Abstract
Background
The COVID-19 pandemic hit all over the world, and cancer patients are more vulnerable for COVID-19. The mortality rate may increase up to 25% in solid malignancies. In parallel to increased mortality rates among cancer patients, safety concerns regarding cancer treatment has increased over time. However, there were contradictory results for the cancer treatment during pandemic. In this study, we assessed the effect of cancer treatment on the severity of COVID-19.
Methods
The MEDLINE database was searched on September 01, 2020. Primary end-points were severe disease and death in the cancer patients treated within the last 30 days before COVID-19 diagnosis. Quality of included studies was assessed by Newcastle–Ottawa scale. The generic inverse-variance method was used to calculate odds ratios (ORs) for each outcome.
Results
Sixteen studies were included for this meta-analysis. Chemotherapy within the last thirty days before COVID-19 diagnosis increased the risk of death in cancer patients after adjusting for confounding variables (OR: 1.85; 95% confidence interval: 1.26–2.71). However, severe COVID-19 risk did not increase. Furthermore, targeted therapies, immunotherapy, surgery and radiotherapy did not increase the severe disease and death risk in cancer patients with COVID-19.
Conclusion
Chemotherapy increased the risk of death from COVID-19 in cancer patients. However, there was no safety concern for immunotherapy, targeted therapies, surgery and radiotherapy.
Keywords: COVID-19, SARS-CoV-2, Cancer treatment, Chemotherapy, Immunotherapy, Targeted therapy
1. Introduction
After reporting the first case of severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2) infection in Wuhan, China, coronavirus disease 2019 (COVID-19) became a pandemic in a short time and hit all over the world. As of September 13, 2020, 28,584,158 confirmed cases and 916,955 deaths from COVID-19 were observed across the world [1]. Cancer patients are more vulnerable to COVID-19. The case fatality rate was 25% for solid organ malignancies; however, it is 2.3% for the general population [2,3]. In this context, since the beginning of the pandemic, there have been some concerns regarding cancer patients' treatment. We observe that the decision-making processes of oncologists are affected globally during the COVID-19 pandemic [4]. However, the European Society for Medical Oncology (ESMO) and the American Society of Clinical Oncology recommended continuing cancer treatment by evaluating and discussing the risk and benefit of cancer treatment with each individual [5,6]. Despite no clear evidence for the safety of using chemotherapy (CT), immunotherapy, targeted therapies, radiotherapy (RT), and performing surgery in the cancer patients during the COVID-19 pandemic, cancer patients are treated in the light of these recommendations.
Over time, the knowledge about cancer treatment in the pandemic increased. Cancer and COVID registries, such as United Kingdom Coronavirus Cancer Monitoring Project, COVID-19 and Cancer Consortium, and numerous studies were published in the last three months particularly [7,8]. However, results from these studies were conflicting.
In the present meta-analysis, we aimed to evaluate cancer treatment's effect on the severity of COVID-19.
2. Methods
This meta-analysis was conducted in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
2.1. Study cohort
The MEDLINE database was searched on September 01, 2020, using the following keywords and boolean operators: ‘((‘COVID-19’ OR ‘SARS-CoV2’ OR ‘SARS-CoV-2’) AND (cancer OR neoplasm OR malignancy))’.
Inclusion criteria to select the studies: (a) patients – cancer patients receiving active cancer treatment and diagnosed with COVID-19; (b) intervention – cancer treatment within the last 30 days before COVID-19 diagnosis (i.e. CT, immunotherapy, targeted therapies, RT and surgery); (c) comparator – patients who do not receive cancer treatment whose effect is evaluated; (d) outcome – severe disease and death; (e) study design – prospective and retrospective studies. Pre-clinical studies, reviews, case reports, articles not in English and articles without full text were excluded.
2.2. Data extraction
According to the inclusion and exclusion aforementioned criteria, full-text articles of studies were assessed independently by two reviewers (E.Y., Y.Ü.). Data including the following headlines were extracted from the database: author names, publishing journals, the year of publication, the total number of patients in each study, the number of male patients, patient subgroups in the comparison groups, median–mean age, cancer treatment intervals before COVID-19 diagnosis, the number of patients in each cancer treatment subtype, unadjusted and adjusted odds ratios (ORs) for the severe disease and death for each cancer treatment subtype, and adjusting variables for multivariable analyses results.
2.3. Assessment quality of included studies
The quality of included studies was assessed independently by two reviewers (E.Y. and Y.Ü.) using the Newcastle–Ottawa scale (NOS) for case-control and cohort studies.
The total score was calculated using the following subsets: 1) selection, 2) comparability and 3) outcome. All subsets in the NOS for the cohort studies have subheadings. Subheadings for study selection: a) representativeness of the exposed cohort, b) selection of the non-exposed cohort, c) ascertainment of exposure, d) demonstration that the outcome of interest was not present at the start of study; the subheading for comparability: a) comparability of cohorts on the basis of the design or analysis; subheadings for outcome: a) assessment of the outcome, b) was follow-up long enough for outcomes to occur?, c) adequacy of follow-up of cohorts.
Similarly, the NOS for case-control studies has three subsets, including selection, comparability and exposure. All subsets also have subheadings. Subheadings for study selection: a) Is the case definition adequate?, b) representativeness of the cases, c) selection of controls, d) definition of controls; the subheading for comparability: a) comparability of cases and controls on the basis of the design or analysis; subheadings for exposure: a) ascertainment of exposure, b) the same method of ascertainment for cases and controls, c) the non-response rate.
Studies can earn four stars from selection, two stars from comparability and three stars from the outcome. Thus, the maximum score for each study can be nine stars [10].
2.4. Statistical analysis
The meta-analysis was performed using the generic inverse-variance method with a random-effects model to calculate each outcome's risk. The effect size was the OR and its 95% confidence interval (CI). The primary comparison was severe disease and death risk between SARS-CoV2 positive patients on active cancer treatment and those who were not on active cancer treatment. All analyses were done using the Review Manager software, version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). The thresholds for statistical significance for overall effect tests were 0.05 and that for the tests of heterogeneity, within the overall, was 0.10. The I [2] coefficient was additionally used to quantify the degree of heterogeneity between the studies.
