Abstract
Purpose
[6]-gingerol is a bioactive compound extracted from ginger, a traditional anti-emetic herb in Chinese medicine. Previous studies have demonstrated that [6]-gingerol can ameliorate chemotherapy-induced pica in rats, although the underlying mechanism has not been elucidated. This study is designed to investigate [6]-gingerol’s antiemetic mechanism focusing on the 5-hydroxytryptamine (serotonin, 5-HT) system by evaluating the synthesis, metabolism and reuptake of 5-HT, as well as the mechanism of 5-hydroxytryptamine type 3 receptor (5-HT3 receptor), in a cisplatin-induced pica model of rats.
Methods
Rats were randomly divided into control group (vehicle + saline, Con), [6]-gingerol control group (50 mg/kg [6]-gingerol + saline, G-con), ondansetron control group (2.6 mg/kg ondansetron + saline, O-con), cisplatin model group (vehicle + cisplatin, Model), ondansetron-treated group (2.6 mg/kg ondansetron + cisplatin, O-treated), high dosage of [6]-gingerol-treated group (100 mg/kg [6]-gingerol + cisplatin, GH-treated), and low dosage of [6]-gingerol-treated group (50 mg/kg [6]-gingerol + cisplatin, GL-treated). The rats were administered with [6]-gingerol, ondansetron, and vehicle (3% Tween-80) by gavage twice (7:00 AM and 7:00 PM). One hour after the first treatment (8:00 AM), rats in groups Model, O-treated, GH-treated and GL-treated were injected intraperitoneally (i.p.) with 6 mg/kg cisplatin, and the other groups were injected i.p. with saline of equal volume. The consumption of kaolin of the rats were measured. All the rats were anesthetized by i.p. injection of pentobarbital sodium at 24 h post-cisplatin. After blood samples were taken, medulla oblongata and ileum were removed. The levels of 5-HT and its metabolite 5-HIAA in ileum, medulla oblongata and serum were determined using high-performance liquid chromatography with electrochemical detection (HPLC-ECD). The mRNA expression levels of 5-HT3 receptor, tryptophan hydroxylase (TPH), monoamine oxidase A (MAO-A) and serotonin reuptake transporter (SERT) were detected by real-time PCR. The protein expression levels and distribution of 5-HT3 receptor, TPH and MAO-A in the medulla oblongata and ileum were measured by Western blotting and immunohistochemistry, respectively.
Results
[6]-gingerol treatment significantly reduced the kaolin ingestion and the increase in 5-HT concentration in rats induced by cisplatin. TPH, MAO-A, SERT, and 5-HT3 receptor are important in 5-HT metabolism, and cisplatin-induced alterations in the associated protein/mRNA levels were restored when treated with [6]-gingerol.
Conclusion
This suggests that the antiemetic effect of [6]-gingerol against cisplatin-induced emesis may be due to 5-HT attenuation via modulating the TPH/MAO-A/SERT/5-HT/5-HT3 receptor system.
Keywords: [6]-gingerol, serotonin, cisplatin, pica, rats
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a common and distressing side effect of anticancer drugs, especially cytotoxic agents such as cisplatin, a highly emetogenic chemotherapeutic drug.1 CINV commonly leads to malnutrition and seriously impairs the quality of life for cancer patients, often reducing their compliance with chemotherapy.2 However, existing antiemetic drugs such as 5-hydroxytryptamine type 3 receptor (5-HT3 receptor) antagonists, Neurokinin 1 (NK1) receptor antagonists, and corticosteroids, when used either alone or in combination can result in unsatisfactory outcomes.3 In addition, antiemetic drugs are often expensive and have side effects, such as diarrhea, headache, asthenia, fatigue, neutropenia, and hypotension.4 Therefore, it is extremely important to explore novel therapies for treating CINV.
The molecular mechanisms that underly CINV involve a large number of neurotransmitters and receptors, and 5-hydroxytryptamine (serotonin, 5-HT) is thought to play a key role in the pathophysiology of CINV. Cytotoxic drugs, such as cisplatin, can cause emesis that is associated with increased 5-HT concentrations in the intestine and brainstem.5 5-HT is released from enterochromaffin (EC) cells that are stimulated by cytotoxic agents, and then 5-HT activates 5-HT3 receptors located on vagal afferent fibers. Activated 5-HT3 receptors act on the vagus nerve, causing the nerve to transmit an impulse to the vomiting center, which evokes an emetic reflex.6 Tryptophan hydroxylase (TPH) is a rate-limiting enzyme in the biosynthesis of 5-HT and the expression level of TPH can be considered to be an indirect marker for 5-HT synthesis. Two TPH isoforms have been identified: TPH-1 is primarily expressed in the EC cells of the gastrointestinal (GI) tract, whereas TPH-2 is expressed in the neuronal tissues.7,8 Studies showed that TPH-1 inhibitors could reduce intestinal serotonin level and decrease emetic response in a cisplatin-induced emesis model in ferret.9 5-HT is mainly oxidized by monoamine oxidase-A (MAO-A) and is transported by serotonin reuptake transporter (SERT), the main carrier of 5-HT. Therefore, TPH, MAO-A and SERT are three enzymes/proteins that are important for determining serotonin concentration and dynamics. CINV is mediated by the 5-HT3 receptor and 5-HT3 receptor antagonists (such as ondansetron) are frequently used for its prevention or treatment. The 5-HT3 receptor is a ligand-gated ion channel composed of five subunits and 5-HT3A receptor subtypes are associated with CINV.10
Ginger (Zingiber officinale) is a traditional Chinese herbal medicine that has been used for treating nausea and emesis for over two thousand years. Some studies have demonstrated that ginger and its multiple active constituents are useful in treating CINV11 and that ginger extracts (acetone, 50% ethanolic, and aqueous) and gingerol possess anti-emetic effects in cisplatin-induced emetic models in dogs12 and mink.13 Furthermore, a pigeon study revealed promising anti-emetic activity of a ginger acetone extract against cisplatin-induced vomiting.14 Gingerol, the main pungent constituents of ginger, is a mixture of gingerols, shogaols, paradols, zingerones, gingerdiones, and gingerdiols.15 Gingerol contains many biologically active constituents, of which [6]-gingerol is the most common compound.16,17 [6]-gingerol has been shown to improve feeding behavior in rats with cisplatin-induced pica, indicating that [6]-gingerol is effective in preventing CINV.18 However, the exact mechanisms have not been characterized. Based on previous studies, we speculated that [6]-gingerol may reduce the increased levels of 5-HT by inhibiting synthesis or accelerating 5-HT degradation during chemotherapy treatment.
Although rats do not vomit,19 they show pica behavior (the consumption of non-nutritive substances, such as kaolin) in response to the various emetic stimuli, which can be used as an index of nausea and vomiting in rat models.19,20 In the present study, we investigated the effect of [6]-gingerol on cisplatin-induced kaolin intake (pica), as well as characterized the underlying mechanisms of the anti-emetic effects of [6]-gingerol by focusing on the 5-HT system: 5-HT, TPH, MAO-A, SERT, and 5-HT3 receptor.
