Abstract
Objective
Observational data can be used to attempt to emulate a target trial of statin use and estimate analogues of intention-to-treat and per protocol effects on dementia risk.
Methods
Using data from a prospective cohort study in the Netherlands, we conceptualized a sequence of “trials” in which eligible individuals ages 55–80 years were classified as statin initiators or noninitiators for every consecutive month between 1993 and 2007 and were followed until diagnosis of dementia, death, loss to follow-up, or the end of follow-up. We estimated 2 types of effects of statin use on dementia and a combined endpoint of dementia or death: the effect of initiation vs no initiation and the effect of sustained use vs no use. We estimated risk by statin treatment strategy over time via pooled logistic regression. We used inverse-probability weighting to account for treatment-confounder feedback in estimation of per-protocol effects.
Results
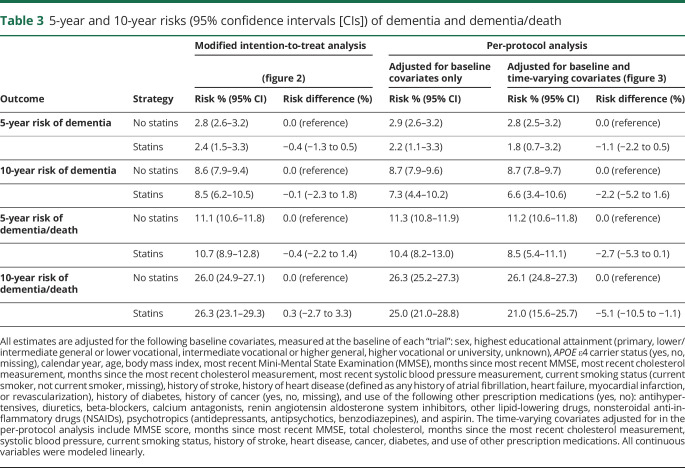
Of 233,526 eligible person-trials (6,373 individuals), there were 622 initiators and 232,904 noninitiators. Comparing statin initiation with no initiation, the 10-year risk differences (95% confidence interval) were −0.1% (−2.3% to 1.8%) for dementia and 0.3% (−2.7% to 3.3%) for dementia or death. Comparing sustained statin use vs no use, the 10-year risk differences were −2.2% (−5.2% to 1.6%) for dementia and −5.1% (−10.5% to −1.1%) for dementia or death.
Conclusions
Individuals with sustained statin use, but not statin initiation alone, had reduced 10-year risks of dementia and dementia or death. Our results should be interpreted with caution due to the small number of initiators and events and potential for residual confounding.
The effect of a commonly prescribed medication on reducing the long-term risk of chronic diseases is often of interest in public health research. However, estimating the effect of a medication for primary prevention can be challenging because it typically requires enrolling disease-free asymptomatic adults and following them for several years. Moreover, the effect of sustained medication use may be of great value for informing personal, clinical, or public health decision-making, even though randomized clinical trials often emphasize or solely estimate an intention-to-treat effect regardless of sustained use.1
For example, use of statin therapy in adults without cognitive impairment could change dementia risk later in life. The effects of statin use for primary and secondary prevention of cardiovascular disease in a general population are well established.2–4 However, randomized trials5,6 and several observational studies assessing the association between statin use and Alzheimer disease or dementia have had conflicting results ranging from statins potentially increasing, decreasing, or having a negligible effect on these outcomes. Overall, the randomized trials have included individuals at high risk of vascular disease, have had relatively short follow-up times (5 years or less), and have reported the intention-to-treat effect only.5,6 Meanwhile, comparable observational studies have notable limitations, as described elsewhere.7 For example, studies that only assess statin use at baseline are by definition limited by the number of individuals who are statin users at baseline,8–13 studies that assess statin use at the time of incident dementia (or with a 1-year or 2-year lag) could be particularly susceptible to reverse causation,14–20 studies that assess statin use as a time-varying variable using stratification-based techniques could be susceptible to bias due to inappropriate adjustment for time-varying confounding,16,17,21–26 and studies that include prevalent statin users could be susceptible to selection bias.8–11,13–15,17–21,23,24 Finally, methods to estimate the effect of sustained statin treatment over follow-up time in addition to the effect of initiating statin treatment are underutilized in analyses of randomized trials and observational studies alike.27
To overcome some of the challenges of randomized clinical trials, we emulate a hypothetical randomized trial—a target trial—for estimating observational analogues to intention-to-treat and per-protocol effects of statin on dementia.28 Through explicitly describing and emulating the target trial, we can leverage the richness of observational data while avoiding selection and residual confounding biases that are consequences of common flaws in observational studies' design and analyses. The advantages of the target trial framework may be particularly pertinent in pharmacoepidemiologic research where time-varying variables can be both causes and consequences of treatment. Here, we describe the protocol of the target trial and then how to emulate it using observational data from the Rotterdam Study. Because few individuals initiate statins in a given calendar month, we emulate a sequence of target trials where, at each calendar month, eligibility criteria are applied anew and eligible individuals are then assigned to statin initiation or noninitiation.29,30
Methods
Standard protocol approvals, registrations, and patient consents
The medical ethics committee of the Erasmus Medical Centre approved the study. Written informed consent was obtained from all patients participating in the study.
Study population
This study is embedded in the Rotterdam Study, a prospective cohort study initially including 7,983 individuals aged 55 years or older living in Ommoord, a district of Rotterdam, the Netherlands.31 Individuals living in the district were invited to participate in the cohort between 1990 and 1993. Home visits and center visits were conducted at enrollment (1990–1993) and again at follow-up visits in 1993–1995, 1997–1999, 2002–2004, 2009–2011, and 2014–2015. Demographic, clinical, and lifestyle factors were measured and recorded at each visit. In 2000, 3,011 individuals who had become 55 years of age or moved into the study district since the start of the study were added to the cohort. Home visits and examinations were conducted at enrollment (2000–2001) and again at follow-up visits in 2004–2005, 2011–2012, and 2014–2015.
