 January 2019. Data were analysed in 5-year age groups.
Results
Of 302 939 women aged 15–45 years, 2651 (0.9%) were prescribed an ACE inhibitor or an ARB in a 3-month period. Of these, 2159 (81.4%) had no advice and no contraception prescription recorded, 35 (1.3%) had preconception advice recorded, and 230 (8.7%) had contraception advice recorded. A total of 100 pregnancies and 21 terminations/miscarriages were recorded in the 12 months preceding the index date (1 January 2019).
Conclusion
This study found that the recording of pre-pregnancy advice and contraception in women of childbearing age who were prescribed an ACE inhibitor or an ARB was suboptimal; this may place women and their babies at risk of exposure to teratogens during pregnancy. The findings indicate that there is a need for improved safety strategies based in primary care.
Keywords: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, contraception, female, pregnancy, primary health care
INTRODUCTION
The global obesity epidemic has caused increasing prevalence of diabetes at younger ages and a sixth of people with hypertension are now aged <45 years.1–4 The National Institute for Health and Care Excellence (NICE) and the British and Irish Hypertension Society (BIHS) currently recommend angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) as first-line management for patients aged <55 years with hypertension.5,6 These medicines are also frequently prescribed for diabetes.7
ACE inhibitors/ARBs are known to be teratogenic and some studies suggest congenital anomalies can occur with exposure in the first trimester only.8–11 With increasing rates of hypertension and diabetes at younger ages, the prescription of ACE inhibitors/ARBs in women of childbearing age is becoming more common; as such, inadvertent use of ACE inhibitors/ARBs during a pregnancy is increasing.8,12,13 It has been reported that 16% of pregnancies in the UK are unplanned.14 Most pregnancies are not recognised until at least halfway through the first trimester, when deleterious effects may already have occurred from ACE inhibitor/ARB exposure.8,9,12,13,15
Indeed, delays in pregnancy confirmation result in ACE inhibitors or ARBs frequently being continued during pregnancy.12,13 Consequently, preventing inappropriate ACE inhibitor/ARB use during a pregnancy has to be an important current consideration, and become a key future consideration, in primary care.5,6
Guideline and regulator advice
The Medicines and Healthcare products Regulatory Agency (MHRA) and NICE advise that clinicians should inform women who are prescribed ACE inhibitors/ARBs of the teratogenic risks of these medicines.5,6,16 UK and US guidelines also advise that, if a woman with hypertension is planning a pregnancy, clinicians should discuss alternative antihypertensive medications prior to conception and, if a pregnancy occurs, the ACE inhibitor/ARB should be stopped or switched immediately.5,6,16,17 This contrasts with national guidelines from other countries, which recommend that these medicines be avoided in women of childbearing age with hypertension or only co-prescribed with contraception.18–24 Consequently, there appears to be little global consensus concerning the prescription of ACE inhibitors/ARBs in women of childbearing age.
Guideline compliance
A 2008 study found that nearly half of women referred to a specialist hypertension clinic were prescribed an ACE inhibitor/ARB; 70% of these were using non-permanent contraception, no contraception, or their contraception status was unknown.25 In a US study, only 11.7% of women of childbearing age with hypertension, hypercholesterolaemia, or diabetes had evidence of giving informed consent to be prescribed an ACE inhibitor, ARB, or statin, having been informed of the risk of adverse fetal effects.26
How this fits in
| Exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers during pregnancy is associated with teratogenic risk to the fetus. This study shows that the recording of pre-pregnancy advice and contraception in women of childbearing age, who are prescribed such medicines, is suboptimal and a number of pregnancies occur in this population. As this may place women and their babies at risk of exposure to teratogens, there is a need for significant improvements in the care of these patients. |
Despite exposure concerns, there have been no studies, of which the authors are aware, to assess guideline compliance in the UK including rates of pre-pregnancy advice and contraception prescriptions or the, possibly associated, pregnancy or miscarriage rate.
The aim of this study was to fill this knowledge deficit, in the context of general practice, because in the UK it is the source of most ACE inhibitor/ARB and contraception prescriptions and, often, the most appropriate environment in which to offer pre-pregnancy advice.
