Abstract
Background
There has been increasing reports associating the coronavirus disease 2019 (COVID-19) with thromboembolic phenomenon including ischemic strokes and venous thromboembolism. Cerebral venous thrombosis (CVT) is a rare neurovascular emergency that has been observed in some COVID-19 patients, yet much remains to be learnt of its underlying pathophysiology.
Objective
We present a case series of local patients with concomitant COVID-19 infection and CVT; and aim to perform a systematic review of known cases in the current literature.
Methods
We describe two patients with concomitant COVID-19 infection and CVT from a nationwide registry in Singapore. We then conducted a literature search in PubMed and Embase using a suitable keyword search strategy from 1st December 2019 to 11th June 2020. All studies reporting CVT in COVID-19 patients were included.
Results
Nine studies and 14 COVID-19 patients with CVT were studied. The median age was 43 years (IQR=36-58) and majority had no significant past medical conditions (60.0%). The time taken from onset of COVID-19 symptoms to CVT diagnosis was a median of 7 days (IQR=6-14). CVT was commonly seen in the transverse (75.0%) and sigmoid sinus (50.0%); 33.3% had involvement of the deep venous sinus system. A significant proportion of patients had raised D-dimer (75.0%) and CRP levels (50.0%). Two patients reported presence of antiphospholipid antibodies. Most patients received anticoagulation (91.7%) while overall mortality rate was 45.5%.
Conclusions
The high mortality rate of CVT in COVID-19 infection warrants a high index of suspicion from physicians, and early treatment with anticoagulation should be initiated.
Key Words: Anticoagulation, Cerebral venous thrombosis, Coronavirus disease 2019, Mortality, Systematic review
Introduction
Cerebral venous thrombosis (CVT) is a rare neurovascular emergency, and its incidence is estimated to be around 1.6 cases per 100,000 per year in a population-based study.1 While the occurrence of arterial acute ischemic stroke (AIS) as a devastating neurologic complication is increasingly being reported on, there have been sporadic case reports and case series examining the association between CVT and coronavirus disease 2019 (COVID-19) infection.2 Several studies have illustrated a higher preponderance to venous thromboembolic manifestations, such as deep vein thrombosis and pulmonary embolism, especially in the critically ill cohorts.3 , 4 However, the true incidence of cerebral venous thrombosis occurrence in COVID-19 patients remains unknown. Furthermore, the underlying mechanism of CVT in COVID-19 patients remains debatable. It has been proposed that COVID-19 may induce a prothrombotic state, as supported by the elevated levels of factor VIII, fibrinogen, D-dimer and circulating prothrombotic microparticles such as antiphospholipid antibodies.5, 6, 7
Given the rapidly rising number of COVID-19 infections globally and the severity of CVT as a complication, it is critical to derive a more in-depth understanding of their association, clinical manifestations, severity and treatment outcomes
In this report, we present a case series of patients with concomitant COVID-19 infection and CVT from Singapore. We subsequently perform a systematic review of the current literature to evaluate patient demographics, clinical characteristics, prothrombotic workup, neuroimaging findings, treatment and outcomes of COVID-19 patients who have suffered CVT.
