Abstract
Purpose
Bardet-Biedl syndrome (BBS) is a ciliopathy with a wide spectrum of symptoms due to primary cilia dysfunction, including genitourinary developmental anomalies as well as impaired reproduction, particularly in males. Primary cilia are known to be required at the following steps of reproduction function: (i) genitourinary organogenesis, (ii) in fetal firing of hypothalamo-pituitary axe, (iii) sperm flagellum structure, and (iv) first zygotic mitosis conducted by proximal sperm centriole. BBS phenotype is not fully understood.
Methods
This study explored all steps of reproduction in 11 French male patients with identified BBS mutations.
Results
BBS patients frequently presented with genitourinary malformations, such as cryptorchidism (5/11), short scrotum (5/8), and micropenis (5/8), but unexpectedly, with normal testis size (7/8). Ultrasonography highlighted epididymal cysts or agenesis of one seminal vesicle in some cases. Sexual hormones levels were normal in all patients except one. Sperm numeration was normal in 8 out of the 10 obtained samples. Five to 45% of sperm presented a progressive motility. Electron microscopy analysis of spermatozoa did not reveal any homogeneous abnormality. Moreover, a psychological approach pointed to a decreased self-confidence linked to blindness and obesity explaining why so few BBS patients express a child wish.
Conclusions
Primary cilia dysfunction in BBS impacts the embryology of the male genital tract, especially epididymis, penis, and scrotum through an insufficient fetal androgen production. However, in adults, sperm structure does not seem to be impacted. These results should be confirmed in a greater BBS patient cohort, focusing on fertility.
Keywords: Bardet Biedl syndrome, male reproduction, male infertility, primitive ciliopathy, genetic
The multisystem involvement of the Bardet-Biedl syndrome (BBS, OMIM 209900) is related to the pathogenesis well recognized as a ciliopathy. Its estimated prevalence in North America and Europe ranges from 1:140 000 to 1:160 000 live births (1). In the last 20 years, 22 genes have been implicated in BBS (2, 3). All of them participate in primary cilia function by coding for proteins involved in the formation of the BBSome (4), the chaperonin complex (5), or the basal body, with an essential role in the intraflagellar traffic (6, 7).
BBS phenotype is characterized by retinal dystrophy, obesity, renal dysfunction, learning difficulties, genital anomalies, and postaxial polydactyly (a pathognomonic sign in this context). Hypogonadism is part of the major diagnosis criteria in males (8, 9). The generic term of hypogenitalism is often used in clinical series and micropenis, cryptorchidism, and delayed puberty are frequently reported (8, 10). However, hormonal and histological data are scarce and the origin of this hypogonadism (primary or hypogonadotropic) remains unclear. Large BBS series stipulate that males are almost invariably infertile (9) and only a few males with descendants are reported (8). By way of a confused extrapolation to the Kartagener syndrome, another ciliopathy (affecting motile cilia), BBS patients are supposed to be infertile by producing immotile spermatozoa (9).
Moreover, the potential impact of primary cilia on fertility is considered to result from the role of cilia structures at different steps of human reproduction function and from a partial common ultrastructural architecture of all cilia (11).
The present study explores the reproduction function in a cohort of BBS male patients with identified mutations within different BBS genes. The results are interpreted in the context of organogenesis of male genital organs, hormonal secretions of the hypothalamic-pituitary-gonadal axis, spermatogenesis, and sexual/reproductive behavior. To our knowledge, this is the first specific study of the reproduction function of BBS male patients, contrasting with the presumption that BBS patients are infertile.
Subjects and Methods
Subjects
Subjects were recruited by the reference center for rare eye diseases at the Strasbourg University Hospital, France (CARGO) and were explored for fertility by the Centre of Medicine and Biology of Reproduction at the Strasbourg University Hospital, France. In total, 11 adult male BBS patients underwent a complete exploration of the gonadotropic axis including clinical examination, comprehensive male hormonal panel testing, including a gonadotropin-releasing hormone (GnRH) stimulation test (0.1 mg Relefact; Sanofi-Aventis, Frankfurt am Main, Germany), urinary-genital ultrasonography, standard sperm analysis with a modified David classification of morphological anomalies (12), and TEM whenever sperm count was high enough. The clinical examination comprised: (i) palpation of testes to evaluate scrotum length and testes volume; (ii) examination of the penis to evaluate the presence of micropenis (when the length of flaccid penis was less than 2.5 SD—measurement taken from the pubic ramus to the distal tip of the gland) (13-15); (3) evaluation of the secondary sexual characteristics; and (4) presence of gynecomastia. This exploration was a part of the French National Research Protocol ethically approved by CPP “EST IV” (Strasbourg, France) (PHRC National Bardet-Biedl 2007 IDRCB 2007-A00868-45); it included also ophthalmic, olfactive, endocrine, and psychological explorations. Furthermore, 1 patient consulted with his wife for infertility and underwent an assisted reproductive technology (ART) program comprising 3 intrauterine artificial inseminations and one in vitro fertilization (IVF) assisted by intracytoplasmic sperm injection (ICSI).
Mutation Analysis
Genomic DNA was isolated and explored as previously described either by direct Sanger sequencing or by high-throughput sequencing (16).
Sperm analysis
All sperm analyses were performed in accordance with the World Health Organization recommendations (17); the morphology was analyzed using David modified criteria (18).
Transmission electron microscopy (TEM)
Spermatozoa were fixed at 4 °C in 2.5% glutaraldehyde for 2 hours, then centrifuged. The pellet was washed in sodium cacodylate buffer (0.1 M), then postfixed in 1% osmium tetroxide in cacodylate buffer and washed again. After a progressive dehydration in ethanol (50°-70°-95°-absolute [3 times]), propylene oxide (3 times), each step 10 minutes at room temperature, the pellet was embedded in epoxy (Epon) resin and ultrathin sections were obtained.
Olfactory evaluation
A senior ear nose throat (ENT) specialist evaluated all BBS patients according to the same protocol as described in Braun et al (19): (i) clinical evaluation of olfaction, (ii) ENT examination with nasal endoscopy and (iii) olfactometry using 2 different psychophysical methods, namely a suprathreshold evaluation of the olfaction and the UPSIT (Sensonics Inc., Haddon Heights, NJ) (20-22).