Statin measurement
Complete information on all prescriptions filled at any of 7 automated pharmacies serving the Ommoord area (>99% of participants) were available for individuals enrolled in the Rotterdam Study starting on January 1, 1991.31 Simvastatin, pravastatin, fluvastatin, atorvastatin, cerivastatin, and rosuvastatin were classified as statins based on Anatomical Therapeutic Chemical codes. Statins were approved in 1990 onwards in the Netherlands, with simvastatin the most commonly prescribed statin.32 The duration of a prescription was calculated as the total number of delivered units divided by the prescribed daily number of units. The date of delivery and duration of prescription were then used to calculate the number of treated days during each month for each individual. In the Netherlands, all statins are available only by prescription.
Dementia measurement
Participants were screened for dementia at baseline and subsequent center visits with the Mini-Mental State Examination (MMSE) and the Geriatric Mental Schedule organic level.33 Those with an MMSE score <26 or Geriatric Mental Schedule score >0 underwent further investigation and informant interview, including the Cambridge Examination for Mental Disorders of the Elderly. All participants also underwent routine cognitive assessment at each center visit. In addition, the entire cohort was continuously under surveillance for clinically diagnosed dementia through electronic linkage of the study database with medical records from general practitioners and the regional institute for outpatient mental health care. Available information on clinical neuroimaging was used when required for diagnosis of dementia subtype. A consensus panel led by a consultant neurologist established the final diagnosis and subtype of dementia according to standard criteria for dementia (DSM-III-R), Alzheimer disease (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association), and vascular dementia (National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l’Enseignement en Neurosciences).
The protocol of the target trial
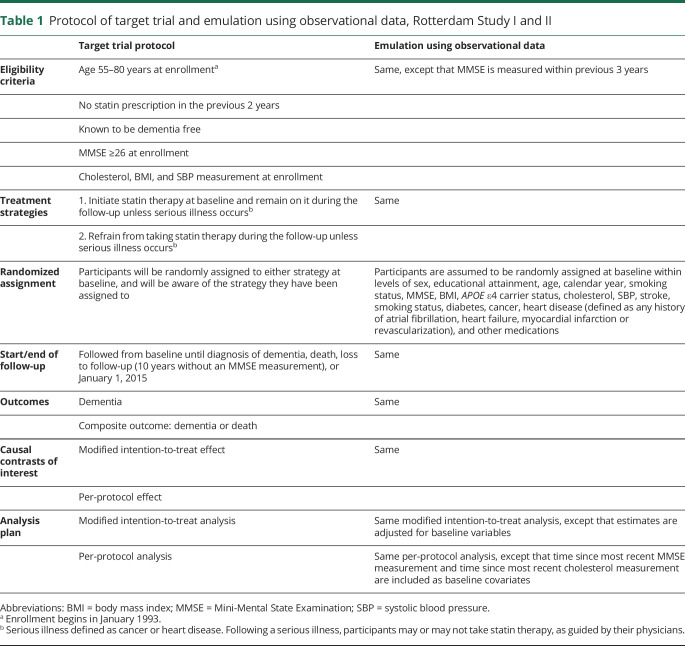
We begin by describing the protocol of our target trial to estimate the effect of statins on incident dementia; subsequently, we describe how we emulate this target trial in our data. A summary of these protocol components can be found in table 1.
Table 1.
Protocol of target trial and emulation using observational data, Rotterdam Study I and II
Eligibility criteria
The target trial includes participants aged 55–80 years with no statin prescription in the previous 2 years and no previous diagnosis of dementia. Individuals receive a cholesterol test and MMSE at enrollment, and are excluded if they have an MMSE score <26. Individuals are enrolled in the target trial starting in January 1993.
Treatment strategies
Two treatment strategies are considered: (1) initiate statin therapy at baseline and remain on statins during the follow-up; or (2) refrain from taking statin therapy during the follow-up. Both strategies allow for deviation if a serious illness occurs, e.g., cancer or heart disease.
Randomized assignment
Eligible individuals are randomized to 1 of the 2 treatment strategies without blinding.
Outcomes
The primary outcomes of interest include incident dementia and a composite outcome of incident dementia or all-cause mortality. Mortality is ascertained through linkage with records of general practitioners and municipality records. Incident dementia is ascertained by combining continuous surveillance through electronic linkage with medical records and MMSE and Geriatric Mental Schedule assessments, using the algorithm described above.
Follow-up period
Individuals are followed from baseline (randomization) until diagnosis of dementia, mortality, loss to follow-up (defined by not attending regular study visits, e.g., 2 years without a study visit), or January 1, 2015, whichever occurs earliest.
Causal contrasts of interest
To compare the 2 treatment strategies, we estimate a modified intention-to-treat effect as well as the per-protocol effect. In the target trial, the modified intention-to-treat effect is the effect of statin initiation vs no statin initiation (the intention-to-treat effect would be the effect of assignment to one of these strategies). The per-protocol effect is the effect of statin initiation and sustained statin use vs no statin initiation and never initiating statin medications.