The study aimed to ascertain:
how many women of childbearing age are prescribed an ACE inhibitor or ARB for any indication, across three clinical commissioning groups (CCGs);
how many of these women are given preconception or contraceptive advice, or are prescribed contraception;
how many pregnancies and terminations/miscarriages occur in this population in association with ACE inhibitor/ARB prescriptions; and
whether patterns in the above rates differ across age groups.
METHOD
Setting
This cross-sectional study included patients from three adjacent urban CCGs in East London: NHS City & Hackney CCG, NHS Newham CCG, and NHS Tower Hamlets CCG. These are among the most deprived and ethnically diverse CCGs in both London and the UK, and are served by 141 GP practices.
Data collection
All 141 GP practices included in the study use EMIS Web, an electronic, integrated clinical IT system. Clinical information and all GP-issued prescriptions are coded and recorded by the healthcare provider, using the Read code system. Read codes are a coded thesaurus of clinical words and phrases that enable information to be coded and searched for in a standard manner across primary and secondary healthcare systems in the UK.27 De-identified Read-coded information was centrally extracted from GPs’ electronic clinical systems and no other healthcare systems were accessed. Free-text information was not available. Data were accessed, encrypted, and securely stored by the Clinical Effectiveness Group (CEG) at Queen Mary University of London. Pawa et al have provided a detailed description of the types of databases held by the CEG, and its role in facilitating information sharing between CCGs and practices.28
Study population
Female patients aged 15–45 years were selected from all patients registered at the 141 practices. A British National Formulary (BNF) code pertaining to an ACE inhibitor/ARB prescription within 3 months prior to the index date (that is, from 1 October 2018 until the index date of 1 January 2019) was searched in the electronic medical record (EMR) for each selected patient. Women who had a recorded ACE inhibitor/ARB prescription within this time frame were selected for inclusion in the study; of those, women with a recorded Read code for a previous hysterectomy or sterilisation procedure were then excluded. The Read codes used to identify patients for exclusion are detailed in Supplementary Table S1.
Read codes for the information elements listed below (see Supplementary Tables S2–S4) were then searched for within a range of time frames, and extracted from the EMR of each patient in the study cohort on the index date (1 January 2019):
ethnicity: most recent code;
comorbidities, including hypertension, diabetes, heart failure, and chronic kidney disease (CKD): most recent code;
ACE inhibitor/ARB name and dose: most recent code;
preconception advice: coded in the 12 months preceding the index date;
contraception advice: coded in the 12 months preceding the index date;
oral contraception, transdermal contraception, or long-acting reversible contraception (LARC): code searched for within various time frames, ranging from a few months to several years (see Supplementary Tables S3 and S4);
pregnancy: coded in the 12 months preceding the index date; and
termination/miscarriage: coded in the 12 months preceding the index date.
Each included patient was allocated a pseudonymised identifier. The Read codes present were recorded against each pseudonymised identifier. There were no updates to the database during the data analysis.
Sex, age, and any prescribed medications are recorded automatically and consistently in the patient’s medical record in the EMIS Web system. Data may be missing for aspects of a patient’s health care, if that care was accessed outside of the primary care setting (for example, in sexual-health centres) or if the data were recorded only as a free-text entry and not coded. This is acknowledged as a common problem in the use of primary care datasets.29
Data analysis
Data were analysed using Microsoft Excel for Mac (version 16.24). Univariate descriptive statistics were used along with 5-year age groups. Averages are presented as the mean, unless otherwise stated.
RESULTS
Study sample
Table 1 outlines the age and ethnicity of the study population. See Supplementary Table S5 for absolute numbers of women in each age group.
Table 1.
Age and ethnicity of the study population (N = 2651)
| Characteristic | n (%) |
|---|---|
| Age groupa | |
| 15–19 years | 19 (0.7) |
| 20–24 years | 34 (1.3) |
| 25–29 years | 93 (3.5) |
| 30–34 years | 238 (9.0) |
| 35–39 years | 626 (23.6) |
| 40–45 years | 1641 (61.9) |
|
| |
| Ethnicityb | |
| Asian/Asian British | 1641 (61.9) |
| White/other white | 342 (12.9) |
| Black African/black Caribbean/black British | 334 (12.6) |
Range: 15–45 years; mean age: 39.6 years.
