Abstract
Objective
To assess cerebrovascular reactivity in response to a visual task in participants with cerebral amyloid angiopathy (CAA), Alzheimer disease (AD), and mild cognitive impairment (MCI) using fMRI.
Methods
This prospective cohort study included 40 patients with CAA, 22 with AD, 27 with MCI, and 25 healthy controls. Each participant underwent a visual fMRI task using a contrast-reversing checkerboard stimulus. Visual evoked potentials (VEPs) were used to compare visual cortex neuronal activity in 83 participants. General linear models using least-squares means, adjusted for multiple comparisons with the Tukey test, were used to estimate mean blood oxygen level–dependent (BOLD) signal change during the task and VEP differences between groups.
Results
After adjustment for age and hypertension, estimated mean BOLD response amplitude was as follows: CAA 1.88% (95% confidence interval [CI] 1.60%–2.15%), AD 2.26% (1.91%–2.61%), MCI 2.15% (1.84%–2.46%), and control 2.65% (2.29%–3.00%). Only patients with CAA differed from controls (p = 0.01). In the subset with VEPs, group was not associated with prolonged latencies or lower amplitudes. Lower BOLD amplitude response was associated with higher white matter hyperintensity (WMH) volumes in CAA (for each 0.1% lower BOLD response amplitude, the WMH volume was 9.2% higher, 95% CI 6.0%–12.4%) but not other groups (p = 0.002 for interaction) when controlling for age and hypertension.
Conclusions
Mean visual BOLD response amplitude was lowest in participants with CAA compared to controls, without differences in VEP latencies and amplitudes. This suggests that the impaired visual BOLD response is due to reduced vascular reactivity in CAA. In contrast to participants with CAA, the visual BOLD response amplitude did not differ between those with AD or MCI and controls.
Cerebral amyloid angiopathy (CAA) is present, to varying degrees, in 82% to 98% of patients with Alzheimer disease (AD).1 Both CAA and AD pathologies coexist on a spectrum in which one end represents parenchymal amyloid deposition causing AD dementia and the other end represents vascular amyloid deposition leading to intracerebral hemorrhage (ICH) and vascular cognitive impairment.2
Accumulating evidence suggests that impaired cerebrovascular reactivity (CVR) in response to a vasodilatory response is an important feature of CAA.3,4 Studies have shown that CAA is associated with reduced amplitude of the blood oxygen level–dependent (BOLD) response to a visual stimulus5–7 and that occipital BOLD response amplitude decreases over a 1-year period in CAA but not in healthy aging.8 It is unclear whether CVR impairment in AD is attributed to parenchymal amyloid or vascular amyloid. Studies measuring blood flow in response to a vasodilatory stimulus in AD showed conflicting results.9–13 fMRI studies have shown decreased BOLD response amplitude to memory tasks in the hippocampus in AD, which could partly reflect impaired CVR in addition to neuronal dysfunction.14,15 However, prior studies have not controlled for the radiological presence of CAA.
The present study aimed to determine whether CVR is impaired in AD or if it is a unique feature of CAA. This was done by using the amplitude of the BOLD response to a visual stimulus as a surrogate marker of CVR. The primary hypothesis was that BOLD response amplitude would be lowest in patients with CAA, followed by those with AD and mild cognitive impairment (MCI), compared to healthy controls. Secondary hypotheses were that visual evoked potential (VEP) amplitudes and latencies, reflecting occipital metabolic activity and function, would not differ between groups and that CVR impairment in CAA would be associated with higher volume of white matter hyperintensities (WMH) of presumed vascular origin.