Methods
Local case series and incidence
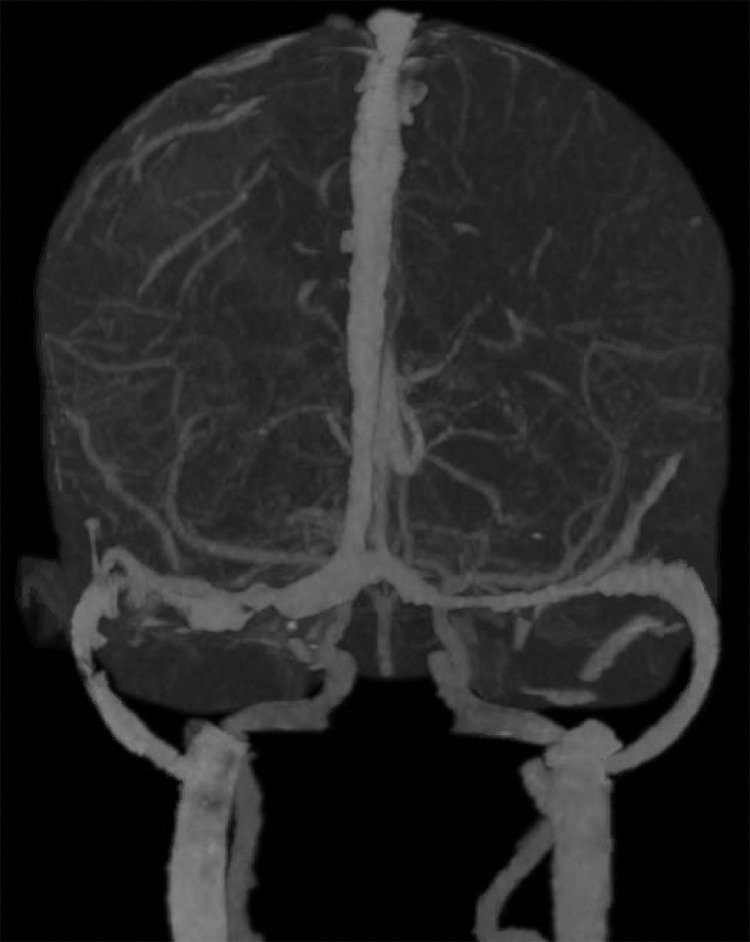
We identified 3 patients with concomitant COVID-19 infection and CVT from a nationwide registry capturing cases of neurological manifestations in COVID-19 from all public healthcare institutions in Singapore. In 1 patient, CVT was found after significant trauma and was excluded from this case series and analyses (Fig. 1). The total number of COVID-19 infections at the time of reporting was obtained from the Ministry of Health, Singapore. Incidence was calculated as the proportion of patients with CVT amongst all COVID-19 cases. We describe the remaining 2 cases and include details on their clinical presentation, prothrombotic workup, neuroimaging findings, treatment and outcomes. Ethics approval was obtained from the Singapore Health Services (CIRB 2020/2410) institutional review board.
Fig. 1.
CT venogram demonstrating a filling defect in the right sigmoid sinus.
Methods of systematic review
Literature search strategy
We conducted the systematic review in accordance with the PRISMA guidelines. A comprehensive literature search was performed on PubMed and Embase from 1st December 2019, before the first case of COVID-19 was reported, to 11th June 2020. The search strategy consisted of different combinations of the following search terms: COVID, Coronavirus, Severe acute respiratory syndrome coronavirus 2, CoV, SARS-CoV-2, cerebral vein thrombosis, cerebral venous thrombosis, cerebro-venous thrombosis, cerebrovenous thrombosis, cerebral sinus thrombosis, cerebral sinus venous thrombosis, cerebral sinovenous thrombosis, venous sinus thrombosis, sinus thrombosis, sinovenous thrombosis, CVT, cavernous sinus thrombosis, lateral sinus thrombosis, superior sinus thrombosis, cortical sinus thrombosis, cortical venous thrombosis, cortical vein thrombosis, dural sinus thrombosis, dural venous thrombosis, dural vein thrombosis, deep cerebral vein thrombosis, deep cerebral venous thrombosis, deep cerebral thrombosis, intracranial sinus thrombosis, intracranial venous thrombosis, intracranial vein thrombosis, thrombus, clot, prothrombotic and coagulopathy. The search was performed by two independent reviewers (YKT & CG). During study screening for relevance, any disagreement was resolved by consensus with the senior author (LY). The reference lists of these articles were also screened, and hand searched to identify further relevant studies.
Study and cohort selection
All studies (case reports, case series and observational cohort studies) that reported cerebral venous thrombosis in COVID-19 patients were included during the initial search. We subsequently excluded all studies that reported only acute ischemic stroke, hemorrhagic stroke and those without an English translation.
Data extraction
Relevant quantitative data were extracted by two authors (YKT & CG) in the form of absolute frequencies of events or absolute numbers when appropriate. Where available, the data included incidence rate, individual case data: patient demographics (age, gender), country of study origin, comorbidities, COVID-19 symptoms, duration of neurologic deficit from symptom onset, National Institutes of Health Stroke Scale (NIHSS), location of thrombosis, relevant laboratory results (white cell count, absolute lymphocyte count, platelets, prothrombin time (PT), activated partial thromboplastin time (aPTT), c-reactive protein (CRP), D-dimer, fibrinogen, lactate dehydrogenase (LDH), and ferritin), treatment (anticoagulation, endovascular treatment, or neurosurgery) and inpatient mortality. Neuroimaging data reported in text or image format was reviewed by two neurologists (TTM & LY).