3. Results
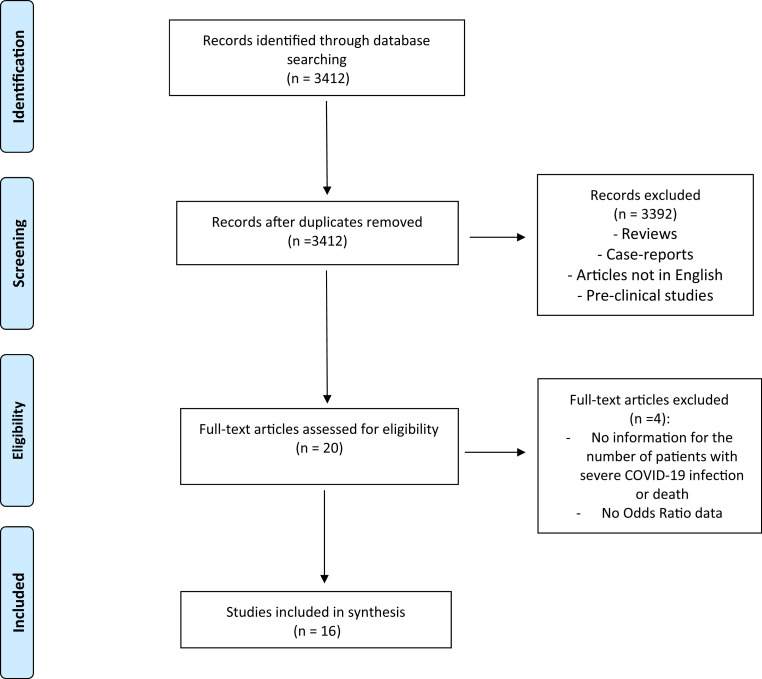
After searching in accordance with the aforementioned criteria, we assessed twenty articles with full text. Finally, we included sixteen studies for this meta-analysis. The PRISMA diagram for the selection process of the included studies is shown in Figure 1 . We assessed the quality of the included studies by using the NOS. Results for the quality assessment of the included studies are shown in Table 1 . Two of sixteen studies were prospective-cohort study [11,12]. Remaining studies were retrospective [7,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. Four thousand five hundred ten cancer patients were included, and 53% of all patients (2421 patients) were male. Baseline characteristics of all the included studies are shown in Table 2 .
Fig. 1.
The PRISMA diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
The Newcastle–Ottawa scale for quality assessment of the studies.
| Author, year | Selection | Comparability | Exposure/outcome | Total |
|---|---|---|---|---|
| Zhang L. et al., Annals of Oncology [13] | ★★ | ★ | ★★ | ★★★★★ |
| Dai et al., Cancer Discovery [14] | ★ | ★★ | ★★★ | ★★★★★★ |
| Stroppa et al., Future Oncology [15] | ★ | ★ | ★★ | ★★★★ |
| Kuderer et al., Lancet [7],a | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Lee LY et al., Lancet [12],a | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Yang et al., Lancet Oncology [16],a | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Zhang H. et al., Cancer [17] | ★ | ★ | ★★ | ★★★★ |
| Robilotti et al., Nature Medicine [18] | ★ | ★★ | ★★ | ★★★★★ |
| Yarza et al., European Journal of Cancer [19] | ★★ | ★★ | ★★ | ★★★★★★ |
| Li et al., Leukemia [20] | ★★ | ★★ | ★★ | ★★★★★★ |
| Jee et al., Journal of Clinical Oncology [21] | ★★ | ★ | ★★ | ★★★★★ |
| Sanchez-Pina et al., European Journal of Haematology [22] | ★★ | ★★ | ★★ | ★★★★★★ |
| Lee LYW, Lancet Oncology [11],a | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Pinato et al., Cancer Discovery [23] | ★★ | ★★ | ★★★ | ★★★★★★★ |
| Assaad et al., European Journal of Cancer [24] | ★★ | ★★ | ★★ | ★★★★★★ |
| Garassino et al., Lancet Oncology [25] | ★★ | ★★ | ★★ | ★★★★★★ |
Quality assessed by using the Newcastle–Ottawa scale (NOS) for cohort studies. Remaining studies assessed by using the NOS for case control studies.
Table 2.
Characteristics of the included trials.