Verbal intelligence quotient evaluation
As described in Braun et al (19), the estimated Verbal Intelligence Quotient (VIQ) was calculated on the basis of the administration of subscales from the Wechsler Adult Intelligence Scale (WAIS-III) (23).
Results
The cohort analyzed in this study was composed of 11 male patients with a clear clinical and molecular diagnosis of BBS. Patients were from 18 to 39 years of age. The main elements of their medical history, clinical examination, and genotype are summarized in Table 1 (24, 25). All patients of this cohort presented primary and secondary recognized features of BBS: all presented with rod-cone dystrophy. Polydactyly or brachydactyly was observed for 9 of the 11. Similarly, 8 out of the 11 patients were obese and 3 of them had morbid obesity. Moreover, 2 patients had hyperinsulinism with glucose intolerance and 1 was diabetic. Interestingly, 7 out of 10 patients evaluated for olfaction presented an anosmia or severe hyposmia (19).
Table 1.
Clinical and Genetic Data for the Cohort
| Patient | Age (years) | Mutation | Clinical and Medical Background | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rod-cone dystrophy | Hands/feet anomalies | BMI | Micropenis | Cryptorchidism | Kidney cysts or ectasia | Diabetes, glucose intolerance |
Olfaction defect | VIQ | |||
| 1 | 22 | BBS1: c.[479G>A];[479G>A], p.[Arg160Gln];[Arg160Gln] | yes | brachydactyly | >30 | NA | no | no | no | mild hyposmia | 60.5 |
| 2 | 25 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | yes | no | <30 | no | no | no | no | normal | 64 |
| 3 | 24 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | yes | no | <30 | no | right | no | no | severe hyposmia | 62 |
| 4 | 36 | BBS1: c.[382C>T];[382C>T], p.[Gln128*];[Gln128*] | yes | polydactyly | >30 | yes | bilateral | no | no | NA | NA |
| 5 | 24 | BBS5: c.[413G>C];[413G>C], p.[Arg138Pro];[Arg138Pro] | yes | polydactyly | >30 | yes moderate | right | yes | no | severe hyposmia | 93 |
| 6 | 35 | BBS10: c.[271dup];[963T>G], p.[Cys91Leufs*5];[Tyr321*] | yes | polydactyly brachydactyly |
>40 | yes | bilateral | yes | no | anosmia | 90 |
| 7 | 38 | BBS1: c.[1169T>G];[1214_1215ins[MT113356]], p.[Met390Arg];[(Ala406Glnfs*47)] | yes | polydactyly | <25 | NA | no | yes | no | severe hyposmia | 62 |
| 8 | 22 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | yes | polydactyly | >40 | yes | no | yes | no | moderate hyposmia | 94.5 |
| 9 | 26 | BBS9: c.[703-?_886+?del];[832C>T], p.[Val235Phefs*6];[Arg278*] | yes | polydactyly | >40 | no but angulation | no | yes, mild form | yes | anosmia | 102 |
| 10 | 18 | BBS3: c.[535G>A];[535G>A], p.[Asp179Asn];[Asp179Asn] | yes | polydactyly | >30 | NA | no | yes | yes | anosmia | 88.5 |
| 11 | 39 | BBS12: c.[1037T>C];[1037T>C], p.[Ile346Thr];[Ile346Thr] | yes | polydactyly | >35 | yes | left | yes | yes | anosmia | 68.5 |
| Total patients | Median 25 | 11/11 | 9/11 | 8/11 | 5/8 | 5/11 | 7/11 | 3/11 | 9/10 | Median 78.5 |
|
Genital examination and hormonal status assessment were part of the protocol; however, 3 patients refused external genital clinical examination. Andrological data are summarized in Table 2 and hormonal status in Table 3: BBS patients frequently presented with a history of nondescended testis (5 out of 11; 3 unilateral and 2 bilateral) and/or a short scrotum at adult age (5 out of 8). The length of penis was much decreased (<−2.5 SD) in 5 out of the 8 examined patients, 3 had a normal length, and 1 presented an abnormally curved penis. Unexpectedly, the size of the testes was normal (7 out of 8). They all reported a normal spontaneous puberty. The systemic androgenic effects, evaluated by the general body hair and sexual androgen dependant body hair, was normal in 7 out of 8 examined patients.
Table 2.
Andrological Data
| Patient | Age (years) | Mutation | Medical background and andrological examination | Genito-urinary ultrasonography | Kidney ultersonography (kidney cysts or ectasia) | |||
|---|---|---|---|---|---|---|---|---|
| Cryptorchidism | micropenis | testis size | scrotum length | |||||
| 1 | 22 | BBS1: c.[479G>A];[479G>A], p.[Arg160Gln];[Arg160Gln] |
no | Examination not performed | Examination not performed | Examination not performed | normal | no |
| 2 | 25 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] |
no | no | normal | normal | not performed | no |
| 3 | 24 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] |
right | no | normal | normal | not performed | no |
| 4 | 36 | BBS1: c.[382C>T];[382C>T], p.[Gln128*];[Gln128*] |
bilateral | yes | Bilateral mild hypotrophy | short | right seminal vesicle non observed; right epididymal cyst | no |
| 5 | 24 | BBS5: c.[413G>C];[413G>C], p.[Arg138Pro];[Arg138Pro] |
right | yes moderate | normal | short | right epididymal cyst | yes |
| 6 | 35 | BBS10: c.[271dup];[963T>G], p.[Cys91Leufs*5];[Tyr321*] |
bilateral | yes | normal right, mild hypotrophy of left testis associated with varicocele; bilateral epididymal cysts | normal | right seminal vesicle non observed; left varicocele; bilateral epididymal cysts | yes |
| 7 | 38 | BBS1: c.[1169T>G]; [1214_1215ins[MT113356], p.[Met390Arg];[Ala406Glnfs*47] |
no | not performed | not performed | not performed | not performed | yes |
| 8 | 22 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | no | yes | normal | very short | enlargement of prostatic utricle | yes |
| 9 | 26 |
BBS9: c.[703-?_886+?del];[832C>T], p.[Val235Phefs*6];[Arg278*] |
no | no but angulation | normal | short | not performed | yes mild form |
| 10 | 18 | BBS3: c.[535G>A];[535G>A], p.[Asp179Asn];[Asp179Asn] | no | not performed | not performed | not performed | not performed | yes |
| 11 | 39 | BBS12: c.[1037T>C];[1037T>C], p.[Ile346Thr];[Ile346Thr] | left | yes | bilateral mild hypotrophy | short | not performed | yes |
Table 3.