Small numbers of women listed under various ethnicities, or women with no recorded ethnicity, made up the remaining 12.6% (n = 334) of the study population’s listed ethnicities.
Population comorbidities and ACE inhibitor/ARB prescriptions
Among the 2651 women included in the study, 2054 (77.5%) had a recorded diagnosis of hypertension, 1000 (37.7%) had one for diabetes, 147 (5.5%) had one for CKD, and 57 (2.2%) had one for heart failure. Ramipril was prescribed to 1538 (58.0%) women, losartan to 470 (17.7%) women, and lisinopril to 212 (8.0%) women; 16.3% of women used others (data not shown).
Preconception and contraception advice, and methods of contraception
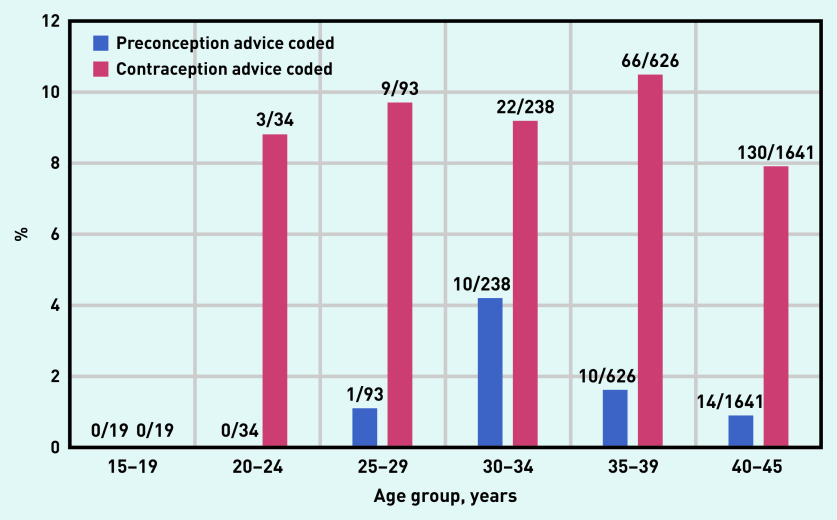
Figure 1 shows the percentage of women in each age group for whom preconception or contraception advice had been recorded. A Read code concerning preconception advice and contraception advice was recorded in the 12 months preceding the index date in 35 (1.3%) women and 230 (8.7%) women, respectively.
Figure 1.
Percentage of each age group for whom preconception or contraception advice was recorded during the 12 months preceding the index date.
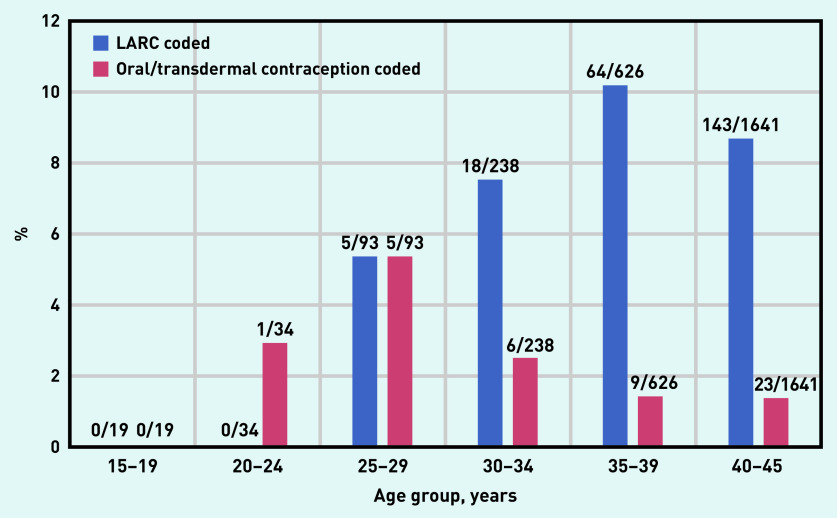
Figure 2 shows the percentage of the population, within each age group, that had a LARC or an oral/transdermal contraceptive recorded in the relevant time frames. In total, 230 (8.7%) women had a LARC prescription recorded in the relevant time frame; for these women, the following methods were used:
intrauterine device (n = 96, 41.7%);
intrauterine system (n = 65, 28.3%);
subcutaneous implant (n = 45, 19.6%); and
depot progestogen injection (n = 24, 10.4%).
