Abstract
Objective
To determine whether performance on the Free and Cued Selective Reminding Test (FCSRT) is associated with PET in vivo markers of brain pathology and whether it can distinguish those who will develop dementia later in life due to autosomal-dominant Alzheimer disease (AD) from age-matched controls.
Methods
Twenty-four cognitively unimpaired Presenilin-1 E280A carriers (mean age 36 years) and 28 noncarriers (mean age 37 years) underwent Pittsburg compound B-PET (amyloid), flortaucipir-PET (tau), and cognitive testing, including the FCSRT (immediate and delayed free and cued recall scores). Linear regressions were used to examine the relationships among FCSRT scores, age, mean cortical amyloid, and regional tau burden.
Results
Free and total recall scores did not differ between cognitively unimpaired mutation carriers and noncarriers. Greater age predicted lower free recall and delayed free and total recall scores in carriers. In cognitively impaired carriers, delayed free recall predicted greater amyloid burden and entorhinal tau, while worse immediate free recall scores predicted greater tau in the inferior temporal and entorhinal cortices. In turn, in all carriers, lower free and total recall scores predicted greater amyloid and regional tau pathology.
Conclusions
FCSRT scores were associated with in vivo markers of AD–related pathology in cognitively unimpaired individuals genetically determined to develop dementia. Difficulties on free recall, particularly delayed recall, were evident earlier in the disease trajectory, while difficulties on cued recall were seen only as carriers neared the onset of dementia, consistent with the pathologic progression of the disease. Findings suggest that the FCSRT can be a useful measure to track disease progression in AD.
Alzheimer disease (AD)–related pathology is evident decades before clinical onset (i.e., the preclinical stage).1,2 Converging evidence suggests that intervening at this stage may afford the most efficacy for modifying the disease. Consequently, several disease-modifying clinical trials are being conducted in cognitively unimpaired adults with the goal of delaying or even preventing cognitive decline onset. For such trials to succeed, it is crucial to have cognitive measures that can aid in the detection and tracking of the earliest disease-related brain changes.
The Free and Cued Selective Reminding Test (FCSRT), a test of associative memory, has been shown to be sensitive to memory decline in cognitively unimpaired older adults with evidence of neurodegeneration.3–7 On this test, words or pictures are paired with semantic cues to facilitate learning and recall. Failure to recall words with cues has been associated with dysfunction of temporolimbic regions that are vulnerable to early tau pathology.7–10 However, to date, it is unclear whether the FCSRT can detect and track disease progression from the preclinical stage of AD and to distinguish who will remain cognitively unimpaired from those who will develop dementia.
We examined the association between free and cued recall scores and in vivo amyloid and tau pathology in individuals without dementia with autosomal-dominant AD (ADAD) from the world's largest kindred who are virtually destined to develop dementia in their 40s.2 We hypothesized that lower scores on total recall (TR; free and cued recall sum) would be associated with higher levels of brain pathology and greater age and would be better at distinguishing performance of carriers from noncarriers than scores on free recall (FR) alone.
Methods
Study design and participants
Presenilin-1 (PSEN-1) E280A carriers and age- and education-matched noncarrier family members were recruited from the Colombian Alzheimer's Prevention Initiative (API) registry, which currently includes >5,800 living members of the kindred, including ≈1,200 mutation carriers.11,12 To be included in the Colombia-Boston (COLBOS) observational longitudinal biomarker study at the Massachusetts General Hospital, participants needed to have a minimum of 5 years of formal education, no cognitive impairments as reported by their most recent neuropsychological assessment, and normal vision or corrected to normal, and they had to agree to travel from Colombia to Boston for a week. Participants were excluded if they had a history of neurologic disorder or medical disorder that affects nervous system functioning, a history of psychiatric disorders, a history of learning disability, a history of cardiovascular disease, metal that would interfere with MRI scanning safety, claustrophobia that would interfere with scanning comfort, or pregnancy. Individuals with dementia were also excluded from this study. To be classified as cognitively unimpaired, participants had to demonstrate no cognitive impairment on the Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery word-list recall and a visuospatial memory test, a Clinical Diagnostic Rating scale score of 0, a Functional Assessment Staging Test score of ≤2, and a Folstein Mini-Mental State Examination score of ≥26. Individuals with mild cognitive impairment (MCI) were diagnosed on the basis of previously established criteria13, which include subjective cognitive concerns, impairment in memory tests (1.5 SD below the mean), intact activities of daily living (Functional Assessment Staging Test score of 3), and essentially preserved general cognitive functioning.
Mutation carriers from this kindred have an onset of MCI at a median age of 44 years (95% confidence interval [CI] 43–45) and of dementia at 49 years (95% CI 49–50).12 Mutation carriers usually exhibit memory impairment first, followed by decline in other cognitive functions, including language and executive function. They also have a well-characterized disease trajectory with cortical amyloid accumulation beginning over a decade before the onset of clinical symptoms (i.e., onset of MCI) and tau burden in medial temporal lobe regions (e.g., entorhinal cortex and inferior temporal gyrus) an average of 6 years before clinical symptom onset, as measured by PET and CSF.2,14,15 PSEN1 E280A carriers and age- and education-matched family noncarriers traveled to Boston for MRI and PET imaging as part of the COLBOS Project, a longitudinal biomarker study being conducted in members of the Colombian kindred. Participants and investigators were blinded to the genetic status of the individual.