Hormonal Status
| Patient | Age (years) | Mutation | Hormonal levels | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basal FSH IU/L [1.5-12] |
FSH at the peak (after GnRH stimulation) [1.5–2 × basal] |
Basal LH IUI/L [1.5-8.5] |
LH at the peak (after GnRH stimulation) [3-4 × basal] |
Basal total testosterone nmol/L [10.4-41.6] |
Testosterone/LH (nMol / IU) | PRL mIU/L [86-324] |
Leptin (µg/L) | |||
| 1 | 22 | BBS1: c.[479G>A];[479G>A], p.[Arg160Gln];[Arg160Gln] | 2.42 | 4.65 | 4.71 | 29.3 | 16.3 | 3.46 | 239 | 21.2 |
| 2 | 25 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | 2.67 | 5.02 | 2.94 | 20.8 | not performed | not performed | 8.83 | |
| 3 | 24 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | 5.37 | 7.15 | 3.21 | 15.7 | 17.7 | 5.51 | 176 | not performed |
| 4 | 36 | BBS1: c.[382C>T];[382C>T], p.[Gln128*];[Gln128*] |
not performed | not performed | not performed | not performed | not performed | not performed | not performed | |
| 5 | 24 | BBS5: c.[413G>C];[413G>C], p.[Arg138Pro];[Arg138Pro] | 3.26 | 5.94 | 3.64 | 20.6 | 13.8 | 3.79 | 134 | 12.6 |
| 6 | 35 | BBS10: c.[271dup];[963T>G], p.[Cys91Leufs*5];[Tyr321*] | 2.08 | 3.69 | 3.38 | 12.5 | 12.8 | 3.77 | 106 | not performed |
| 7 | 38 | BBS1: c.[1169T>G]; [1214_1215ins[MT113356], p.[Met390Arg];[Ala406Glnfs*47]. | 3.51 | 6.37 | 4.11 | 26.9 | 11.1 | 2.70 | 94.7 | 4.78 |
| 8* | 22 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] | 0.85 | not performed | 0.71 | not performed | 18.7* | Not applicable | 295 | 19.8 |
| 9 | 26 | BBS9: c.[703-?_886+?del];[832C>T], p.[Val235Phefs*6];[Arg278*] |
1.56 | 3.16 | 1.66 | 12 | 6.9 | 4.15 | normal | 50.4 |
| 10 | 18 | BBS3: c.[535G>A];[535G>A], p.[Asp179Asn];[Asp179Asn] |
2.84 | 8.46 | 3.57 | 51.8 | 8.6 | 2.4 | 72.1 | not performed |
| 11 | 39 | BBS12: c.[1037T>C];[1037T>C], p.[Ile346Thr];[Ile346Thr] | 9.41 | 20.5 | 7.02 | 43 | 11.1 | 1.58 | 209 | not performed |
*Patient 8 undergoing testosterone therapy
Genito-urinary ultrasonography was performed in 5 patients and highlighted abnormalities, such as a cyst of prostatic utricle (1 case), epididymal cysts (1 case), or unilateral agenesis of seminal vesicle (3 out of 5 cases), that could be related to extreme hypospermia (<0.5 mL in 2 cases). Kidney cysts or ectasia were found in 7 out of 11 patients (2 with normal semen volume, 4 with decreased semen volume, and 1 patient with failure of semen collection).
Testosterone and gonadotropin levels (Table 3) were not interpretable in patient 8, since he was undergoing testosterone therapy: he was then considered as having a hypogonadism (previous hormonal status not available). For the other patients, testosterone was low only in 2 cases out of 8 (2 missing data) and basal gonadotrophins were all in normal range (1 missing data). For all tested patients, the pituitary response to a GnRH stimulation test was normal (9/9). The testosterone/luteinizing hormone (LH) ratio was calculated for 8 out of 11 patients and ranged from 1.58 to 5.51.
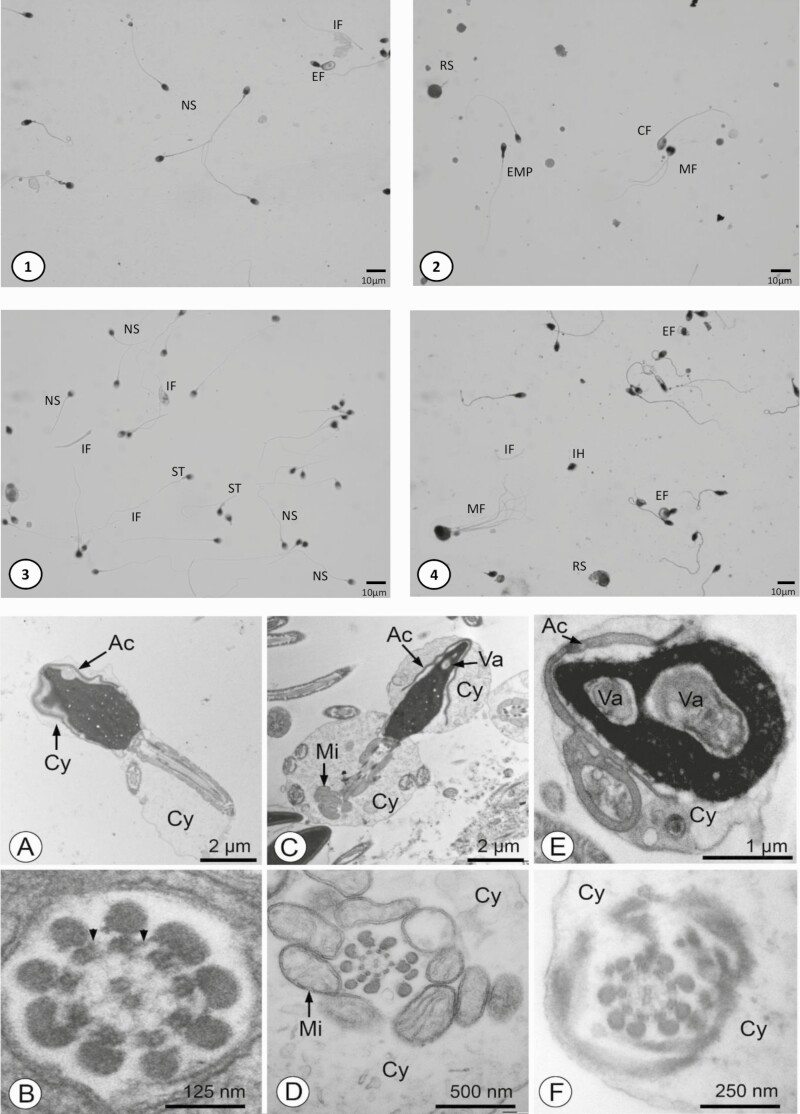
Sperm analysis was performed in 10 of the 11 patients (Table 4) as 1 failed to collect semen (only 0.12 mL of glandular secretion without epididymal fraction of ejaculate). A decreased semen volume was observed in 6 out of the 10 patients (median of 1.2 mL (0.1, 12.8), and a very low semen volume (<0.5 mL) was observed in 3 out of 10 patients performing a semen collection. Sperm numeration was normal in 8 out of 10 patients (median of 72 × 106 [0, 485 × 106]), and sperm progressive motility ranged from 5% to 45% (median of 23%). The teratozoospermia was moderately increased (median 8% of typical forms [2, 28]), and abnormalities were heterogeneous. These findings were confirmed by TEM analysis: more than 80% of the spermatozoa displayed severe morphological alterations of the head (eg, large nuclear vacuoles) and/or the midpiece (eg, disorganized mitochondrial sheath), which were apparently combined at random. TEM analysis also revealed that the normal microtubule pattern of the axoneme, with nine doublets surrounding a pair of singlets was preserved in most of transverse sections through the midpiece or principal piece. Therefore, these spermatozoa were without any specific anomaly of the axoneme, having notably a central pair of microtubules as well as para-microtubular proteins like dynein arms (Fig. 1).