Figure 2.
Percentage of each age group for whom a LARC or an oral/transdermal contraceptive was recorded in the relevant time frames. LARC = long-acting reversible contraception.
Of the total cohort of 2651 women, 44 (1.7%) had a prescription for an oral or transdermal contraceptive recorded in the 6 months preceding the index date; of these, 42 (95.5%) had the combined oral contraceptive pill recorded and two (4.5%) had a transdermal contraceptive recorded.
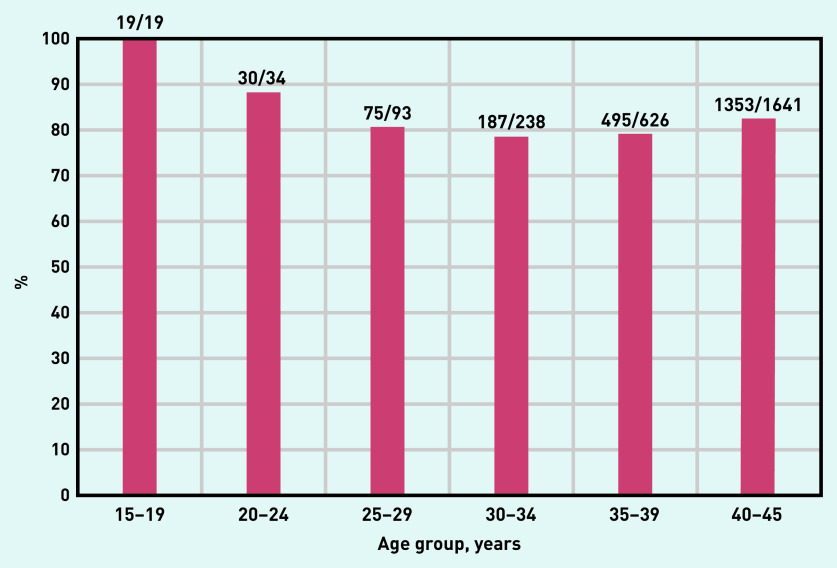
Figure 3 shows the percentage of women in each age group for whom no preconception advice and no contraception advice had been recorded in the 12 months preceding the index date, and no contraception had been recorded in the time frames outlined in Supplementary Tables S3 and S4.
Figure 3.
Percentage of each age group for whom no preconception advice, contraception advice, or contraception was recorded in the relevant time frames.
Pregnancy and termination of pregnancy
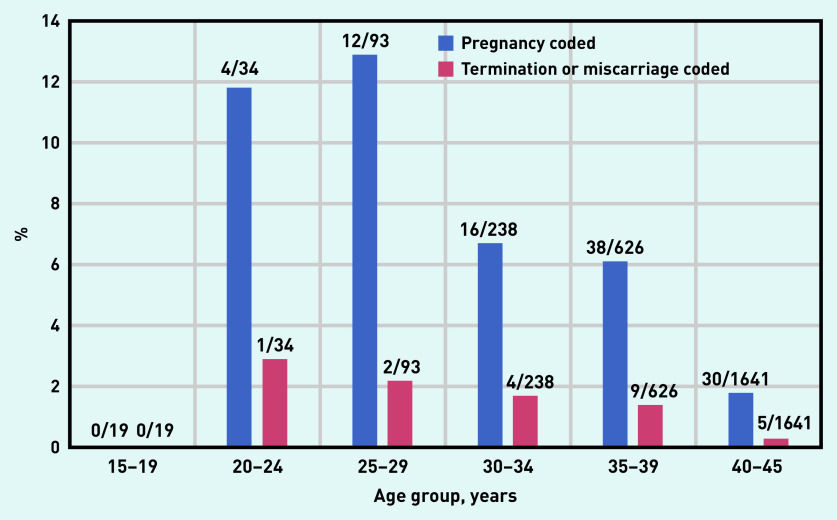
In total, 100 pregnancies and 21 terminations/miscarriages were recorded in the whole study population (n = 2651) during the 12 months preceding the index date (Table 2). Based on the Read codes, 58 pregnancies were ‘definite’ and 42 ‘inferred’ (Supplementary Table S6). Thirteen pregnancies and four terminations/miscarriages followed the most recent prescription of an ACE inhibitor or ARB (Table 2).