Methods
Study population
This cross-sectional study included fMRI data from 40 patients with CAA, 22 with AD, 27 with MCI, and 25 healthy aging controls who did not have stroke or dementia. Inclusion and exclusion criteria have been published elsewhere.6 Study participants were recruited as part of a prospective longitudinal study. Probable CAA was defined as MRI evidence of lobar ICH, microbleeds, or cortical superficial siderosis (cSS) without other evident cause, consistent with the validated Boston criteria.16,17 Specifically, the CAA population contained 18 patients who presented with ICH, 6 with cognitive symptoms without dementia, and 16 with transient focal neurologic episodes. Five participants with CAA presented with headache or cognitive impairment with neuroimaging evidence of CAA-related inflammation (all of whom were studied in a phase of remission without MRI fluid-attenuated inversion recovery [FLAIR] evidence of acute vasogenic edema at the time of study). Participants with CAA were recruited from a cognitive clinic or stroke prevention clinic. Patients with recent symptomatic stroke (<90 days) were excluded to avoid any acute effects of ICH. Patients with MRI evidence of ICH in the occipital pole were excluded if their hemorrhagic lesion extended into the occipital region of interest used to calculate the BOLD response amplitudes. AD with mild dementia was diagnosed with the National Institute of Aging criteria by a neurologist, blinded to fMRI results, using information from clinical history, a neuropsychological test battery,18 and a validated questionnaire on activities of living completed by an informant.19 The participants with AD were required to have mild dementia, as defined by a Clinical Dementia Rating score of 0.5 or 1 and a Folstein Mini-Mental Status Examination score of ≥20.20–22 MCI was diagnosed with National Institute of Aging criteria.23 Healthy aging controls were recruited from the community by advertising in a newsletter or poster and did not have a history of stroke or dementia as determined by neurologist assessment. In all groups, participants were excluded if they resided in a nursing home or long-term care facility, had moderate to severe dementia (defined as a Clinical Dementia Rating score >1.0), had abnormal visual acuity (<20/50 Snellen visual acuity), or were not fluent in English (because English language cognitive testing was part of the study). Healthy controls and participants with MCI and AD were excluded if their MRI showed lobar microbleeds or superficial siderosis indicating CAA. Participants or authorized caregivers provided written consent to participate in the study, which was approved by our Institutional Review Board.
MRI acquisition
Functional echo planar images, T2-weighted FLAIR, susceptibility-weighted, and 3D T1-weighted inversion-recovery spoiled gradient recall images were acquired with a 3.0T magnetic resonance (MR) scanner (either GE Signa VH/i or Discovery MR750; GE Healthcare, Waukesha, WI) with a 12-channel phased-array neurovascular coil. Because of an MR scanner upgrade, 22 of 114 participants were scanned on the Signa VH/i scanner and the remaining on the Discovery MR750 scanner. A T2-weighted, 2D FLAIR sequence was used to measure the WMH of presumed vascular origin volume (repetition time/echo time/inversion time 9,000/149/2,250 milliseconds, voxel size 0.9 × 0.9 × 3.5 mm3, 39 slices, 256 × 256 matrix). A T1-weighted, 3D inversion-recovery spoiled gradient recalled sequence was used to register fMRI data to standard Montreal Neurologic Institute space (repetition time/echo time 6/2.4 milliseconds, flip angle 8°, voxel size 0.94 × 0.94 × 1.0 mm3, 256 × 256 acquisition matrix). Susceptibility-weighted imaging was used to detect the number of cerebral microbleeds (repetition time/echo time 30/20 milliseconds, flip angle 15°, voxel size 0.5 × 0.5 × 1.0 mm3, 120 slices, 256 × 256 acquisition matrix). The entire imaging protocol took ≈1 hour and included diffusion tensor imaging and arterial spin labeling acquisitions, which were not used in this study.
All T2*-weighted fMRI scans were acquired with a gradient-recalled echo, echo planar imaging sequence (repetition time/echo time 2,000/30 milliseconds, voxel size 3.75 × 3.75 × 4.0 mm3, 34 slices, 64 × 64 acquisition matrix). During fMRI scans, participants viewed 4 repetitions of 40-second blocks of an 8-Hz contrast-reversing black-and-white checkerboard visual stimulus followed by 40 seconds of a gray screen with a central fixation cross.
Image analysis
All fMRI data were processed with the FMRIB Software Library (FSL version 5.0.1, Oxford, UK). After brain extraction,24 fMRI data were corrected for interleaved slice timing, corrected for motion with the MCFLIRT tool,25 spatially smoothed (using a 5-mm full-width half-maximum gaussian kernel), and temporally filtered using a high-pass temporal filter with a cutoff of 0.01 Hz. A voxel-by-voxel analysis of each participant’s fMRI data was then performed using a time-series general linear model (GLM) as implemented in the fMRI Expert Analysis Tool (FEAT)26,27 in FSL. The regressor of interest was a time-series model consisting of the binary timing of the visual stimulus convolved with a canonical hemodynamic response function. Estimates of brain activity magnitude in response to the stimulus were then computed with FEAT and converted to a z statistic. The 200 most active voxels (11.3 cm3) exhibiting the greatest z statistic within the primary visual cortex were selected as the region of interest, and the amplitude of the BOLD signal (calculated as the percent change in the MR signal between visual fixation and the checkerboard stimulus, as estimated with the Featquery tool of FSL) was compared across the 4 groups.