| Author; journal | Type of the study | Number of patients | Number of male patients | Median age (IQR) (years) | Cancer treatment tnterval before COVID-19 infection (days) | Comparison group | Number of patients in chemotherapy group | Number of patients in immunotherapy group | Number of patients in targeted therapy group | Number of patients in surgery group | Number of patients in radiotherapy group | Number of patients in cancer treatment group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang L. et al., Annals of Oncology [13] | Retrospective | 28 | 17 | 65 (56–70) | 30 | Cancer patients with no treatment | N/A | N/A | N/A | N/A | N/A | 12 |
| Dai et al., Cancer Discovery [14] | Retrospective | 105 | 57 | 64 (57–71) | 40 | Non-cancer patients | 17 | 6 | 4 | 8 | 13 | 48 |
| Stroppa et al., Future Oncology [15] | Retrospective | 25 | 20 | 71a | N/A | Cancer patients with no treatment | 8 | 4 | N/A | N/A | N/A | 12 |
| Kuderer et al., Lancet [7] | Retrospective | 928 | 468 | 66 (57–76) | 28 | Cancer patients with no treatment | 160 | 38 | 75 | 32 | 12 | 366 |
| Lee LY et al., Lancet [12] | Prospective cohort | 800 | 449 | 69 (59–76) | 28 | Cancer patients with no treatment | 281 | 44 | 72 | 29 | 76 | 528 |
| Yang et al., Lancet Oncology [16] | Retrospective | 205 | 96 | 63 (56–70) | 28 | Cancer patients with no treatment | 31 | 4 | 12 | 4 | 9 | 54 |
| Zhang H. et al., Cancer [17] | Retrospective | 107 | 60 | 66 (36–98) | N/A | Cancer patients with no treatment | N/A | 6 | N/A | N/A | N/A | 37 |
| Robilotti et al., Nature Medicine [18] | Retrospective | 423 | 212 | N/A | 30 | Cancer patients with no treatment | 191 | 31 | N/A | N/A | N/A | N/A |
| Yarza et al., European Journal of Cancer [19] | Prospective cohort | 63 | 34 | N/A | 28 | Cancer patients treated other options | 36 | 8 | 7 | N/A | N/A | N/A |
| Li et al., Leukemia [20] | Retrospective | 59 | 31 | 63 (54–70) | 30 | Cancer patients with no treatment | 12 | N/A | 6 | 1 | 1 | 20 |
| Jee et al., Journal of Clinical Oncology [21] | Retrospective | 309 | 159 | N/A | 35 | Cancer patients with no treatment | 102 | 18 | 49 | N/A | N/A | 170 |
| Sanchez-Pina et al., European Journal of Haematology [22] | Retrospective | 39 | 23 | 64a | N/A | Cancer patients with no treatment | 4 | N/A | 5 | N/A | N/A | 24 |
| Lee LYW, Lancet Oncology [11] | Prospective cohort | 227 | 148 | 69 | 28 | Cancer patients with no treatment | 108 | N/A | N/A | N/A | N/A | N/A |
| Pinato et al., Cancer Discovery [23] | Retrospective | 890 | 503 | 68a | 19 (mean) | Cancer patients with no treatment | 206 | 56 | 93 | N/A | N/A | N/A |
| Assaad et al., European Journal of Cancer [24] | Retrospective | 302 | 144 | 58a | 30 | Cancer patients with no treatment | 137 | 26 | N/A | N/A | N/A | 137 |
| Garassino et al., Lancet Oncology [25] | Retrospective | 200 | 141 | 68 (61–75) | 7 (median) | Cancer patients with no treatment | 48 | 34 | 28 | N/A | N/A | 142 |
Abbreviations: COVID-19 = coronavirus disease 2019, IQR= interquartile range, N/A = data not available.
Mean age.
3.1. Chemotherapy and severity of COVID-19
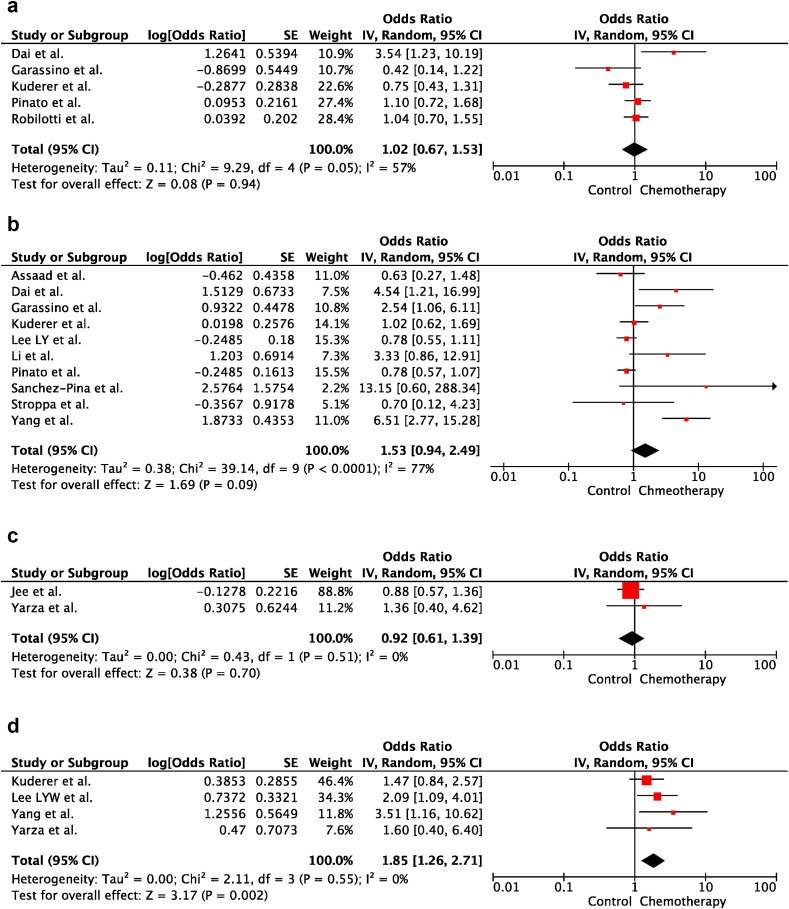
According to the analysis of univariable ORs, CT administration within the last 30 days before COVID-19 diagnosis did not increase the risk of severe disease and death in cancer patients (OR:1.02; 95% CI:0.67–1.53; p = 0.94 and OR:1.53; 95% CI:0.94–2.49; p = 0.09, respectively). There was heterogeneity between the included studies for severe disease and death (p = 0.05; I2 = 57% and p < 0.001; I2 = 77%, respectively). Forest plots of unadjusted ORs for the effect of CT on severe COVID-19 and risk of death from COVID-19 are shown in Figure 2 a and b, respectively. Furthermore, in multivariable analysis, there was no significant difference between the CT and control groups in severe COVID-19 risk (OR:0.92; 95% CI:0.61–1.39). In contrast, risk of death from COVID-19 was higher in the CT group than that in the control group after adjusting for confounding variables (i.e. age, sex, cancer type, cancer treatment subtype, duration of cancer diagnosis, smoking status, obesity, performance status, presence of metastasis and comorbidities) (OR: 1.85; 95% CI:1.26–2.71). There was no heterogeneity between the included studies for adjusted results of severe disease and death (p = 0.55; I2 = 0% and p = 51; I2 = 0%, respectively). Forest plots of adjusted ORs for the effect of CT on severe COVID-19 and death of risk from COVID-19 are shown in Figure 2c and d, respectively.