Analysis plan
Modified intention-to-treat effect
For each outcome, we fit the pooled logistic regression model 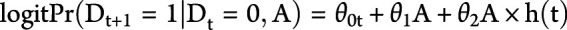 , where
, where 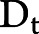 is an indicator for developing the outcome during time t (1: yes, 0: no), A is an indicator for treatment initiation (1: statins; 0: no statins), θ0t is a time-varying intercept, and h(t) is follow-up time, modeled as restricted cubic splines. The model's predicted values are then used to estimate 10-year dementia-free and 10-year dementia-free survival curves and 5- and 10-year risks of dementia and the combined endpoint of dementia or death.
is an indicator for developing the outcome during time t (1: yes, 0: no), A is an indicator for treatment initiation (1: statins; 0: no statins), θ0t is a time-varying intercept, and h(t) is follow-up time, modeled as restricted cubic splines. The model's predicted values are then used to estimate 10-year dementia-free and 10-year dementia-free survival curves and 5- and 10-year risks of dementia and the combined endpoint of dementia or death.
Per-protocol effect
Individuals are artificially censored when they deviate from their assigned treatment strategy. Specifically, individuals assigned to initiation and sustained statin use are censored after 1 calendar month with no treated days, and individuals assigned to refrain from statins are censored after 1 calendar month with 1 or more treated days. Individuals can no longer be artificially censored after a diagnosis of heart disease or cancer.
For each outcome, we fit the weighted pooled logistic regression model  , where Ct is an indicator for artificial censoring at time t (1: yes, 0: no) and V is a vector of covariates measured at baseline. To adjust for time-varying selection bias induced by the artificial censoring, we weight each individual at each time t by the inverse probability of receiving his or her own time-varying treatment history,34,35 i.e., the weight
, where Ct is an indicator for artificial censoring at time t (1: yes, 0: no) and V is a vector of covariates measured at baseline. To adjust for time-varying selection bias induced by the artificial censoring, we weight each individual at each time t by the inverse probability of receiving his or her own time-varying treatment history,34,35 i.e., the weight 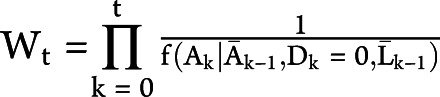 , where
, where 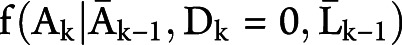 is the conditional probability mass function
is the conditional probability mass function 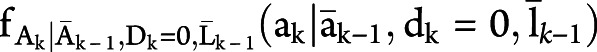 with
with 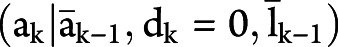 evaluated at the random argument
evaluated at the random argument 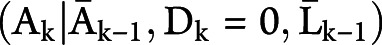 . We use overbars to denote an individual's covariate or treatment history. For an individual who is uncensored through time t, note that the contribution to the denominator of the weight is equal to the probability that the individual remains uncensored through time t conditional on not developing the outcome by time t, covariate history, and treatment history.36 An individual's weight at each time is therefore the cumulative product of the conditional probability of remaining uncensored. The weights are estimated by fitting a pooled logistic model including a time-specific intercept, the baseline covariates, and the most recent measurement of several time-varying covariates.
. We use overbars to denote an individual's covariate or treatment history. For an individual who is uncensored through time t, note that the contribution to the denominator of the weight is equal to the probability that the individual remains uncensored through time t conditional on not developing the outcome by time t, covariate history, and treatment history.36 An individual's weight at each time is therefore the cumulative product of the conditional probability of remaining uncensored. The weights are estimated by fitting a pooled logistic model including a time-specific intercept, the baseline covariates, and the most recent measurement of several time-varying covariates.
Baseline covariates included variables measured at the clinic and home visits: sex, highest educational attainment, calendar year, age, APOE ε4 carrier status, body mass index (BMI), most recent MMSE, most recent total cholesterol measurement, most recent systolic blood pressure measurement, and current smoking status; variables measured using clinical diagnoses: history of stroke, history of heart disease, and history of cancer; variables measured using continuous pharmacy dispensing records: antihypertensives, diuretics, beta-blockers, calcium blockers, renin angiotensin aldosterone system (RAAS) inhibitors, non-statin cholesterol-lowering medications, nonsteroidal anti-inflammatory drugs (NSAIDs), psychotropic medications (antidepressants, antipsychotics, and benzodiazepines), and aspirin (yes, no); and variables measured using a combination of pharmacy records and clinical diagnoses: history of diabetes. Time-varying covariates that could be common causes of statin initiation (or discontinuation) and incident dementia included MMSE score, months since most recent MMSE, total cholesterol, months since the most recent cholesterol measurement, systolic blood pressure, current smoking status, history of stroke, heart disease, cancer, and diabetes, and use of other prescription medications. All continuous variables were modeled linearly. The contributions to the weights are set to 1 after diagnosis of heart disease or cancer. Weights are truncated at the 99th percentile37 (∼7.8, appendix table 3, available from Dryad, doi:10.5061/dryad.kprr4xh19).
Subgroup analyses
We explore effect modification by calendar year, cohort, and age in subgroup analyses restricted to (1) calendar years in 2000 or later; (2) the initial cohort only; (3) individuals younger than 70 years at baseline; and (4) individuals 70 years or older at baseline.
