Abstract
Objective
To evaluate the race-stratified state-level prevalence of health determinants and the racial disparities in coronavirus disease 2019 (COVID-19) cumulative incidence and mortality in the United States.
Patients and Methods
The age-adjusted race-stratified prevalence of comorbidities (hypertension, diabetes, dyslipidemia, and obesity), preexisting medical conditions (pulmonary disease, heart disease, stroke, kidney disease, and malignant neoplasm), poor health behaviors (smoking, alcohol abuse, and physical inactivity), and adverse socioeconomic factors (education, household income, and health insurance) was computed in 435,139 American adult participants from the 2017 Behavioral Risk Factor Surveillance System survey. Correlation was assessed between health determinants and the race-stratified COVID-19 crude mortality rate and infection-fatality ratio computed from respective state public health departments in 47 states.
Results
Blacks had a higher prevalence of comorbidities (63.3%; 95% CI, 62.4% to 64.2% vs 55.1%; 95% CI, 54.7% to 55.5%) and adverse socioeconomic factors (47.0%; 95% CI, 46.0% to 47.9% vs 30.9%; 95% CI, 30.6% to 31.3%) than did whites. The prevalence of preexisting medical conditions was similar in blacks (30.4%; 95% CI, 28.8% to 32.1%) and whites (30.8%; 95% CI, 30.2% to 31.4%). The prevalence of poor health behaviors was higher in whites (57.2%; 95% CI, 56.3% to 58.0%) than in blacks (50.2%; 95% CI,46.2% to 54.2%). Comorbidities and adverse socioeconomic factors were highest in the southern region, and poor health behaviors were highest in the western region. The cumulative incidence rate (per 100,000 persons) was 3-fold higher in blacks (1546.4) than in whites (540.4). The crude mortality rate (per 100,000 persons) was 2-fold higher in blacks (83.2) than in whites (33.2). However, the infection-fatality ratio (per 100 cases) was similar in whites (6.2) and blacks (5.4). Within racial groups, the geographic distribution of health determinants did not correlate with the state-level COVID-19 mortality and infection-fatality ratio (P>.05 for all).
Conclusion
Racial disparities in COVID-19 are largely driven by the higher cumulative incidence of infection in blacks. There is a discordance between the geographic dispersion of COVID-19 mortality and the regional distribution of health determinants.
Abbreviations and Acronyms: BRFSS, Behavioral Risk Factor Surveillance System; COVID-19, coronavirus disease 2019; IFR, infection-fatality ratio
A higher prevalence of coronavirus disease 2019 (COVID-19) cases and mortality in black individuals is being increasingly reported nationally.1, 2, 3, 4 Racial inequities in the mortality from COVID-19 have been reported from several states in the United States (US).1, 2, 3, 4 These racial disparities in the fatal implications of the novel pandemic are hypothesized to be a consequence of the higher prevalence of comorbidities, preexisting medical conditions, poor health behaviors, and adverse socioeconomic factors in black individuals.1, 2, 3, 4
The demographic characteristics of the populations in the respective US states and the response of the various state public health agencies to the containment of the pandemic have varied widely.5, 6, 7 There is a heterogeneous geographic distribution of the biological, behavioral, and socioeconomic health determinants in the overall population.8,9 However, there are limited contemporary data on the race-stratified state-level prevalence of various health determinants.8, 9, 10, 11, 12 Data pertaining to comorbidities, preexisting medical conditions, poor health behaviors, and adverse socioeconomic factors may help provide insight into the public health implications of the COVID-19 pandemic, especially in black individuals.
We hypothesized that racial disparities exist in the state-level prevalence of health determinants, and these relate to the racial disparities and geographic distribution of COVID-19 cumulative incidence and fatality. We evaluated the 2017 Behavioral Risk Factor Surveillance System (BRFSS) and the respective state COVID-19 public health databases to determine the race-stratified state-level prevalence of health determinants in the US and the racial disparity in the COVID-19 cumulative incidence, crude mortality, and infection-fatality ratio (IFR).
Patients and Methods
Data Sources
The BRFSS was used to quantify state-level health behaviors and risk factors.13 The BRFSS is one of the largest continuously conducted health-related surveys, collecting data from more than 400,000 American adults annually.13 The respective state public health department databases were used to quantify the race-stratified COVID-19 cases and mortality. The latest state-level population data were obtained from the US Census Bureau.14
All American adults 18 years and older who participated in the 2017 BRFSS survey were included in the study. Data on all health determinants were obtained from participants from all 50 states and Washington, District of Columbia, and included in this study. Pregnant women and those with missing data were excluded from the analyses. Pregnant women were excluded because of the difficulty in attributing various health measures as being a chronic condition or being associated with pregnancy only. Mortality analyses included data from residents from the 47 states that are reporting race-stratified COVID-19 cases and mortality.
Measures
The self-reported race from each respondent was used to stratify them as either white or black individuals. The baseline characteristics assessed for BRFSS participants included age, sex, annual household income, and highest educational level obtained. The health determinants were grouped into 4 strata, as follows: (1) comorbidities (any of diabetes, hypertension, hypercholesterolemia, or obesity), (2) preexisting medical conditions (any of stroke, cardiac disease [myocardial infarction/coronary heart disease], malignant neoplasm, or pulmonary disease), (3) poor health behaviors (any of alcohol abuse, smoking, or physical inactivity), and (4) adverse socioeconomic factors (any of lack of health insurance, low annual household income, or less than a high school education). The definition of the individual determinants is given in Supplemental Methods (available online at http://www.mcpiqojournal.org).
The cumulative incidence rate, crude mortality rate, and IFR was computed for those states reporting race-stratified COVID-19 cases and deaths reported until August 16, 2020.