Each participant's preprocessed fMRI dataset was registered to the standard Montreal Neurologic Institute brain template to permit a voxel-by-voxel statistical analysis of the estimates of activation for each group. The analysis was performed within FEAT using a GLM.28,29 The FMRIB Local Analysis of Mixed Effects was used for modeling and estimating the random effects between participants. Age and hypertension were added as covariates to the model. Resulting comparisons between groups were computed as a z statistic, with clusters of significantly activated voxels (z > 2.3) corrected for multiple comparisons at p = 0.05 (using AlphaSim, part of the AFNI image analysis package, afni.nimh.gov/afni/doc/manual/AlphaSim). This analysis was performed to determine whether there were common brain regions between groups that exhibited changes in BOLD response amplitude.
WMH, microbleeds, and cSS were defined according to Standards for Reporting Vascular Changes on Neuroimaging.30 Microbleeds and cSS were identified visually by a neurologist with >10 years of experience using susceptibility-weighted imaging combined phase and magnitude images. WMH volume was measured on T2-weighted FLAIR images by a single rater using Quantomo, a custom-designed software application (Cybertrials, Inc, Calgary, Alberta, Canada). Quantomo software uses a semiautomated threshold-based seed-growing algorithm to detect the volume of hyperintense signal by a trained rater.31 To account for differences in head sizes between participants, WMH volumes were normalized to the average intracranial volume of participants in a population-based study such that the reported WMH volume in this study represented the volume of WMH in a participant with an average intracranial volume (1,449 cm3).32 WMH volumes were measured by investigators blinded to the fMRI results.
A subset of 27 participants with CAA, 20 with MCI, 12 with AD, and 25 healthy controls had occipital lobe VEPs to assess surface neuronal activity between groups using the Queen's Square standard electrode placement, consistent with American Clinical Neurophysiology Society33 guidelines. The main reason that VEPs were not done in all participants was that they were removed from the study protocol after our prior analysis showed that evoked potentials were not altered in CAA, despite a reduction in BOLD amplitudes, and did not correlate with fMRI responses.6 Participants were seated 1.5 m from a monitor that presented a 2-Hz contrast-reversing black-and-white checkerboard pattern. One hundred responses were averaged and repeated at least once to ensure reproducibility. The amplitude of the P100 potential (in microvolts) was calculated by determining the difference between the N75 and P100 peaks. VEP P100 amplitudes are known to correlate well with fMRI and ultrasound measures of brain metabolic activity,34,35 while the P100 peak latency (in milliseconds) has been shown to vary among disease groups and with age.36
Statistical analysis
Participant characteristics across the 4 groups (those with CAA, MCI, and AD and controls) were tabulated and compared by use of analysis of variance, Kruskal-Wallis test, or χ2 test as appropriate. Comparisons between pairs of groups were by t test, Wilcoxon rank-sum test, or χ2 test. BOLD response amplitudes were normally distributed and were compared across groups with a GLM with least squares means. Multivariable-adjusted GLMs with least-squares means were used to determine whether group status was an independent predictor of BOLD response amplitudes, controlling for age (as a linear variable) and hypertension. In these models, BOLD response amplitude was the dependent (outcome) variable, with group status, age, and history of hypertension as covariates. In this small study, data were complete for all covariates. Within-group pairwise comparisons with 95% confidence intervals (CIs) were calculated using least-squares means, with the Tukey method used to control for multiple comparisons. Associations of group with VEP amplitude and latency were tested using GLMs adjusted for the same covariates. WMH volumes had right-skewed distributions and were log transformed when used as a dependent variable in regression models. To determine whether BOLD response amplitude was associated with WMH volume, we first tested for an interaction of BOLD response with group in a model also adjusted for age and hypertension. Because the interaction p value was significant, indicating that the association of BOLD response with WMH volume varied by group, we then created separate models of WMH volume for each group. Statistical analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, NC). For all statistical tests, a value of p < 0.05 was considered to be significant.
Standard protocol approvals, registrations, and patient consents
Participants provided written informed consent to participate in the study, which was approved by the Institutional Review boards of the University of Calgary.
Data availability
Anonymized data will be made available to other qualified researchers on request to the corresponding author.