Competing events
In our setting, death is a competing event for dementia because an individual cannot get dementia once he or she has died. The definition and interpretation of the causal estimand for an effect of statins on dementia is tied to the analytic choice to define the competing event death as a censoring event (i.e., an event that prevents observation of the counterfactual outcome of interest) or not.38 In our dementia analyses, we consider the competing event death to be a censoring event. That is, our modified intention-to-treat and per-protocol estimates are interpreted as the effect of statin initiation and sustained statin use on incident dementia in a hypothetical population in which death does not occur or in which death is independent of risk factors for dementia (conditional on measured covariates). This estimate can also be interpreted as the controlled direct effect of statins on dementia not mediated by the competing event death.39 To adjust for potential bias due to competing events in the per-protocol analysis, we compute inverse probability weights for death in a sensitivity analysis. Each individual receives a time-varying weight inversely proportional to the probability of not dying. To compute the weights, we fit a pooled logistic model including a time-specific intercept, the baseline covariates and time-varying covariates listed previously, and an indicator for the treatment arm. The product of the estimated conditional probabilities at each time is then used to estimate the time-varying weight for each person at each time. Another approach for dealing with competing risks is to consider a composite outcome of dementia or death. Although this approach addresses some issues with the interpretation and valid estimation of our primary approach, it remains difficult to interpret because a non-null estimate can occur due to an effect of statins on dementia alone, death alone, or a combination thereof.
Loss to follow-up
To adjust for potential selection bias due to nondifferential loss to follow-up between the treatment arms, we compute inverse probability of censoring weights in a sensitivity analysis. Each individual receives a time-varying weight inversely proportional to the probability of not being lost to follow-up.
Emulation of the target trial using observational data
We emulated the target trial by using Rotterdam Study data from the initial and first extended cohort. We will first describe emulating a target trial with baseline in January 1993, and then describe how we can emulate a series of target trials with baseline months ranging from January 1993 to December 2007.
Eligibility criteria
We identified individuals aged 55–80 years with no statin prescription (operationalized as at least 2 years enrolled in the Rotterdam Study with no recorded statin prescription) and no previous diagnosis of dementia. Individuals were excluded if they did not have a recent cholesterol measurement (within the previous 3 years), if they did not have a recent MMSE score ≥26 (within the previous 3 years), or if they did not have any BMI or SBP measurement.
Treatment strategies
Eligible individuals were classified as statin initiators if they initiated statins in January 1993 and as noninitiators if they did not initiate statins in January 1993.
Randomized assignment
To emulate the randomization component of the target trial, we adjusted for the baseline variables listed in our analysis (see below).
Follow-up and outcome
Same as in the target trial, except we define loss to follow-up as 10 years without an MMSE measurement (due to infrequent scheduled visits in the later years of the Rotterdam Study).
Causal contrasts
Same as in the target trial.
Analysis plan
The modified intention-to-treat analysis was the same as in the target trial except that we included the previously described baseline covariates in the pooled logistic regression model. Because individuals in the observational study did not necessarily have MMSE and cholesterol measurements taken exactly at baseline, we also included time since most recent MMSE measurement and time since most recent cholesterol measurement as baseline covariates. The per-protocol analysis was the same as in the target trial (individuals are artificially censored when they deviate from their assigned treatment strategy) except that we included the 2 additional baseline covariates time since most recent MMSE measurement and time since most recent cholesterol measurement as baseline covariates.
Sensitivity analyses and subgroup analyses
We performed the same sensitivity and subgroup analyses as in the target trial. We also conducted several additional sensitivity analyses. To evaluate residual confounding by indication, we excluded individuals who (1) had a history of heart disease or history of stroke at baseline; (2) were taking vascular medications at baseline (non-statin cholesterol-lowering medications, diuretics, beta-blockers, calcium blockers, RAAS inhibitors, NSAIDs, or aspirin); (3) had high cholesterol (>6.2 mmol/L) at baseline; and (4) did not have high cholesterol at baseline. To evaluate whether our results were sensitive to overly strict inclusion criteria that could decrease our sample size, we required a 6-month statin washout period at baseline (rather than 2-year) and required an MMSE ≥26 at baseline but did not require it to be in the previous 3 years.
Creating a sequence of target trials using observational data
Enrollment in the target trial began in January 1993 and continues until the desired sample size has been attained. In the emulation of the target trial described above, the eligibility criteria are applied once, in January 1993. However, if only a small number of eligible individuals initiated statin therapy in January 1993, a meaningful comparison of statin initiators and noninitiators will not be possible. To increase the sample size in our trial emulation, we apply the same eligibility criteria anew in February 1993 and each month thereafter until December 2007. That is, we emulate a series of 180 “trials,” each of them with a 1-month enrollment period.30 For example, individuals eligible to enroll in the January 1993 “trial” who do not initiate statins that month may be eligible to enroll in the February 1993 “trial,” assuming they continue to meet the other eligibility criteria that month.
In the analysis, baseline variables are updated at the start of each “trial.” For example, the baseline variables for the February 1993 “trial” are the most recent measurements of the covariates at that time. We pooled data from all 180 “trials” into a single model and include “month at the trial's baseline” (taking values from 1 to 180) and month of follow-up in each “trial” in our models (both modeled using restricted cubic splines).
All analyses were performed in SAS using publicly available macros (hsph.harvard.edu/causal/software/). Ninety-five percent confidence intervals (CIs) were obtained using bootstrapping with 200 samples.
Data availability
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.