Materials and Methods
Drugs and Chemicals
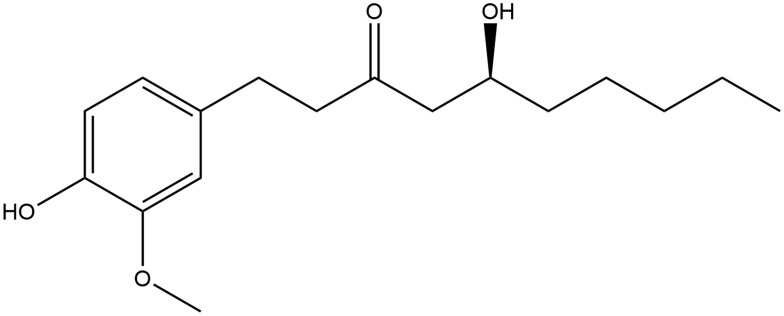
[6]-gingerol (≥98% purity, measured by HPLC) was obtained from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China) and dissolved in 3% Tween 80. The chemical structure of [6]-gingerol is shown in Figure 1. Cisplatin (powder injection) and ondansetron (hydrochloride injection) were acquired from Qilu Pharmaceutical Co., Ltd. (China). Cisplatin was dissolved in saline. Arabic gum and kaolin powder were acquired from China Pharmaceutical Chemical Reagents Co., Ltd. (China). 5-hydroxytryptamine (serotonin, 5-HT) and 5-hydroxyindole acetic acid (5-HIAA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-5HT3A receptor antibody (ab13897), anti-MAO-A antibody (ab126751), anti-SERT antibody (ab130130), anti-TPH-1 antibody (ab 52954), and anti-TPH-2 antibody (ab184505) were obtained from Abcam (Cambridge, UK). Anti-rabbit secondary antibodies, anti-mouse secondary antibodies, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody were purchased from Beyotime Biotechnology Co., Ltd. (Haimen, Jiangsu, China).
Figure 1.
Chemical structure of [6]-gingerol.
Preparation of Kaolin Pellets
Kaolin was prepared as previously described.18 A 2% Arabic gum solution was slowly added to kaolin powder, stirred to thick paste, and shaped into pellets similar to the rats’ normal laboratory diet, then dried completely at room temperature.
Animals
All animal experiments were approved by the Committee on Laboratory Animal Care and Use of Guangdong Pharmaceutical University (Guangzhou, China), in accordance with the National Institutes of Health guide for the care and use of laboratory animals. Adult male Sprague-Dawley (SD) rats (200–230 g) were obtained from the Laboratory Animal Center of Guangzhou University of Chinese Medicine, Guangzhou, China. Rats were housed in individual cages covered with steel grids under controlled environmental conditions (23 ± 2°C, relative humidity 50±10%, 12 h dark/light cycles, food and water ad libitum). The kaolin and food pellets were provided in their respective divided food hopper on the steel grids.
Measurement of Kaolin and Food Consumption and Drug Administration
Kaolin was provided for 3 days before cisplatin treatment in order to acclimate the rats to the presence of kaolin in the cage. Most of the rats stopped kaolin consumption by the third day, while rats that were still consuming kaolin were excluded from the study. The remaining rats were randomly divided into seven groups of eight rats each: Group 1 was the control group (vehicle + saline, Con); group 2 was the [6]-gingerol control group (50 mg/kg [6]-gingerol + saline, G-con); group 3 was the ondansetron control group (2.6 mg/kg ondansetron + saline, O-con); group 4 was the cisplatin model group (vehicle + cisplatin, Model); group 5 was the ondansetron-treated model group (2.6 mg/kg ondansetron + cisplatin, O-treated); group 6 was the high dosage of [6]-gingerol-treated model group (100 mg/kg [6]-gingerol + cisplatin, GH-treated), and group 7 was the low dosage of [6]-gingerol-treated model group (50 mg/kg [6]-gingerol + cisplatin, GL-treated). [6]-gingerol doses were selected based on our previous studies.18
The rats were weighed prior to treatment and then administered with [6]-gingerol, ondansetron, and vehicle (3% Tween-80) by gavage twice (7:00 AM and 7:00 PM). One hour after the first treatment (8:00 AM), groups Model, O-treated, GH-treated and GL-treated were injected intraperitoneally (i.p.) with 6 mg/kg cisplatin, and the other groups were injected i.p. with saline of equal volume. The general conditions of the rats were closely observed, including physical activity, fur, appetite, breathing, and stool. The consumption of kaolin and food and body weight of the rats were measured every 24 h (8:00 AM) both before and after cisplatin injection. Spilt kaolin and food were collected and weighed to correct the actual consumption. All the rats were anesthetized by i.p. injection of pentobarbital sodium (45 mg/kg) at 24 h post-cisplatin.
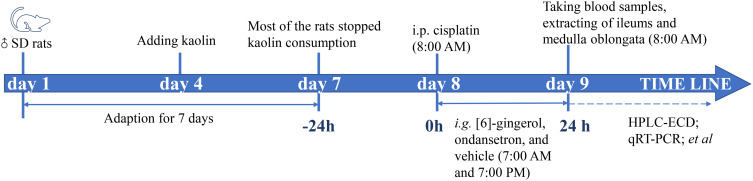
Two rats from each group were processed by cardiac perfusion with 150 mL of saline and then 150 mL of 4% phosphate-buffered paraformaldehyde, while the medulla oblongata was removed and fixed in 4% phosphate-buffered paraformaldehyde. From the remaining six rats in each group, blood samples were taken from the abdominal aorta and collected in vacutainers, while serum samples were collected by centrifuging the blood samples and discarding the cells and storing the supernatants at −20°C for measurement of 5-HT and 5-HIAA levels. After blood samples were taken, medulla oblongata and ileum were removed and divided into two sections: one section was fixed in 4% phosphate-buffered paraformaldehyde and embedded in paraffin for immunohistochemical examinations, and the other section was immediately flash-frozen with liquid nitrogen and stored at −80°C to be used for measurement of 5-HT and 5-HIAA levels, quantitative real time-polymerase chain reaction (qRT-PCR) and Western blotting analysis. The schematic presentation of the experimental design is shown in Figure 2.
Figure 2.
The schematic presentation of the experimental design.
Determination of the Levels of 5-HT and Its Metabolite 5-HIAA Using HPLC-ECD
Sample Preparation
Briefly, tissues from the medulla oblongata and ileum were weighed and homogenized in 300 µL of 5% perchloric acid. The homogenates were centrifuged at 10,100 g for 15 min at 4°C and the supernatant was separated and filtered through a 0.22 µm membrane filter pore. The serum (200 µL) was collected in a polypropylene tube containing 50 μL of 10% perchloric acid and mixed for 2 min to ensure complete precipitate protein. Samples were then centrifuged at 10,100 g for 15 min at 4°C and the supernatant was separated and filtered through a 0.22 µm membrane filter.