Statistical Analyses
All statistical analyses were performed using the survey procedures in SAS 9.4 (SAS Institute Inc.) to account for the intricate stratified survey sampling design.15 The national, regional, and state-level population estimates were generated using sample weights to account for participant nonresponse and noncoverage in the sample design.16,17 The race-stratified demographic and socioeconomic details of the study population were summarized. The 2010 US Census population proportions for the age groups of 18 to 44, 45 to 64, 65 years and older were used to generate race-stratified age-standardized prevalence estimates for the biological, behavioral, and socioeconomic determinants of health.15 The estimates were generated at the level of state and geographic region, as defined by the US Census Bureau. States and regions with unreliable estimates (wide CIs crossing zero) due to low population counts were not reported. The states of Alaska, Hawaii, Idaho, Montana, New Hampshire, New Jersey, South Dakota, Utah, Vermont, and Wyoming each had 1 or more parameters that were not reported because of low counts. The cumulative incidence rate was calculated as the number of cases per 100,000 persons of the given race living in the state. The COVID-19 crude mortality rate was computed as the number of deaths per 100,000 persons of the particular race living in the state. The 2018 post-census population estimates provided by the US Census Bureau based on the annual American Community Survey were used to compute the crude mortality rate.18 The IFR was computed as the number of deaths per 100 confirmed cases of COVID-19 in respective states. We assessed the correlation (Pearson correlation) of the race-stratified state-level crude mortality rate, IFR, and the state-level race-stratified prevalence of various health determinants. The correlation of the COVID-19 measures with the prespecified health determinant groups (comorbidities, preexisting medical conditions, poor health behaviors, and adverse socioeconomic factors) was assessed.
Results
We included 435,139 individuals from the 2017 BRFSS survey, in which self-reported data on race were available. The demographic distribution and socioeconomic measures of the study population across the census regions for the respective racial groups are described in Supplemental Table 1 (available online at http://www.mcpiqojournal.org).
Prevalence of Comorbidities
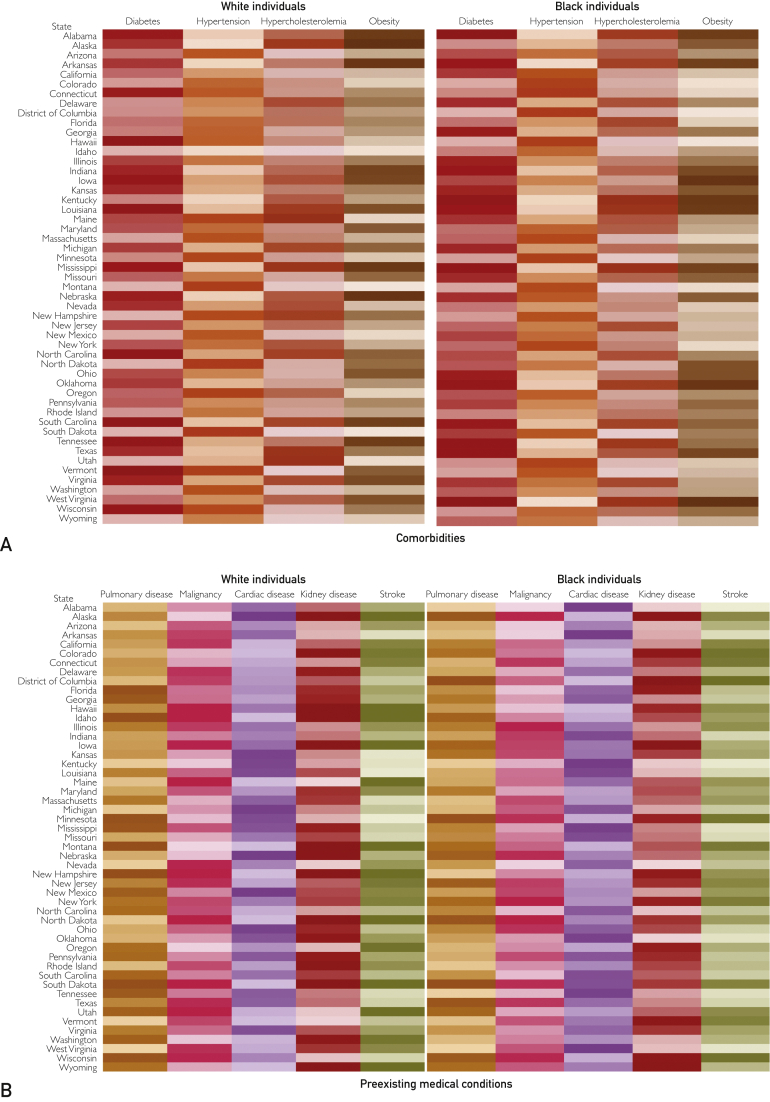
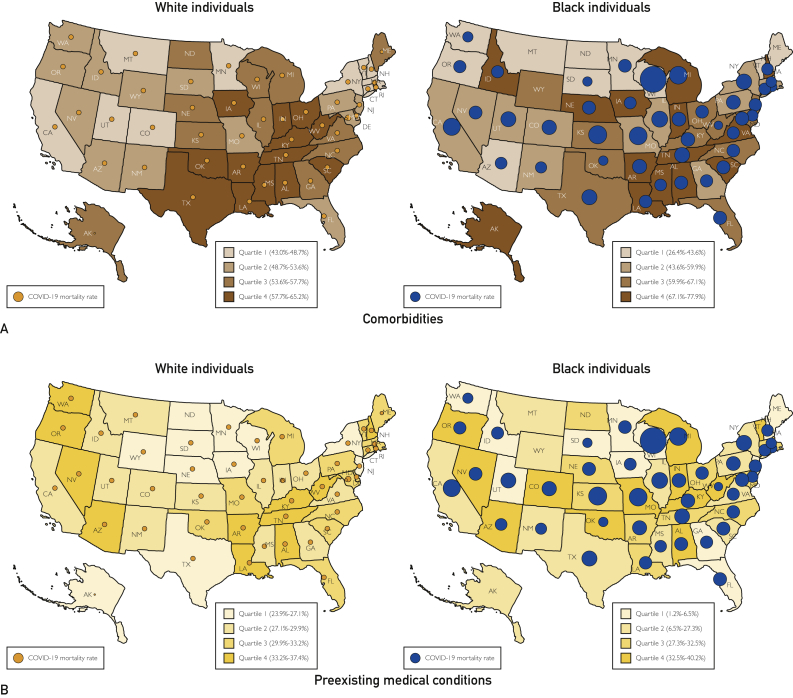
Nationally, the prevalence of 1 or more comorbidities (any of diabetes, hypertension, hypercholesterolemia, and obesity) was 63.3% (95% CI, 62.4% to 64.2%) in blacks and 55.1% (95% CI, 54.7% to 55.5%) in whites. In black individuals, the prevalence of 1 or more comorbidities was highest in the southern region (64.7%; 95% CI, 63.4% to 66.0%) (Table). There was a high prevalence of hypertension (42.6%; 95% CI, 41.5% to 43.8%), diabetes (15.3%; 95% CI, 14.4% to 16.2%), hypercholesterolemia (31.4%; 95% CI, 30.1% to 32.7%), and obesity (40.6%; 95% CI, 39.2% to 41.9%) nationally and this was highest in the southern region in black individuals for all 4 comorbidities. In white individuals, the prevalence of 1 or more comorbidities was also highest in the southern region (57.5%; 95% CI, 56.8% to 58.2%) (Table). The prevalence of hypertension (32.6%; 95% CI, 32.0% to 33.2%), diabetes (10.2%; 95% CI, 9.9% to 10.6%), and hypercholesterolemia (33.0%; 95% CI, 32.3% to 33.6%) was highest in the southern region, and the prevalence of obesity was highest in the midwestern region (31.2%; 95% CI, 30.5% to 31.8%). Figure 1 and Supplemental Tables 2 to 5 (available online at http://www.mcpiqojournal.org) depict the state-level distribution of various health determinants for both black and white individuals.