Risk of bias assessment
The quality of the included case reports and case series was assessed using the guidelines recommended by the Johanna Briggs Institute. For case series, each study was assessed if there were clear inclusion criteria, measured the condition in a reliable way, had a valid method to identify condition, had consecutive or complete inclusion and reported patient demographics, clinical information, and outcomes. For case reports, each study was assessed if the following was well described: patient demographics, medical history, clinical conditions, diagnostic tests, treatment, post-treatment clinical condition, adverse events and takeaway lessons.
Statistical analyses
All data analysis was conducted using IBM SPSS Statistics 25.0 software. Individual case data were pooled with patient demographics, COVID-19 symptoms, stroke characteristics, treatment and clinical outcomes.
Results
Local case series and incidence
As of 3 July 2020, Singapore had reported 44,479 confirmed COVID-19 cases. Of these COVID-19 cases, we describe 2 patients with concomitant CVT and COVID-19 infection, deriving an incidence of 2/44,479 (0.0045%) or 4.5 per 100,000 COVID-19 cases.
Case 1:
A mid-thirties man with no past medical history of note and a non-smoker, presented with pleuritic left sided chest pain of 4 days duration. He also complained of fever and chills of 1-day duration. He visited his general practitioner and was subsequently referred to the emergency department. He was living in a dormitory with COVID-19 positive patients. Clinical examination at the emergency department was unremarkable and a chest radiograph was normal. Despite his lack of physical findings, the nasal and pharyngeal swabs were positive for COVID-19 via RT-PCR test. Other laboratory results, including the full blood count, C-reactive protein, D-dimer levels, and anticardiolipin IgG were within normal limits (Fig. 1 ).
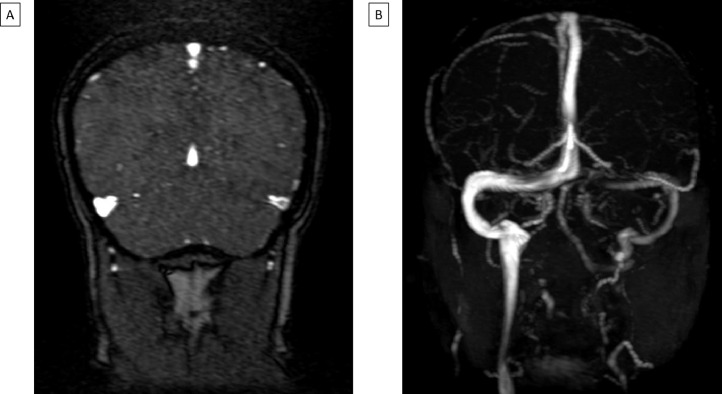
The patient started to develop a generalized non-remitting headache on the day of presentation, which did not improve with analgesia. Magnetic resonance imaging (MRI) of the brain with contrast and magnetic resonance venogram (MRV) was performed which demonstrated a left transverse and sigmoid sinus thrombosis (Fig. 2 ). No abnormal susceptibility signal to denote an intracranial hemorrhage was noted on the GRE sequence. Lumbar puncture was performed to exclude meningitis in view of the presence of headache and fever. Cerebrospinal fluid (CSF) analysis demonstrated 3 nucleated cells per mm3 (normal 0-5 cells/mm3), 1000 red blood cells per mm3 (normal 0-5 cells/mm3) and raised protein at 0.76 g/L (normal 0.1-0.4g/L). CSF was negative for COVID-19 on RT-PCR test. The patient was initiated with dabigatran for the treatment of his cerebral venous thrombosis. He was discharged well. A follow up repeat CT venogram was performed four weeks after the initial MRV which demonstrated complete resolution of the venous thrombosis.
Fig. 2.
MR venogram demonstrating the following: A - Filling defect in the left transverse sinus; B - Left transverse and sigmoid sinus CVT.