Fig. 2.
(a) The forest plot of unadjusted severe COVID-19 risk due to chemotherapy. (b) The forest plot of unadjusted risk of death from COVID-19 due to Chemotherapy. (c) The forest plot of adjusted severe COVID-19 risk due to chemotherapy. ∗Adjusted variables for the study of Jee et al. [21]: age, BMI, sex, performance score, smoking, comorbidities, malignancy type, cancer remission status, neutropenia and lymphopenia. ∗Adjusted variables for the study of Yarza et al. [19]: age, sex, performance score, presence of metastasis, previous venous thromboembolic event, chronic obstructive pulmonary disease. (d) The forest plot of adjusted risk of death from COVID-19 due to chemotherapy. ∗ Adjusted variables for the study of Kuderer et al. [7]: age, sex, smoking status and obesity. ∗ Adjusted variables for the study of Lee et al. [8]: cancer treatment subtype. ∗ Adjusted variables for the study of Yang et al. [16]: sex, cancer type, duration of cancer diagnosis. ∗ Adjusted Variables for the study of Yarza et al. [19]: age, sex, performance score, presence of metastasis, previous venous thromboembolic event, chronic obstructive pulmonary disease. CI, confidence interval; IV, inverse variance; SE, standard error.
3.2. Immunotherapy and severity of COVID-19
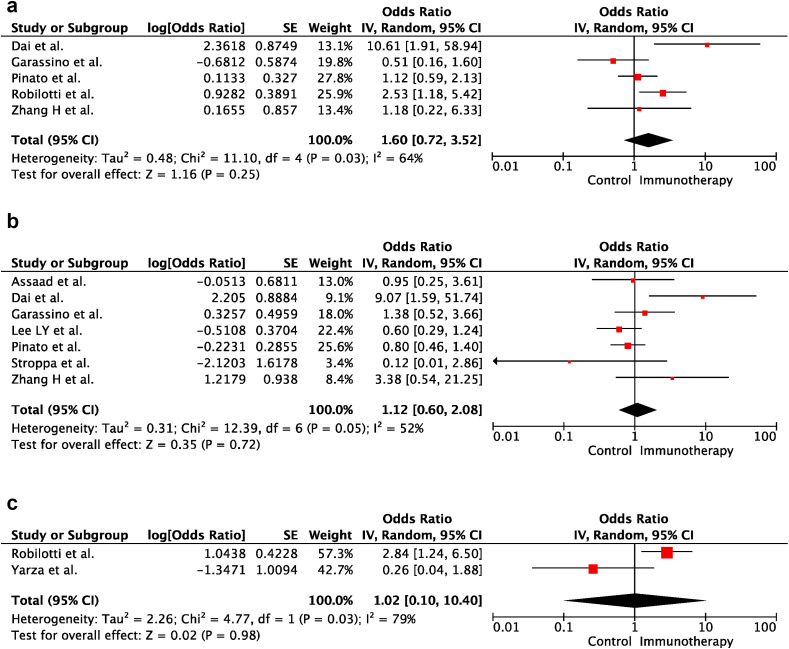
In univariable analyses, immunotherapy within the last 30 days before COVID-19 diagnosis did not increase the risk of severe disease and death in cancer patients (OR:1.60; 95% CI:0.72–3.52; p = 0.25 and OR:1.12; 95% CI:0.60–2.08; p = 0.72, respectively). There was heterogeneity between the included studies for severe disease and death (p = 0.03; I2 = 64% and p = 0.05; I2 = 52%, respectively). Forest plots of unadjusted ORs for the effect of immunotherapy on severe COVID-19 and risk of death from COVID-19 are shown in Figure 3 a and b. Furthermore, there was no significant difference between the immunotherapy and control groups in the severe disease risk after adjusting for confounding variables (i.e. age, sex, race, performance score, smoking status, cancer type, corticosteroid use, lymphopenia and presence of comorbidities and metastasis) (OR:1.02; 95% CI:0.10–10.40; p = 0.98). There was heterogeneity between the included studies for adjusted results of severe disease (p = 0.03; I2 = 79%). The forest plot of adjusted ORs for the effect of immunotherapy on severe COVID-19 is shown in Figure 3c.
Fig. 3.
(a) The forest plot of unadjusted severe COVID-19 Risk Due to Immunotherapy. (b) The forest plot of unadjusted risk of death from COVID-19 due to immunotherapy. (c) The forest plot of adjusted severe COVID-19 risk due to immunotherapy. ∗Adjusted variables for the study of Robilotti et al.: age, race, smoking, asthma, cancer type, comorbidities, corticosteroid use/lymphopenia. ∗ Adjusted variables for the study of Yarza et al.[19]: age, sex, performance score, presence of metastasis, previous venous thromboembolic event, chronic obstructive pulmonary disease. CI, confidence interval; IV, inverse variance; SE, standard error.
3.3. Targeted therapies and severity of COVID-19
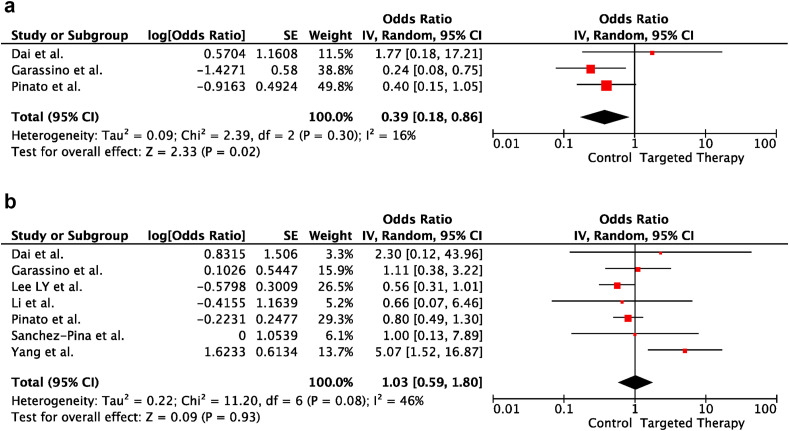
In univariable analyses, targeted therapies within the last 30 days before COVID-19 diagnosis did not increase the risk of severe disease and death in cancer patients (OR:0.39; 95% CI:0.18–0.86; p = 0.02 and OR:1.03; 95% CI:0.59–1.80; p = 0.93, respectively). There was heterogeneity between the included studies for death (p = 0.08; I2 = 46%). In contrast, there was no heterogeneity between the included trials for severe disease (p = 0.30; I2 = 16%). Forest plots of unadjusted ORs for the effect of targeted therapies on severe COVID-19 and risk of death from COVID-19 are shown in Figure 4 a and b, respectively.