Standard protocol approvals, registrations, and participant consents
The study was approved by the institutional ethics review boards of both the University of Antioquia in Colombia and Massachusetts General Hospital in Boston. All participants provided signed informed consent before participating in any procedures.
Procedures
Neuropsychological tasks
Participants completed the FCSRT in their native language (i.e., Spanish). Cognitive testing was conducted at the University of Antioquia within 2 months of brain imaging.
The FCSRT is a multimodal associative memory measure.3,16 During the test, 16 pictured objects are paired with semantic categories to facilitate learning. In the encoding phase, participants learn 16 object-category pairs, which are presented in groups of 4. In this phase, the examiner names a category (e.g., animal), and the participant is asked to name which of the 4 objects belongs to the category the corresponding pictured object that is presented (e.g., bear). Once the person has named the 4 objects presented at a time, the stimulus is taken away, and the person is given the semantic cue for each object and asked to name the object that was paired with that category. If the person fails to recall an item, then s/he is presented with the semantic cue and item together. If the individual misses >1 item per card, then the examiner reteaches the card 1 more time. In the testing phase, the examiner first asks the participant to freely recall all 16 items. For those items not freely recalled, the semantic cue is provided. If the participant is still unable to retrieve the word, then the participant is reminded of the item that was paired with the cue. This procedure is repeated 3 times, with a 20-second distracter task between trials. Two scores are then generated: FR, the sum of items freely recalled across 3 trials, and TR, the sum of items recalled freely and items retrieved by cued recall (48 items), which is considered a measure of cued recall. We modified the standard protocol to further test consolidation and added 30-minute FR and total delayed recall (dTR) conditions. Two additional scores are then generated: free delayed recall (dFR; 16 items) and dTR (16 items). In addition to FR, TR, dFR, and dTR scores, we examined free and cued recall scores in trial 1 (16 items), as well as the learning slope (trial 3 − trial 1).
Imaging acquisition and processing
All participants underwent tau and amyloid PET imaging at Massachusetts General Hospital in Boston.
As reported previously,2 11C-Pittsburg compound B (PiB) PET was acquired with a 8.5- to 15-mCi bolus injection followed immediately by a 60-minute dynamic acquisition in 69 frames (12 × 15 seconds, 57 × 60 seconds). 18F-flortaucipir (FTP) was acquired between 80 and 100 minutes after a 9.0- to 11.0-mCi bolus injection in 4 separate 5-minute frames.
11C-PiB-PET data were expressed as the distribution volume ratio with cerebellar gray as reference tissue; regional time-activity curves were used to compute regional distribution volume ratios for each region of interest (ROI) using the Logan graphic method applied to data obtained between 40 and 60 minutes after injection.17 11C-PiB retention was assessed with a large cortical ROI aggregate that included frontal, lateral temporal, and retrosplenial cortices as described previously.18
FTP-specific binding was expressed in FreeSurfer ROIs as the standardized uptake value ratio (SUVR) to cerebellum, similar to a previous report.19 The spatially transformed SUVR PET data were smoothed with an 8-mm gaussian kernel to account for individual anatomic differences.20 SUVR values were represented graphically on vertices at the pial surface. Regional FTP-SUVR analyses were conducted in the entorhinal cortex and the inferior temporal cortex because these regions were found to be associated with early FTP-SUVR increases over age in the PSEN1 E280A kindred.
Statistical analyses
All statistical analyses were conducted with SPSS Statistics, version 24.0 (IBM Corp, Armonk, NY) Analyses used a significance threshold of p < 0.05. We used the Mann-Whitney U test to examine differences in age and education and in cognitive performance between PSEN1 mutation carriers and noncarriers. Between-group sex differences were examined with the χ2 test. Effect sizes (i.e., r) were calculated by dividing z by the squared root of the sample size. We conducted a Bonferroni test to correct for multiple comparisons. Because age in PSEN1 E280A mutation carriers is predictive of clinical onset, cross-sectional assessments can be considered analogous to what might be expected from the assessment of longitudinal trajectories of cognitive change. Therefore, we conducted linear regressions to test the association among the FCSRT scores, age, and mean cortical amyloid and regional tau pathology (entorhinal and inferior temporal cortices). We reported 95% CIs for each test.
We first examined cognitively unimpaired carriers only to examine how FCSRT scores related to markers of pathology at the earliest stages of the disease. We then conducted the same analyses with all carriers (i.e., cognitively unimpaired and mildly impaired) to obtain a better understanding of the sensitivity of the test to pathology throughout the spectrum of the disease before the onset of dementia.