Case 2:
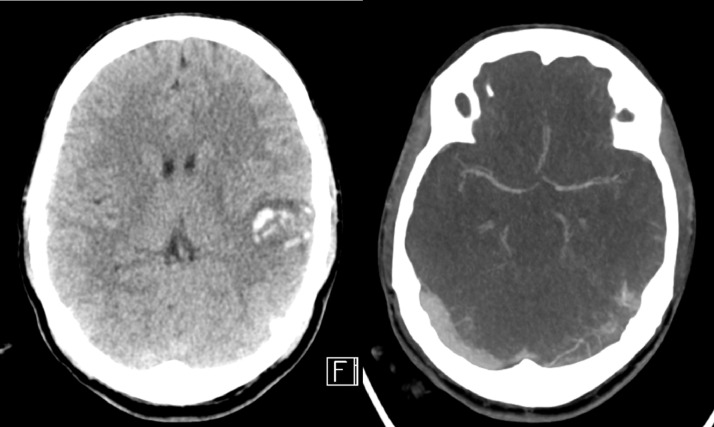
A man in his late thirties with no significant past medical history presented with a first-onset seizure. He stayed in the dormitory and was witnessed to have a generalized tonic-clonic convulsion that lasted around 5 min by his friends. During this episode, he fell and struck the back of his head on the floor. An ambulance transferred him to the emergency department where he was found to be confused and restless, with a GCS of 13. Clinical examination revealed a right parietal scalp hematoma, but no fever or any abnormal respiratory findings. The chest radiograph was normal. An urgent CT brain and CT venogram revealed a thrombosis in the left transverse and sigmoid sinuses, extending into the left internal jugular vein, as well as an acute left temporal lobe intraparenchymal hematoma (Fig. 3 ). Laboratory tests showed raised D-dimer (4.6 mg/L), CRP (7.3 mg), elevated homocysteine levels (119.2 umol/L, normal values 5.0 - 15.0 umol/L), presence of lupus anticoagulant and low protein C activity (53%, normal 83-144%). Anticardiolipin and anti-B2-glycoprotein I IgG and IgM were within normal limits. In view of the outbreak situation in the Singapore dormitories, COVID-19 screening was performed, where nasal and pharyngeal swabs were positive for the reverse transcriptase-polymerase chain reaction (RT-PCR) test. Given the extensive cerebral venous thrombosis, the patient was initiated on anticoagulation with IV heparin, in addition to IV levetiracetam for seizures and cobalamin replacement. A week into his admission, the patient deteriorated neurologically. Urgent CT brain demonstrated increased size of hemorrhage and edema with mid-line shift, and decompressive craniectomy was performed emergently. Despite surgical intervention, he passed away the following day.
Fig. 3.
CT brain and CT venogram revealed an acute left temporal lobe intraparenchymal hematoma and an underlying thrombosis of the left transverse sinus.
Systematic review results
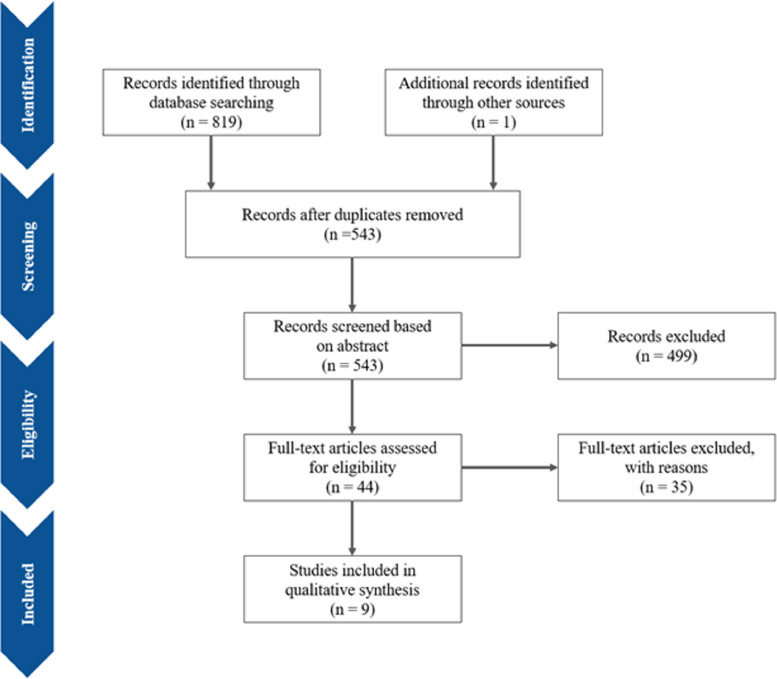
The electronic search strategy yielded 819 studies, with one additional study found after screening through reference lists. After removing duplicates, 543 records were screened based on abstracts, of which a further 499 studies were excluded as they reported only acute ischemic stroke, hemorrhagic stroke or did not have an English translation. Lastly, 35 articles were excluded after full-text review (reported only on coagulopathy (n=13), venous thromboembolism events unrelated to CVT (n=11), were editorials/opinion letters to editors (n=5), were reviews (n=2), reported only on acute ischemic stroke (n=2), pulmonary embolism (n=1), cardiopulmonary complications (n=1)). Overall, nine studies with 12 patients from the existing literature were included in this systematic review. The study selection process is shown in the PRISMA flowchart (Fig. 4 )8, 9, 10, 11, 12, 13, 14, 15, 16, while Supplementary Table 1 presents the grading of the studies. Adding 2 patients from our current local case series, we included a total of 14 patients with COVID-19 infection and CVT for descriptive analyses.