Table 2.
Recorded preconception advice, contraception advice, pregnancies, and terminations/miscarriages
| Population | Time frame | Recorded pregnancies, n (%) | Recorded terminations/miscarriages, n (%) |
|---|---|---|---|
| All women (n = 2651) | 12 months preceding the index date | 100 (3.8) | 21 (0.8) |
| All women (n = 2651) | Following most-recent ACE inhibitor/ARB prescription | 13 (0.5) | 4 (0.2) |
| Women with no recorded advice or contraception (n = 2159) | 12 months preceding the index date | 63 (2.9) | 16 (0.7) |
| Women with no recorded advice or contraception (n = 2159) | Following most recent ACE inhibitor/ARB prescription | 11 (0.5) | 3 (0.1) |
ACE = angiotensin-converting enzyme. ARB = angiotensin receptor blocker.
A total of 2159 (81.4%) of the entire study population had neither preconceptual or contraception advice recorded in the 12 months preceding the index date, nor any form of contraception recorded within the relevant time frames. Within these women, there were 63 recorded pregnancies and 16 recorded terminations/miscarriages. In those women for whom no advice or contraception was recorded, 11 pregnancies and three terminations/miscarriages were recorded, respectively, following the most-recent prescription for an ACE inhibitor or ARB.
Figure 4 shows the percentage of women in each age group for whom a pregnancy or a termination/miscarriage had been recorded in the 12 months preceding the index date.
Figure 4.
Percentage of each age group, for whom either a pregnancy or a termination/miscarriage was recorded in the 12 months preceding the index date.
DISCUSSION
Summary
Among 2651 women of childbearing age with a prescription for an ACE inhibitor or ARB, 81.4% had no recorded preconception or contraception advice in the 12 months preceding the index date and no recorded contraception prescription. Just 1.3% and 8.7% of the population had preconception and contraception advice recorded, respectively, in the 12 months preceding the index date and 8.7% and 1.7% had a prescription for a LARC or oral/transdermal contraceptive recorded, respectively, in the relevant time frames. This apparent lack of provision for delivering pre-pregnancy safety advice may increase the risk of a woman conceiving while prescribed an ACE inhibitor or ARB and, as such, also increase the risk of the fetus being exposed to teratogens.
Indeed, women without pregnancy-prevention interventions are likely to be most at risk of conceiving.30 A total of 100 pregnancies and 21 terminations/miscarriages were recorded during the 12 months examined, while 13% and 19% of these pregnancies and terminations/miscarriages, respectively, were recorded within 3 months of the woman receiving a prescription for an ACE inhibitor or ARB. As such, pregnancy exposure to ACE inhibitors or ARBs remains a current and important hazard to women and their unborn babies.
Strengths and limitations
This study provides a contemporary perspective on pregnancy preparation in women of childbearing age who have been prescribed an ACE inhibitor or ARB. Although the sample was drawn from a relatively large socially and ethnically diverse population without prior selection — thereby providing a real-world cohort — the population was drawn from areas of deprivation and was ethnically diverse, therefore the results may not be generalisable, especially to non-urban settings, and the study requires replication elsewhere. In spite of this, the patient demographics and comorbidities were comparable with those in existing literature.25,26
The use of the Read code system — a single, electronic, consistent coding system that allows for universal access to patients’ EMRs — is a significant strength. Furthermore, all prescribed medicines are electronically prescribed by GPs; therefore, the data presented is an accurate representation of prescribing. However, a small number of patients may choose not to have their medicine dispensed by the pharmacy — the authors were unable to account for these patients in this study. GPs may not always code the advice they give, and some women may access care elsewhere; as a result, the findings presented here may underestimate the advice offered about potential pregnancy/pregnancy risks, whether planned or not.
Indeed, the results highlight a key medicolegal issue: if advice is not being recorded or coded correctly, should an adverse event occur, the clinician would have very little evidence supporting their claim that advice was given.