Table 4.
Sperm Analysis
| Patient | Age (years) | Semen volume (mL) | pH | Viscosity | Numeration spz/Ejaculate (N > 39 × 106) | Progressive motility (%) (N > 33%) | Total motility (%) (N > 50%) | Vitality (%) (N > 58%) | Morphology according to David modified criteria (N TF > 12%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | 12.8 | 6 | normal | 0.09 × 106 | 0 | 0 | Not performed | 10/19 enrolled tail, 18/19 abnormal acrosome, 1/20 TF |
| 2 | 25 | 1.2 (da = 2j) | 8.1 | Normal | 324 × 106 | 45 | 50 | 69 | 6% enrolled tail, 68% abnormal acrosome; 11% TF; isolated tails: 17% of observed spermatozoa |
| 3 | 24 | 2.3 | 7.8 | Normal | 138 × 106 | 25 | 30 | 61 | 32% enrolled tail, 86% abnormal acrosome, 2% TF; isolated tails: 85% of observed spermatozoa |
| 4 | 36 | 6.5 | 6.8 | Normal | 15 | 0 | 0 | 0 | not interpretable |
| 5* | 24 | 0.12 * | 6.3 | Normal | 0 | Not applicable | Not applicable | Not applicable | not applicable |
| 6 | 35 | 0.3 | 7.8 | Normal | 79.2 × 106 | 37 | 52 | >60 | 50% abnormal tail, 11 à 20% TF |
| 7 | 38 | 0.3 | 8.1 | Normal | 217 × 106 | 20 | 30 | >30 | 28% TF |
| 8 | 22 | 2.7 | 6.6 | Normal | 32.1 × 106 | 9 | 11 | 33 | 13% multitailed sperm; 14%enrolled tail; 15% isolated tails; 4% TF |
| 9 | 26 | 0.9 (DA = 2j) | 7.5 | Normal | 62.3 × 106 | 5 | 10 | 66 | 8% TF; 6% isolated tails |
| 10 | 18 | 2.1 | 7.5 | Normal | 481 × 106 | 26 | 35 | 60 | 39% abnormal tail; 8%TF; <5% isolated tails |
| 11 | 39 | 0.45 | 8.1 | normal | 72 × 106 | 26 | 31 | 48 | 7% TF 82% abnormal acrosome; 12% isolated tails |
Abbreviations: spz, spermatozoa; TF, typical form.
*Patient 5 failed to produce ejaculate.
Figure 1.
Sperm morphology in BBS patients. Panels 1-4: Harris Shorr staining, from different patients, (1) Patient 2; (2) Patient 8; (3) Patient 6; (4) Patient 9. Abbreviations: CF, coiled flagellum; EMP, enlarged middle piece; IF, isolated flagellum; IH, isolated head; MF, multiflagellar sperm; NS, normal spermatozoa; RS, round spermatid; ST, short tail. The proportion of isolated flagella is increased. Coiled flagellum, short tail, enlarged middle piece suggest an impaired spermiogenesis. Panels A-F. TEM analysis of spermatozoa. (A and B) and (C-F) correspond to 2 different patients. (A, C-F) Commonly observed defects include: acrosomes (Ac) detached from the nucleus (in A, C, E) and malformed (in E), excess of cytoplasm (Cy) in the head (in A, C, E) and flagellum (in D, F), disorganized mitochondrial sheaths (Mi) (in C, D) and large nuclear vacuoles (Va) (in C, E). (B, D, F) The majority of the cross sections of axonemes show a normal 9 plus 2 microtubule pattern and well-preserved dynein arms (arrowheads in B). Note that abnormalities of axonemes in the spermatozoa (eg, absence of the central pair of microtubules; not illustrated here) are almost systematically associated with poor preservation of the adjacent mitochondria, therefore strongly suggesting that they reflect a state of cellular necrosis.
The BBS1 gene was implicated in 6 out of the 11 cases. Other cases comprised homozygous mutation in BBS3/ARL6, BBS5, BBS9, BBS12 or compound heterozygous mutations in BBS10. Regarding the reproductive function, no genotype to phenotype association could be observed in our cohort. Even between siblings, variations of the phenotype were observed: only 1 of the siblings, with the same homozygote mutation of BBS1 (c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg]), reported a cryptorchidism history. Another patient bearing the same mutation presented a severe decrease of semen volume (0.3 mL).
The psychological evaluation of patients (Tables 1 and 5) uncovered a moderate mental retardation with a Verbal Intellectual Quotient varying from 60.5 to 102 (median 78.5) associated with a frequent lack of self-confidence mainly due to their obesity. Two patients lived with a partner; only 1 consulted for infertility and the couple underwent ART.