Fig. 4.
(a) The forest plot of unadjusted severe COVID-19 risk due to targeted therapy. (b) The forest plot of unadjusted risk of death from COVID-19 due to targeted therapy. CI, confidence interval; IV, inverse variance; SE, standard error.
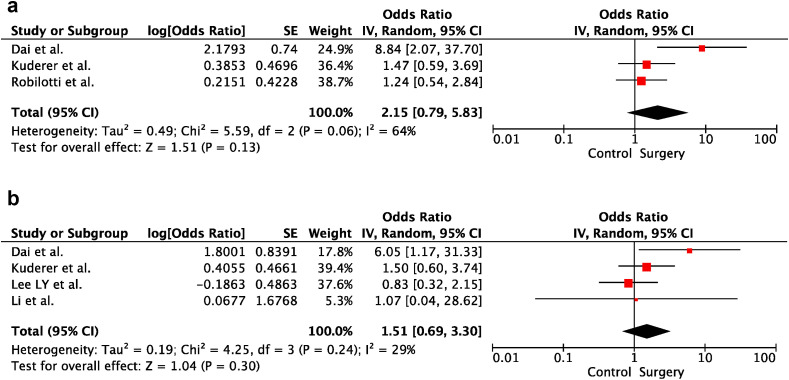
3.4. Cancer surgery and severity of COVID-19
In univariable analyses, cancer surgery within the last 30 days before COVID-19 diagnosis did not increase the risk of severe disease and death in cancer patients (OR:2.15; 95% CI:0.79–5.83; p = 0.13 and OR:1.51; 95% CI:0.69–3.30; p = 0.30, respectively). There was heterogeneity between the included studies for severe disease (p = 0.06; I2 = 64%). In contrast, there was no heterogeneity between the included trials for death (p = 0.24; I2 = 29%). Forest plots of unadjusted ORs for the effect of cancer surgery on severe COVID-19 and risk of death from COVID-19 are shown in Figure 5 a and b, respectively.
Fig. 5.
(a) The forest plot of unadjusted severe COVID-19 risk due to surgery. (b) The forest plot of unadjusted risk of death from COVID-19 due to surgery. CI, confidence interval; IV, inverse variance; SE, standard error.
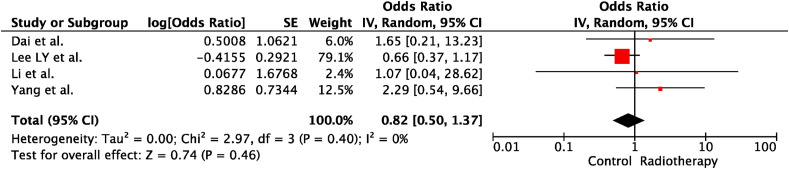
3.5. RT and severity of COVID-19
In univariable analyses, RT within the last 30 days before COVID-19 diagnosis did not increase cancer patients' risk of death (OR:0.82; 95% CI:0.50–1.37; p = 0.46). There was no heterogeneity between the included trials (p = 0.40; I2 = 0%). The forest plot of unadjusted ORs for the effect of RT on the risk of death from COVID-19 is shown in Figure 6 .
Fig. 6.
The forest plot of unadjusted risk of death from COVID-19 due to radiotherapy. IV, inverse variance; SE, standard error
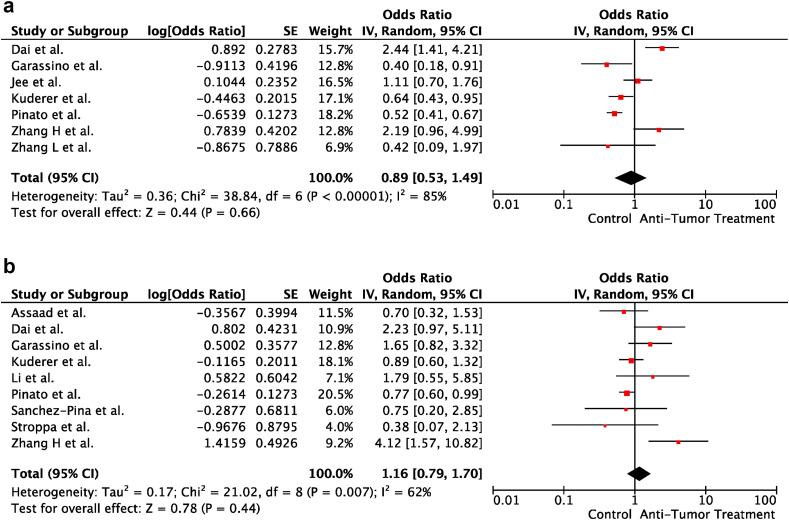
3.6. Cancer treatment and severity of COVID-19
In univariable analyses, cancer treatment within the last 30 days before COVID-19 diagnosis did not increase the risk of severe disease and death in cancer patients (OR:0.89; 95% CI:0.53–1.49; p = 0.66 and OR:1.16; 95% CI:0.79–1.70; p = 0.44, respectively). There was heterogeneity between the included studies for severe disease and death (p < 0.001; I2 = 85% and p = 0.007; I2 = 62%, respectively). Forest plots of unadjusted ORs for the effect of cancer treatment on severe COVID-19 and risk of death from COVID-19 are shown in Figure 7 a and b, respectively.