As this study only examined prescribing between 1 October 2018 and 1 January 2019 inclusive, those women who were prescribed an ACE inhibitor or ARB earlier in the year, and may have had a pregnancy exposed to these teratogens, were not included; this may underestimate potential exposure. The authors searched for parent Read codes rather than individual daughter Read codes, for example, the ‘gravida’ parent code was searched for as an ‘inferred’ pregnancy code, which includes all values of gravida, including gravida 0. Therefore, it should be noted that use of a parent code in this instance may have resulted in a slightly higher number of pregnancies being recorded than actually occurred.
Comparison with existing literature
The findings of this study are in agreement with previous international studies that suggest care is suboptimal.8,13,16,26,31 The most recent UK study, of which the authors are aware, dates from 2008; it found low levels of contraception use in a hospital clinic population of hypertensive women of childbearing age taking ACE inhibitors or ARB who potentially could become pregnant (that is, they were pre-menopausal and neither they nor their partners were sterilised).25 The findings presented here suggest there has been little improvement in pre-pregnancy care for women prescribed an ACE inhibitor or ARB in the decade since then.
The study presented here found that younger women were least likely to receive preconception advice or prescribed contraception (Figure 3).
A recent British population-wide survey found that those aged <25 years have the highest rates of unplanned pregnancy and potentially women in this age group who are taking ACE inhibitors or ARBs are similar to this age group as a whole.14 The recording of advice and contraception also decreased for women aged ≥35 years, who formed a larger at-risk group as they are more likely to be prescribed an ACE inhibitor/ARB. UK pregnancy rates in those aged >40 years are increasing rapidly.32 The findings presented here show that women aged 40–45 years had the second highest absolute number of pregnancies and terminations/miscarriages (although not the highest proportion).
Although some age differences were observed, overall, all women of childbearing age who were prescribed an ACE inhibitor or ARB had very low levels of recorded pregnancy advice, associated with considerable numbers of pregnancies and potential exposure to teratogenic medications. Previous studies suggest a lack of time, financial incentive, and clinician knowledge are responsible for poor pregnancy preparation.33
International guidelines recommend that the prescription of an ACE inhibitor or ARB is avoided in young women, contraception is co-prescribed, or ‘adequate precautions’ are taken against pregnancy.18–22 European Society of Cardiology guidelines suggest the use of ACE inhibitors and ARBs in women without reliable contraception is a possible contraindication.23,24
New NICE guidance includes no recommendation that pre-pregnancy planning/advice or contraception should be provided, and recommends the use of enalapril (an ACE inhibitor) as a first-line management option in the post-partum period for chronic hypertension;5 this is despite an MHRA warning, advising against ACE inhibitor or ARB use in the early post-partum period because of possible neonatal hypotension.34 Furthermore, NICE advises that, in women prescribed an ACE inhibitor or ARB for hypertension, clinicians should discuss suitable alternative medicines if pregnancy is planned.5 In contrast, for women prescribed an ACE inhibitor or ARB for other conditions such as diabetes, a discussion about alternatives is recommended, irrespective of pregnancy plans.5 Inconsistency in national and international guidelines may also preclude optimal delivery of effective pregnancy preparation provisions to these women, and guidelines and improvement programmes require system-wide interventions for maximum impact.35
Trials of decision-support systems and ad hoc patient reviews have demonstrated no improvement in prescribing of potentially teratogenic medications to women who could become pregnant.36 The failure to provide stronger system-wide measures to prevent pregnancy exposure to ACE inhibitors and ARBs is in sharp contrast with recent MHRA safety initiatives regarding the highly teratogenic drug, valproate, which have generated a system-wide approach to active engagement by patients, their hospital specialists, and GPs.37
Implications for research and practice
As mentioned previously, low levels of recorded pre-pregnancy advice and contraception may place women who are prescribed an ACE inhibitor or ARB at high risk of pregnancy exposure to these teratogens. Teratogenic risks of ACE inhibitors and ARBs are well documented, and the prescribing of these medications in women of childbearing age in primary care is becoming increasingly frequent. As this study found that the recording of pre-pregnancy advice and contraception in such women was suboptimal and has not improved over the last decade, primary-care-based improvement strategies are required. Patient-held records, signed consent relating to the understanding of pregnancy risks, electronic-alert and virtual review systems, clinician education, prescribing incentives, and patient education materials may contribute to safer systems for women of childbearing age prescribed an ACE inhibitor or an ARB.