Table.
Health Determinants Across the United States Regions, Stratified by Race
| Variable | Prevalence (95% CI) |
|||
|---|---|---|---|---|
| Northeast | Midwest | South | West | |
| Black individuals | ||||
| Comorbidities | ||||
| ≥1 Comorbidities | 60.6 (58.8-62.5) | 63.5 (61.8-65.3) | 64.7 (63.4-66.0) | 59.7 (56.4-62.9) |
| Diabetes | 13.8 (12.5-15.1) | 14.4 (13.3-15.6) | 15.3 (14.4-16.2) | 13.2 (10.3-16.1) |
| Hypertension | 38.4 (36.5-40.2) | 40.3 (38.7-42.0) | 42.6 (41.5-43.8) | 39.6 (36.3-43.0) |
| Hypercholesterolemia | 29.8 (27.9-31.6) | 28.6 (27.0-30.3) | 31.4 (30.1-32.7) | 25.6 (22.0-29.1) |
| Obesity | 35.1 (33.1-37.2) | 40.3 (38.4-42.2) | 40.6 (39.2-41.9) | 32.7 (28.9-36.5) |
| Preexisting medical conditions | ||||
| ≥1 Medical conditions | 27.5 (25.6-29.3) | 30.4 (28.8-32.1) | 27.8 (26.6-29.0) | 30.1 (26.7-33.6) |
| Pulmonary disease | 19.8 (18.1-21.4) | 22.1 (20.6-23.6) | 19.0 (17.9-20.0) | 22.3 (19.3-25.3) |
| Malignant Neoplasm | 5.7 (4.7-6.6) | 5.7 (5.0-6.5) | 5.4 (4.9-5.8) | 5.4 (3.4-7.3) |
| Cardiac disease | 6.1 (5.1-7.0) | 7.3 (6.5-8.2) | 6.0 (5.4-6.5) | 3.6 (2.4-4.9) |
| Kidney disease | 3.4 (2.7-4.1) | 4.1 (3.3-5.0) | 3.6 (3.1-4.1) | 4.1 (2.3-5.9) |
| Stroke | 3.5 (2.8-4.2) | 5.0 (4.3-5.7) | 5.0 (4.4-5.5) | 3.0 (1.5-4.5) |
| Poor health behaviors | ||||
| ≥1 Poor health behaviors | 42.8 (40.7-45.0) | 42.8 (40.9-44.7) | 48.0 (47.3-48.7) | 50.2 (46.2-54.2) |
| Alcohol | 4.7 (3.7-5.6) | 5.0 (4.1-5.9) | 4.7 (4.0-5.4) | 4.7 (3.2-6.2) |
| Smoking | 45.1 (42.8-47.3) | 44.1 (42.1-46.1) | 42.1 (40.6-43.6) | 53.0 (48.8-57.2) |
| Physical inactivity | 15.7 (14.2-173) | 22.5 (20.9-24.1) | 18.9 (17.8-20.0) | 18.7 (15.6-21.8) |
| Adverse socioeconomic factors | ||||
| ≥1 Adverse socioeconomic factors | 42.4 (40.4-44.5) | 50.0 (48.1-51.9) | 48.7 (47.3-50.0) | 40.2 (36.4-43.9) |
| Education status | 15.1 (13.4-16.7) | 16.5 (14.8-18.2) | 15.5 (14.5-16.5) | 10.9 (8.5-13.4) |
| Household income | 37.0 (34.8-39.2) | 44.7 (42.7-46.7) | 42.6 (41.1-44.1) | 36.2 (32.2-40.2) |
| Lack of health insurance | 11.0 (9.7-12.3) | 13.6 (12.3-14.9) | 17.6 (16.5-18.8) | 10.5 (8.4-12.7) |
| White individuals | ||||
| Comorbidities | ||||
| ≥1 Comorbidities | 53.1 (52.3-53.9) | 56.0 (55.4-56.5) | 57.5 (56.8-58.2) | 51.9 (51.4-52.7) |
| Diabetes | 8.2 (7.9-8.6) | 9.2 (8.9-9.5) | 10.2 (9.9-10.6) | 8.4 (7.9-8.8) |
| Hypertension | 27.8 (27.1-28.4) | 30.1 (29.7-30.6) | 32.6 (32.0-33.2) | 27.4 (26.8-28.1) |
| Hypercholesterolemia | 30.1 (29.4-30.8) | 30.4 (29.9-30.9) | 33.0 (32.3-33.6) | 29.3 (28.6-30.1) |
| Obesity | 27.4 (26.7-28.2) | 31.9 (31.3-32.4) | 31.2 (30.5-31.8) | 27.0 (26.2-27.7) |
| Preexisting medical conditions | ||||