Fig. 7.
(a) The forest plot of unadjusted severe COVID-19 risk due to cancer treatment. (b) The forest plot of unadjusted risk of death from COVID-19 due to cancer treatment. CI, confidence interval; IV, inverse variance; SE, standard error.
4. Discussion
To the best of our knowledge, this was the first comprehensive meta-analysis regarding the effect of cancer treatment on the clinical course of COVID-19.
In the present meta-analysis, we showed that active cancer treatment was not associated with an increased risk for severe disease and death from COVID-19. Furthermore, immunotherapy, targeted therapies, cancer surgery and RT did not increase the risk of severe disease and death from COVID-19 in cancer patients. According to multivariable analyses, although there was no increased risk for severe disease, the risk of death from COVID-19 was higher in the cancer patients administered CT.
During the COVID-19 pandemic, we learnt that age and male sex are associated with higher severe disease and mortality rates [26,27]. Furthermore, comorbidities and smoking affect the prognosis of COVID-19 [28,29]. In univariable analysis for the effect of CT on COVID-19 mortality, there was no increased risk of death from COVID-19 in cancer patientsadministered CT within the last 30 days before COVID-19 diagnosis. However, after adjusting for confounding variables, such as age, sex and comorbidities, we showed that CT increased the risk of death from COVID-19. In other words, CT was an independent risk factor for death in cancer patients with COVID-19.
At first look, it seems that there was a discrepancy in the results of the present meta-analysis. In the usual clinical course of a COVID-19, it is expected that severe pulmonary dysfunction and septic shock cause death from COVID-19. Indeed, dyspnoea is the most common symptom of COVID-19 [30]. Furthermore, acute respiratory distress syndrome (ARDS) and septic shock were the most common causes of death [31]. However, it is well known that cytokine storm and macrophage-activation syndrome (MAS) led by SARS-CoV-2 is the main reason for ARDS and septic shock in patients with COVID-19. Uncontrolled inflammatory response against viral infection causes severe organ damage and, thus, pulmonary and cardiac dysfunction [32,33]. To make this robust and surge inflammatory response, individuals should have an effective immune system. However, the negative impact of CT on the immune system is well known for years [34]. Furthermore, after the mitigation of immune functions by CT, recovery of the immune system might take a long time [35]. An impaired immune system might cause a decreased inflammatory response against SARS-CoV-2 and, thus, protecting from cytokine storm and MAS [36]. Immune dysfunction led by CT might cause COVID-19 without apparent clinical manifestations. On the other hand, studies established that immunocompromised patients had similar clinical findings with immunocompetent patients [37]. Furthermore, although no apparent clinical findings of COVID-19, patients with immune dysfunction have an increased mortality [38]. It is worth noting that there is no convincing data to conclude a strict result regarding the immune system's effect on the severity of COVID-19. There was no clear reason to explain the increased mortality without severe disease in cancer patients administered CT. Besides the typical clinical course of severe COVID-19, the sudden death due to cardiac arrhythmia and injury was also reported [39]. However, there is no obvious evidence for the increased risk of sudden cardiac death in cancer patients with COVID-19. Furthermore, increased risk of death in cancer patients administered CT within the last 30 days before COVID-19 diagnosis might be associated with the increased adverse event (AEs) rates of CT regardless of COVID-19. Neutropenic fever and sepsis are the most common causes of death from CT administered within the last 30 days [40]. However, there was no additional information regarding deaths from CT-related AEs in the included studies. In addition, there was no information for the primary administration purpose of CT. If most patients administered CT have advanced disease and are administered palliative CT, it is expected that mortality rates are higher than the control arm. In this regard, it could be explained why mortality rates were higher in the CT group without severe disease manifestations of COVID-19.
Targeted therapies have less risk for neutropenia or leukopenia than CT [41]. In this context, the immunocompromising effect of targeted agents is lower than that of CT. On the other hand, targeted therapies have various effects on the immune functions. They may enhance T-cell immunity or suppress the immune system [42]. There has been no security concern regarding targeted agents in treating cancer patients during the pandemic. Furthermore, the ESMO did not recommend the interruption of targeted therapies [5]. Our results were consistent with the previous expectations. Furthermore, we showed that targeted therapy decreased the risk of severe COVID-19. Although this result needs more evidence, it may be associated with the enhancing effect of targeted therapeutic agents, such as imatinib, sunitinib, axitinib and trametinib, on the immune system [42]. Besides the immune-enhancing effects of targeted agents, Weisberg et al. [43] recommended the repurposing of these agents for treating COVID-19 due to their antiviral, immunomodulatory and antifibrotic effects. Indeed, particularly Abelson (Abl) kinase inhibitors (i.e. imatinib, dasatinib, nilotinib) showed antiviral effect on severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) infections [44].
Immunotherapeutic agents, such as programmed death-1, programmed death ligand-1 or cytotoxic T lymphocyte associated–antigen-4 inhibitors show their effect enhancing T-cell functions against cancer cell or virus [[45], [46], [47]]. In our previous case report, we observed that nivolumab within the last seven days before diagnosis provided a good clinical course of COVID-19 in a seventy-five-year-old female malign melanoma patient [61]. Furthermore, there are still ongoing clinical trials investigating the effect of nivolumab on COVID-19 (NCT04413838, NCT04343144 and NCT04356508). One of the main concerns regarding the use of immune checkpoint inhibitors (ICIs) during pandemic is that the clinical findings of pneumonitis due to ICIs or pneumonia of COVID-19 may confuse the patient management. Clinical symptoms of COVID-19 pneumonia and ICI-related pneumonitis are very similar [49]. However, the present meta-analysis established that ICIs can be used in treating cancer patients during pandemic without concerns about the risk of severe disease and mortality.