Results
The clinical characteristics of each group are outlined in table 1. Participants with CAA were older and more likely to have a history of hypertension. However, there were similar distributions of female sex, coronary artery disease, atrial fibrillation, hypercholesterolemia, diabetes mellitus, and smoking history. Patients with CAA presented with ICH (18), transient focal neurologic episodes (16), or cognitive symptoms without dementia (6). The median microbleed count in the participants with CAA was 22, and 15 of 40 (38%) had cSS. Among the participants not in the CAA group, 16.4% (3 of 21 with AD, 5 of 27 with MCI, and 4 of 25 controls) had microbleeds not in a CAA pattern; none had cSS.
Table 1.
Study population characteristics
Mean BOLD response amplitudes for participants with CAA, AD, and MCI and healthy controls are displayed graphically in figure 1. An unadjusted GLM revealed that BOLD response amplitudes in the 200 most active voxels differed significantly across the 4 groups (p = 0.006). BOLD response amplitudes were 29% lower in participants with CAA compared to healthy controls (mean 1.88% [95% CI 1.62%–2.13%] vs 2.64% [95% CI 2.31%–2.97%], p = 0.003). BOLD response amplitudes in participants with MCI or AD did not differ from that in healthy controls.
Figure 1. BOLD signal change across all groups.

Blood oxygen level–dependent (BOLD) signal change in the 200 most active voxels in response to a visual stimulus was 29% lower in participants with cerebral amyloid angiopathy (CAA) compared to healthy controls after adjustment for age and hypertension (p = 0.01). There were no differences between participants with mild cognitive impairment (MCI) (p = 0.17) or Alzheimer disease (AD) (p = 0.39) and healthy controls. Error bars represent SD.
In a multivariable analysis adjusted for the effects of age and hypertension (table 2), BOLD response amplitudes remained significantly different across the 4 groups. After adjustment for multiple comparisons, the BOLD response amplitudes in patients with CAA remained 29% lower than in healthy controls (mean 1.88% [95% CI 1.60%–2.15%] vs 2.65% [95% CI 2.29%–3.00%], p = 0.01).
Table 2.
GLM comparing BOLD response amplitudes across groups adjusted for age and hypertension

The BOLD response amplitudes did not differ between participants with MCI and controls or those with AD and controls when adjusted for age and hypertension (table 2).
Voxel-wise group contrast analysis showed that the area of fMRI activation was significantly smaller in the primary visual cortex of each participant group compared to the healthy control group, cluster corrected for multiple comparisons (figure 2). The greatest volume of impaired visual cortex activation compared to healthy controls was in participants with CAA, followed by those with MCI and then those with AD.
Figure 2. BOLD activation across all groups.

Voxel-wise comparisons of blood oxygen level–dependent (BOLD) activation between patients with (A) cerebral amyloid angiopathy (CAA), (B) Alzheimer disease (AD), or (C) mild cognitive impairment (MCI) compared to healthy controls. The greatest volume of impaired visual cortex activation compared to healthy controls was in CAA, followed by AD and then MCI. Models were adjusted for age and hypertension. Significance was determined by a z statistic >2.3 (p < 0.05) and was cluster corrected for multiple comparisons.
The mean amplitude of the P100 potentials did not differ across all groups, but the mean latency did (table 1). However, after adjustment for age and hypertension, there were no group differences for either the amplitude (p = 0.48) or latency (p = 0.48) of P100 potentials. Higher age was associated with lower P100 amplitude (−0.093 per additional 1 year of age [95% CI −0.180 to −0.006]) and longer P100 latency (0.34 per additional year of age [95% CI 0.14–0.55]).
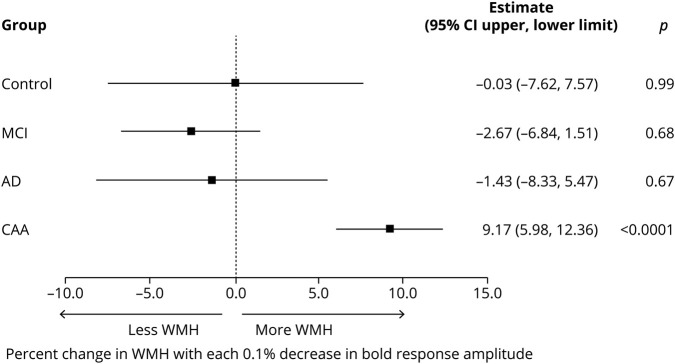
WMH volumes differed between groups (table 1). Post hoc analyses adjusted for age, hypertension, and multiple comparisons showed that WMH volumes were greater in participants with CAA compared to healthy controls (p < 0.0001), MCI (p < 0.0001), or AD (p = 0.02) and in participants with AD compared healthy controls (p = 0.03), but not in participants with MCI compared to either healthy controls (p = 0.68) or AD (p = 0.31). The association of BOLD response amplitude with WMH volume varied by group (figure 3). Lower BOLD amplitude response was associated with higher WMH volume only in participants with CAA; for each 0.1% lower BOLD response amplitude, the predicted WMH volume was 9.2% higher (95% CI 6.0%–12.4%). BOLD amplitude responses were not associated with WMH volume in participants with AD, those with MCI, or controls (figure 3).