| ≥1 Medical conditions | 29.1 (28.4-29.8) | 28.8 (28.4-29.3) | 30.8 (30.2-31.4) | 30.6 (29.8-31.3) |
| Pulmonary disease | 18.7 (18.0-19.3) | 17.8 (17.3-18.2) | 18.5 (18.0-19.1) | 18.4 (17.8-19.0) |
| Malignant Neoplasm | 11.3 (10.9-11.7) | 11.2 (11.0-11.5) | 12.9 (12.5-13.3) | 12.6 (12.2-13.1) |
| Cardiac disease | 5.5 (5.2-5.9) | 6.0 (5.8-6.2) | 6.7 (6.4-7.0) | 5.0 (4.6-5.3) |
| Kidney disease | 2.4 (2.2-2.7) | 2.8 (2.6-2.9) | 3.0 (2.8-3.2) | 3.1 (2.8-3.3) |
| Stroke | 2.4 (2.2-2.6) | 2.7 (2.6-2.9) | 3.3 (3.1-3.5) | 2.4 (2.2-2.6) |
| Poor health behaviors | ||||
| ≥1 Poor health behaviors | 52.8 (52.0-53.6) | 51.4 (50.9-52.0) | 40.3 (38.9-41.6) | 57.2 (56.3-58.0) |
| Alcohol | 7.2 (6.8-7.6) | 7.3 (6.9-7.6) | 6.9 (6.5-7.3) | 7.3 (6.9-7.8) |
| Smoking | 53.2 (52.3-54.0) | 51.0 (50.4-51.6) | 48.2 (47.5-49.0) | 58.2 (57.3-59.0) |
| Physical inactivity | 16.6 (15.9-17.2) | 18.9 (18.4-19.4) | 19.0 (18.5-19.6) | 14.0 (13.4-14.7) |
| Adverse socioeconomic factors | ||||
| ≥1 Adverse socioeconomic factors | 25.1 (24.3-25.9) | 27.8 (27.2-28.3) | 35.0 (34.3-35.7) | 32.1 (31.3-32.9) |
| Education status | 9.1 (8.5-9.8) | 9.2 (8.8-9.6) | 12.8 (12.3-13.4) | 13.5 (12.8-14.3) |
| Household income | 19.1 (18.5-19.8) | 21.1 (20.6-21.6) | 26.5 (25.8-27.3) | 23.9 (23.1-24.7) |
| Lack of health insurance | 7.1 (6.6-7.5) | 8.4 (8.1-8.8) | 14.8 (14.3-15.4) | 10.6 (10.1-11.2) |
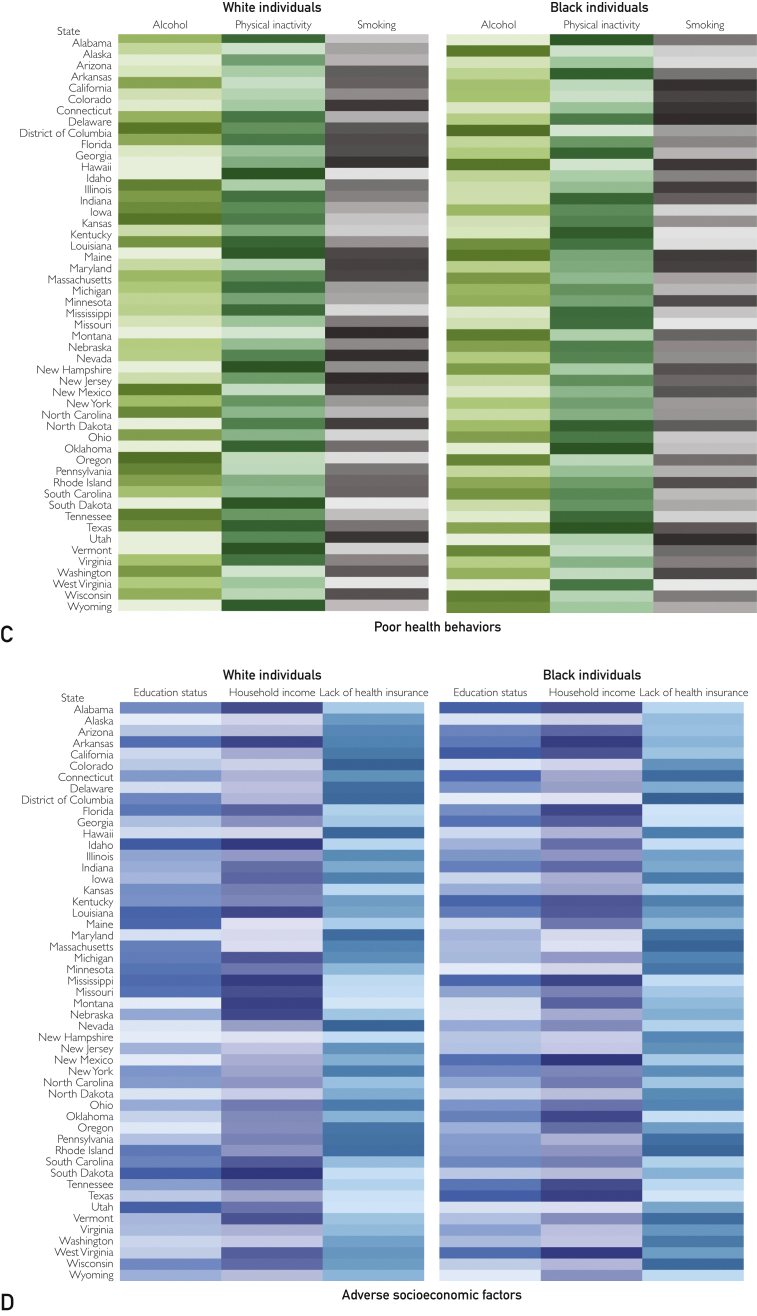
Figure 1.
Heatmap of the prevalence of health determinants, stratified by race. Darker shades represent higher prevalence, and lighter shades represent lower prevalence. Age-adjusted prevalence is reported along with 95% CI. A, Comorbidities. B, Preexisting medical condition. C, Poor health behaviors. D, Adverse socioeconomic factors. The estimates and CIs are given in Supplemental Tables 4 and 5.
Prevalence of Preexisting Medical Conditions
Nationally, the prevalence of 1 or more preexisting medical conditions (any of pulmonary disease, malignant neoplasm, kidney disease, or cardiac disease) was 28.4% (95% CI, 27.5% to 29.3%) in blacks and 30.0% (95% CI, 29.7% to 30.3%) in whites. In black individuals, the prevalence of 1 or more preexisting medical conditions was highest in the midwestern region (30.4%; 95% CI, 28.8% to 32.1%). In blacks, the prevalence of pulmonary disease was highest in the western region (22.3%; 95% CI, 19.3% to 25.3%) and the prevalence of cardiac disease was highest in the midwestern region (7.3%; 95% CI, 6.5% to 8.2%). In white individuals, the prevalence of 1 or more preexisting medical conditions was highest in the southern region (30.8%; 95% CI, 30.2% to 31.4%).