In follow-up analyses, we carried out an exploratory whole-brain analysis examining the relationship between tau pathology burden and TR scores in all mutation carriers. Regions were p < 0.01 after cluster size correction for multiple comparisons (minimum cluster extent k = 100 mm2).
Data availability
The dataset used in the current study is available from the corresponding author on reasonable request, as long as data transfer is in agreement with US regulations on data protection regulation.
Results
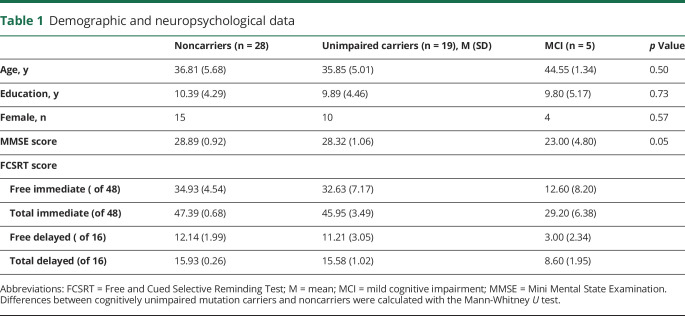
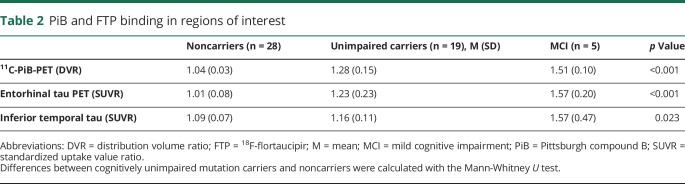
Demographic and neuropsychological data are presented in table 1. Groups did not significantly differ in sex, age, or educational attainment. PiB and FTP binding data for each group are presented in table 2. Mutation carriers, with and without symptoms, had greater mean cortical amyloid and regional tau compared to noncarriers.
Table 1.
Demographic and neuropsychological data
Table 2.
PiB and FTP binding in regions of interest
Differences between groups in FCSRT performance
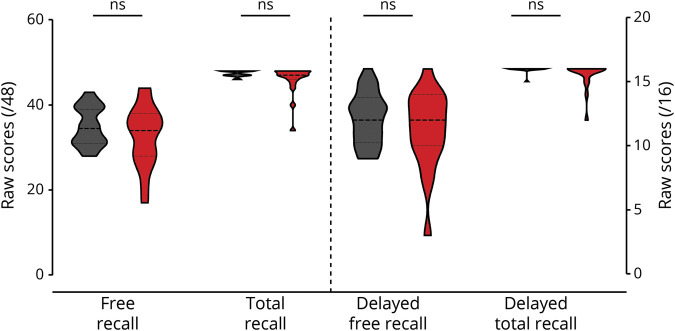
FCSRT scores did not significantly differ between cognitively unimpaired carriers and noncarriers (figure 1). In turn, cognitively unimpaired and impaired carriers together had significantly lower scores in FR (U = 218, p = 0.030, r = −0.301), TR (U = 201, p = 0.009, r = −0.362), dFR (U = 227, p = 0.044, r = −0.279), and dTR (U = 227, p = 0.005, r = −0.388) compared to noncarriers. Only TR and dTR survived multiple comparisons.
Figure 1. FCSRT performance in mutation carriers and noncarriers.
No significant group-differences were seen in Free and Cued Selective Reminding Test (FCSRT) scores between cognitively unimpaired mutation carriers (red) and noncarriers (black). ns = Nonsignificant.
Associations between age and FCSRT scores
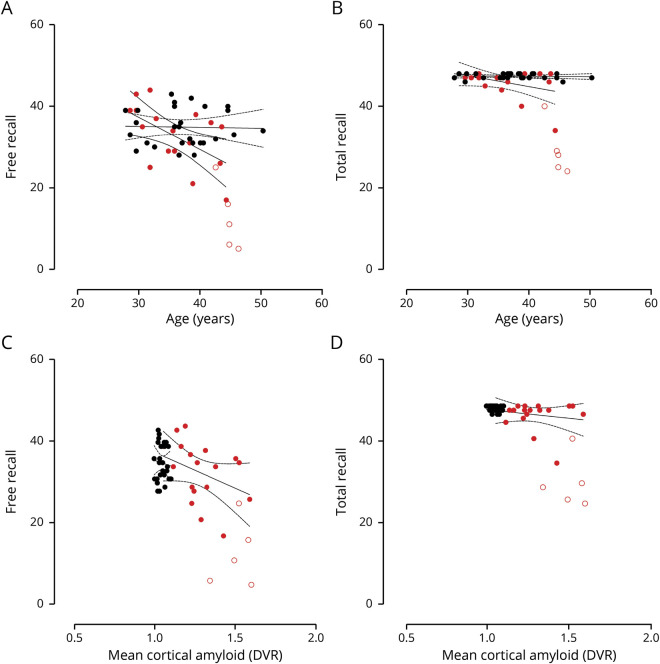
In cognitively unimpaired mutation carriers, greater age was a significant predictor of lower FR (B = −0.786, p = 0.015, 95% C [−1.38, −0.18]), dFR (B = −0.378, p = 0.004, 95% C [−0.62, −0.13]), and dTR (B = −0.096, p = 0.014, 95% C [−0.19, −0.004]) scores such that those who were closer to clinical onset performed worse (figure 2, A and B).