Chromatographic Conditions
The mobile phase consisted of a mixture of an aqueous solution (71 mM citric acid, 78 mM citrate, 0.8 mM sodium octanesulfonate, and 0.1 mM Na2EDTA) and methanol at 90:10 (v/v). The mobile phase was filtered through a 0.2 μm Millipore filter. An Agilent C18 column (4.6 mm × 250 mm, 5 µm) was used for separation, the oven temperature was 33°C, and the injector temperature was 4°C. The analytical cell potentials were E 1 −150 mV and E 2 +750mV. The flow rate was 0.8 mL/min and the injection volume was 20μL. Same quantification was achieved by comparison with standard solutions of known concentrations and analysis using HPLC software (N2000 Workstation). The filtrate sample was used for quantification of 5-HT (Y = 43.749X+ 422.87, r = 0.9994) and 5-HIAA (Y = 2.0325X + 144.42, r= 0.9994) in ileum by HPLC coupled with electrochemical detection. The filtrate sample was used for quantification of 5-HT (Y = 23.028X + 41.919, r = 0.9999) and 5-HIAA (Y= 2.1474X + 16.53, r = 0.9992) in medulla oblongata by HPLC coupled with electrochemical detection. The filtrate sample was used for quantification of 5-HT (Y = 27.62X + 295.95, r= 0.9997) and 5-HIAA (Y = 22.986X +75.494, r = 0.9999) in serum by HPLC coupled with electrochemical detection. Neurotransmitter and metabolite concentrations were calculated as ng/mg protein (ng/mg pro).
qRT-PCR of the 5-HT3 Receptor, TPH, MAO-A and SERT Receptor Gene Expression
Trizol reagent (Thermo Fisher Scientific, No.15596018) was used to extract total RNA from ileum and medulla oblongata samples according to the manufacturer’s protocol. The purity and integrity of total RNA were assessed by UV-Vis spectrometry, calculating the ratio of absorbance at 260 nm and 280 nm (1.6–2.0). cDNA was generated using a PrimeScriptTM RT reagent Kit with gDNA Eraser per the manufacturer’s protocol (Takara, No. RR047A). The reverse transcription reaction was completed as follows: 42°C for 2 min, 37°C for 15 min, and 85°C for 5 s. A CFX96 Real-Time PCR Detection System was used to perform the real-time PCR analysis. The concentration of 5-HT3 receptor, TPH, MAO-A, and SERT mRNA were normalized against the concentration of GAPDH mRNA in the same sample. qRT-PCR was performed using TB Green Premix Ex Taq TM II Kit (Takara, No. RR820A) according to the manufacturer’s protocol. Primer sequences, product size, and accession number for the 5-HT3 receptor, TPH, MAO-A, and SERT genes are reported in Table 1. The conditions for PCR were 95°C for 30 s, followed by 40 amplification cycles of 95°C for 5 s, 60°C for 30 s, and 95°C for 10 s. Target gene expression was computed by the comparative threshold cycle (Ct) method. The mRNA levels were normalized to the housekeeping gene, GAPDH. The fold change in the expression of each target gene was calculated by the following formula: Relative quantitation (RQ)= 2 −ΔΔCt.
Table 1.
Primers for Gene Amplification
| Primer | Sequence | Product Size/bp | Accession Number |
|---|---|---|---|
| 5-HT3 receptor | F:5ʹ-CTGTCCTCCATCCGCCACTCC-3ʹ R: 5ʹ-CAGCAGCCTGTCCAGCACATATC-3’ |
96 | NM_024394.2 |
| MAO-A | F: 5ʹ-AGTGGAGTGGCTACATGGAAGGAG-3ʹ R: 5ʹ-AGCAGACCAGGCACGGAAGG-3’ |
187 | NM_033653.1 |
| TPH-1 | F: 5ʹ-GCACAGCCAGGCATGAGGATG-3ʹ R: 5ʹ-GGCTACACTGCTGACACCACAC-3’ |
195 | NM_001100634.2 |
| TPH-2 | F: 5ʹ-CCGCTCACCTTCCTCCTACATCTC-3ʹ R: 5ʹ-GCTCTTCTGGCACCGCTGAATC-3’ |
165 | NM_173839.2 |
| SERT | F: 5ʹ-ATAGCCAACATGCCAGCATCCAC-3ʹ R: 5ʹ-ACCACGATGAGCACGAACCATTC-3’ |
170 | NM_013034.4 |
| GAPDH | F: 5ʹ-GACATGCCGCCTGGAGAAAC-3ʹ R: 5ʹ-AGCCCAGGATGCCCTTTAGT-3’ |
20 | NM_017008.4 |
Measurement of 5-HT3 Receptor, TPH, and MAO-A Protein Expression by Immunoblotting
Total protein was extracted with Total Protein Extraction Reagents (Beyotime, No. P0013B) according to the protocols suggested by the manufacturer. After centrifugation at 10,100 g at 4°C for 30 min, supernatants were collected and total protein concentrations were determined by Enhanced BCA Protein Assay Kit (Beyotime Biotechnology, No. P0010S) according to the manufacturer’s instructions. After separated by vertical sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were then transferred to polyvinylidene fluoride membranes. Subsequently, membranes were blocked with 5% skim milk about 2 h at room temperature and washed three times with 0.02% Tris-buffered saline-Tween 20 for 10 min each. The membranes then were incubated with primary antibodies for GAPDH (1:1000 dilution, Beyotime Biotechnology, AG019), 5-HT3A receptor (1:1000 dilution, Sigma, ab13897), MAO-A (1:1000 dilution, Sigma, ab126751), TPH-1 (1:500 dilution, Sigma, ab52954), and TPH-2 (1:1000 dilution, Sigma, ab184505) overnight at 4°C. After washing with 0.02% Tris-buffered saline-Tween 20 three times, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (1:1000, HRP-labeled Goat Anti-Rabbit IgG, A0208; HRP-labeled Donkey Anti-Goat IgG, A0181; HRP-labeled Goat Anti-Mouse IgG, A0216, Beyotime Biotechnology). Finally, immunocomplexes were visualized by chemiluminescence using an ECL Western blotting kit (BeyoECL Star, Beyotime Biotechnology, P0018AS) according to the manufacturer’s instructions. Quantification of band intensity was performed by using Image J software. Relative protein expression levels were expressed as the ratio of band intensity of target protein compared to GAPDH.
Immunohistochemical Analyses of 5-HT3 Receptor, TPH, MAO-A, and SERT
Paraffin-embedded tissue sections (4 μm thick) were deparaffinized first with xylene then hydrated using graded ethanol solutions and heated in 0.5 M EDTA (pH 8.0) for 15 min. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min at room temperature, and three washes were performed with PBS (pH 7.4) for 5 min. After that, 5% bovine serum albumin in tris-buffered saline was used to block the tissue sections for 20 min. They were then incubated overnight at 4°C with one of the following specific primary antibodies: anti-5HT3A receptor antibody, anti-MAO-A antibody, anti-SERT antibody, anti-TPH-1 antibody, and anti-TPH-2 antibody. After washing the slides with Tris-buffered saline, the sections were incubated with the corresponding secondary antibody. The protocol described for a DAKO kit was used for color development. The slides were counterstained with hematoxylin for 3 min, following by rinsing with deionized water, and then immersion in ammonia blue for 2 min. Images were acquired with a confocal microscope. At least three sections per rat were investigated, and immunohistochemical quantification was carried out using Image J analysis software.