Fig. 4.
PRISMA flowchart.
The clinical characteristics of the 14 patients were shown in Table 1 . The majority of the patients were middle-aged (median 43 years, IQR=36-58 years) and male (64.3%). Most patients had no significant past medical conditions prior to the CVT episode (57.1%). Symptoms related to COVID-19 infection were commonly observed in these patients including fever (75.0%), acute respiratory infection (ARI) symptoms (50.0%), and dyspnea (41.7%). Two patients (14.3%) did not display any symptoms related to COVID-19 infection. CVT was observed in patients across a spectrum of COVID-19 disease severity, with 35.7% having mild infection, 28.6% of moderate severity and 35.7% with severe or critical illness. In addition, patients with CVT displayed a range of neurological symptoms attributed to the CVT such as altered mental status (38.5%), headache (30.8%), hemiparesis (30.8%), and reduced consciousness level (30.8%). The time taken from onset of COVID-19 symptoms to diagnosis of CVT was a median of 7 days (IQR=6-14 days).
Table 1.
COVID-19 patients with cerebral venous thrombosis.
| Study | Patient | Country | Age (years) | Sex | Co-morbidities | COVID-19 symptoms | COVID-19 severity* | Neurological symptoms | Days from COVID-19 symptoms | Location of CVT | ICH on Imaging | Prothrombotic work-up | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavalcanti | 1 | US | 38 | M | Mild ASD | Diarrhea, vomiting Fever Headache | Critical | AMS | 10 | Cortical veins, R internal cerebral vein, straight sinus, distal superior sagittal sinus, torcular and R transverse sinus | Nil | EVT ACC | Death | |
| 2 | 41 | F | Nil | Mild | AMS Aphasia | Vein of Galen, internal cerebral veins, distal straight sinus | Yes | Raised D-dimer | EVD ACC | Death | ||||
| 3 | 23 | M | Nil | ARI, fever Headache Lethargy | Critical | GCS drop | 7 | Yes | Raised D-dimer, ferritin | Nil | Death | |||
| Klein | 4 | 29 | F | Nil | ARI, dyspnea, fever Headache | Mild | Post-ictal AMS Aphasia Facial palsy Seizure | >7 | Distal L transverse and sigmoid sinus | Yes | Raised CRP, D-dimer, LDH, anti-CL IgM Low ferritin | ACC AED | Alive | |
| Garaci | 5 | Italy | 44 | F | Nil | ARI, dyspnea, fever | Severe | AMS Aphasia Headache R hemiparesis | 14 | Vein of Galen, L internal cerebral vein, straight sinus | Nil | Raised D-dimer Normal anti-CL, anti-B2gp1, anti-dsDNA IgM | ACC | |
| Malentacchi | 6 | 81 | M | Prostate CA CLL | Dyspnea | Critical | AMS GCS drop | R sigmoid sinus | Nil | Raised CRP, D-dimer, LDH Normal fibrinogen | ACC | Death | ||
| Hughes | 7 | UK | 59 | M | Obesity HTN DM | Fever Headache | Moderate⁎⁎ | Aphasia Dysarthria R hemiparesis R hypoesthesia | 4 | R transverse and sigmoid sinuses | Nil | Raised fibrinogen, CRP, ESR | ACC | Discharged |
| Dahl-Cruz | 8 | Spain | 53 | M | Nil | Anosmia, dysgeusia ARI, dyspnea, fever Headache | Moderate⁎⁎ | Ataxia R hemiparesis R hypoesthesia | 7 | Superior sagittal and R transverse sinus | Yes | Raised CRP, D-dimer | ACC AED | Discharged |
| Poillon | 9 | France | 62 | F | Obesity | ARI, dyspnea, fever | Moderate | Blurry vision GCS drop Headache R hemiparesis | 15 | Vein of Galen, internal cerebral vein, straight sinus, L transverse sinus | Yes | Raised D-dimer | ||
| 10 | 54 | F | Breast CA | ARI, fever Lethargy | Moderate | Headache | 14 | L transverse sinus | Yes | Raised CRP, D-dimer Normal LDH | ||||
| Hemasian | 11 | Iran | 65 | M | Nil | Nil | Mild | GCS drop Seizure | R transverse and sigmoid sinuses | Yes | Raised LDH Normal CRP, ESR | ACC AED | Discharged | |
| Li | 12 | China | 32 | M | Smoking | Severe | 15 | ACC | Alive | |||||
| Tu | 13 | SG | 30s | M | Nil | Fever | Mild | Headache | 1 | L transverse and sigmoid sinuses | Nil | Normal CRP, D-dimer, anti-CL IgM and IgG | ACC | Discharged |
| 14 | 30s | M | Nil | Nil | Mild | Seizure | 1 | L transverse and sigmoid sinuses, extending into the internal jugular vein. | Yes | Raised CRP, D-dimer, homocysteine, LAC, low protein C activity Normal protein S, anti-CL, anti-β2gp1 IgM and IgG | ACC AED | Death |