Table 5.
Psychological Concerns
| Patient | Age (years) | Mutation | Clinical data | Lifestyle | ||||
|---|---|---|---|---|---|---|---|---|
| BMI | blindness | VIQ | Profession | Living with a partner | Child wish | |||
| 1 | 22 | BBS1: c.[479G>A];[479G>A], p.[Arg160Gln];[Arg160Gln] |
>30 | yes | 60.5 | baker | no | no |
| 2 | 25 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] |
<30 | yes | 64 | telephone advisor | no | no |
| 3 | 24 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] |
<30 | No, residual visual acuity | 62 | telephone advisor | yes | yes |
| 4 | 36 | BBS1: c.[382C>T];[382C>T], p.[Gln128*];[Gln128*] |
>30 | yes | not performed | metallurgy worker | yes | no |
| 5 | 24 | BBS5: c.[413G>C];[413G>C], p.[Arg138Pro];[Arg138Pro] |
>30 | yes | 93 | groom | no | no |
| 6 | 35 | BBS10: c.[271dup];[963T>G], p.[Cys91Leufs*5];[Tyr321*] |
>40 | yes | 90 | office worker | no | no |
| 7 | 38 | BBS1: c.[1169T>G];[1214 _1215ins[MT113356]], p.[Met390Arg];[(Ala406 Glnfs*47)] | <25 | yes | 62 | professionnal sportman | no | no |
| 8 | 22 | BBS1: c.[1169T>G];[1169T>G], p.[Met390Arg];[Met390Arg] |
>40 | yes | 94.5 | economy student | no | no |
| 9 | 26 |
BBS9: c.[703-?_886+?del];[832C>T], p.[Val235Phefs*6];[Arg278*] |
>40 | yes | 102 | telephone advisor | no | no |
| 10 | 18 | BBS3: c.[535G>A];[535G>A], p.[Asp179Asn];[Asp179Asn] |
>30 | yes | 88.5 | musician (guitarist) | no | no |
| 11 | 39 | BBS12: c.[1037T>C];[1037T>C], p.[Ile346Thr];[Ile346Thr] |
>35 | yes | 68.5 | wood sawmill worker | no | no |
Discussion
Among the main phenotypic features of BBS, hypogenitalism/hypogonadism is often used as a generic term, encompassing cryptorchidism, short scrotum, micropenis, and low testicular volume that also has suggested associated infertility (9). If the literature frequently reports genital anomalies (8-10, 26), in contrast, hormonal assessment is rarely performed. In some cases of BBS, the presence of hypogonadotropic hypogonadism was concluded (27-29), while in others, the presence of hypogonadism of testicular origin (30-33), or both mechanisms were reported (34). Of note, in these reports, the diagnosis of BBS was only clinically assessed, and some patients were prepubertal or young adolescents.
In order to document this aspect of the syndrome, we have systematically analyzed the reproductive function of 11 male BBS patients. As illustrated in Tables 2, 3, and 4, BBS patients present an important variability of features (clinical and biological) linked to fertility.
Five patients had a history of bilateral (n = 2) or unilateral cryptorchidism and 4 of them exhibited a micropenis and short scrotum. This association strongly suggests a congenital hypogonadotropic hypogonadism (CHH) (35, 36) with insufficient hypothalamic-pituitary–induced androgen secretion after midgestation that is normally responsible for inguino-scrotal testes descent and penile growth. The neuro-endocrine regulation of GnRH hypothalamic release is quite complex and results from the interplay of activating and inhibitory inputs to GnRH neurons during fetal life. The recent description of the fundamental role of hypothalamic kisspeptin and its receptor Kiss1r (previously named GPR54) (37) on GnRH secretion brought a major advance in the understanding in this field (for an extensive review, see (37)). The regulation of GnRH release by kisspeptin signaling appears crucial for the functional integrity of the gonadotropic axis during fetal life, for pubertal onset, and to maintain fertility in adults (37, 38). Interestingly, in the mouse brain, primary cilia of GnRH neurons are enriched in Kiss1r and Kiss1r activity, in response to kisspeptin binding, is enhanced by the presence of cilia on GnRH neurons (39). This signal amplification appears determinant in the firing rate of fetal GnRH neurons, especially in males. Koemeter-Cox et al (39) also showed that the percentage of GnRH neurons displaying at least 1 Kiss1r-positive cilium was 75% in both sexes at birth and was stable throughout the lifetime. However, quantifying the percentage of GnRH neurons possessing more than 1 Kiss1r positive cilium revealed that the frequency of multiciliate GnRH neurons significantly increases during postnatal development (from 10% at birth to 42% at P60) in parallel with sexual maturation. In humans, as well as in rodents, signaling in the GnRH neurons is the result of clustering of receptors in the ciliary membrane (11, 39). Any dysfunction of primitive cilia in BBS patients could explain a decreased activity of KISS1R signaling pathway during fetal and early postnatal life leading to micropenis and undescended testis.
This CHH seems reversible in most cases, since all patients presented a spontaneous puberty, normal secondary sexual characteristics, and normal testicular volume. Testosterone levels at adult age were indeed normal in all except 2 patients with relatively low testosterone and 1 who had been undergoing testosterone replacement for years. Reversal of CHH occurs in 10% to 20% of patients, which challenges the dogma that the condition is lifelong (35). Desai et al have described a case of reversible hypogonadotropic hypogonadism in a BBS man (40). We propose the hypothesis that in BBS patients, the increase of cilia number on GnRH neurons during puberty, as observed in mice, leading to an increase in KISS1Rs number, might overcome the individual signal reduction and allow a normal puberty and normal adult gonadotropic axis in most of cases. Many case reports focused on pubertal delay (32, 34, 40). Unfortunately, exact timing and tempo of puberty could not be assessed in our patients since it was a retrospective declarative data. Following along the lifespan testicular function in these patients could be of interest, since cryptorchidism and obesity can both impact on gonadal function, as suggested by the lowest ratio of testosterone/LH observed in the oldest patient of our series.