Figure 2. Age, Pittsburgh B binding, and Free and Cued Selective Reminding Test scores.
Black circles represent noncarriers; red circles represent cognitively unimpaired PSEN1 mutation carriers. Unfilled red circles represent mutation carriers with mild cognitive impairment. Lines represent 95% CI. (A and B) Age significantly predicts free recall (FR) scores, but not total recall (TR) scores, in cognitively unimpaired mutation carriers. (C and D) There is no association between FR and TR scores and cortical amyloid burden in cognitively unimpaired carriers. DVR = distribution volume ratio.
Similarly, greater age predicted lower FR (B = −0.43, p < 0 .001, 95% C [−1.99, −0.87]), dFR (B = −0.609, p < 0 .001, 95% C [−0.82, −0.40]), dTR (B = −0.381, p < 0 .001, 95% C [−0.55, −0.21]), and TR (B = −0.963, p < 0 .001, 95% C [−1.41, −0.51]) scores in all mutation carriers. Age did not predict FCSRT scores in noncarriers.
PiB binding and FCSRT scores
In cognitively unimpaired mutation carriers, worse dFR significantly predicted greater cortical PiB retention (B = −0.027, p = 0.017, 95% C [−0.048, −0.005]) (Figure 2, C and D).
When cognitively unimpaired were combined with mildly impaired mutation carriers, worse FR (B = −0.009, p = 0.003, 95% C [−0.014, −0.003]), TR (B = −0.011, p = 0.006, 95% C [−0.02, −0.004]), dFR (B = −0.026, p < 0 .001, 95% C [−0.04, −0.013]), and dTR (B = −0.032, p = 0.002, 95% C [−0.05, −0.01]) scores predicted greater cortical amyloid burden.
FTP binding and FCSRT scores
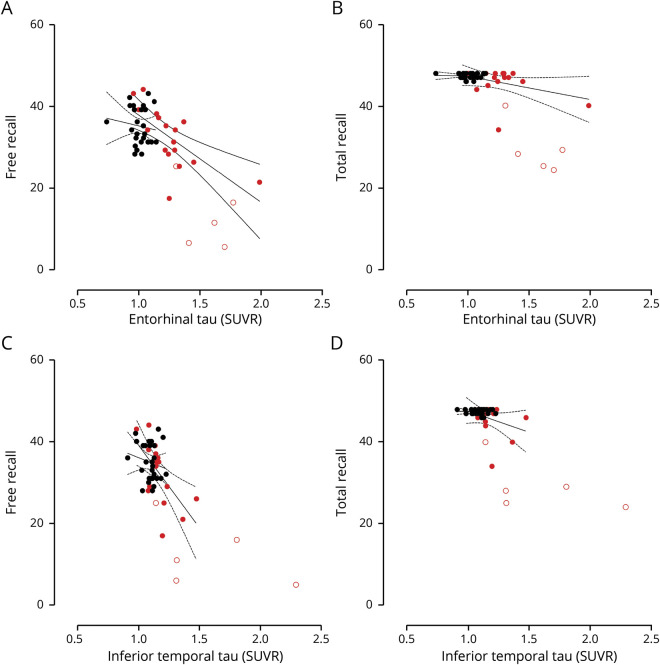
Lower scores in FR (B = −0.022, p = 0.001, 95% C [−0.03, −0.01]) and dFR (B = −0.38, p = 0.028, 95% C [−0.07, −0.005]) predicted greater FTP binding in the entorhinal cortex in cognitively unimpaired mutation carriers (figure 3, A and B). Similarly, lower scores in FR (B = −0.009, p = 0.006, 95% C [−0.02, −0.003]) predicted greater FTP binding in the inferior temporal cortex in cognitively unimpaired mutation carriers (figure 3, C and D).
Figure 3. 18F-flortaucipir binding and Free and Cued Selective Reminding Test score.
Black circles represent noncarriers; red circles represent cognitively unimpaired PSEN1 mutation carriers. Unfilled red circles represent mutation carriers with mild cognitive impairment. (A) Free recall (FR) scores significantly predict tau burden in the entorhinal cortex. (B) There is no association between total recall (TR) and tau in the entorhinal cortex. (C) FR scores significantly predict tau burden in the inferior temporal cortex. (D) There is no association between TR and tau burden in the inferior temporal cortex.