Abbreviations: ACC – anticoagulation; AED – anti-epileptic drug; AMS – altered mental status; ARI – acute respiratory infection symptoms; ASD – autism spectrum disorder; CA – cancer; COVID-19 – coronavirus disease 2019; CVT – cerebral venous thrombosis; DM – diabetes mellitus; EVD – external ventricular drain; EVT – endovascular thrombectomy; F – female; GCS – Glasgow coma scale; ICH – intracranial hemorrhage; LAC – lupus anticoagulant; M – male; US – United States; UK – United Kingdom
ARI includes sore throat, rhinorrhoea, blocked nose, cough, myalgia; Prothrombotic work-up includes CRP, D-dimer, ESR, ferritin, fibrinogen, LDH, anti-dsDNA / antiphospholipid antibodies where available
Severity according to “Clinical management of COVID-19” Interim guidance 27/5/2020 from WHO
Moderate but has bilateral infiltrates on CXR/CT thorax
Presence of CVT in the transverse sinus (75.0%) and sigmoid sinus (50.0%) was seen in the majority of the cases (Figure 1). Involvement of the deep venous sinus system was also commonly observed, with 33.3% having internal cerebral vein or straight sinus thrombosis. Intracranial bleeding was seen on initial neuroimaging in 61.5% of patients.
In terms of laboratory findings, a significant proportion of patients had raised D-dimer (75.0%) and CRP levels (50.0%). Antibody testing was reported in four patients. Patient 4 had raised anti-cardiolipin IgM, while Patient 14 had positive lupus anticoagulant but normal anti-cardiolipin and anti-B2-glycoprotein-I IgM and IgG titers. In contrast, Patient 5 had normal anti-cardiolipin, anti-B2-glycoprotein-I and anti-dsDNA IgM titers while Patient 13 had normal anti-cardiolipin IgM and IgG titers.
In the 12 patients with information provided on CVT treatment, most received anticoagulation (91.7%). Of note, Patient 1 underwent percutaneous venous mechanical thrombectomy with adjunct catheter-directed thrombolysis, while Patient 2 had an external ventricular drain inserted due to interval development of venous infarction with hemorrhagic transformation, intraventricular hemorrhage, and obstructive hydrocephalus. Overall, mortality rate reported amongst COVID-19 patients with CVT in this study was 45.5%, while mortality rate amongst the subgroup of mild COVID-19 patients was 40.0%.
Discussion
This systematic review highlights the occurrence of CVT in patients across the spectrum of severity of COVID-19 infection. Of note, the majority of these patients were middle-aged and without significant comorbidities. The COVID-19 pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first recognized in Wuhan after a cluster of atypical pneumonia was reported.17 Since then, the pandemic has grown rapidly and reached global proportions, overwhelming healthcare systems around the world. Being a novel coronavirus, much is to be learnt about its various clinical manifestations. Interestingly, multiple studies have reported neurological manifestations of the COVID-19 infection, whereby 36.4% of patients were found to have neurological symptoms and that those with severe infection were further associated with strokes and coagulopathy.7 , 18 Indeed, new evidence seems to suggest a tendency for severe COVID-19 infection to trigger cytokine storms which leads to a proinflammatory and prothrombotic state.19 These patients may thus suffer from thromboembolic events, both arterial and venous13 , 20 , 21. However, it is surprising that in this systematic review of patients with concomitant COVID-19 and CVT, a large majority were relatively young and healthy patients with relatively few co-morbidities. In addition, a significant proportion of CVT patients only displayed mild to moderate severity of COVID-19 infection, thus indicating that a hypercoagulable state may be present even in mild infection.