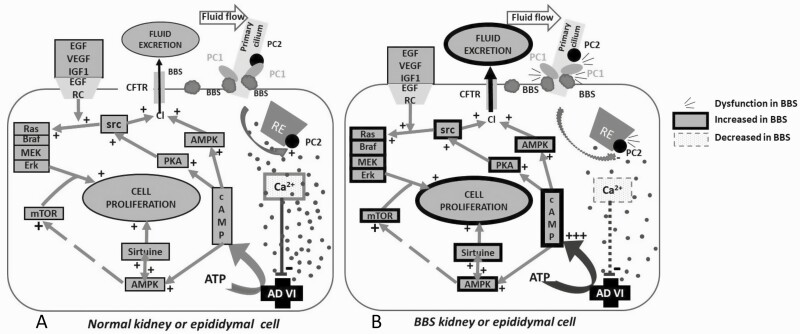
The frequent occurrence of cystic formation in male genital tract, seminal vesicles and kidneys can be explained by the fact that epithelial cells in these structures share a similar embryonic origin from the intermediate mesoderm and expression of primitive cilia. The high rate of kidney cysts observed in BBS patients is in favor of this hypothesis (7 out of 11 in our series, in accordance with the literature (16)). We hypothesize that cyst formation in the male genital tract of BBS patients results from a physio-pathological mechanism similar to that described in polycystic kidney disease: cyst formation would result from a dysfunction of the dimer polycystin 1–polycystin 2 (PC1-PC2) (11, 41, 42), inducing excess cell growth, proliferation, and secretion. In accordance with a recent discussion about the role of BBS proteins in the cystogenesis (43), we presume that, in ciliated epididymal cells like in kidney cells, BBSome interacts with PC1 to stabilize the complex (Fig. 2). Expression of a pathogenic BBS3/Arl6 mutant (T31R) locks Arl6 in the GDP form leading to stunted cilia and inhibition of PC1 on primary cilia (44).
Figure 2.
Main mechanisms involved in the cystogenesis. A: In epithelial kidney or epididymal cells, primary cilia constitute a reserve of polycystin 1 (PC1) and polycystin 2 (PC2). PC1 assembly with PC2 to form a dimer inducing from the endoplasmic reticulum an exit of calcium. A high concentration of cytoplasmic Ca2+ represses adenylate cyclase VI (AC VI). The AC VI produces cAMP stimulating AMPk pathway and PKA pathway which are resulting in a stimulation of cell proliferation and CFTR-linked chloride secretion respectively (42). BBSome of the primary cilia stabilizes the PC1 complex and allows a functional dimerization with PC2 (43). B: An anomaly of BBSome decreases the stability of PC1-PC2 dimer (43), resulting further in an insufficient Ca2+ intracytoplasmic concentration, an insufficient repression of AC VI and finally to an excessive cell proliferation and excretion of chloride and fluid through the CFTR. Excessive cell proliferation and excessive fluid secretion lead to cyst formation.
The diminution of semen volume appears to be due more to a partial obstruction of the genital duct by cysts of the genital tract, rather than to hypogonadotropic hypogonadism not observed in our series. In patient 8, ultrasonography revealed the presence of a cyst of prostatic utricle, which is an embryologic Müllerian remnant and should not be confused with other ciliopathy-related cysts of the genital tract, which could also affect the prostate. Another cause of hypovolemia is the unilateral agenesis of a seminal vesicle, potentially related to the prenatal embryogenesis of this gland (45, 46), which begins by GW 14 to 16 from the Wolffian duct and is dependent on testosterone. The prenatal defect of fetal testosterone production linked to prenatal hypogonadotropic hypogonadism in BBS patients could explain this rather frequent abnormality (2 patients out of 5 patients explored by ultrasonography).
Furthermore, this study discloses the conserved motility of the spermatozoa in a great proportion of the BBS patients studied as the integrity of the 9 peripheral + 1 central pair of microtubules is conserved. The variable asthenozoospermia observed (5%-45% of progressive motility) in the series could rather be due to the poor spermatogenesis conditions due to the short scrotum and the obesity disturbing scrotal thermoregulation (47). The notion of infertility in BBS patients linked to a suspected major asthenozoospermia results from a confusion between motile cilia and primary cilia. Conserved sperm motility as well as results of ART performed with spermatozoa of 1 patient suggest a normal functionality of sperm flagellum and proximal centriole, even if all cilia structure share common some structural elements and common mechanism of genesis (11, 33).
The functionality of the sperm centriole has been confirmed in patient 3, through the IVF procedure which succeeded in the birth of a healthy child.
The absence of obvious correlation between genotype and phenotype of BBS patients has been already mentioned in a more general context, not focused on reproduction (16). The most frequent mutation, a single missense mutation of exon 12 of BBS1 (p.M390R) known to induce sometimes a mild phenotype, seems to lead, in our series, to moderate abnormalities of genitalia, suggesting a moderate fetal hypothalamo-pituitary impairment and finally a moderate fetal androgen deficit.
Abnormal tail morphologies of the spermatozoa were observed often in our series (patient 1, patient 3, patient 6, and patient 10) without significant impact on their motility. In addition, the structure of the axoneme in our patients was not specifically disturbed in TEM analysis. These observations contrast with those in mice carrying similar mutations: spermatozoa of Bbs1 (M390R/M390R) knockout mice presented no flagellum even if the cilia of these mice presented a normal axonemal structure, including 9 peripheral microtubules doublets + 1 central pair arrangement of axonemal microtubules, and elongated cilia with abnormally swollen distal ends suggesting the mutation may impair the completion of flagella assembly (48). Similarly, Bbs4-null mice develop normally their somatic motile and primary cilia, suggesting that Bbs4 is dispensable for global cilia genesis, but interestingly, male Bbs4-null mice do not form spermatozoa flagella, suggesting a difference in the formation of the different types of tails (49). Other Bbs null mice (Bbs2, Bbs7) present also disturbed sperm flagellum synthesis (50, 51). Although BBS6 belongs to another family of BBS proteins, characterized as chaperonins, Bbs6-null mice present also impaired spermatozoa tail synthesis (52). In 2008, Walsh et al highlighted an important role of chaperonin proteins in the fusion mechanism of acrosomal function (acrosomal building by proacrosomal vesicles fusion and acrosomal reaction by acrosome membrane fusion to plasma membrane of sperm head) (53). Since several BBS proteins interact with other chaperonin proteins, it is possible that mutations of BBS proteins of the chaperonin-like family have an impact on sperm acrosomal function and thus on spontaneous fertility. In this case, the ART solution would consist in carrying out an ICSI-IVF (which is what was performed for the patient consulting for infertility in our series).