When mildly impaired mutation carriers were considered, lower FR (B = −0.018, p < 0 .001, 95% C [−0.03, −0.01]), TR (B = −0.021, p = 0.001, 95% C [−0.03, −0.01]), dFR (B = −0.038, p = 0.001 [−0.06, −0.02]), and dTR (B = −0.05, p = 0.002 [−0.08, −0.02]) scores predicted greater tau in the entorhinal cortex. Consistently, greater FR (B = −0.018, p < 0 .001, 95% CI [−0.03, −0.01]), TR (B = −0.025, p < 0 .001, 95% CI [−0.03, −0.01]), dFR (B = −0.036, p = 0.003, 95% CI [−0.06, −0.01]), and dTR (B = −0.057, p = 0.001, 95% CI [−0.09, −0.03]) scores predicted less FTP binding in the inferior temporal cortex. Notably, we also examined the relationship between FTP binding in the whole brain and TR score. Consistent with findings with regions selected a priori, TR scores were related to higher tau burden in the inferior temporal cortex and medial temporal regions (figure 4).
Figure 4. Whole-brain analysis of the relationship between tau pathology and TR score.
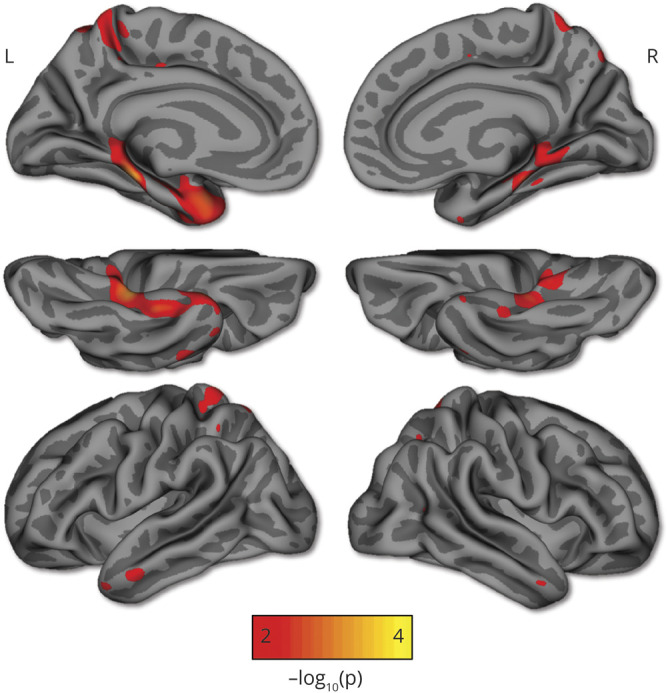
Greater total recall (TR) scores were related to 18F-flortaucipir binding in mostly the inferior temporal cortex and medial temporal regions in mutation carriers. Regions shown are p < 0.01 after cluster size correction for multiple comparisons (minimum cluster extent k = 100 mm2).
Trial 1 and learning slope scores
We also examined group differences in trial 1 free and cued performances, as well as in the learning slope (trial 3 − trial 1) between cognitively unimpaired mutation carriers and noncarriers and their relationship with pathology.
We found that cognitively unimpaired mutation carriers performed significantly worse than noncarriers on trial 1 cued recall only (U = 183, p = 0.046, r = −0.290). In all mutation carriers, greater age predicted worse performance in trial 1 FR (B = −0.261, p = 0.021, 95 % CI [−0.48, −0.04]). Worse performance in trial 1 FR, but not cued recall, predicted greater cortical PiB retention (B = −0.029, p = 0.036, 95 % CI [−0.06, −0.002]), tau in the entorhinal cortex (B = −0.059, p = 0.003, 95 % CI [−0.09, −0.02]), and tau in the inferior temporal cortex (B = −0.023, p = 0.019, 95% CI [−0.42, −0.004]). Learning slopes did not significantly differ between groups, nor did they predict pathology burden.
Finally, we calculated an index (TR − FR/TR + FR) to examine more closely the benefits of the category-exemplar association to memory performance. Similar to our previous findings, we found that there were no significant differences between cognitively unimpaired carriers and noncarriers or significant associations with age and pathology. When both cognitively unimpaired and impaired carriers were considered, we found that there were significant group differences (U = 208, p = 0.019, r = −0.367), as well as relationships with age (B = −0.531, p < 0 .001, 95% CI [−0.78, −0.28]), amyloid burden (B = −0.013, p = 0.006, 95% CI [−0.02, −0.004]), and tau in the entorhinal cortex (B = −0.023, p = 0.001, 95% CI [−0.03, −0.01]) and inferior temporal cortex (B = −0.026, p < 0 .001, 95% CI [−0.04, −0.01]).
Discussion
There is an urgent need to have tools that can detect and track subtle cognitive decline and AD-related brain changes in the preclinical stage of the disease. We leveraged data from the world's largest ADAD kindred with a single-gene mutation (E280A) in PSEN1 and dementia onset at a median age of 49 years12,21 to examine whether the FCSRT, an associative memory test known to be sensitive to early AD-related brain changes, can distinguish mutation carriers from age-matched noncarriers years before dementia onset. Furthermore, we characterized the relationships between FCSRT and in vivo measures of amyloid and tau pathology and between FCSRT and age (a proxy of disease progression) in mutation carriers.