Statistical Analysis
The data are expressed as mean ± SEM and analyzed using GraphPad Prism 6.0 software. One-way ANOVA and two-way ANOVA were performed when more than two groups were compared. Tukey’s test was performed as post-hoc test. In all of the tests, P<0.05 was considered statistically significant.
Results
Effects of [6]-Gingerol on Kaolin Consumption, Food Consumption, and Body Weight in Rats
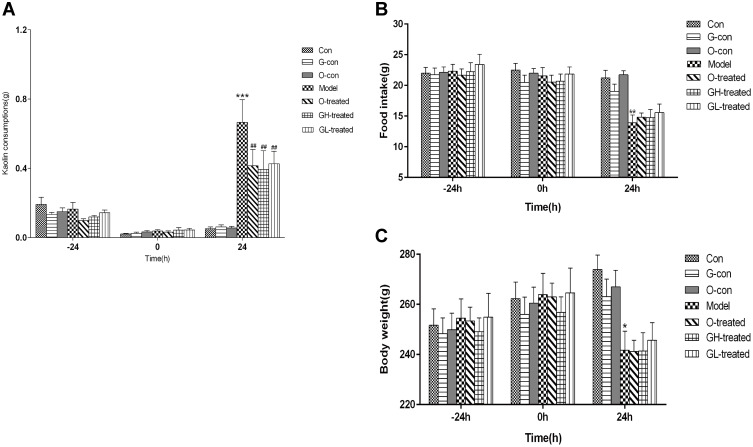
In order to understand the antiemetic activity of [6]-gingerol in response to CINV, we established a vomiting model of rats by i.p. injection of cisplatin, followed by assessing the amount of kaolin consumption as an index of vomiting. On the first day of adaptation, most rats consumed kaolin, followed by a gradual decrease in kaolin consumption to zero on the third day. The kaolin intake of rats injected i.p. with cisplatin was significantly increased compared with the control group (P<0.001). Kaolin consumption in the ondansetron- and [6]-gingerol-treatment groups decreased to differing extents 24 h after treatment (P<0.05), indicating that both ondansetron and [6]-gingerol can ameliorate pica that is induced by cisplatin in rats (Figure 3). The food consumption and body weight of rats in the cisplatin model group decreased after i.p. injection of cisplatin (P <0.05 vs the control group, Figure 3A). [6]-gingerol or ondansetron treatment did not reverse decreases in body weight and food consumption (P <0.05 vs the cisplatin model group, Figure 3B). Together, these data indicated that ondansetron and [6]-gingerol had no significant effect on the body weight of cisplatin-treated rats (Figure 3C).
Figure 3.
Pica induced by cisplatin. Measurements in treated rats over time: (A) Kaolin consumption, (B) Food intake, (C) Body weight.
Notes: Values are expressed as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.01, compared to the control group; ##P < 0.01, compared to the cisplatin model group. t=0, i.p. 6 mg/kg cisplatin.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
Determination of 5-HT and 5-HIAA Levels in Ileum, Medulla Oblongata, and Serum
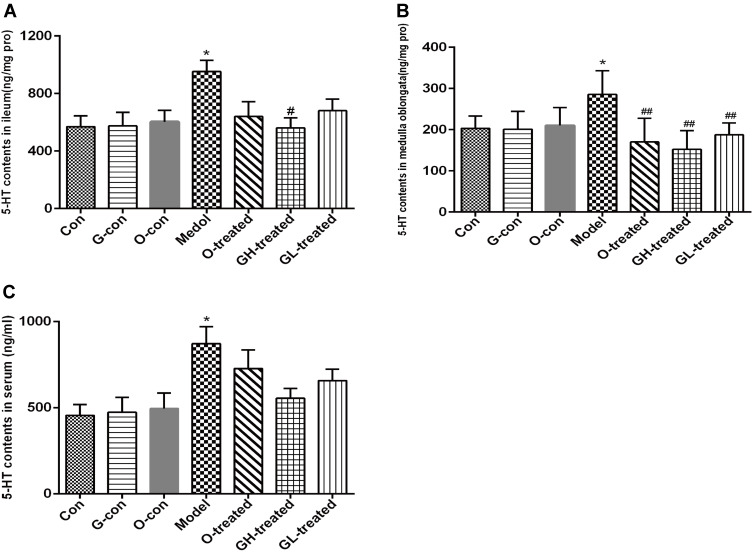
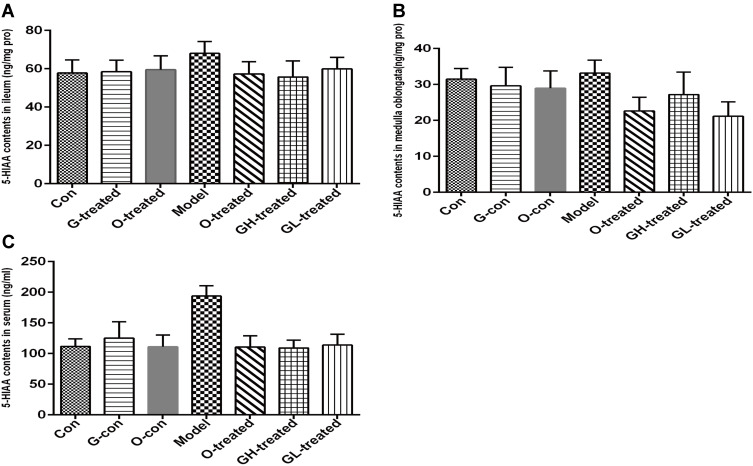
As increases in 5-HT concentration are associated with cisplatin-induced emesis, the levels of 5-HT and its metabolite 5-HIAA in the ileum, medulla oblongata, and serum were measured. The levels of 5-HT in serum, medulla oblongata, and ileum of rats treated with cisplatin were significantly increased in comparison to the control group (P<0.05, Figure 4A–C). Compared to the cisplatin model group, the rats treated with ondansetron and [6]-gingerol had a reduction in 5-HT levels, although statistical significance was only observed for treatment with ondansetron and two dosages of [6]-gingerol in medulla oblongata (P<0.01) and of high dosage of [6]-gingerol in ileum (P<0.05). The levels of 5-HIAA in serum, medulla oblongata, and ileum of rats treated with cisplatin had an increasing trend, but did not reach statistical significance (Figure 5A–C). Compared to the cisplatin model group, [6]-gingerol and ondansetron inhibited the increased 5-HIAA levels, but also did not reach statistical significance (Figure 5A–C).
Figure 4.
5-HT Levels in ileum, medulla oblongata and serum. (A) Ileum, (B) Medulla Oblongata, (C) serum.
Notes: Values are expressed as mean ± SEM (n = 6). *P < 0.05, compared to the control group; #P < 0.05, ##P < 0.01, compared to the cisplatin model group.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
Figure 5.
5-HIAA Levels in ileum, medulla oblongata and serum. (A) Ileum, (B) Medulla Oblongata, (C) serum.
Note: Values are expressed as mean ± SEM (n = 6).