Figure 3. Association of visual BOLD response amplitude with WMH volume.
Estimated percent increase in white matter hyperintensities (WMH) per 0.1% decrease in visual blood oxygen level–dependent (BOLD) response is graphed for each group. The association between visual BOLD response amplitude and WMH volume varied by group (interaction p = 0.002). AD = Alzheimer disease; CAA = cerebral amyloid angiopathy; CI = confidence interval; MCI = mild cognitive impairment.
Discussion
BOLD amplitude responses to a visual stimulus were lower in participants with CAA compared to controls but were not different when those with AD or MCI were compared to controls. In contrast, despite the reduced BOLD responses, the VEP amplitude did not differ between participants with CAA and controls. The preserved VEP amplitudes in CAA suggest that occipital metabolic activity is preserved, which implies that the reduced BOLD responses are due to lower blood flow from impaired CVR. Therefore, our study suggests that CVR in response to a visual stimulus may be impaired in CAA but not in AD. The association of WMH volume with BOLD response amplitude differed by group. Only in CAA was lower BOLD response amplitudes associated with higher WMH volume, suggesting that in CAA impaired CVR may be one of the mechanisms that is associated with white matter ischemia.
Our findings in CAA are consistent with previous studies that have implicated reduced BOLD response amplitudes to a visual task in sporadic CAA5,6 and a hereditary form of CAA.37 Participants with CAA had the largest volume of reduced primary visual cortex activation compared to controls (figure 2). Our previous study of 18 participants with CAA showed decreased BOLD response amplitudes to a visual stimulus despite normal neuronal function as measured by VEP6; the current study, adding 22 participants with CAA, showed consistent results in a larger sample size.
Prior literature on the presence of impaired CVR in AD has been somewhat inconsistent. A systematic review of transcranial Doppler (TCD) ultrasound studies found altered cerebral hemodynamics in individuals with AD compared to healthy controls.38 However, 2 studies using MRI with carbon dioxide inhalation as a vasodilatory stimulus gave conflicting results, with 1 study revealing no difference in whole-brain CVR between patients with AD and healthy controls39 and the other study finding slower BOLD time to peak with preserved amplitudes in individuals with AD compared to healthy controls.40 A limitation of all of these studies of AD is that none of them considered the degree to which comorbid CAA was present (e.g., by identifying CAA-related microbleeds, which are present in up to 20%–30% of patients with AD)41 and how it could affect the results. Our study compared CVR in patients with CAA to patients with AD without CAA-related microbleeds, and we found that CVR in AD did not differ from normal (even though reduction in resting perfusion in temporal-parietal regions is well recognized in AD and is felt to be due to atrophy with reduced metabolism).42 This suggests that CVR is more impaired in CAA. Prior studies that detected lower CVR in patients with AD may have instead been detecting changes related to comorbid CAA.
Results in participants with MCI were similar to those in individuals with AD in that visual BOLD did not differ from that of controls. There are few studies of CVR in patients with MCI. A recent study using TCD observed impaired CVR in the middle cerebral artery of participants with amnestic MCI who progressed to AD within 24 months of follow-up compared to those who did not progress.43 However, another study comparing controls to those with MCI using TCD did not show impairment of CVR.44 Similar to the AD studies, neither used MRI to see whether CAA-related microbleeds were present.
Our study showed that WMH volumes were associated with group status: they were highest in CAA, followed by AD, compared to healthy controls, which is consistent with previous studies.45 Higher WMH volumes were associated with lower BOLD response amplitudes only in patients with CAA. This suggests that impaired CVR leading to ischemia may cause WMH in CAA, a vascular disease, whereas other mechanisms (such as inflammation, altered blood brain permeability, or microembolic disease) may be at play in AD, MCI, and healthy brains.