Prevalence of Poor Health Behaviors
Nationally, the prevalence of 1 or more poor health behaviors (any of alcohol abuse, current smoking, or physical inactivity) was 53.2% (95% CI, 52.2% to 54.2%) in blacks and 60.7% (95% CI, 60.4% to 61.1%) in whites. In black individuals, the prevalence of poor health behaviors was highest in the western region (50.2%; 95% CI, 46.2% to 54.2%) (Table). The prevalence of alcohol abuse (5.0%; 95% CI, 4.1% to 5.9%) and smoking (22.5%; 95% CI, 20.9% to 24.1%) was highest in blacks in the midwestern region. Black individuals in the western region had the highest prevalence of physical inactivity (53.0%; 95% CI, 48.8% to 57.2%). In white individuals, the prevalence of alcohol abuse was highest in the midwestern (7.3%; 95% CI, 6.9% to 7.6%) and western (7.3%; 95% CI, 6.9% to 7.8%) regions. Smoking was highest in whites in the southern region (19.0%; 95% CI, 18.5% to 19.6%), and physical inactivity was highest in whites in the western region (58.2%; 95% CI, 57.3% to 59.0%).
Prevalence of Adverse Socioeconomic Factors
Nationally, the prevalence of 1 or more adverse socioeconomic factors (any of lack of health insurance, low annual household income, or less than high school education) was 47.0% (95% CI, 46.0% to 47.9%) in blacks and 30.9% (95% CI, 30.6% to 31.3%) in whites. In black individuals, the prevalence of 1 or more adverse socioeconomic factors was highest in the midwestern region (50.0%; 95% CI, 48.1% to 51.9%). The prevalence of poor educational status (16.5%; 95% CI, 14.8% to 18.2%) and low annual household income (44.7%; 95% CI, 42.7% to 46.7%) was highest in black individuals in the midwestern region (Table). The lack of health insurance coverage was highest in black individuals in the southern region (17.6%; 95% CI, 16.5% to 18.8%). In white individuals, the prevalence of 1 or more adverse socioeconomic factors was highest in the southern region (35.0%; 95% CI, 34.3% to 35.7%). In white individuals, the prevalence of lack of health insurance (14.8%; 95% CI, 14.3% to 15.4%) and that of low annual household income (26.5%; 95% CI, 25.8% to 27.3%) were highest in the southern region. The prevalence of lower educational status was highest in white individuals in the western region (13.5%; 95% CI, 12.8% to 14.3%).
Coronavirus Disease 2019 Mortality Rates and Relationship With Determinants of Health
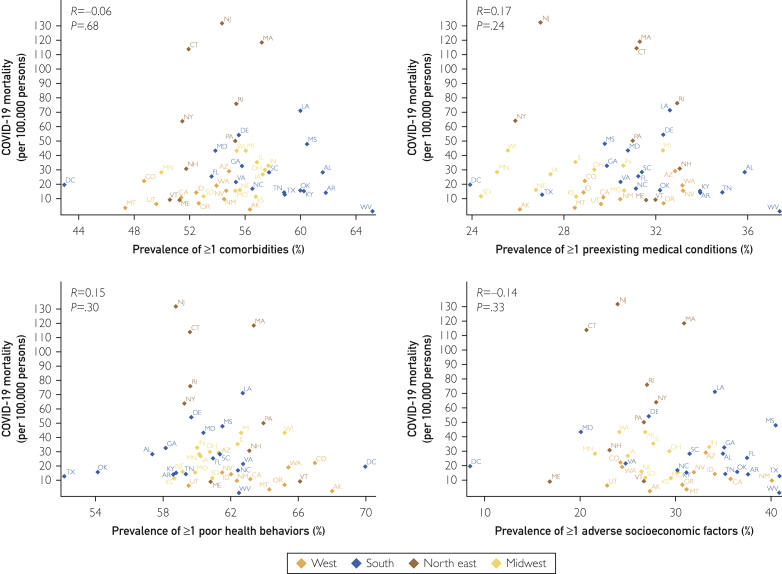
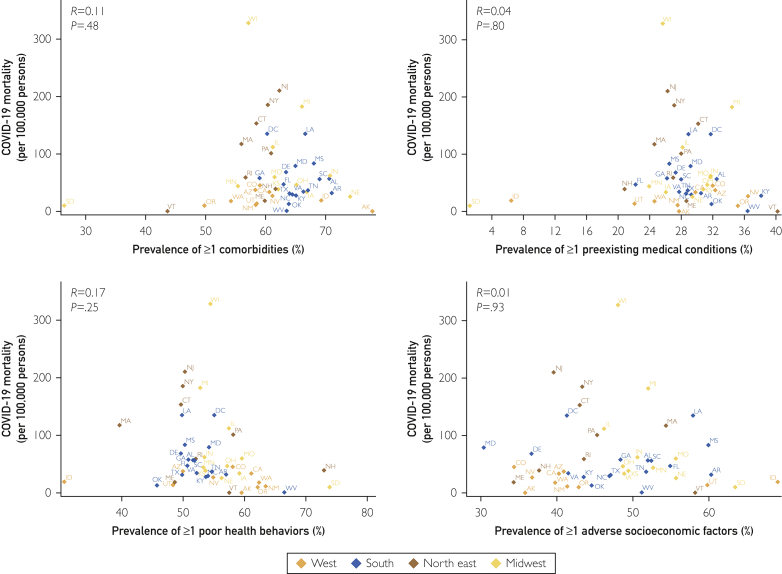
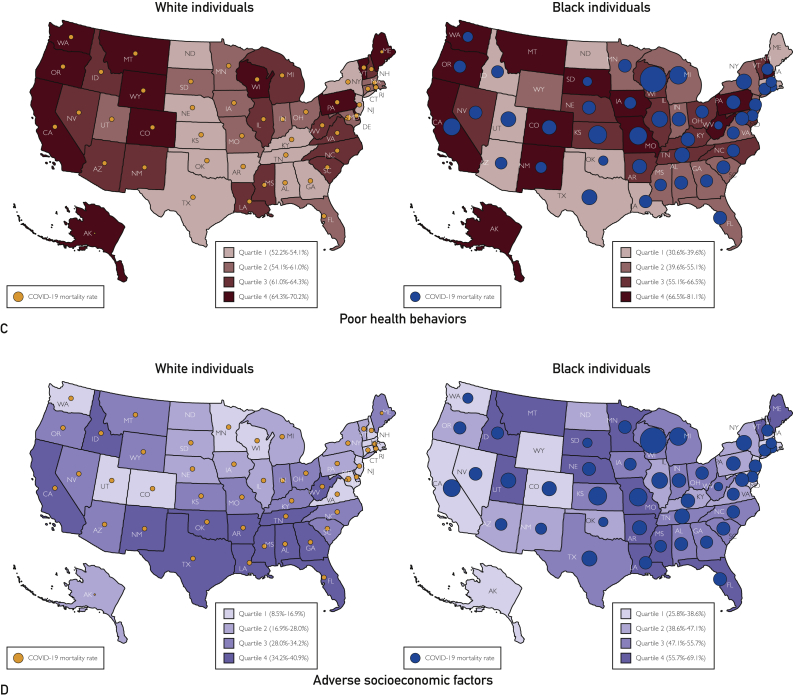
In the states reporting race-stratified data, there were a total of 623,177 COVID-19 cases in black individuals and 1,272,675 cases in white individuals. There were 33,513 total deaths in black individuals and 78,272 deaths in whites. The cumulative incidence rate in blacks (1546.4 per 100,000 persons) was nearly 3 times higher than in whites (540.4 per 100,000 persons). The crude mortality rate in blacks (83.2 per 100,000 persons) was more than 2 times higher than that in whites (33.2 per 100,000 persons). The IFR in blacks and whites was 5.4 and 6.2 per 100 cases, respectively. There was no significant correlation of the race-stratified state-level crude mortality and IFR with the prevalence of comorbidities, preexisting medical conditions, poor health behaviors, and adverse socioeconomic factors (P >.05 for all) (Figure 2, Figure 3, Figure 4; Supplemental Figures 1 and 2, available online at http://www.mcpiqojournal.org).