Results
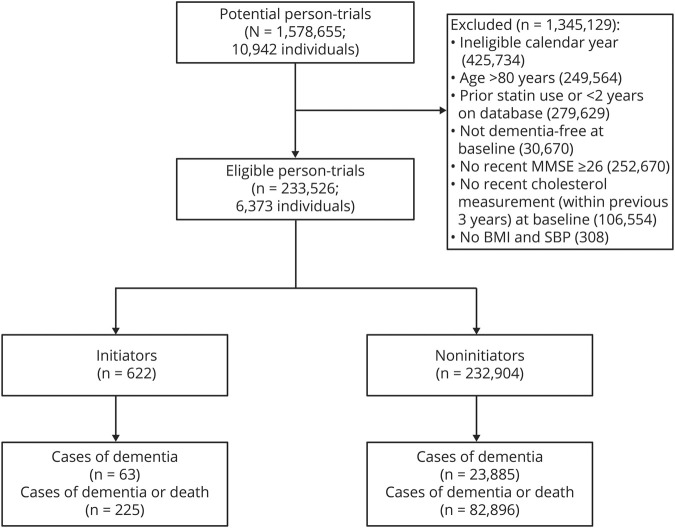
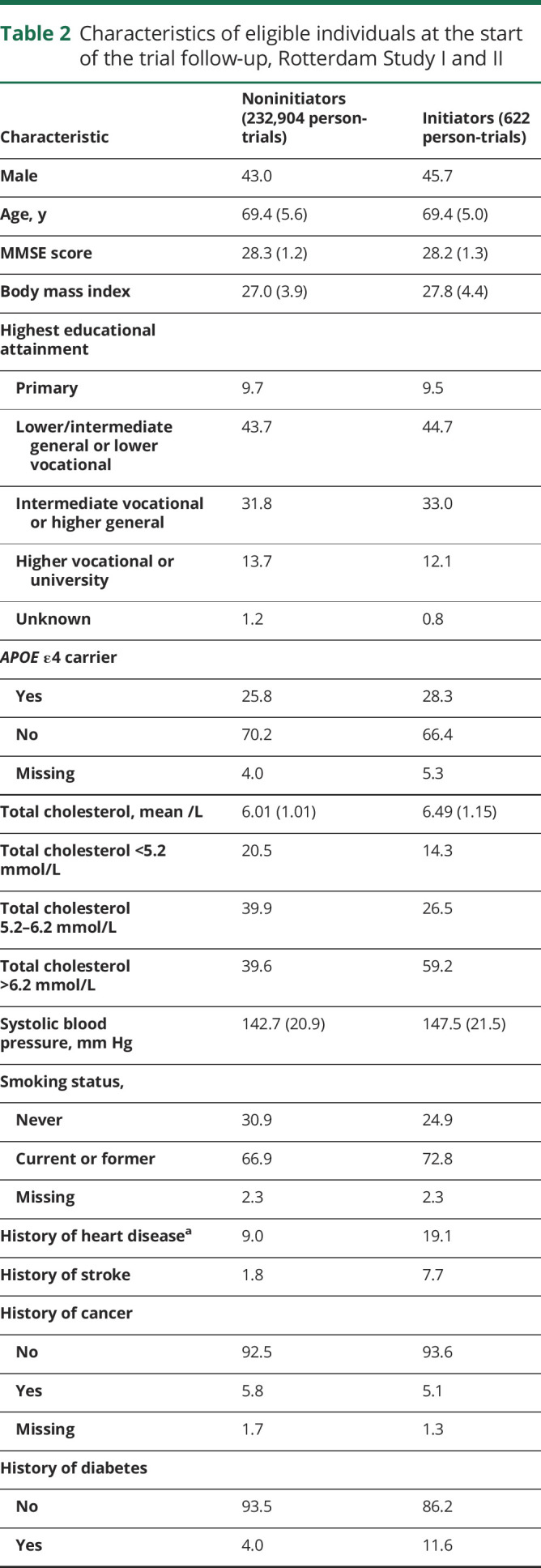
One hundred eighty “trials” were conducted from January 1993 to December 2007. Of 1,578,655 potential person-“trials” (10,942 individuals), 233,526 person-trials (6,373 individuals) met the eligibility criteria. There were 622 initiators and 232,904 noninitiators (figure 1). Across the 180 months of trials, the mean number of participants in a given month was 1,297 with a mean number of 3.5 initiators. The trial beginning in March 2006 had the most initiators (15 initiators, 1,915 noninitiators) (appendix table 1, available from Dryad, doi:10.5061/dryad.kprr4xh19). Initiators and noninitiators were similar in age, MMSE, and educational attainment. Compared with noninitiators, initiators had higher baseline total cholesterol measurements and systolic blood pressure measurements and were more likely to be current or former smokers, have a history of heart disease, stroke, and diabetes, and use other prescription medications (table 2).
Figure 1. Flowchart of person-trials in the modified intention-to-treat analysis, Rotterdam Study.
BMI = body mass index; MMSE = Mini-Mental State Examination; SBP = systolic blood pressure.
Table 2.
Characteristics of eligible individuals at the start of the trial follow-up, Rotterdam Study I and II
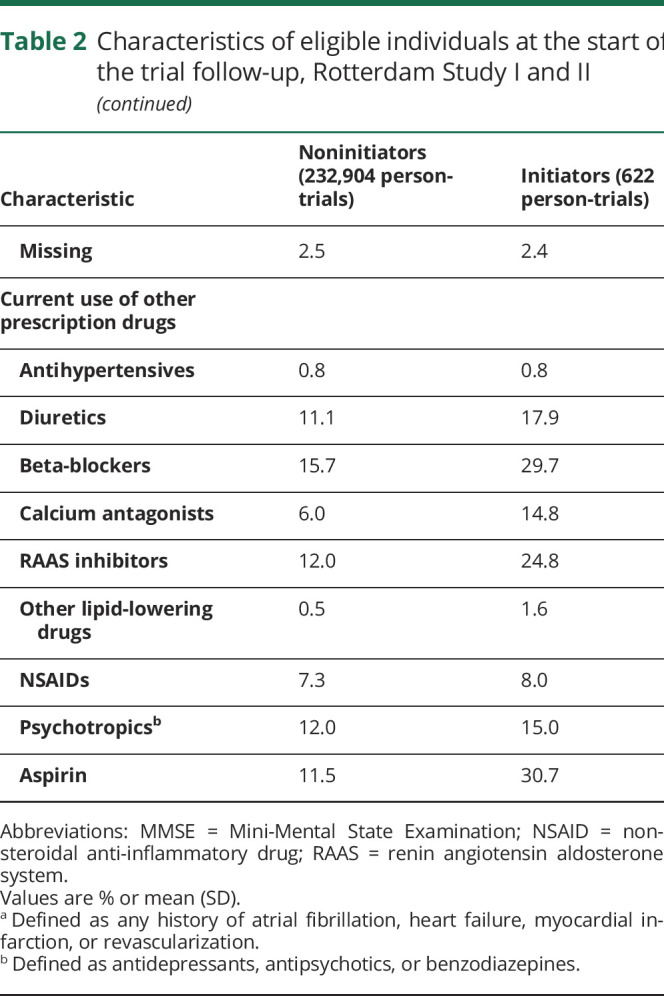
Of the 622 initiators, 63 developed dementia and 225 died or developed dementia over the follow-up. Of the 232,904 noninitiators, 23,885 developed dementia and 82,896 died or developed dementia over the follow-up. The median (interquartile range [IQR]) follow-up time was 9.3 (7.8–11.9) years for initiators and 9.9 (7.8–13.5) years for noninitiators in the dementia analysis. The median (IQR) time to dementia was 7.5 (5.4–11.4) years for initiators and 8.3 (4.9–11.4) years for noninitiators.