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
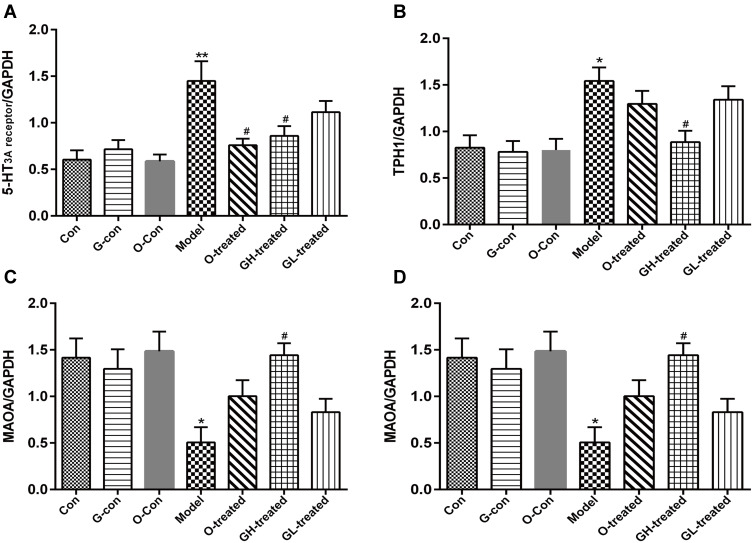
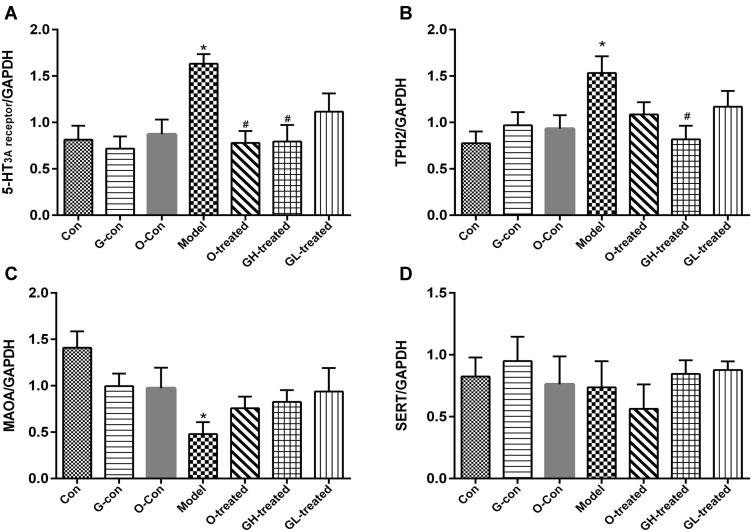
RNA Expression of TPH, MAO-A, SERT, and 5-HT3 Receptor in Ileum and Medulla Oblongata
The reduction in 5-HT levels by [6]-gingerol against cisplatin-induced emesis in rats might be due to the regulation of 5-HT synthesis, metabolism, and/or reuptake, as well as 5-HT3A receptor activity. In order to assess these metrics, the RNA expression of TPH, MAO-A, SERT, and 5-HT3 receptor were measured. After cisplatin administration, TPH-1 and TPH-2 expression increased significantly in ileum and medulla oblongata, respectively, compared to the control group (P<0.05, Figures 6B and 7B). [6]-gingerol significantly downregulated TPH-1 and TPH-2 expression after cisplatin treatment as compared to the cisplatin control (P<0.05). 5-HT3 receptor mRNA expression was increased in medulla oblongata and ileum (P<0.05). Combined treatment with [6]-gingerol reduced 5-HT3 receptor expression in medulla oblongata and ileum (P<0.05, Figures 6A and 7A). However, cisplatin administration significantly reduced MAO-A mRNA content in ileum and medulla oblongata compared to the control group (Figures 6C and 7C, P<0.05), while the addition of [6]-gingerol significantly increased MAO-A mRNA in ileum when compared to the cisplatin model group (P<0.05). SERT expression in ileum was significantly reduced by cisplatin treatment (P<0.05). Combined treatment with [6]-gingerol increased ileum SERT expression (P<0.05, Figures 6D and 7D).
Figure 6.
TPH-1, MAO-A, SERT, and 5-HT3 receptor mRNA expression in ileum. (A) 5-HT3 receptor, (B) TPH-1, (C) MAOA, (D) SERT.
Notes: Values are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, compared to the control group; #P < 0.05, compared to the cisplatin model group.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
Figure 7.
TPH-2, MAO-A, SERT, and 5-HT3 receptor mRNA expression in medulla oblongata. (A) 5-HT3 receptor, (B) TPH-1, (C) MAOA, (D) SERT.
Notes: Values were expressed as mean ± SEM (n = 6). *P < 0.05, compared to control group; #P < 0.05, compared to cisplatin model group.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
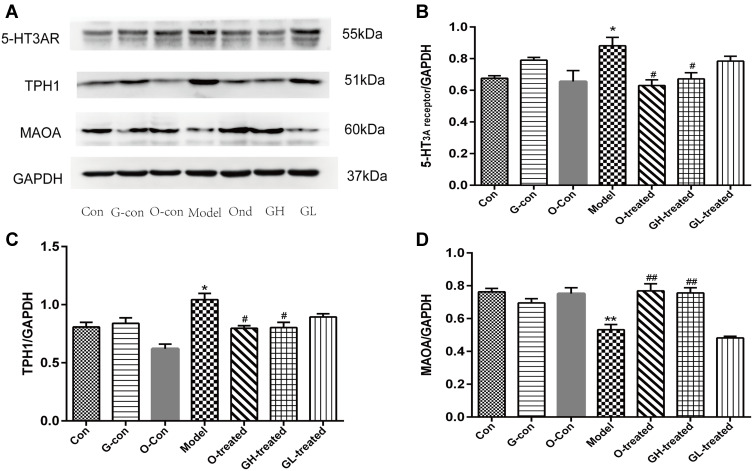
Protein Expression of TPH, MAO-A, and 5-HT3 Receptor in Ileum and Medulla Oblongata
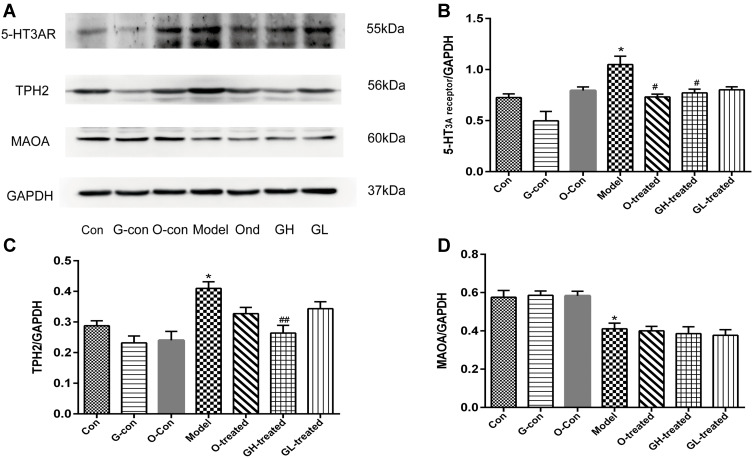
After the RNA expression of TPH, MAO-A, SERT, and 5-HT3 receptor had been assessed, protein expression was measured. Protein samples from the treatment groups were analyzed for protein content (Figures 8A and 9A). After cisplatin treatment, TPH-1 levels in ileum and TPH-2 levels in medulla oblongata were significantly increased compared to the blank group (P<0.05, Figures 8C and 9C), while the addition of [6]-gingerol significantly reduced the noted increases in TPH-1 and TPH-2 levels (P<0.01, P<0.05). Ileum and medulla oblongata MAO-A were significantly reduced in the cisplatin group compared to the blank group (P<0.01, Figure 8D and P<0.05, Figure 9D), whereas ondansetron and high dosage of [6]-gingerol significantly increased the expression levels of MAO-A in medulla oblongata (P<0.05, Figure 9D). The protein expression of 5-HT3 receptor in medulla oblongata and ileum increased significantly with cisplatin administration (P<0.05), and this effect was reduced in groups treated with ondansetron and high dosage of [6]-gingerol (P<0.05, Figure 8B and P<0.05, Figure 9B).