We propose that the presence of COVID-19 should be screened in patients presenting with neurological complications CVT without prominent respiratory symptoms nor known prothrombotic risk factors. From our systematic review, four (28.6%) patients (case 2, 11, 13 and 14) presented initially with neurological symptoms and all had no prothrombotic risk factors. Moreover, COVID-19 could have been potentially missed as all 4 had mild respiratory symptoms. For our local 2 cases (13, 14), COVID-19 was only diagnosed due to pro-active testing with RT-PCR, as they were living in workers dormitories where other cases of COVID-19 were known to occur. Additionally, the incidence of CVT in COVID-19 appears to be approximately 3 times higher than previously published population incidence (4.5 per 100,000 vs 1.6 per 100,000).
The presence of concomitant COVID-19 infection and CVT seems to portend a much poorer prognosis than each condition individually. This study found an unusually high mortality rate of 45.5% in COVID-19 patients diagnosed with CVT, in contrast to the known mortality rates of CVT at 15% and current estimates of COVID-19 case fatality rate of 5.6%.22 , 23 One possibility for the poorer outcome in these patients may be due to the location of the occluded sinuses, which showed a higher predilection for the deep venous sinuses to be involved. Thrombus in the internal cerebral veins or straight sinus was seen in 33.3% of the patients and these sites of thrombosis are typically rare and have a higher morbidity and mortality rate24 Consequently, this may be a contributing factor to the high mortality reported in our series, where 50% of the patients who died had involvement of the deep cerebral veins.
Many studies in the current COVID-19 literature have reported cases of arterial and venous thromboembolism, attributed to deranged coagulopathy, extensive systemic inflammation and endothelial dysfunction that are associated with severe COVID-19 [25]. The incidence of venous thromboembolism reported thus far has ranged from 4.4% to 19.7%, with a remarkably high rate seen in the subgroup of severe COVID-19 patients in ICU reaching up to 69%.4 , 26 , 27 This has led to several recommendations being made for the use of anticoagulation in hospitalised COVID-19 patients, where those treated with anticoagulation were observed to have improved outcomes.19 , 28 At the same time, anticoagulation with low molecular weight or unfractionated heparin remains the mainstay of treatment in CVT.29 Given the higher mortality rates of CVT seen in COVID-19 infection, there may be a role for early initiation of anticoagulation in patients suspected to have CVT or predisposed to its formation. Amongst the patients treated with anticoagulation, 60.0% survived which is an encouraging sign. Lastly, thrombectomy is a therapeutic option in patients with severe CVT, not unlike those with concomitant COVID-19.24 In this study, while the only patient (case 1) who underwent endovascular treatment died, this may be related to the need for a salvage endovascular maneuver in a deteriorating patient despite anticoagulation rather than a reflection of the inability of endovascular treatment in COVID-19 patients with CVT.
Limitations
The following limitations of this study should be acknowledged. Firstly, the patients included in this study were from case reports and case series which are retrospective in nature and may be limited by data availability or accuracy. Secondly, the number of COVID-19 patients with CVT included in the analysis is small (n=14), which did not allow for further comparative analysis. The actual number of COVID-19 patients with CVT may be significantly higher but not detected as clinical suspicion may be low in a large number of COVID-19 patients who are intubated and without appropriate imaging done. Thirdly, there was insufficient data available from published studies to evaluate the overall incidence of CVT. However, we were able to calculate the local incidence of CVT in COVID-19 patients (4.5 per 100,000), which is higher than the previously published incidence of 1.6 per 100,000 in other population-based studies.1 Lastly, while the aforementioned factors limit the generalizability of the results, it is nevertheless useful in highlighting a likely association between COVID-19 infection and CVT, serving as a basis for future large cohort studies.
Conclusion
Whilst infrequently reported, CVT has been found to occur in patients with COVID-19 infection. The unusually high mortality rate warrants a high index of suspicion from physicians, and early treatment with anticoagulation should be initiated in these settings.
Declaration of Competing Interest
There are no conflict of interests.
Acknowledgments
Funding
None
Availability of data and material
All data relevant to the study are included in the article or uploaded as supplementary information.