Figure 2.
Relationship of the geographic distribution of coronavirus disease 2019 (COVID-19) mortality and the prevalence of health determinants in black individuals. Pink diamonds represent the northeastern region states, green diamonds represent the midwestern states, red diamonds represent the states in the southern region, and blue diamonds represent the states in the western region.
Figure 3.
Relationship of the geographic distribution of coronavirus disease 2019 (COVID-19) mortality and the prevalence of health determinants in white individuals. Pink diamonds represent the northeastern region states, green diamonds represent the midwestern states, red diamonds represent the states in the southern region, and blue diamonds represent the states in the western region.
Figure 4.
Geographic distribution of coronavirus disease 2019 (COVID-19) mortality and the prevalence of health determinants, stratified by race. The figure represents the race-stratified state-level prevalence of comorbidities (Panel A), preexisting medical conditions (Panel B), poor health behaviors (Panel C), and adverse socioecomic factors (Panel D). The size of bubbles is proportional to COVID-19 mortality. Purple bubbles represent COVID-19 mortality in whites, and red bubbles represent COVID-19 mortality in blacks.
Discussion
In this study, we observed that the prevalence of comorbidities and adverse socioeconomic factors was higher in black individuals, and these determinants were concentrated in southern and midwestern US states for both black and white individuals. Substantial geographic heterogeneity in the prevalence of clinical and social determinants of health was noted within the racial groups. In states reporting race-stratified data on COVID-19 cases and mortality, the COVID-19 cumulative incidence rate was nearly 3-fold higher and the COVID-19 mortality was more than 2-fold higher in black individuals than in their white counterparts. Notably, the IFR in black and white individuals was similar across all the states with available data. Although the pernicious influence of comorbidities and adverse socioeconomic factors may explain the higher proportion of COVID-19 infections and mortality burden in black individuals, the known health determinants did not track with the current geographic pattern of COVID-19 fatality within the racial groups.
We found a higher prevalence of comorbidities in black individuals universally across the US states, with a considerably higher concentration of comorbidities in the southern and midwestern regions. Although the prevalence of preexisting medical conditions was comparable between racial groups, white individuals had a higher prevalence of poor health behaviors. There are inherent differences in the geographic distribution of these biological determinants within the black and white racial groups. A higher prevalence of comorbidities has been previously reported in the deep south, which is often attributed to the higher proportion of black individuals and clustering of the socioeconomic factors in the region.16,19, 20, 21, 22 We substantiate these reports and provide perspective into these regional differences by highlighting that both blacks and whites in the southern region have a higher comorbidity burden compared with their counterparts in the rest of the United States.
The population-level COVID-19 mortality burden is presumed to follow the population prevalence of comorbidities given that comorbidity burden and concomitant illnesses are critical factors in predicting the risk of fatal outcomes from COVID-19 in inpatient settings.23, 24, 25, 26, 27, 28, 29, 30 However, our investigation indicates that within the racial groups, the higher comorbidity burden does not track with the geographic dispersion of COVID-19 fatality. Although black individuals are evidently at a higher risk of getting COVID-19 and bearing a higher mortality burden, once they get the disease, they may be deemed to be at a similar risk as their white counterparts. This is evident in the higher population-level COVID-19 mortality burden in blacks. However, Blacks have a similar or lower IFR compared with Whites. Our ecological investigation aligns with the clinical reports from inpatient settings, in which the risk of COVID-19 mortality is similar in black and white patients after adjusting the clinical and socioeconomic health determinants.31 There may be other plausible biological and environmental factors governing the severity and fatality from the novel and ubiquitous virus above and beyond the population prevalence of comorbidities.32,33
Racial disparities in exposure, susceptibility, and mortality due to a respiratory viral pandemic have been previously reported during the H1N1 pandemic.34,35 Although the effect of a higher prevalence of comorbidities in black individuals increases their susceptibility to severe infection and adverse outcomes, the malignant effect of adverse socioeconomic determinants of health is heightened during the pandemic.34, 35, 36, 37, 38 Numerous adverse socioeconomic factors prevent the at-risk black population from assiduously adhering to social distancing measures.1, 2, 3, 4,38,39 Disproportionately higher numbers of blacks are socioeconomically restricted and are at risk for COVID-19 exposure because of increased housing density, crowded living conditions, reduced ability to work from home or endure long unpaid furloughs, dependence on public transportation, and decreased access to healthy food.1, 2, 3, 4,38,39 Racial minorities also make up most of the workforce in areas such as health care, government, transportation, and food supply that are deemed as essential services.1, 2, 3, 4,38,39 The socioeconomic constraints affecting the high-risk black population presents a vexing challenge to the adequate implementation of preventive measures.1, 2, 3, 4,38,39 However, the racial effect of these determinants may be attenuated once individuals are infected.