In this study, we targeted activation of the occipital lobe by visual stimulation because CAA preferentially affects posterior brain regions. However, a drawback of this approach is that one can only investigate brain regions that are strongly activated by tasks. Future studies should investigate whether CVR can be measured using a global stimulus such as carbon dioxide inhalation, which dilates arteries through the entire brain. This would allow investigation of regional associations between impaired vascular reactivity and vascular lesions (not limited to WMH but also including microbleeds, cSS, microinfarcts, and large perivascular spaces) in finer detail at higher spatial resolution.
The primary limitation of our study is the small sample size of participants with AD. We used clinical criteria to define AD, without using amyloid-PET or CSF β-amyloid biomarkers because these markers are also altered by CAA.46 Finally, although amyloid-PET could have been useful to determine whether the amount of amyloid deposition correlated with CVR, it was not used in this study.
Our finding that BOLD amplitude in response to a visual stimulus was lower in those with CAA compared to healthy controls but not in participants with AD (without evidence of CAA) compared to controls suggests that decreased occipital lobe CVR may be a feature that is unique to CAA. This finding could be exploited in the future to help distinguish the effects of CAA from AD because they usually coexist to some degree and current β-amyloid markers (CSF β-amyloid and amyloid-PET) do not distinguish well between them. The amount of decreased occipital CVR may serve as an index of the amount of CAA in persons with AD, but this requires pathologic validation. Decreased BOLD amplitude may also be a useful marker for clinical trials in CAA and indeed has already been used for this purpose.47 As a clinical trial marker, BOLD response has several potential advantages over other markers such as ICH or WMH that reflect mostly irreversible structural damage. Abnormal BOLD response is present at an early stage of disease preceding major hemorrhages (being seen in presymptomatic carriers of CAA-causing mutations37); it declines over time, and this decline is detectable over a 1-year time period even in a relatively small sample8; and it is plausible that it could be improved or reversed by interventions that restore more normal vascular function.
Glossary
- AD
Alzheimer disease
- BOLD
blood oxygen level–dependent
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- cSS
cortical superficial siderosis
- CVR
cerebrovascular reactivity
- FEAT
fMRI Expert Analysis Tool
- FLAIR
fluid-attenuated inversion recovery
- GLM
general linear model
- ICH
intracerebral hemorrhage
- MCI
mild cognitive impairment
- MR
magnetic resonance
- TCD
transcranial Doppler
- VEP
visual evoked potential
- WMH
white matter hyperintensities
Appendix. Authors


Footnotes
Editorial, page 415
Study funding
Supported by the Canadian Institutes of Health Research, Brain Canada, Canadian Stroke Network, Heart and Stroke Foundation of Canada, and Alzheimer Society of Canada.
Disclosure
A. Switzer, I. Cheema, C. McCreary, A. Zwiers, A. Charlton, A. Alvarez-Veronesi, C. Zerna, and R. Stafford report no disclosures relevant to the manuscript. R. Frayne reports grant funding from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. B. Goodyear reports no disclosures relevant to the manuscript. E. Smith reports grant funding from the Canadian Institutes of Health Research and Brain Canada; is an assistant editor for Stroke and a member of the Editorial Board for Neurology and the Journal of the American Heart Association; has received consulting fees from Portola and Alnylam Pharmaceuticals; and has received royalties from Up to Date. Go to Neurology.org/N for full disclosures.