Figure 8.
Protein content of TPH-1, MAO-A and 5-HT3 receptor in ileum. The protein expression was detected by immunoblot. (A) Protein immunoblot, (B) 5-HT3 receptor, (C) TPH-1, (D) MAOA.
Notes: Values were expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, compared to the control group; #P < 0.05, ##P < 0.01, compared to the cisplatin model group.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group. GAPDH was used as an internal control for grayscale analyses.
Figure 9.
Protein expression of TPH-2, MAO-A, and 5-HT3 receptor in medulla oblongata. The protein expression was detected by immunoblot. (A) Protein immunoblot, (B) 5-HT3 receptor, (C) TPH-2, (D) MAOA.
Notes: Values were expressed as mean ± SEM (n = 6). *P < 0.05, compared to the control group; #P < 0.05, ##P < 0.01, compared to the cisplatin model group.
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group. GAPDH was used as an internal control for grayscale analyses.
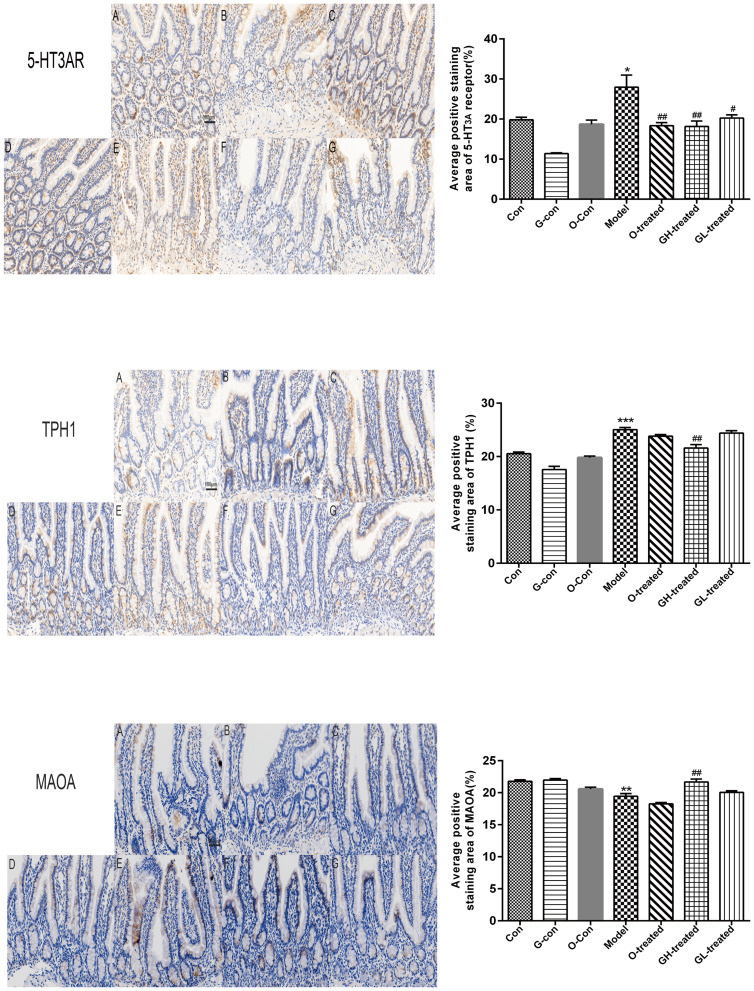
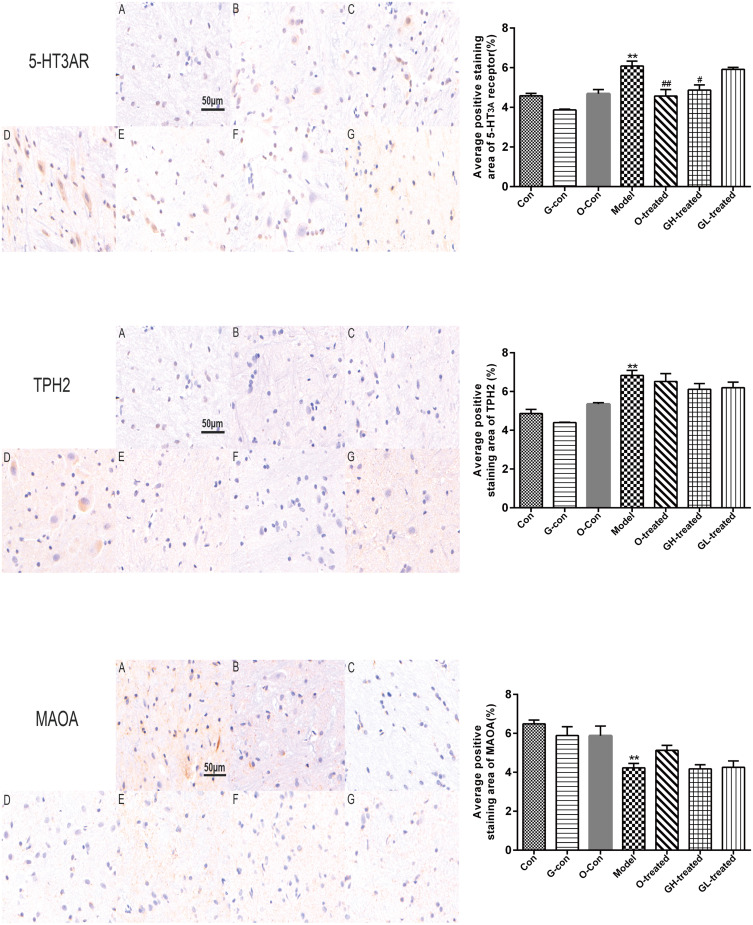
TPH, MAO-A, and 5-HT3 Receptor Immunostaining Expression in Ileum and Medulla Oblongata
5-HT3 receptor, TPH, and MAO-A staining intensities (red-brown deposits indicate positive staining) were mainly present in the mucosa and submucosa of ileum, as well as in the neurons of medulla oblongata. As previously noted, cisplatin had significant impacts on 5-HT3 receptor, TPH, and MAO-A protein levels in both medulla oblongata and ileum, which was reversed by combined treatment with [6]-gingerol (Figures 10 and 11). Importantly, the observed effect of [6]-gingerol on 5-HT3 receptor, TPH, and MAO-A protein levels was in a dose-dependent manner.