Postoperative pulmonary complications are challenging for all patients. The spectrum of these complications changes from minimal atelectasis to severe respiratory failure. Pulmonary embolism, pleural effusion, infections, pneumothorax and aspiration pneumonitis are the other pulmonary complications of the postoperative setting [50]. Amid the COVID-19 pandemic, we have a new infectious agent, SARS-CoV-2. Nepogodiev et al. [51]showed that the postoperative complications and mortality risk were higher in the patients infected by SARS-Cov-2 in the peri-operative setting. In compliance with the concerns in this study's results, most elective surgical procedures were postponed across the world. However, articles published recently did not show increased mortality and severe disease risk in patients who had undergone elective surgery [52,53]. Similarly, according to the results of the present meta-analysis, it seems that cancer surgery can be performed without increased risk for severe disease and death during the pandemic.
During pandemic, authors and guidelines recommended the assessment of each patient for the risk and benefit of RT. They also recommended that hypofractionated regimens should be encouraged. Furthermore, active surveillance may be a good option in the low-risk prostate cancer patients [54]. The mitigation of patient admissions to the hospitals is the main purpose of all these recommendations. On the other hand, postponing elective surgeries during the pandemic increased the administration of CT and RT as well [55]. RT-induced lymphopenia (RTIL) is the main concern for the use of RT during pandemic because of lymphopenia is the poor prognostic indicator for COVID-19 [[56], [57], [58]]. Despite the lymphopenia effect of RT, stereotactic procedures can be used safely without an increased risk for RTIL [59]. Indeed, during the pandemic, stereotactic body RT is a good alternative for surgery. Furthermore, the authors recommended that RT can be performed safely during the pandemic [60]. In parallel to this recommendation, we did not show a safety concern regarding RT administration within the last 30 days before the COVID-19 diagnosis.
We had some limitations in the present meta-analysis. First, fourteen of sixteen studies included retrospective data. Only two trials assessed patients prospectively. Second, we only evaluated multivariable results in the severe disease risk and death risk for CT and severe disease risk for immunotherapy. Because of confounding factors, such as age, sex and comorbidities are crucial on the prognosis of COVID-19, univariable analyses may not show the real effect of cancer treatment on the severity of COVID-19. Third, there was no information in the articles regarding cancer treatment, whether palliative or not. Indeed, the rates of advanced cancer patients may affect the severity and mortality of COVID-19. Fourth, there was also no information regarding death from treatment-related complications or COVID-19. At that point, if we know the rate of cancer treatment related-death in the studies, it may explain the effect of CT on the mortality risk despite without any increased risk for severe disease. Fifth, the numbers of included studies were different for each outcome, and this led to heterogeneity between the outcomes. Sixth, we only assessed the risk of death from COVID-19 in the patients administered RT. There was no information for the severe disease risk in the included studies. Seventh, comparison arms were different in the included studies. Fourteen studies included patients without cancer treatment in the comparison group. In contrast, one study included remaining cancer patients except for evaluated treatment type, and one study included no cancer patients in the comparison groups. Eight, we did no assessed the effect of hormonal therapy on the severity of COVID-19.
In conclusion, cancer treatment did not increase the severe disease and mortality risk in cancer patients treated within the last 30 days before COVID-19 diagnosis. However, being on active CT was associated with an increased mortality risk in cancer patients with the COVID-19. Though, for each patient, expected benefit and toxicity of treatment should be discussed widely with the patient, and, when necessary, a decision should be made in a multidisciplinary tumour board according to the treatment goal.
Author contribution
All authors contributed equally to this manuscript.
This manuscript is a meta-analysis and not required an ethical approval.
All authors confirm that there is a data set available when needed.
Conflict of interest statement
All authors confirm that there is no conflict of interest.
Funding
There is no funding for this meta-analysis.
Author statement
Emre Yekedüz: Conceptualization, Methodology, Software, Data Extraction, Writing; Güngör Utkan: Conceptualization, Methodology, Software, Data Extraction, Writing; Yüksel Ürün: Conceptualization, Methodology, Software, Writing.
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/ Published.
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Canc Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urun Y., Hussain S.A., Bakouny Z., Castellano D., Kilickap S., Morgan G., et al. Survey of the impact of COVID-19 on oncologists' decision making in cancer. JCO Glob Oncol. 2020;6:1248–1257. doi: 10.1200/GO.20.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curigliano G., Banerjee S., Cervantes A., Garassino M., Garrido P., Girard N., et al. Managing cancer patients during the COVID-19 pandemic: an ESMO interdisciplinary expert consensus. Ann Oncol. 2020;31(10):1320–1335. doi: 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASCO 2020. https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdfhttps://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdf Published.
- 7.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L.Y.W., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G.A.S.B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyse. 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Published.