The Cumberlege report has recently expressed concern about exposure to teratogenic medicines in pregnancy.38
Future research could be undertaken in large representative national general practice datasets such as QResearch, The Health Improvement Network (THIN), or Clinical Practice Research Datalink (CPRD) to further estimate the scale of possible pregnancy exposure to ACE inhibitors/ARBs, as well as additional research on GPs’ knowledge of prescribing hazards in pregnancy.
Acknowledgments
The authors would like to thank Ms Isabel Dostal for completing the data extraction and are grateful to the GPs and their registered populations in East London who made this study possible by contributing to data sharing with the Clinical Effectiveness Group, Queen Mary University of London; this allowed the group to extract the data and make it available for the purposes of improved patient care and population benefit.
Funding
The study was unfunded.
Ethical approval
No ethical approval was required.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Public Health England Health matters: combating high blood pressure. 2017 https://www.gov.uk/government/publications/health-matters-combating-high-blood-pressure/health-matters-combating-high-blood-pressure (accessed 28 Sep 2020).
- 2.Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113(3 Pt 1):475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 3.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Editor Diabetes prevalence. Diabetes.co.uk. 2019 Jan 15; https://www.diabetes.co.uk/diabetes-prevalence.html (accessed 28 Sep 2020). [Google Scholar]
- 5.National Institute for Health and Care Excellence . Hypertension in pregnancy: diagnosis and management NG133. London: NICE; 2019. https://www.nice.org.uk/guidance/NG133 (accessed 28 Sep 2020). [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence . Hypertension in adults: diagnosis and management CG127. London: NICE; 2011. https://www.nice.org.uk/guidance/cg127 (accessed 28 Sep 2020). [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence . Drugs affecting the renin-angiotensin system Angiotensin-converting enzyme inhibitors. London: NICE; 2020. https://bnf.nice.org.uk/treatment-summary/drugs-affecting-the-renin-angiotensin-system.html (accessed 28 Sep 2020). [Google Scholar]
- 8.Bullo M, Tschumi S, Bucher BS, et al. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012;60(2):444–450. doi: 10.1161/HYPERTENSIONAHA.112.196352. [DOI] [PubMed] [Google Scholar]
- 9.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 10.Moretti ME, Caprara D, Drehuta I, et al. The fetal safety of angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers. Obstet Gynecol Int. 2012;2012:658310. doi: 10.1155/2012/658310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pucci M, Sarween N, Knox E, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in women of childbearing age: risks versus benefits. Expert Rev Clin Pharmacol. 2015;8(2):221–231. doi: 10.1586/17512433.2015.1005074. [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension. 2012;60(4):913–920. doi: 10.1161/HYPERTENSIONAHA.112.197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cea Soriano L, Bateman BT, García Rodríguez LA, Hernández-Díaz S. Prescription of antihypertensive medications during pregnancy in the UK. Pharmacoepidemiol Drug Saf. 2014;23(10):1051–1058. doi: 10.1002/pds.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellings K, Jones KG, Mercer CH, et al. The prevalence of unplanned pregnancy and associated factors in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Lancet. 2013;382(9907):1807–1816. doi: 10.1016/S0140-6736(13)62071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen ME, Ray WA, Arbogast PG, et al. Increasing exposure to angiotensin-converting enzyme inhibitors in pregnancy. Am J Obstet Gynecol. 2008;198(3):291.e1–e5. doi: 10.1016/j.ajog.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Medicines and Healthcare products Regulatory Agency ACE inhibitors and angiotensin II receptor antagonists: not for use in pregnancy. 2014 https://www.gov.uk/drug-safety-update/ace-inhibitors-and-angiotensin-ii-receptor-antagonists-not-for-use-in-pregnancy (accessed 28 Sep 2020).