Performance on the FCSRT has been shown to be particularly sensitive to subtle cognitive changes in preclinical AD and associated with postmortem neuropathology as defined by a Braak stage IV or greater.22 The test was recommended by an International Working Group as a criterion for the diagnosis of AD and is part of the preclinical Alzheimer cognitive composite score, which is used as a primary outcome measure in the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) study5,23 and as a secondary outcome measure in the API ADAD preclinical trial.24 Yet, little is known about how FCSRT scores relate to in vivo measures of pathology, particularly tau, in cognitively unimpaired individuals at increased risk for dementia.
Our findings showed that the FCSRT FR, TR, dFR and dTR do not distinguish cognitively unimpaired mutation carriers from age- and education-matched noncarriers, but they do distinguish mutation carriers without dementia (i.e., cognitively unimpaired and mildly impaired) from noncarriers. Cognitively unimpaired mutation carriers performed worse in trial 1 cued recall. Furthermore, greater age, a proxy for disease progression in this cohort, significantly predicted lower performance on free and delayed recall scores, as well as dTR scores in cognitively unimpaired carriers, such that those who were closer to age at dementia onset had the most difficulty recalling words with and without semantic cues. In terms of AD-related pathology in cognitively unimpaired mutation carriers, worse performance on dFR predicted greater cortical amyloid and tau burden in the entorhinal cortex, one of the initial sites of tau neurofibrillary tangles formation.2,25,26 Similarly, worse immediate FR and dFR scores significantly predicted tau burden in both the entorhinal cortex and the inferior temporal cortex, which also shows tau accumulation early in the preclinical stage. This supports that FR scores are the most sensitive to early pathology burden in the preclinical stage of AD. In turn, in mutation carriers with MCI, worse immediate and delayed free recall and total recall (i.e., cued recall) scores, and an index score of benefits to memory of the category-exemplar association, predicted greater mean cortical amyloid and tau burden in the entorhinal and inferior temporal cortices, suggesting that the inability to benefit from semantic cues reflects a greater extent of brain damage and proximity to dementia onset.
Furthermore, we examined whether trial 1 of the FCSRT and the learning slope from trial 1 to trial 3, the ability to encode information, were also sensitive to early pathology accumulation. No group differences or relationships with pathology were seen with the amount of additional words that cognitively unimpaired and impaired carriers recalled between trials 1 and 3. However, cognitively unimpaired carriers showed worse cued recall in trial 1. This is consistent with data showing that temporal regions necessary for memory, and less for executive functioning, are the first ones to degenerate in AD.
Our findings are consistent with prior studies of older adults at risk for late-onset AD, which showed that free immediate recall declines first, followed by impairment in cued recall.4,27 For instance, Schindler et al.6 reported that of 9 neuropsychological measures of language, executive functioning, global cognition, and episodic memory (FCSRT, Logical Memory 1 and 2, and Verbal Paired Associates), FR from the FCSRT was the most sensitive to early cognitive decline in older adults with high CSF tau/β-amyloid42. Similarly, Papp and colleagues4,27 reported that clinically normal older adults with brain amyloidosis performed worse in FR compared to their peers who were amyloid negative, while those with both evidence of amyloidosis and neurodegeneration (preclinical AD stage 2) had worse performance on FR and TR scores. In conjunction with prior research,3,28 our findings suggest the temporal sequencing of memory decline in preclinical AD in which FR declines first, followed by decline in cued recall when individuals are closer to the onset of dementia.
As previously noted, it has been argued that the FCSRT is particularly sensitive to early brain changes in AD because, contrary to tests that require learning a story or a word list, it controls for increased demands on attention and working memory by presenting semantic cues during the encoding phase.4,8,27,29 Thus, the TR scores appear to isolate memory deficits that are associated with regions that are vulnerable to tau burden early in AD30 such as the entorhinal cortex and inferior temporal cortex, which are crucial for associative memory.31–33 We now provide evidence showing that difficulties with cued recall (i.e., TR) after only 1 learning trial may be an early indicator of both subtle cognitive changes and AD-related pathology burden, while cued recall after 3 learning trials (i.e., TR) may be the most indicative of imminent risk for clinically meaningful decline and conversion to dementia.
The current study has multiple strengths. First, we did not rely on presenting symptoms or cognitive data to infer whether individuals will go on to develop dementia. Instead, we examined cognitive changes in a group of individuals who have a well-characterized clinical trajectory with MCI starting at a median age of 44 years and dementia at 49 years.12,21,34 In addition, we examined in vivo amyloid and tau pathology using PET imaging, which is considered the gold standard for quantifying and examining brain pathology in AD. Mutation carriers were also young and otherwise healthy, which minimized potential confounding variables occurring with age that contribute to cognitive decline (e.g., cardiovascular risk). Finally, the nearly homogeneous clinical profile of mutation carriers allows us to infer how free and cued recall performances decline as the disease progresses, supporting the utility of this test for tracking disease progression in clinical trials.