- 11.Lee L.Y.W., Cazier J.B., Starkey T., Briggs S.E.W., Arnold R., Bisht V., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee L.Y., Cazier J.B., Angelis V., Arnold R., Bisht V., Campton N.A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Canc Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroppa E.M., Toscani I., Citterio C., Anselmi E., Zaffignani E., Codeluppi M., et al. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy) Future Oncol. 2020;16(20):1425–1432. doi: 10.2217/fon-2020-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Wang L., Chen Y., Wu Q., Chen G., Shen X., et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126(17):4023–4031. doi: 10.1002/cncr.33042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarza R., Bover M., Paredes D., Lopez-Lopez F., Jara-Casas D., Castelo-Loureiro A., et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Canc. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q., Chen L., Li Q., He W., Yu J., Chen L., et al. Cancer increases risk of in-hospital death from COVID-19 in persons <65 years and those not in complete remission. Leukemia. 2020;34(9):2384–2391. doi: 10.1038/s41375-020-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee J., Foote M.B., Lumish M., Stonestrom A.J., Wills B., Narendra V., et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020 doi: 10.1200/JCO.20.01307. JCO2001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Pina J.M., Rodriguez Rodriguez M., Castro Quismondo N., Gil Manso R., Colmenares R., Gil Alos D., et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105(5):597–607. doi: 10.1111/ejh.13493. [DOI] [PubMed] [Google Scholar]
- 23.Pinato D.J., Zambelli A., Aguilar-Company J., Bower M., Sng C., Salazar R., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Canc Discov. 2020;10(10) doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assaad S., Avrillon V., Fournier M.L., Mastroianni B., Russias B., Swalduz A., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Canc. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Saez J., Lauer S.A., Kaiser L., Regard S., Delaporte E., Guessous I., et al. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhopal S.S., Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–533. doi: 10.1016/S0140-6736(20)31748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundy∗ E.J., Suddek∗ T., Filippidis F.T., Majeed A., Coronini-Cronberg S. Smoking, SARS-CoV-2 and COVID-19: a review of reviews considering implications for public health policy and practice. Tob Induc Dis. 2020;18 doi: 10.18332/tid/124788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner R.V. Long term hematopoietic damage after chemotherapy and cytokine. Front Biosci. 1999;4:e47–57. doi: 10.2741/A479. [DOI] [PubMed] [Google Scholar]
- 35.Kang D.H., Weaver M.T., Park N.J., Smith B., McArdle T., Carpenter J. Significant impairment in immune recovery after cancer treatment. Nurs Res. 2009;58(2):105–114. doi: 10.1097/NNR.0b013e31818fcecd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D., Chen Y., Liu H., Jia Y., Li F., Wang W., et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduct Target Ther. 2020;5(1):62. doi: 10.1038/s41392-020-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beri A., Kotak K. Cardiac injury, arrhythmia, and sudden death in a COVID-19 patient. HeartRhythm Case Rep. 2020;6(7):367–369. doi: 10.1016/j.hrcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salih O.S.M., McLaren B.R., Jackson C. Mortality within 30 days of receiving chemotherapy: a single regional experience from New Zealand. Eur J Canc. 2016;60:e18. [Google Scholar]
- 41.Zhou J.G., Tian X., Cheng L., Zhou Q., Liu Y., Zhang Y., et al. The risk of neutropenia and leukopenia in advanced non-small cell lung cancer patients treated with erlotinib: a prisma-compliant systematic review and meta-analysis. Medicine (Baltim) 2015;94(40):e1719. doi: 10.1097/MD.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allegrezza M.J., Conejo-Garcia J.R. Targeted therapy and immunosuppression in the tumor microenvironment. Trends Cancer. 2017;3(1):19–27. doi: 10.1016/j.trecan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Weisberg E., Parent A., Yang P.L., Sattler M., Liu Q., Liu Q., et al. Repurposing of kinase inhibitors for treatment of COVID-19. Pharm Res (N Y) 2020;37(9):167. doi: 10.1007/s11095-020-02851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman C.M., Sisk J.M., Mingo R.M., Nelson E.A., White J.M., Frieman M.B. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus fusion. J Virol. 2016;90(19):8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeidi A., Zandi K., Cheok Y.Y., Saeidi H., Wong W.F., Lee C.Y.Q., et al. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. 2018;9:2569. doi: 10.3389/fimmu.2018.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galvao D.A., Spry N.A., Taaffe D.R., Newton R.U., Stanley J., Shannon T., et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44–47. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 47.Baranski A.C., Schafer M., Bauder-Wust U., Wacker A., Schmidt J., Liolios C., et al. Improving the imaging contrast of (68)Ga-PSMA-11 by targeted linker design: charged spacer moieties enhance the pharmacokinetic properties. Bioconjugate Chem. 2017;28(9):2485–2492. doi: 10.1021/acs.bioconjchem.7b00458. [DOI] [PubMed] [Google Scholar]
- 49.Rossi E., Schinzari G., Tortora G. Pneumonitis from immune checkpoint inhibitors and COVID-19: current concern in cancer treatment. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miskovic A., Lumb A.B. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–334. doi: 10.1093/bja/aex002. [DOI] [PubMed] [Google Scholar]
- 51.Nepogodiev D., Bhangu A., Glasbey J.C., Li E., Omar O.M., Simoes J.F.F., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrikhande S.V., Pai P.S., Bhandare M.S., Bakshi G., Chaukar D.A., Chaturvedi P., et al. Outcomes of elective major cancer surgery during COVID 19 at tata memorial centre: implications for cancer care policy. Ann Surg. 2020;272(3):e249–e252. doi: 10.1097/SLA.0000000000004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Couto R.A., Wiener T.C., Adams W.P. Evaluating postoperative outcomes of patients undergoing elective procedures in an ambulatory surgery center during the COVID-19 pandemic. Aesthetic Surg J. 2020 doi: 10.1093/asj/sjaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lancia A., Bonzano E., Bottero M., Camici M., Catellani F., Ingrosso G. Radiotherapy in the era of COVID-19. Expert Rev Anticancer Ther. 2020;20(8):625–627. doi: 10.1080/14737140.2020.1785290. [DOI] [PubMed] [Google Scholar]
- 55.Vordermark D. Shift in indications for radiotherapy during the COVID-19 pandemic? A review of organ-specific cancer management recommendations from multidisciplinary and surgical expert groups. Radiat Oncol. 2020;15(1):140. doi: 10.1186/s13014-020-01579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y., et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkatesulu B.P., Mallick S., Lin S.H., Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Joseph N., Choudhury A. Lymphocytopenia and radiotherapy treatment volumes in the time of COVID-19. Clin Oncol. 2020;32(7):420–422. doi: 10.1016/j.clon.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wild A.T., Herman J.M., Dholakia A.S., Moningi S., Lu Y., Rosati L.M., et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):571–579. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagar H., Formenti S.C. Cancer and COVID-19 - potentially deleterious effects of delaying radiotherapy. Nat Rev Clin Oncol. 2020;17(6):332–334. doi: 10.1038/s41571-020-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yekedüz E., Dursun B., Aydın G.C., Yazgan S.C., Öztürk H.H., Azap A., et al. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J Oncol Pharm Pract. 2020;26:1289–1294. doi: 10.1177/1078155220924084. [DOI] [PubMed] [Google Scholar]