We noted that a lack of health insurance coverage was more prevalent in black individuals in all US states. Lack of access to health care is a critical factor that impedes minority groups from seeking medical attention for symptoms. Early in the pandemic, limited testing capacity for COVID-19 led to a requirement that referral from health care workers was necessary to be tested. Those without health insurance were less likely to seek physician consultation, rendering them undiagnosed with possible COVID-19 infection, and a source of potential viral spread within the community. Additionally, the nationwide response to the pandemic has varied because of the differential burden of COVID-19 across states and the heterogeneity in responses of local public health agencies.5,6 The complex equipoise of the various factors may explain the geographic mismatch in the risk factor prevalence and COVID-19 fatality within the racial groups.
Our study has crucial public health implications by contextualizing the racial disparities seen with COVID-19. The persistent racial disparities in health care, as underlined by our study, may predispose black individuals to bear the hefty share of the COVID-19 pandemic. Within race, individuals are at a higher COVID-19 risk, notwithstanding the population prevalence of comorbidities and socioeconomic factors. The observed racial differences in COVID-19 cumulative incidence and mortality may be due to variation in the physical environment, health behaviors, access to clinical care, low penetration of evidence-based treatment strategies, social discrimination, poor socioeconomic status, and education, which will continue to persist, if not increase, after the effect of the ongoing pandemic.20,40, 41, 42, 43, 44, 45, 46, 47 Our data call for the prioritization of public health initiatives explicitly targeted at minority populations with region-specific approaches to stem these disparities. The COVID-19 cumulative incidence and mortality do not conform to the geographic distribution of health and social determinants, which is worrisome and indicate that other modulating factors need to be explored. The economic recession secondary to the pandemic may disproportionately affect racial minorities, with more people losing health insurance coverage and further worsening of the preexisting medical conditions. The relaxation of social distancing may critically affect racial minorities who are already at a higher risk for adverse outcomes because of a large comorbidity burden, unemployment, food insecurity, and limited access to health care. Such conditions may perpetuate the deeply entrenched racial disparities in health outcomes48,49 and make the mitigation of these disparities even more challenging.
Our investigation may have several limitations. The BRFSS includes self-reported cross-sectional survey data from which true disease incidence cannot be discerned. However, BRFSS data have been previously validated in numerous studies.8,9 The small population size of other racial subgroups at the state level lead to unreliable estimates, restricted their inclusion in the study. Moreover, because of several incongruences, lack of uniformity, and large missing data, in the reporting of race-stratified COVID-19 cumulative incidence and mortality reported by respective states, we were restricted unable to make a uniform assessment. Mortality due to COVID-19 has been assessed both at the population level (per 100,000 individuals in the area) and at the case level (IFR). We report both the mortality metrics that quantify the population mortality burden (crude mortality rate) and case-associated fatality (IFR). The COVID-19 pandemic is ongoing and at various stages in different states, and the current mortality statistics provide only a cross-sectional picture of the current status of the pandemic. The currently observed patterns of COVID-19 mortality may also be affected by physical mobility and the ability of the existing health care infrastructure to sustain the increased pandemic burden. The ecological study design with no data on individual-level measurements in patients with COVID-19 may be susceptible to ecological fallacies if conclusions are drawn at the individual level. Because of a lack of individual-level measures of comorbidities, preexisting conditions, poor health behaviors, and socioeconomic factors in patients with COVID-19, we were not able to assess a multivariable-adjusted relationship of the various race-stratified state-level measures. We did not assess the correlation of individual health determinants with COVID-19 mortality, as it may lead to the ecological fallacy of the derivation of exposure-outcome inference at the patient level. Additionally, COVID-19 outcome data are still accumulating and are lagging. As the understanding of the disease advances, the accuracy of our assumptions may be reevaluated when further research examines individual-level data from patients with COVID-19. Notwithstanding these limitations, our investigation provides a robust account of race-stratified geographic disparity in health determinants and COVID-19 mortality.
Conclusion
Black individuals have a higher prevalence of comorbidities, adverse socioeconomic factor, and higher COVID-19 mortality burden but similar IFR compared with their white counterparts in all regions of the United States. Within both racial groups, there was a discordance between COVID-19 mortality burden, IFR, and the prevalence of various health determinants, indicating that there are novel factors that contribute to the population-level mortality burden of COVID-19.
Footnotes
Grant Support: The work was supported by grant U54MD000502 (P.A.) from the Minority Health and Health Disparities Research Center, National Institute on Minority Health and Health Disparities and grant 5K23 HL146887-02 (Mentored-Patient Oriented Research Award; P.A.) from the National Institutes of Health.
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Chowkwanyun M., Reed A.L., Jr. Racial health disparities and Covid-19—caution and context. N Engl J Med. 2020;383(3):201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 3.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Equity Considerations and Racial and Ethnic Minority Groups Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
- 5.Schuchat A., CDC COVID-19 Response Team Public Health response to the initiation and spread of pandemic COVID-19 in the United States, February 24-April 21, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):551–556. doi: 10.15585/mmwr.mm6918e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019-February 4, 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020;69(6):173] MMWR Morb Mortal Wkly Rep. 2020;69(5):140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin S.M., Crist M.B., Perkins K.M., Perz J.F. Outbreak response capacity assessments and improvements among public health department health care-associated infection programs—United States, 2015-2017. https://doi.org/10.1097/PHH.0000000000001148 [published online ahead of print April 17, 2020]. J Public Health Manag Pract. [DOI] [PMC free article] [PubMed]