Patient consent for publication
Waiver of consent was granted by the Singapore Health Services institutional review board as the data collected was based on routine clinical care and in view of the nature of the pandemic.
Acknowledgements
None
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2020.105379.
Appendix. Supplementary materials
References
- 1.Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 2016;47(9):2180–2182. doi: 10.1161/STROKEAHA.116.013617. [DOI] [PubMed] [Google Scholar]
- 2.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, Shah S. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip MJ, Van Der Meer NJ, Arbous MS, Gommers DA, Kant KM, Kaptein FH, van Paassen J, Stals MA, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thrombosis Res. 2020 doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thrombosis Res. 2020 doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Tripodi A. Hypercoagulability of COVID‐19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thrombosis Haemostasis. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valderrama E.V., Humbert K., Lord A., Frontera J., Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020 doi: 10.1161/strokeaha.120.030153. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Zhang S. (2020). Coagulopathy and antiphospholipid antibodies in patients with Covid-19. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161262/ [DOI] [PMC free article] [PubMed]
- 8.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA, Radmanesh A, Nelson PK. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl-Cruz F, Guevara-Dalrymple N, López-Hernández N. [Cerebral venous thrombosis and SARS-CoV-2 infection] Rev Neurol. 2020;70(10):391–392. doi: 10.33588/rn.7010.2020204. [DOI] [PubMed] [Google Scholar]
- 10.Garaci F, Di Giuliano F, Picchi E, Da Ros V, Floris R. Venous cerebral thrombosis in COVID-19 patient. J Neurol Sci. 2020;414 doi: 10.1016/j.jns.2020.116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemasian H, Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol (Paris) 2020 doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein D.E., Libman R., Kirsch C., Arora R. Cerebral venous thrombosis: atypical presentation of COVID-19 in the Young. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Wang M., Zhou Y., Chang J., Xian Y., Mao L., Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malentacchi M, Gned D, Angelino V, Demichelis S, Perboni A, Veltri A, Bertolotto A, Capobianco M. Concomitant brain arterial and venous thrombosis in a COVID-19 patient. Eur J Neurol. 2020 doi: 10.1111/ene.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poillon G, Obadia M, Perrin M, Savatovsky J, Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol = J de Neuroradiologie. 2020 doi: 10.1016/j.neurad.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO | World Health Organization; 2020. Timeline of who's response to COVID-19.https://www.who.int/news-room/detail/29-06-2020-covidtimeline [Google Scholar]
- 18.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurology. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connors JM, & Levy JH. (2020). Thromboinflammation and the hypercoagulability of COVID-19. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32302453. [DOI] [PMC free article] [PubMed]
- 20.Tang N, Bai H, Chen X, Gong J, Li D, & Sun Z. (2020, April 27). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Retrieved from: https://onlinelibrary.wiley.com/doi/full/10.1111/jth.14817 [DOI] [PMC free article] [PubMed]
- 21.Tan YK, Goh C, Leow AS, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LL, Chan BP, Tan BY. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thrombosis Thrombolysis. 2020:1–9. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. The Lancet. Infectious Dis. 2020;20(7):773. doi: 10.1016/S1473-3099(20)30195-X. 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bousser M.G., Ferro J.M. Cerebral venous thrombosis: an update. The Lancet. Neurol. 2007;6(2):162–170. doi: 10.1016/S1474-4422(07)70029-7. 10.1016/S1474-4422(07)70029-7 [DOI] [PubMed] [Google Scholar]
- 24.Yeo L.L., Lye P.P., Yee K.W., Cunli Y., Ming T.T., Ho A.F., Sharma V.K., Chan B.P., Tan B.Y., Gopinathan A. Deep cerebral venous thrombosis treatment. Clin Neuroradiol. 2020 doi: 10.1007/s00062-020-00920-3. 10.1007/s00062-020-00920-3 [DOI] [PubMed] [Google Scholar]
- 25.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. 10.1007/s12975-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M., Bouman C., Beenen L., Kootte R.S., Heijmans J., Smits L.P., Bonta P.I., van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thrombosis Haemostasis: JTH. 2020 doi: 10.1111/jth.14888. 10.1111/jth.14888 Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thrombosis Haemostasis: JTH. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am College Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferro J.M., Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Current Cardiol Rep. 2014;16(9):523. doi: 10.1007/s11886-014-0523-2. 10.1007/s11886-014-0523-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.