The present study also had some limitations. First, our sample size is relatively small compared to studies with older adults. However, individuals with these mutations are rare, and all our participants had a single mutation in PSEN1. Furthermore, there is uncertainty about whether our findings in ADAD generalize to late-onset sporadic AD, even when they are consistent with data from studies with older adults at risk for AD. Thus, our findings should be validated with other independent cohorts. Lastly, longitudinal studies are needed to track over time how FR and TR scores change in relation to the accumulation of AD pathology. We are currently conducting the first longitudinal biomarker with this kindred and plan to examine these relationships in the future.
Our data suggests significant difficulties with benefiting from semantic cues do not become evident until the individuals have overt cognitive symptoms and are thus closer to age at dementia onset. Our findings are consistent with what is known about the neural correlates of associative memory in that temporal regions do not become affected by tau pathology in this kindred until close to the clinical onset of the disease. Therefore, poor performance on the FCSRT, particularly TR, indicates that the person likely has pathology in temporal regions important for memory and is at imminent risk for developing dementia. Together, these findings suggest that the FCSRT is a useful tool for tracking progression of the disease in individuals at high risk for dementia, which may have important implications for clinical trials.
Acknowledgment
The authors thank the Colombian families for contributing their valuable time and effort, without which this study would not have been possible. They also thank Francisco Piedrahita, Alex Navarro, and Claudia Ramos from Grupo de Neurociencias, Universidad de Antioquia in Medellín, Colombia, as well as Fred Uquillas, Olivia Hampton, Heirangi Torrico-Teave, Enmanuelle Pardilla-Delgado, and Josh Fuller from the Massachusetts General Hospital in Boston, MA, for helping coordinate visits to Boston and assisting with data collection and processing.
Glossary
- AD
Alzheimer disease
- ADAD
autosomal-dominant Alzheimer disease
- A4
Anti-Amyloid Treatment in Asymptomatic Alzheimer's
- API
Alzheimer's Prevention Initiative
- CI
confidence interval
- COLBOS
Colombia-Boston
- dFR
free delayed recall
- dTR
total delayed recall
- FCSRT
Free and Cued Selective Reminding Test
- FR
free recall
- FTP
18F-flortaucipir
- MCI
mild cognitive impairment
- PiB
Pittsburg compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- TR
total recall
Appendix. Authors
Study funding
This research was supported by the NIH National Institute of Aging (NIA) (RO1AG054671 [Y.T.Q.]) and Office of the Director (DP5OD019833 [Y.T.Q.]), Massachusets General Hospital (MGH) Executive Committee On Research Claflin Distinguished Scholar Award (Y.T.Q.), MGH Physician/Scientist Development Award (YTQ), COLCIENCIAS (Colombia) (F.L), and MGH Executive Committee on Research Fund for Medical Clinical Fellowship Award (E.G.-V.).
Disclosure
E. Guzmán-Vélez receives research funding from the NIA K23AG061276 and NIH Research Supplement for Diversity (DP5OD019833). J. Martínez, K. Papp, and A. Baena report no disclosures relevant to the manuscript. C. Vila-Castelar receives research support from an Alzheimer's Association–Research Fellowship (grant 2019-AARF-644631). A. Artola, A.P. Schultz, Y. Bocanegra, and J. Sanchez report no disclosures relevant to the manuscript. D. Rentz has provided consulting services to the Neurotrack Scientific Advisory Board and Biogen. P.N. Tariot serves on the scientific board and consults for commercial entities (Abbott Laboratories, AbbVie, AC Immune, Amgen, BioGen, Insys, Intracellular Therapeutics, Lilly, Lundbeck, Boehringer-Ingelheim, Chase Pharmaceuticals, Otsuka, T3D, Takeda, Pfizer, Merck, Roche) and nonprofits (W Garfield Weston Foundation, and the California Pacific Medical Center). He is a member of the International Journal of Geriatric Psychiatry Editorial Board. He receives research support from Avanir, Lilly, Merck, Roche, AstraZeneca, Functional Neuromodulation, Genentech, Toyama. Abbott Laboratories, AbbVie, Amgen, BioGen, Lundbeck, Takeda, Pfizer, Novartis, and the NIA (1RFAG041705-01A1 and NIA 1UF1AG046150). E.M. Reiman reports research funding from Genentech/Roche, Novartis/Amgen and Avid/Lilly AVID, as well as NIA RF1 AG041705, NIA UF1 AG046150, NIA R01 AG031581, NIA P30 AG19610, NIA R01 AG055444, NIA R01 AG031581, NIA P30 AG19610, NIA RF1 AG041705, PI, and NIA 1UF1 AG046150, Arizona Department of Health Services (Arizona Alzheimer's Consortium) and state of Arizona (Arizona Alzheimer's Consortium), the Banner Research, University of Arizona, Arizona State University, Translational Genomics Research Institute, Arizona Alzheimer's Consortium, Anonymous Foundation, Flinn Foundation, and NOMIS Foundation. He serves on the advisory board of and consults for Alkahest, Alzheon, Biogen, Denali, Green Valley, United Neuroscience, Zinfandel Roche, and Roche Diagnostics (expenses only). R. Sperling receives research support for NIH grants P01AG036694, P50AG005134, 2009–2020, and U19 AG10483, as well as from Eli Lilly (clinical trial) and the Alzheimer's Association. She is a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. She receives travel funding and honoraria from AC Immune, Janssen, and Roche. She consults for Biogen, Roche, AC Immune, Eisai, Takeda, Neurocentria, and Janssen. K.A. Johnson has provided consulting services for Novartis, Biogen, and Eli Lilly; received support from a joint NIH-Lilly–sponsored clinical trial (A4 Study; U19AG10483); and received research support from NIH grants R01 AG027435, P50 AG00513421, AG036694, R01 AG046396, R13 AG042201174210, U19AG10483, and U01AG024904, as well as the Alzheimer Association and Marr Foundation. F. Lopera receives research support from philanthropy, NIA, Genentech, and Roche for the API ADAD Trial. Y.T. Quiroz receives research funding from NIH for RO1AG054671 and DP5OD019833 and from MGH. Go to Neurology.org/N for full disclosures.