- 17.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: executive summary — a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–e483. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. Latin American Expert Group. J Hypertens. 2009;27(5):905–922. doi: 10.1097/HJH.0b013e32832aa6d2. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Malaysian Society of Hypertension. Ministry of Health Malaysia. Academy of Medicine of Malaysia . Clinical practice guidelines. Management of hypertension. 5th edn. Malaysian Society of Hypertension; 2018. [Google Scholar]
- 21.Chiang C-E, Wang T-D, Li Y-H, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109(10):740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 22.Chiang C-E, Wang T-D, Ueng K-C, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78(1):1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 24.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 25.Martin U, Foreman MA, Travis JC, et al. Use of ACE inhibitors and ARBs in hypertensive women of childbearing age. J Clin Pharm Ther. 2008;33(5):507–511. doi: 10.1111/j.1365-2710.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 26.Force RW, Keppel GA, Guirguis-Blake J, et al. Contraceptive methods and informed consent among women receiving medications with potential for adverse fetal effects: a Washington, Wyoming, Alaska, Montana, Idaho (WWAMI) region study. J Am Board Fam Med. 2012;25(5):661–668. doi: 10.3122/jabfm.2012.05.120056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS Digital Read codes. 2018 https://digital.nhs.uk/services/terminology-and-classifications/read-codes (accessed 28 Sep 2020).
- 28.Pawa J, Robson J, Hull S. Building managed primary care practice networks to deliver better clinical care: a qualitative semi-structured interview study. Br J Gen Pract. 2017 doi: 10.3399/bjgp17X692597. [DOI] [PMC free article] [PubMed]
- 29.Petersen I, Welch CA, Nazareth I, et al. Health indicator recording in UK primary care electronic health records: key implications for handling missing data. Clin Epidemiol. 2019;11:157–167. doi: 10.2147/CLEP.S191437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among US adolescents, 2007–2014. J Adolesc Health. 2018;63(2):253–256. doi: 10.1016/j.jadohealth.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrical-Kline KA, Walton AM, Guildenbecher TM. Teratogen use in women of childbearing potential: an intervention study. J Am Board Fam Med. 2011;24(3):262–271. doi: 10.3122/jabfm.2011.03.100198. [DOI] [PubMed] [Google Scholar]
- 32.Office for National Statistics Conceptions in England and Wales: 2018–2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/conceptionandfertilityrates/bulletins/conceptionstatistics/2018 (accessed 28 Sep 2020).
- 33.Schwarz EB, Santucci A, Borrero S, et al. Perspectives of primary care clinicians on teratogenic risk counseling. Birth Defects Res A Clin Mol Teratol. 2009;85(10):858–863. doi: 10.1002/bdra.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medicines and Healthcare products Regulatory Agency ACE inhibitors and angiotensin II receptor antagonists: recommendations on how to use for breastfeeding. 2014 https://www.gov.uk/drug-safety-update/ace-inhibitors-and-angiotensin-ii-receptor-antagonists-recommendations-on-how-to-use-for-breastfeeding (accessed 28 Sep 2020).
- 35.Jeffries M, Phipps DL, Howard RL, et al. Understanding the implementation and adoption of a technological intervention to improve medication safety in primary care: a realist evaluation. BMC Health Serv Res. 2017;17(1):196. doi: 10.1186/s12913-017-2131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz EB, Parisi SM, Handler SM, et al. Clinical decision support to promote safe prescribing to women of reproductive age: a cluster-randomized trial. J Gen Intern Med. 2012;27(7):831–838. doi: 10.1007/s11606-012-1991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medicines and Healthcare products Regulatory Agency Valproate medicines (Epilim▼, Depakote▼): contraindicated in women and girls of childbearing potential unless conditions of Pregnancy Prevention Programme are met. 2018 https://www.gov.uk/drug-safety-update/valproate-medicines-epilim-depakote-contraindicated-in-women-and-girls-of-childbearing-potential-unless-conditions-of-pregnancy-prevention-programme-are-met (accessed 28 Sep 2020).
- 38.Independent Medicines and Medical Devices Safety Review. First do no harm The report of the Independent Medicines and Medical Devices Safety Review. 2020 doi: 10.1177/00369330211058467. https://www.immdsreview.org.uk/downloads/IMMDSReview_Web.pdf (accessed 28 Sep 2020). [DOI] [PubMed]