- 8.Pilkerton C.S., Singh S.S., Bias T.K., Frisbee S.J. Changes in cardiovascular health in the United States, 2003-2011. J Am Heart Assoc. 2015;4(9):e001650. doi: 10.1161/JAHA.114.001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J., Yang Q., Hong Y., Loustalot F. Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia, 2009. J Am Heart Assoc. 2012;1(6):e005371. doi: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James C.V., Moonesinghe R., Wilson-Frederick S.M., Hall J.E., Penman-Aguilar A., Bouye K. Racial/ethnic health disparities among rural adults—United States, 2012-2015. MMWR Surveill Summ. 2017;66(23):1–9. doi: 10.15585/mmwr.ss6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer P.A., Penman-Aguilar A., Campbell V.A., Graffunder C., O’Connor A.E., Yoon P.W., Centers for Disease Control and Prevention (CDC) Conclusion and future directions: C.D.C. Health Disparities and Inequalities Report—United States, 2013. MMWR Suppl. 2013;62(3):184–186. [PubMed] [Google Scholar]
- 12.Meyer P.A., Yoon P.W., Kaufmann R.B., Centers for Disease Control and Prevention (CDC) Introduction: CDC Health Disparities and Inequalities Report—United States, 2013. MMWR Suppl. 2013;62(3):3–5. [PubMed] [Google Scholar]
- 13.Rolle-Lake L., Robbins E. StatPearls Publishing; Treasure Island, FL: 2020. Behavioral Risk Factor Surveillance System (BRFSS) [PubMed] [Google Scholar]
- 14.2019 national and state estiamtes. United States Census Bureau website. https://www.census.gov/newsroom/press-kits/2019/national-state-estimates.html
- 15.Module 8: age standardization and population estimates. Centers for Disease Control and Prevention website. https://wwwn.cdc.gov/nchs/nhanes/tutorials/module8.aspx
- 16.Gebreab S.Y., Davis S.K., Symanzik J., Mensah G.A., Gibbons G.H., Diez-Roux A.V. Geographic variations in cardiovascular health in the United States: contributions of state- and individual-level factors. J Am Heart Assoc. 2015;4(6):e001673. doi: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendy V.L., Vargas R. Trends in major risk factors for cardiovascular disease among adults in the Mississippi Delta region, Mississippi Behavioral Risk Factor Surveillance System, 2001-2010. Prev Chronic Dis. 2015;12:E21. doi: 10.5888/pcd12.140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Community Survey (ACS). United States Census Bureau website. https://www.census.gov/programs-surveys/acs
- 19.Mujib M., Zhang Y., Feller M.A., Ahmed A. Evidence of a “heart failure belt” in the southeastern United States. Am J Cardiol. 2011;107(6):935–937. doi: 10.1016/j.amjcard.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker L.E., Kirtland K.A., Gregg E.W., Geiss L.S., Thompson T.J. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Howard G., Howard V.J. Twenty years of progress toward understanding the stroke belt. Stroke. 2020;51(3):742–750. doi: 10.1161/STROKEAHA.119.024155. [DOI] [PubMed] [Google Scholar]
- 22.Mensah G.A., Cooper R.S., Siega-Riz A.M. Reducing cardiovascular disparities through community-engaged implementation research: a National Heart, Lung, and Blood Institute Workshop Report. Circ Res. 2018;122(2):213–230. doi: 10.1161/CIRCRESAHA.117.312243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harcourt J., Tamin A., Lu X. Severe acute respiratory syndrome coronavirus 2 from patient with 2019 novel coronavirus disease, United States. Emerg Infect Dis. 2020;26(6):1266–1273. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 29.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 31.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giudicessi J.R., Roden D.M., Wilde A.A.M., Ackerman M.J. Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm. 2020;17(9):1487–1492. doi: 10.1016/j.hrthm.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y., Li L., Feng Z. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn S.C., Kumar S., Freimuth V.S., Musa D., Casteneda-Angarita N., Kidwell K. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health. 2011;101(2):285–293. doi: 10.2105/AJPH.2009.188029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Placzek H., Madoff L. Effect of race/ethnicity and socioeconomic status on pandemic H1N1-related outcomes in Massachusetts. Am J Public Health. 2014;104(1):e31–e38. doi: 10.2105/AJPH.2013.301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham T.J., Croft J.B., Liu Y., Lu H., Eke P.I., Giles W.H. Vital signs: racial disparities in age-specific mortality among blacks or African Americans—United States, 1999-2015 [published correction appears in MMWR Morb Mortal Wkly Rep. 2017;66(18):490] MMWR Morb Mortal Wkly Rep. 2017;66(17):444–456. doi: 10.15585/mmwr.mm6617e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fothergill A., Maestas E.G., Darlington J.D. Race, ethnicity and disasters in the United States: a review of the literature. Disasters. 1999;23(2):156–173. doi: 10.1111/1467-7717.00111. [DOI] [PubMed] [Google Scholar]
- 38.Jackson S.A., Anderson R.T., Johnson N.J., Sorlie P.D. The relation of residential segregation to all-cause mortality: a study in black and white. Am J Public Health. 2000;90(4):615–617. doi: 10.2105/ajph.90.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall W.J., Chapman M.V., Lee K.M. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y., Greenlund K.J., Croft J.B., Keenan N.L., Giles W.H. Factors explaining excess stroke prevalence in the U.S. Stroke Belt. Stroke. 2009;40(10):3336–3341. doi: 10.1161/STROKEAHA.109.561688. [DOI] [PubMed] [Google Scholar]
- 41.Le A., Judd S.E., Allison D.B. The geographic distribution of obesity in the U.S. and the potential regional differences in misreporting of obesity. Obesity (Silver Spring) 2014;22(1):300–306. doi: 10.1002/oby.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillum R.F., Mehari A., Curry B., Obisesan T.O. Racial and geographic variation in coronary heart disease mortality trends. BMC Public Health. 2012;12:410. doi: 10.1186/1471-2458-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamin E.J., Muntner P., Alonso A., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Heart Disease and Stroke Statistics—2019 Update: a report from the American Heart Association [published correction appears in Circulation. 2020;141(2):e33] Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 44.Voeks J.H., McClure L.A., Go R.C. Regional differences in diabetes as a possible contributor to the geographic disparity in stroke mortality: the REasons for Geographic And Racial Differences in Stroke Study. Stroke. 2008;39(6):1675–1680. doi: 10.1161/STROKEAHA.107.507053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughan A.S., Kramer M.R., Casper M. Geographic disparities in declining rates of heart disease mortality in the southern United States, 1973-2010. Prev Chronic Dis. 2014;11:E185. doi: 10.5888/pcd11.140203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidney S., Go A.S., Jaffe M.G., Solomon M.D., Ambrosy A.P., Rana J.S. Association between aging of the U.S. population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4(12):1280–1286. doi: 10.1001/jamacardio.2019.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingram D.D., Montresor-Lopez J.A. Differences in stroke mortality among adults aged 45 and over: United States, 2010-2013. NCHS Data Brief. 2015;(207):1–8. [PubMed] [Google Scholar]
- 48.Kalra R., Patel N., Arora P., Arora G. Cardiovascular health and disease among Asian-Americans (from the National Health and Nutrition Examination Survey) Am J Cardiol. 2019;124(2):270–277. doi: 10.1016/j.amjcard.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Patel N., Kalra R., Bhargava A., Arora G., Arora P. Ideal cardiovascular health among American adults after the economic recession of 2008-2009: insights from NHANES. Am J Med. 2019;132(10):1182–1190.e1185. doi: 10.1016/j.amjmed.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.