Psychology and Implications for Sexual Behavior in BBS
Intellectual disability is a variable feature of BBS and like in earlier reports (54, 55), our patients had Verbal Intellectual Quotient close to 80 (60.5-102; mean and median = 78.5). Nevertheless, all except 1 had a professional activity (from groom to telephone advisor).
Similar to the observation made by Kerr et al (54), we observed emotional immaturity in some BBS patients, with frequent inappropriate emotional outbursts but, in contrast, no disinhibited behavior or inability to recognize social cues.
Disability linked to blindness, frequent lack of self-confidence mainly due to obesity, associated with these psychological traits could explain the low proportion of BBS patients living with a partner (2 out of 11 in our series) and consultation for reproductive assistance (only 1 out of 11).
Conclusion
BBS is a pleiotropic syndrome affecting reproductive prognostic through: (i) fetal hypogonadotropic hypogonadism resulting in micropenis, short scrotum, and cryptorchidism, (ii) variable impairment in spermatogenesis due to the proximity of testes to abdomen (iii) susceptibility to cystic formation of genital tract leading to partial obstruction of genital ducts, and (iv) sexual/reproductive behavior linked to psychological traits of BBS patients.
However, in contrast with the literature we suggest that: (i) the hypothalamic-pituitary-gonadal axis can function normally in adults despite a frequent severe obesity, (ii) the sperm motility can be normal, and (iii) sperm is able to fertilize mature oocyte and the centriole is able to conduct the first zygote division.
Very few cases of spontaneous fatherhood are reported, maybe more linked to an impaired sexual behaviour. Our study is reassuring about the possibility for male BBS patients to benefit from reproductive medicine care.
Acknowledgments
First, we would like to thank the patients and their families for taking part in this study. This work was funded by the study PHRC National Bardet Biedl 2007 IDRCB 2007-A00868-45. We would like also to acknowledge the members of the diagnostic laboratory (Manuela Antin, Anne-Sophie Leuvrey, and Elsa Nourisson) and Nicolas Becker of the laboratory of Biology of Reproduction at the University Hospital in Strasbourg for technical assistance. We would like to thank to Shyue-fang Battaglia-Hsu for her language assistance.
Financial Support: This study was supported by the Direction Générale des Soins as a part of the French National Research Protocol Bardet-Biedl registred under the No: 2007 IDRCB 2007-A00868-45.
Clinical Trial Information: Registration no. PHRC 2007 – ref HUS No. 4056.
Glossary
Abbreviations
- ART
assisted reproductive technology
- BBS
Bardet-Biedl syndrome
- CHH
congenital hypogonadotropic hypogonadism
- GnRH
gonadotropin-releasing hormone
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- LH
luteinizing hormone
- TEM
Transmission Electron Microscopy
- VIQ
verbal intelligence quotient
Additional Information
Conflict of Interest: The authors have noting to disclose and state there are no competing interest.
Disclosure Summary: This study explores each step of reproduction in a cohort of 11 Bardet-Biedl male patients, from embryology of genitalia to hormonal status, sperm analysis and psychological profile, discussing the hypothetical impact of the ciliopathy.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Waters AM, Beales PL. Bardet-Biedl syndrome. GeneReviews.:1993–2003 (Updated 2020). [Google Scholar]
- 2. Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S. Genetics of human Bardet-Biedl syndrome, an updates. Clin Genet. 2016;90(1):3-15. [DOI] [PubMed] [Google Scholar]
- 3. Schaefer E, Delvallée C, Mary L, et al. Identification and characterization of known biallelic mutations in the IFT27 (BBS19) gene in a novel family with Bardet-Biedl syndrome. Front Genet. 2019;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loktev AV, Zhang Q, Beck JS, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15(6):854-865. [DOI] [PubMed] [Google Scholar]
- 5. Seo S, Baye LM, Schulz NP, et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010;107(4):1488-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taschner M, Lorentzen E. The intraflagellar transport machinery. Cold Spring Harb Perspect Biol. 2016;8(10):a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wingfield JL, Lechtreck KF, Lorentzen E. Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 2018;62(6):753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437-446. [PMC free article] [PubMed] [Google Scholar]
- 9. Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21(1):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore SJ, Green JS, Fan Y, et al. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132A(4):352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12(4):222-234. [DOI] [PubMed] [Google Scholar]
- 12. Auger J, Jouannet P, Eustache F. Another look at human sperm morphology. Hum Reprod. 2016;31(1):10-23. [DOI] [PubMed] [Google Scholar]
- 13. Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. J Pediatr. 1999;134(5):579-583. [DOI] [PubMed] [Google Scholar]
- 14. Custer J, Rau RIn: The Harriet Lane Handbook. Vol Endocrinology. Ballel S, McIntosh P, eds. 18th ed. Elsevier Mosby; 2008:269-300. [Google Scholar]
- 15. Schonfeld W, Beebe G. Normal growth and variation in the male genitalia from birth to maturity. J Urol. 1942;48:759-777. [Google Scholar]
- 16. Mary L, Chennen K, Stoetzel C, et al. Bardet-Biedl syndrome: antenatal presentation of forty-five fetuses with biallelic pathogenic variants in known Bardet-Biedl syndrome genes. Clin Genet. 2019;95(3):384-397. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization; 2010:7-113. [Google Scholar]
- 18. Auger J, Eustache F, David G. Standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie. 2000;10(4): 358–373. [Google Scholar]
- 19. Braun JJ, Noblet V, Durand M, et al. Olfaction evaluation and correlation with brain atrophy in Bardet-Biedl syndrome. Clin Genet. 2014;86(6):521-529. [DOI] [PubMed] [Google Scholar]
- 20. Doty RL. Intranasal trigeminal detection of chemical vapors by humans. Physiol Behav. 1975;14(6):855-859. [DOI] [PubMed] [Google Scholar]