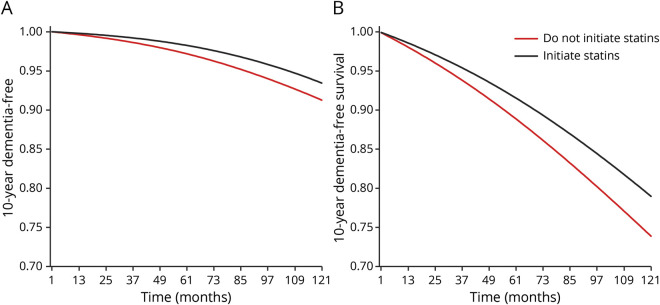
Figure 2 shows the modified intention-to-treat 10-year dementia-free and dementia-free survival curves by statin initiation. Comparing statin initiation with no initiation, the 10-year risk differences (95% CI) were −0.1% (−2.3% to 1.8%) for dementia and 0.3% (−2.7% to 3.3%) for dementia or death (table 3). Unadjusted estimates were similar for dementia but larger for dementia or death (data not shown).
Figure 2. Modified intention-to-treat 10-year dementia-free and dementia-free survival curves.
(A) Dementia-free and (B) dementia-free survival curves. The curves are standardized to the following covariates, measured at the baseline of each “trial”: sex, educational attainment, APOE ε4 carrier status, calendar year, age, body mass index, most recent Mini-Mental State Examination (MMSE), months since most recent MMSE, most recent cholesterol measurement, months since the most recent cholesterol measurement, most recent systolic blood pressure measurement, current smoking status, history of stroke, history of heart disease (defined as any history of atrial fibrillation, heart failure, myocardial infarction, or revascularization), history of diabetes, history of cancer, and use of the following other prescription medications: antihypertensives, diuretics, beta-blockers, calcium antagonists, renin angiotensin aldosterone system inhibitors, other lipid-lowering drugs, nonsteroidal anti-inflammatory drugs, psychotropics (antidepressants, antipsychotics, benzodiazepines), and aspirin.
Table 3.
5-year and 10-year risks (95% confidence intervals [CIs]) of dementia and dementia/death
After 1 year of follow-up, 459 of the initiators remained on statin therapy. This number was 385, 274, and 100 after 2 years, 5 years, and 10 years, respectively. Compared with individuals who did not stop statin use over follow-up, individuals who stopped statin use over follow-up were more likely to be female, have lower baseline MMSE scores, and be smokers and were less likely to be on other prescription medications. In the per-protocol analysis, 27 of the 622 initiators developed dementia and 122 died or developed dementia over the follow-up. Of the 232,904 noninitiators, 20,379 developed dementia and 72,207 died or developed dementia over the follow-up. The median (IQR) time of follow-up was 3.8 (0.9–8.7) years for initiators and 9.0 (5.9–12.2) years for noninitiators. Figure 3 shows the per-protocol 10-year dementia-free and dementia-free survival curves by statin initiation. Comparing sustained statin use with no use, the 10-year risk difference (95% CI) was −2.2% (−5.2% to 1.6%) for dementia and −5.1% (−10.5% to −1.1%) for dementia or death (table 3).
Figure 3. Per-protocol 10-year dementia-free and dementia-free survival curves.
(A) Dementia-free and (B) dementia-free survival curves. The curves are standardized to the following covariates, measured at the baseline of each “trial”: sex, educational attainment, APOE ε4 carrier status, calendar year, age, body mass index, most recent Mini-Mental State Examination (MMSE), months since most recent MMSE, most recent cholesterol measurement, months since the most recent cholesterol measurement, most recent systolic blood pressure measurement, current smoking status, history of stroke, history of heart disease (defined as any history of atrial fibrillation, heart failure, myocardial infarction, or revascularization), history of diabetes, history of cancer, and use of the following other prescription medications: antihypertensives, diuretics, beta-blockers, calcium antagonists, renin angiotensin aldosterone system inhibitors, other lipid-lowering drugs, nonsteroidal anti-inflammatory drugs, psychotropics (antidepressants, antipsychotics, benzodiazepines), and aspirin.