References
- 1.Weller R, Boche D, Nicoll JR. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol 2009;118:87–102. [DOI] [PubMed] [Google Scholar]
- 2.Cupino T, Zabel M. Alzheimer's silent partner: cerebral amyloid angiopathy. Transl Stroke Res 2013;5:330–337. [DOI] [PubMed] [Google Scholar]
- 3.Shin HK, Jones PB, Garcia-Alloza M, et al. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain 2007;130:2310–2319. [DOI] [PubMed] [Google Scholar]
- 4.Park L, Koizumi K, El Jamal S, et al. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 2014;45:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol 2012;72:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peca S, McCreary CR, Donaldson E, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiography. Neurology 2013;81:1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RJ, Goodyear BG, Peca S, et al. Identification of neurovascular changes associated with cerebral amyloid angiopathy from subject-specific hemodynamic response functions. J Cereb Blood Flow Metab 2017;37:3433–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Switzer AR, McCreary C, Batool S, et al. Longitudinal decrease in blood oxygenation level dependent response in cerebral amyloid angiopathy. NeuroImage Clin 2016;11:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bär K-J, Boettger MK, Seidler N, Mentzel HJ, Terborg C, Sauer H. Influence of galantamine on vasomotor reactivity in Alzheimer's disease and vascular dementia due to cerebral microangiopathy. Stroke 2007;38:3186–3192. [DOI] [PubMed] [Google Scholar]
- 10.Cantin S, Villien M, Moreaud O, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. NeuroImage 2011;58:579–587. [DOI] [PubMed] [Google Scholar]
- 11.Hajjar I, Sorond F, Lipsitz LA. Apolipoprotein E, carbon dioxide vasoreactivity, and cognition in older adults: effect of hypertension. J Am Geriatr Soc 2015;63:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagust WJ, Eberling JL, Reed BR, Mathis CA, Budinger TF. Clinical studies of cerebral blood flow in Alzheimer's disease. Ann NY Acad Sci 1997;826:254–262. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi F, Meyer JS, Yamamoto M, Sakai F, Shaw T. Noninvasive regional cerebral blood flow measurements in dementia. Arch Neurol 1980;37:410–418. [DOI] [PubMed] [Google Scholar]
- 14.Small SA, Perera GM, DeLapaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol 2001;45:466–472. [DOI] [PubMed] [Google Scholar]
- 15.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 17.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Case NF, Charlton A, Zwiers A, et al. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke 2016;47:2010–2016. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 23.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkinson M, Bannister P, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 26.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 2001;14:1370–1386. [DOI] [PubMed] [Google Scholar]
- 27.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, eds. Functional MRI: An Introduction to Methods. Oxford: Oxford University Press, 2001. [Google Scholar]
- 28.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using bayesian inference. NeuroImage 2004;21:1732–1747. [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosior JC, Idris S, Dowlatshahi D, et al. Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke 2011;6:302–305. [DOI] [PubMed] [Google Scholar]
- 32.Smith EE, O'Donnell M, Dagenais G, et al. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol 2015;77:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Clinical Neurophysiology Society. Guideline 9B: guidelines on visual evoked potentials. J Clin Neurophysiol 2006;23:138–156. [DOI] [PubMed] [Google Scholar]
- 34.Bonmassar GA, Anami K, Ives J, Belliveau JW. Visual evoked potentials (VEP) measured by simultaneous 64-channel EEG and 3T fMRI. Neuroreport 1999;10:1893–1897. [DOI] [PubMed] [Google Scholar]
- 35.Zaletel MS, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity responses. Funct Neurol 2005;20:115–120. [PubMed] [Google Scholar]
- 36.Walsh P, Kane N, Butler S. The clinical role of evoked potentials. J Neurol Neurosurg Psychiatry 2005;76:ii16–ii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Opstal AM, van Rooden S, van Harten T, et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: a case-control study. Lancet Neurol 2017;16:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabayan B, Jansen S, Oleksik AM, et al. Cerebrovascular hemodynamics in Alzheimer's disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev 2012;11:271–277. [DOI] [PubMed] [Google Scholar]
- 39.Lajoie I, Nugent S, Debacker C, et al. Application of calibrated fMRI in Alzheimer's disease. NeuroImage Clin 2017;15:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richiardi J, Monsch AU, Haas T, et al. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2015;36:33–41. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Ramirez S, Greenberg SM, Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimers Res Ther 2014;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000;47:93–100. [PubMed] [Google Scholar]
- 43.Lim E-Y, Yang D-W, Cho AH, Shim YS. Cerebrovascular hemodynamics on transcranial Doppler ultrasonography and cognitive decline in mild cognitive impairment. J Alzheimers Dis 2018;65:651–657. [DOI] [PubMed] [Google Scholar]
- 44.Shim Y, Yoon B, Shim DS, Kim W, An JY, Yang DW. Cognitive correlates of cerebral vasoreactivity on transcranial Doppler in older adults. J Stroke Cerebrovasc Dis 2015;24:1262–1269. [DOI] [PubMed] [Google Scholar]
- 45.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 2008;39:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leurent C, Goodman JA, Zhang Y, et al. Immunotherapy with ponezumab for probable cerebral amyloid angiopathy. Ann Clin Transl Neurol 2019;6:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be made available to other qualified researchers on request to the corresponding author.