Figure 10.
TPH-1, MAO-A, and 5-HT3 receptor immunostaining in ileum.
Notes: Values were expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.01, compared to the control group; #P < 0.05, ##P < 0.01, compared to the cisplatin model group. (magnification × 200, scale bars = 100 μm).
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
Figure 11.
TPH-2, MAO-A, and 5-HT3 receptor immunostaining in medulla oblongata.
Notes: Values were expressed as mean ± SEM (n = 6). **P < 0.01, compared to the control group; #P < 0.05, ##P < 0.01, compared to the cisplatin model group. (magnification × 200, scale bars = 100 μm).
Abbreviations: Con, normal control group; G-con, the [6]-gingerol control group; O-con, the ondansetron control group; Model, the cisplatin model group; O-treated, the ondansetron-treated model group; GH-treated, the high dosage of [6]-gingerol-treated model group; GL-treated, the low dosage of [6]-gingerol-treated model group.
Discussion
As a major debilitating side effect of many chemotherapy drugs, CINV severely affects the quality of life for cancer patients. Despite a significant proportion of patients utilize anti-emetic drugs, up to 40% of cancer patients receiving chemotherapy fail to achieve complete nausea and vomiting control.21 Cisplatin-induced emesis is often caused by 5-HT from EC cells within the GI tract following exposure to cytotoxic drugs. Therefore, one method of inhibiting cytotoxic drug-induced emesis would be to reduce EC cells 5-HT release.
Ginger has a long history as a traditional Chinese medicine for emesis and gastrointestinal discomfort. Ginger has been screened for postoperative nausea and vomiting in clinics and subsequently found to be superior to placebo and equally effective as metoclopramide.22 A double-blind, randomized clinical study showed that ginger root powder was effective in reducing the severity of acute and delayed CINV as an additional antiemetic to ondansetron and dexamethasone in cancer patients receiving high emetogenic chemotherapy.23 Ullah et al reported that cisplatin-induced vomiting in pigeons was accompanied by an increase in 5-HT in the area postrema, brain stem and intestine, while ginger acetone extract could attenuate cisplatin-induced emesis and reduced 5-HT concentration in the central and peripheral system.14 Gingerol, the main pungent component of ginger, is a mixture of various substances, consisting of [6]-gingerol, [8]-gingerol, [10]-gingerol, [6]-shogaol, etc.15 Qian et al13,24,25 and Tian et al26 reported that gingerol has an activity against cisplatin-induced emesis in mink and cisplatin-induced pica in rats by downregulating 5-HT, dopamine (DA) and substance P (SP) expression and regulating the central and peripheral 5‑HT, SP, and DA systems. Unfortunately, the purity and composition proportion of gingerol were not given in their reports. [6]-gingerol is the most bioactive compound of gingerol, so it is often used as an indicator of ginger quality.15 [6]-gingerol’s anti-emetic activity has been confirmed in a cisplatin-induced pica model of rats.18 Previous in vitro and animal studies have demonstrated that [6]-gingerol is likely to exert 5-HT3 antagonistic effects, while a further study demonstrated that ginger extracts and its active principles [6]-gingerol and [6]-shogaol could block the human 5-HT3A receptor in a non-competitive manner.27 Another study28 showed that the ginger water extract and its three major pungent constituents, including [6]-gingerol, could non-competitively inhibit serotonin currents on acutely dispersed visceral afferent neurons as assessed by patch-clamp. These results indicate that ginger and its constituents exert antiemetic effects by blocking 5-HT-induced emetic signal transmission in vagal afferent neurons.28
In this study, the level of 5-HT in rat serum, medulla oblongata, and ileum were significantly increased after cisplatin treatment, confirming that cisplatin-induced emesis is accompanied by an increase in 5-HT levels. This effect was reversed when rats were treated with [6]-gingerol or ondansetron, suggesting that the antiemetic effect of these compounds is due to a decrease in 5-HT levels. In this experiment, the effects of [6]-gingerol on the cisplatin-induced changes are similar to that of ondansetron, but there are slight differences between them. Ondansetron significantly inhibited the increase of 5-HT levels from medulla oblongata but not from ileum. [6]-gingerol significantly inhibited the increase of 5-HT levels in medulla oblongata and ileum, probably because [6]-gingerol inhibit 5-HT synthesis or accelerate 5-HT metabolism. Compared to the cisplatin model group, [6]-gingerol inhibited the increased 5-HIAA levels in ileum, but did not reach statistical significance. From the lumen of the gut outward, the wall of small intestine has the basic arrangement of four layers, ie, the mucosa, the submucosa, the muscularis and the serosa. It is well accepted that the majority of serotonin (>95%) in the body is synthesized within the intestinal mucosa.29 The reason for no significant difference in 5-HIAA level among the different groups might be the whole intestinal tissue, instead of the intestinal mucosa (the main source of 5-HT), was homogenized and analyzed in our experiment. The inhibition of 5-HIAA by [6]-gingerol in the ileum might reach statistical significance if the mucosa had been isolated and analysed separately from other layers of small intestine.
More than 90% of the 5-HT in the body is produced and released into circulation by EC cells.30 In the rate-limiting and first step of serotonin production, TPH converts tryptophan to 5-hydroxytryptophan, followed by reduction to 5-HT by amino acid decarboxylase. After cisplatin administration, the TPH-1 and TPH-2 content increased in ileum and medulla oblongata, respectively, suggesting that cisplatin could accelerate 5-HT synthesis by upregulating TPH expression. Interestingly, [6]-gingerol significantly reduced TPH-1 and TPH-2 levels, which may be a mechanism by which the compound attenuates cisplatin-induced 5-HT levels.
5-HT is released into the synaptic cleft as a neurotransmitter, followed by binding to 5-HT3 receptor in the postsynaptic membrane. The excess 5-HT binds to SERT, which transports the neurotransmitter to presynaptic terminals, and is stored in vesicles or metabolized by MAO-A.31–33 As 5-HIAA is a metabolite of 5-HT, it is an important indicator in 5-HT metabolism and MAO-A levels can be an indicator of 5-HT levels. Cisplatin administration reduced MAO-A content in ileum and medulla oblongata, but this was reversed by [6]-gingerol, providing further evidence that this compound reduces 5-HT level during chemotherapy.
Conclusion
In summary, our results showed that when treated with [6]-gingerol, the intake of kaolin of rats injected intraperitoneally with cisplatin was significantly reduced compared with the model group, indicating that [6]-gingerol was effective in the prevention of CINV. [6]-gingerol attenuated cisplatin-induced emesis in a pica model of rats via a putative mechanism by which 5-HT levels are modulated by the TPH/MAO-A/SERT/5-HT/5-HT3 receptor system.