Inverse-probability weighting to adjust for censoring due to infrequent follow-up or censoring due to death did not materially change estimates. The 10-year per-protocol risk difference estimates for dementia or death were attenuated when excluding individuals age 70 years or older (−1.5%, 95% CI −7.4% to 3.5%) and when excluding individuals with high cholesterol at baseline (−1.3%, 95% CI −7.2% to 6.3%), but were larger when excluding individuals with a history of heart disease or stroke at baseline (−7.1%, 95% CI −12.9% to −0.1%). The 10-year per-protocol risk difference estimates for dementia were also attenuated when excluding individuals age 70 years or older and larger when excluding individuals with a history of heart disease or stroke at baseline (data not shown). None of the other subgroup and sensitivity analyses yielded appreciably different results (appendix table 2, available from Dryad, doi:10.5061/dryad.kprr4xh19).
Discussion
Our study is the first to explicitly emulate a hypothetical randomized trial of statin use in older adults and incident dementia. We leveraged the rich data of the Rotterdam Study while mitigating some common limitations often associated with observational studies. While the protective effect of statin use on cardiovascular disease prevention is well established, we found little evidence for a difference in the risk of dementia or dementia or death after initiating statins compared with not initiating statins in an analysis that was agnostic about statin discontinuation (the modified intention-to-treat analysis analogue). This finding was consistent with 2 previous randomized trials that found no difference in cognitive decline or dementia in intention-to-treat analyses.5,6 Our findings suggest a potential decreased risk of dementia and dementia or death after sustained statin use compared with no statin use (the per-protocol analysis analogue), but residual confounding could not be ruled out and our CIs were wide. The attenuation of the 10-year per-protocol risk-difference estimates when excluding individuals age 70 years or older suggests statins likely have less absolute benefit (within 10 years) when initiated during midlife. In addition, the attenuation of the 10-year per-protocol estimate for the combined endpoint of dementia or death when excluding individuals with high baseline total cholesterol (and variability of the per-protocol 10-year dementia or death risk difference in general) suggests our analyses may not have successfully adjusted for confounding by cardiovascular disease risk. Finally, since statin use may delay death due to cardiovascular disease, estimates of the effect of statin initiation and statin use on dementia could be biased due to the competing event death.
A causal interpretation of all of our estimates relies on the untestable assumption that the measured baseline covariates were sufficient to adjust for confounding (i.e., to emulate randomization). Confounding by indication might partly explain our estimates, given that individuals who initiated statins were more likely to have other risk factors for dementia, including high blood pressure and APOE ε4 carrier status. We were nevertheless able to adjust for several vascular risk factors (e.g., total cholesterol and systolic blood pressure and history of heart disease, stroke, and diabetes) in addition to demographic factors (e.g., smoking) and prescription medication use that could be key confounding variables in estimating effects of statin use on dementia. Unmeasured confounding by a prodromal dementia stage (which may affect likelihood of initiating or maintaining medication use) could remain despite adjustment for a recent MMSE score. In addition, data on low-density lipoprotein cholesterol were not available, a strong indication for statin initiation and strongly associated with death due to cardiovascular disease (but perhaps not with dementia). In general, confounding by indication is a more substantial issue when evaluating primary outcomes for the treatment indication (in this case, cardiovascular disease) compared with secondary outcomes (in this case, dementia).40
In addition to unmeasured or residual confounding, all of our estimates could also be biased if there are diagnostic delays for dementia that are differential with respect to statin use. For example, perhaps individuals taking statins are more likely to visit the doctor and receive a dementia diagnosis more quickly after onset of symptoms compared with someone not taking statins who does not visit a doctor as frequently. This differential outcome diagnosis would yield an underestimate of a protective effect of statins on dementia. While adjudication of dementia diagnoses in our study is based on continuous linkage to medical records in addition to information systematically gathered during the study, diagnostic delays will still occur for individuals who stop attending study visits and do not regularly see a doctor. We are interested in the effects of statins on dementia; however, estimates of the effects of statins on dementia diagnoses are not susceptible to this bias.
Protocols typically allow treatment discontinuation due to clinical reasons such as contraindications, serious diagnoses, side effects, or toxicity. Accordingly, a per-protocol analysis should estimate an effect of adhering to a protocol that allows treatment discontinuations for appropriate reasons. Estimating an appropriate per-protocol effect analogue is challenging when, as in our study, data on reasons for discontinuation are not available. We dealt with this challenge by allowing individuals to discontinue (or initiate) statins after diagnosis of cancer or heart disease, but this is likely an insufficient attempt to allow clinically supported deviations.
A causal interpretation of the per-protocol effect analogues' estimates further relies on the additional assumption that the measured time-varying covariates were sufficient to adjust for time-varying confounding. This assumption would not be met if there were reasons for discontinuing (or initiating) statins over time related to dementia risk for which we did not adequately measure or adjust. Individuals who discontinued statins had lower baseline MMSE scores, further suggesting the possibility of residual confounding by subclinical dementia. In addition, since linkage with pharmacy dispending records is lost when individuals enter nursing homes in the Rotterdam Study, these individuals will be censored from the sustained statin use strategy even though they may not have discontinued statins. Because these individuals may be sicker, this could lead to an overestimation of the beneficial effect of sustained statin use. Finally, because many of the measured time-varying covariates in our analysis could only be updated when a Rotterdam Study visit occurred, we may not have been able to sufficiently adjust for time-varying confounding by measured covariates. Instrumental variable approaches that do not rely on measuring exposure–outcome confounders but instead make different strong assumptions41 may offer a complementary way to estimate per-protocol effect analogues in some studies, but these methods are not well suited for estimating sustained treatment strategies.41,42
Altogether, our findings suggest a potential decreased 10-year risk of dementia and dementia or death after sustained statin use compared with no statin use in older adults. However, this decreased risk relied heavily on data from few individuals (resulting in wide CIs), and certain plausible biases (such as residual confounding by cardiovascular disease risk) cannot be ruled out. Our study may be useful to inform the design and analyses of future observational studies and randomized clinical trials to estimate the effect of sustained statin use on dementia.