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quiroz YT, Sperling RA, Norton DJ, et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol 2018;75:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grober E, Veroff AE, Lipton RB. Temporal unfolding of declining episodic memory on the free and cued selective reminding test in the predementia phase of Alzheimer's disease: implications for clinical trials. Alzheimers Dement (Amst) 2018;10:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp KV, Amariglio RE, Mormino EC, et al. Free and cued memory in relation to biomarker-defined abnormalities in clinically normal older adults and those at risk for Alzheimer's disease. Neuropsychologia 2015;73:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC. Optimizing the preclinical Alzheimer's cognitive composite with semantic processing: the PACC5. Alzheimers Dement 2017;3:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schindler SE, Jasielec MS, Weng H, et al. Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging 2017;56:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner M, Wolf S, Reischies FM, et al. Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology 2012;78:379–386. [DOI] [PubMed] [Google Scholar]
- 8.Lemos R, Duro D, Simões MR, Santana I. The free and cued selective reminding test distinguishes frontotemporal dementia from Alzheimer's disease. Arch Clin Neuropsychol 2014;29:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology 2007;69:1859–1867. [DOI] [PubMed] [Google Scholar]
- 10.Slachevsky A, Barraza P, ornberger M, et al. Neuroanatomical comparison of the “word” and “picture” versions of the free and cued selective reminding test in Alzheimer's disease. J Alzheimers Dis 2018;61:589–600. [DOI] [PubMed] [Google Scholar]
- 11.Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer's prevention initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26(suppl) 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol 2011;10:213–220. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 14.Fuller JT, Cronin-Golomb A, Gatchel JR, et al. Biological and cognitive markers of presenilin1 E280A autosomal dominant Alzheimer's disease: a comprehensive review of the Colombian kindred. J Prev Alzheimers Dis 2019;6:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol 2015;72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado C, Muñoz-Neira C, Soto A, et al. Comparison of the psychometric properties of the “word” and “picture” versions of the free and cued selective reminding test in a Spanish-speaking cohort of patients with mild Alzheimer's disease and cognitively healthy controls. Arch Clin Neuropsychol 2016;31:165–175. [DOI] [PubMed] [Google Scholar]
- 17.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990;10:740–747. [DOI] [PubMed] [Google Scholar]
- 18.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology 2015;85:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien DT, Szardenings AK, Bahri S, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis 2014;38:171–184. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre-Acevedo DC, Lopera F, Henao E, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol 2016;73:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grober E, Lipton R, Wang C. The free and cued selective reminding test predicts Alzheimer's disease neuropathology (P4.1-026). Neurology 2019;92:P4.1–026. [Google Scholar]
- 23.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014;71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tariot PN, Lopera F, Langbaum JB, et al. The Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer's disease, including a placebo-treated noncarrier cohort. Alzheimers Dement 2018;4:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging 1997;18:S85–S88. [DOI] [PubMed] [Google Scholar]
- 27.Papp KV, Rentz DM, Mormino EC, et al. Cued memory decline in biomarker-defined preclinical Alzheimer disease. Neurology 2017;88:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auriacombe S, Helmer C, Amieva H, Berr C, Dubois B, Dartigues JF. Validity of the free and cued selective reminding test in predicting dementia: the 3C Study. Neurology 2010;74:1760–1767. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc 1995;1:525–536. [DOI] [PubMed] [Google Scholar]
- 30.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007;30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr VA, Bernstein JD, Favila SE, Rutt BK, Kerchner GA, Wagner AD. Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proc Natl Acad Sci USA 2017;114:12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks SM, Lockhart SN, Baker SL, Jagust WJ. Tau and beta-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci 2017;37:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganath C, Cohen MX, Dam C, D'Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 2004;24:3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guzman-Velez E, Jaimes S, Aguirre-Acevedo DC, et al. A three-factor structure of cognitive functioning among unimpaired carriers and non-carriers of autosomal-dominant Alzheimer's disease. J Alzheimers Dis 2018;65:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in the current study is available from the corresponding author on reasonable request, as long as data transfer is in agreement with US regulations on data protection regulation.