Funding Statement
This work was supported by the National Natural Science Foundation of China; Grant number (81673779).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. J Lancet Oncol. 2005;6(2):93–102. doi: 10.1016/S1470-2045(05)01735-3 [DOI] [PubMed] [Google Scholar]
- 2.Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85–93. doi: 10.1097/PPO.0b013e31816a0f07 [DOI] [PubMed] [Google Scholar]
- 3.Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. J Eur J Cancer. 2003;39(8):1074–1080. doi: 10.1016/S0959-8049(02)00674-3 [DOI] [PubMed] [Google Scholar]
- 4.Haniadka R, Popouri S, Palatty PL, Arora R, Baliga MS. Medicinal plants as antiemetics in the treatment of cancer: a review. Integr Cancer Ther. 2012;11(1):18–28. doi: 10.1177/1534735411413266 [DOI] [PubMed] [Google Scholar]
- 5.Minami M, Endo T, Hirafuji M, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99(2):149–165. doi: 10.1016/S0163-7258(03)00057-3 [DOI] [PubMed] [Google Scholar]
- 6.Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. J Ann Oncol. 2011;22(1):30–38. doi: 10.1093/annonc/mdq600 [DOI] [PubMed] [Google Scholar]
- 7.Lelyveld NV, Linde JT, Schipper MEI, Samsom M. Regional differences in expression of TPH-1, SERT, 5-HT3 and 5-HT4 receptors in the human stomach and duodenum. J Neurogastroenterol Motil. 2007;19(5):342–348. doi: 10.1111/j.1365-2982.2006.00891.x [DOI] [PubMed] [Google Scholar]
- 8.Carkaci-Salli N, Salli U, Kuntz-Melcavage KL, et al. TPH2 in the ventral tegmental area of the male rat brain. Brain Res Bull. 2011;84(6):376–380. doi: 10.1016/j.brainresbull.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu QY, Yang Q, Sun WM, et al. Discovery and Characterization of Novel Tryptophan Hydroxylase Inhibitors That Selectively Inhibit Serotonin Synthesis in the Gastrointestinal Tract. J Pharmacol Exp Therapeutics. 2008;325(1):47–55. doi: 10.1124/jpet.107.132670 [DOI] [PubMed] [Google Scholar]
- 10.Niesler B, Kapeller J, Hammer C, Rappold GJP. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. J Pharmacogenomics. 2008;9(5):501–504. doi: 10.2217/14622416.9.5.501 [DOI] [PubMed] [Google Scholar]
- 11.Ryan JL, Heckler CE, Roscoe JA, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20(7):1479–1489. doi: 10.1007/s00520-011-1236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol. 1997;57(2):93–96. doi: 10.1016/S0378-8741(97)00054-8 [DOI] [PubMed] [Google Scholar]
- 13.Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. J Arch Pharm Res. 2009;32(4):565–573. doi: 10.1007/s12272-009-1413-9 [DOI] [PubMed] [Google Scholar]
- 14.Ullah I, Subhan F, Ayaz M, et al. Anti-emetic mechanisms of zingiber officinale against cisplatin induced emesis in the pigeon; behavioral and neurochemical correlates. BMC Complement Altern Med. 2015;15(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Liu J, Zhang Y. Zingiber officinaleResearch Progress on Chemical Constituents of Roscoe. J BioMed Res Int. 2019;1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu XX, Liu X, Chu Y, Chen WX, Zhang KW, Wu H. Antiemetic activity of effective extract and bioactive compounds in ginger. J Zhongguo Zhong Yao Za Zhi. 2016;41(5):904–909. [DOI] [PubMed] [Google Scholar]
- 17.Konmun J, Danwilai K, Ngamphaiboon N, Sripanidkulchai B, Sookprasert A, Subongkot S. A Phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med Oncol. 2017;34(4):69–78. doi: 10.1007/s12032-017-0931-4 [DOI] [PubMed] [Google Scholar]
- 18.Feng X, Cheng Q, Meng Q, Yang Y, Nie K. Effects of ondansetron and [6]-gingerol on pica and gut microbiota in rats treated with cisplatin. J Drug Des Devel Ther. 2019;13:2633–2641. doi: 10.2147/DDDT.S211845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn CC, Kimball BA, Hong W, et al. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. J Plos One. 2013;8(4):1–16. doi: 10.1371/annotation/1c75cd5d-9dde-4ace-8524-a4980745e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45(4):817–821. doi: 10.1016/0091-3057(93)90126-E [DOI] [PubMed] [Google Scholar]
- 21.Dranitsaris G, Molassiotis A, Clemons M, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. J Ann Oncol. 2017;28(6):1260–1267. doi: 10.1093/annonc/mdx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips S, Ruggier R, Hutchinson SE. Zingiber officinale (ginger)–an antiemetic for day case surgery. Anaesthesia. 1993;48(8):715–717. doi: 10.1111/j.1365-2044.1993.tb07188.x [DOI] [PubMed] [Google Scholar]
- 23.Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56(2):234–238. doi: 10.1002/pbc.22778 [DOI] [PubMed] [Google Scholar]
- 24.Qian QH, Yue W, Chen WH, Yang ZH, Liu ZT, Wang YX. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. J Chin Med J. 2010;123(4):478–484. [PubMed] [Google Scholar]
- 25.Qian W, Cai X, Wang Y, et al. Effect of Gingerol on Cisplatin-Induced Pica Analogous to Emesis Via Modulating Expressions of Dopamine 2 Receptor, Dopamine Transporter and Tyrosine Hydroxylase in the Vomiting Model of Rats. J Yonago Acta Medica. 2016;59(2):100–110. [PMC free article] [PubMed] [Google Scholar]
- 26.Tian L, Qian WB, Qian QH, Zhang W, Cai XR. Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J Nat Med. 2020;74(2):353–370. doi: 10.1007/s11418-019-01372-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walstab J, Krüger D, Stark T, et al. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT receptors of enteric neurons. Neurogastroenterol Motil. 2013;25(5):439. doi: 10.1111/nmo.12107 [DOI] [PubMed] [Google Scholar]
- 28.Jin Z, Lee G, Kim S, Park CS, Park YS, Jin YH. Ginger and its pungent constituents non-competitively inhibit serotonin currents on visceral afferent neurons. J Korean J. 2014;18(2):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer NJ, Keating DJ. Is There a Role for Endogenous 5-HT in Gastrointestinal Motility? How Recent Studies Have Changed Our Understanding. Vol. 11 Springer International Publishing; 2016. [DOI] [PubMed] [Google Scholar]
- 30.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003;100(23):13525–13530. doi: 10.1073/pnas.2233056100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keating E, Lemos C, Monteiro R, Azevedo I, Martel F. The effect of a series of organic cations upon the plasmalemmal serotonin transporter, SERT. J Life Sci. 2004;76(1):103–119. doi: 10.1016/j.lfs.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 32.Trisha J, Jason N, Kate P, Paul B. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Best J, Nijhout HF, Reed M. Serotonin synthesis, release and reuptake in terminals: a mathematical model. Theor Biol Med Model. 2010;7(1):34–59. doi: 10.1186/1742-4682-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]