Acknowledgment
The authors thank Miguel Hernán for comments on an earlier version of this manuscript.
Glossary
- BMI
body mass index
- CI
confidence interval
- DSM-III
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition
- IQR
interquartile range
- MMSE
Mini-Mental State Examination
- NSAID
nonsteroidal anti-inflammatory drug
- RAAS
renin angiotensin aldosterone system
Appendix. Authors
Study funding
This research was supported by NIH grant R01-AI073127 and T32 AI007433. This study was partly funded by ZonMW Memorabel (project 73305095005) and Alzheimer Nederland through the Netherlands Consortium of Dementia Cohorts in the context of Deltaplan Dementie. Further funding was obtained from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 Heart Brain Connection Cross-roads), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 2012;9:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol 2013;20:641–657. [DOI] [PubMed] [Google Scholar]
- 3.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;1:Cd004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udell JA, Ray JG. Primary and secondary prevention of heart failure with statins. Expert Rev Cardiovasc Ther 2006;4:917–926. [DOI] [PubMed] [Google Scholar]
- 5.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 6.Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly: results of the PROSPER study. J Neurol 2010;257:85–90. [DOI] [PubMed] [Google Scholar]
- 7.Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia: systematic review and methodological commentary. Nat Rev Neurol 2015;11:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology 2008;70:1795–1802. [DOI] [PubMed] [Google Scholar]
- 9.Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology 2007;69:1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 2005;62:217–224. [DOI] [PubMed] [Google Scholar]
- 11.Ancelin ML, Carriere I, Barberger-Gateau P, et al. Lipid lowering agents, cognitive decline, and dementia: the Three-City Study. J Alzheimers Dis 2012;30:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009;67:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 2007;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity: The Rotterdam Study. J Neurol Neurosurg Psychiatry 2009;80:13–17. [DOI] [PubMed] [Google Scholar]
- 15.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 2008;71:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the Ginkgo Evaluation of Memory Study. J Stroke Cerebrovasc Dis 2012;21:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beydoun MA, Beason-Held LL, Kitner-Triolo MH, et al. Statins and serum cholesterol's associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health 2011;65:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Higdon R, Kukull WA, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology 2004;63:1624–1628. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Shofer JB, Rhew IC, et al. Age-varying association between statin use and incident Alzheimer's disease. J Am Geriatr Soc 2010;58:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 2005;62:1047–1051. [DOI] [PubMed] [Google Scholar]
- 21.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology 2005;65:1388–1394. [DOI] [PubMed] [Google Scholar]
- 22.Sparks DL, Kryscio RJ, Sabbagh MN, Connor DJ, Sparks LM, Liebsack C. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 2008;5:416–421. [DOI] [PubMed] [Google Scholar]
- 23.Steenland K, Zhao L, Goldstein FC, Levey AI. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc 2013;61:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr JM, McGurn B, Whiteman M, Pattie A, Whalley LJ, Deary IJ. Lifelong changes in cognitive ability are associated with prescribed medications in old age. Int J Geriatr Psychiatry 2004;19:327–332. [DOI] [PubMed] [Google Scholar]
- 25.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou CY, Chou YC, Chou YJ, Yang YF, Huang N. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol 2014;173:305–310. [DOI] [PubMed] [Google Scholar]
- 27.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 28.Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danaei G, Garcia Rodriguez LA, Cantero OF, Logan RW, Hernan MA. Electronic medical records can be used to emulate target trials of sustained treatment strategies. J Clin Epidemiol 2018;96:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danaei G, Rodriguez LA, Cantero OF, Logan R, Hernan MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res 2013;22:70–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofman A, Brusselle GG, Darwish Murad S, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantel-Teeuwisse AK, Klungel OH, Verschuren WM, Porsius AJ, de Boer A. Time trends in lipid lowering drug use in The Netherlands. Has the backlog of candidates for treatment been eliminated? Br J Clin Pharmacol 2002;53:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bruijn RF, Bos MJ, Portegies ML, et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med 2015;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cain LE, Saag MS, Petersen M, et al. Using observational data to emulate a randomized trial of dynamic treatment-switching strategies: an application to antiretroviral therapy. Int J Epidemiol 2016;45:2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 36.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernan MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat 2010;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, Hernán MA. A causal framework for classical statistical estimands in failure-time settings with competing events. Stat Med 2020;39:1199–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stensrud MJ, Young JG, Didelez V, Robins JM, Hernan MA. Separable effects for causal inference in the presence of competing risks. 2019. Available at: semanticscholar.org/paper/Separable-Effects-for-Causal-Inference-in-the-of-Stensrud-Young/568bf867f52e94d0585bc992e203529e45601e8ahttps://www.semanticscholar.org/paper/Separable-Effects-for-Causal-Inference-in-the-of-Stensrud-Young/568bf867f52e94d0585bc992e203529e45601e8a. Accessed June 1, 2019.
- 40.Swanson SA, Hernandez-Diaz S, Palmsten K, Mogun H, Olfson M, Huybrechts KF. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol Drug Saf 2015;24:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist's dream? Epidemiology 2006;17:360–372. [DOI] [PubMed] [Google Scholar]
- 42.Swanson SA, Tiemeier H, Ikram MA, Hernan MA. Nature as a trialist? Deconstructing the analogy between Mendelian randomization and randomized trials. Epidemiology 2017;28:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.