- 21. Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007;21(4):460-473. [DOI] [PubMed] [Google Scholar]
- 22. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34(4):222-226. [PubMed] [Google Scholar]
- 23. Wechsler D Echelle d’intelligence adulte. Les éditions du centre de psychologie appliquée; 1997. [Google Scholar]
- 24. Hichri H, Stoetzel C, Laurier V, et al. Testing for triallelism: analysis of six BBS genes in a Bardet-Biedl syndrome family cohort. Eur J Hum Genet. 2005;13(5):607-616. [DOI] [PubMed] [Google Scholar]
- 25. Gouronc A, Zilliox V, Jacquemont ML, et al. High prevalence of Bardet-Biedl syndrome in La Réunion Island is due to a founder variant in ARL6/BBS3. Clin Genet. 2020;98(2):166-171. [DOI] [PubMed] [Google Scholar]
- 26. M’hamdi O, Redin C, Stoetzel C, et al. Clinical and genetic characterization of Bardet-Biedl syndrome in Tunisia: defining a strategy for molecular diagnosis. Clin Genet. 2014;85(2):172-177. [DOI] [PubMed] [Google Scholar]
- 27. Guran T, Ekinci G, Atay Z, Turan S, Akcay T, Bereket A. Radiologic and hormonal evaluation of pituitary abnormalities in patients with Bardet-Biedl syndrome. Clin Dysmorphol. 2011;20(1):26-31. [DOI] [PubMed] [Google Scholar]
- 28. Pérez-Palacios G, Uribe M, Scaglia H, et al. Pituitary and gonadal function in patients with the Laurence-Moon-Biedl syndrome. Acta Endocrinol (Copenh). 1977;84(1): 191-199. [DOI] [PubMed] [Google Scholar]
- 29. Reinfrank RF, Nichols FL. Hypogonadotrophic hypogonadism in the Laurence-Moon syndrome. J Clin Endocrinol Metab. 1964;24:48-53. [DOI] [PubMed] [Google Scholar]
- 30. Leroith D, Farkash Y, Bar-Ziev J, Spitz IM. Hypothalamic-pituitary function in the Bardet-Biedl syndrome. Isr J Med Sci. 1980;16(7):514-518. [PubMed] [Google Scholar]
- 31. Mozaffarian G, Nakhjavani MK, Farrahi A. The Laurence-Moon-Bardet-Biedl syndrome: unresponsiveness to the action of testosterone, a possible mechanism. Fertil Steril. 1979;31(4):417-422. [DOI] [PubMed] [Google Scholar]
- 32. Toledo SP, Medeiros-Neto GA, Knobel M, Mattar E. Evaluation of the hypothalamic-pituitary-gonadal function in the Bardet-Biedl syndrome. Metabolism. 1977;26(12):1277-1291. [DOI] [PubMed] [Google Scholar]
- 33. Green JS, Parfrey PS, Harnett JD, et al. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321(15):1002-1009. [DOI] [PubMed] [Google Scholar]
- 34. Soliman AT, Rajab A, AlSalmi I, Asfour MG. Empty sellae, impaired testosterone secretion, and defective hypothalamic-pituitary growth and gonadal axes in children with Bardet-Biedl syndrome. Metabolism. 1996;45(10):1230-1234. [DOI] [PubMed] [Google Scholar]
- 35. Boehm U, Bouloux PM, Dattani MT, et al. Expert consensus document: European consensus statement on congenital hypogonadotropic hypogonadism–pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11(9):547-564. [DOI] [PubMed] [Google Scholar]
- 36. Young J, Xu C, Papadakis GE, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40(2):669-710. [DOI] [PubMed] [Google Scholar]
- 37. Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: inside and outside the hypothalamus. J Reprod Dev. 2016;62(6):537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silveira LG, Tusset C, Latronico AC. Impact of mutations in kisspeptin and neurokinin B signaling pathways on human reproduction. Brain Res. 2010;1364:72-80. [DOI] [PubMed] [Google Scholar]
- 39. Koemeter-Cox AI, Sherwood TW, Green JA, et al. Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A. 2014;111(28):10335-10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desai A, Jha O, Iyer V, Dada R, Kumar R, Tandon N. Reversible hypogonadism in Bardet-Biedl syndrome. Fertil Steril. 2009;92(1):391.e13-391.e15. [DOI] [PubMed] [Google Scholar]
- 41. Torra R, Sarquella J, Calabia J, et al. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2008;3(3):790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cornec-Le Gall E, Le Meur Y. [Autosomal dominant polycystic kidney disease: is the treatment for tomorrow?]. Néphrologie Thérapeutique. 2014;10(6):433–440. [DOI] [PubMed] [Google Scholar]
- 43. Adamiok-Ostrowska A, Piekiełko-Witkowska A. Ciliary genes in renal cystic diseases. Cells. 2020;9(4):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su X, Driscoll K, Yao G, et al. Bardet-Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum Mol Genet. 2014;23(20):5441-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jirásek JE Human Fetal Endocrines. Springer Netherlands; 1980. [Google Scholar]
- 46. Jirásek JE. Morphogenesis of the genital system in the human. Birth Defects Orig Artic Ser. 1977;13(2):13-39. [PubMed] [Google Scholar]
- 47. Mieusset R, Fouda PJ, Vaysse P, Guitard J, Moscovici J, Juskiewenski S. Increase in testicular temperature in case of cryptorchidism in boys. Fertil Steril. 1993;59(6):1319-1321. [DOI] [PubMed] [Google Scholar]
- 48. Davis RE, Swiderski RE, Rahmouni K, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104(49):19422-19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mykytyn K, Mullins RF, Andrews M, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101(23):8664-8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nishimura DY, Fath M, Mullins RF, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101(47):16588-16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Q, Nishimura D, Vogel T, et al. BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci. 2013;126(Pt 11):2372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shah AS, Farmen SL, Moninger TO, et al. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci U S A. 2008;105(9):3380-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walsh A, Whelan D, Bielanowicz A, et al. Identification of the molecular chaperone, heat shock protein 1 (chaperonin 10), in the reproductive tract and in capacitating spermatozoa in the male mouse. Biol Reprod. 2008;78(6):983-993. [DOI] [PubMed] [Google Scholar]
- 54. Kerr EN, Bhan A, Héon E. Exploration of the cognitive, adaptive and behavioral functioning of patients affected with Bardet-Biedl syndrome. Clin Genet. 2016;89(4):426-433. [DOI] [PubMed] [Google Scholar]
- 55. Barnett S, Reilly S, Carr L, Ojo I, Beales PL, Charman T. Behavioural phenotype of Bardet-Biedl syndrome. J Med Genet. 2002;39(12):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.