Abstract
Objective
Surgery is an effective but costly treatment for many patients with drug-resistant temporal lobe epilepsy (DR-TLE). We aim to evaluate whether, in the United States, surgery is cost-effective compared to medical management for patients deemed surgical candidates and whether surgical evaluation is cost-effective for patients with DR-TLE in general.
Methods
We use a semi-Markov model to assess the cost-effectiveness of surgery and surgical evaluation over a lifetime horizon. We use second-order Monte Carlo simulations to conduct probabilistic sensitivity analyses to estimate variation in model output. We adopt both health care and societal perspectives, including direct health care costs (e.g., surgery, antiepileptic drugs) and indirect costs (e.g., lost earnings by patients and care providers.) We compare the incremental cost-effectiveness ratio to societal willingness to pay (∼$100,000 per quality-adjusted life-year [QALY]) to determine whether surgery is cost-effective.
Results
Epilepsy surgery is cost-effective compared to medical management in surgically eligible patients by virtue of being cost-saving ($328,000 vs $423,000) and more effective (16.6 vs 13.6 QALY) than medical management in the long run. Surgical evaluation is cost-effective in patients with DR-TLE even if the probability of being deemed a surgical candidate is only 5%. From a societal perspective, surgery becomes cost-effective within 3 years, and 89% of simulations favor surgery over the lifetime horizon.
Conclusion
For surgically eligible patients with DR-TLE, surgery is cost-effective. For patients with DR-TLE in general, referral for surgical evaluation (and possible subsequent surgery) is cost-effective. Patients with DR-TLE should be referred for surgical evaluation without hesitation on cost-effectiveness grounds.
The clinical effectiveness of surgery in well-selected patients with drug-resistant temporal lobe epilepsy (DR-TLE) has been well established by at least 2 large randomized controlled trials,1,2 a major systematic review by the American Academy of Neurology,3 and a model-based comparative effectiveness analysis.4 In addition, it has been suggested that successful surgery may reduce some of the costs associated with epilepsy, especially in the long run.5 While surgery may be cost-saving for individual patients who achieve seizure freedom after surgery, it has yet to be established that evaluation for surgery is cost-effective for an entire cohort of patients with DR-TLE given that a meaningful proportion of patients will not be offered surgery and a substantial subset of those who receive surgery will not achieve seizure remission. Consequently, practitioners may be hesitant to refer patients for evaluation in light of the substantial upfront cost associated with presurgical testing and the lack of guarantee that the patient will be found eligible for surgery. Early investigation into the cost-effectiveness of epilepsy surgery in the United States was conducted in the 1990s,6 but there were important limitations. Data were not available to model long-term outcomes after surgery, necessitating use of expert opinions, and societal costs of the disease were not included. More recent investigations have been conducted in countries with nationalized health care systems,7,8 but the cost-analyses are of limited utility in the US health care context.
The objectives of this study are to evaluate the cost-effectiveness of surgery for patients deemed surgical candidates and to further evaluate whether referral for surgical evaluation is cost-effective for patients who may be potential surgical candidates. The target population is adult patients with DR-TLE, and the definitive interventions being compared are surgical resection and continued medical management. We use a model-based analysis with a lifetime time horizon, from both health care and societal perspectives.
When comparing 2 treatment strategies for a disease, we may determine that a given strategy is cost-effective if it meets 1 of the following criteria: (1) the strategy is more effective than the alternative and reduces costs, or (2) the strategy is more effective than the alternative, at a higher cost, which is deemed reasonable. To adjudicate whether the higher cost associated with a treatment strategy is reasonable, it is possible to compare the incremental cost-effectiveness ratio (ICER) for the strategy to the societal willingness to pay (WTP), both of which are expressed as dollars per additional quality-adjusted life year (QALY). A single QALY represents a year of life in perfect health. The ascribing of specific QALY values to individual health states (e.g., having uncontrolled seizures) is based on patient surveys in which patients are asked to assign utility scores to different states of disease/health. While there is no universal figure for societal WTP, a range should be considered.9 In the United States, figures between $50,000 and $150,000 per QALY are reasonable.10,11
Methods
Patient population
Our analyses are based on meta-analysis of existing literature concerning adult patients with DR-TLE. Parameter estimates are derived from pooled estimates from multiple component studies with some heterogeneity in the patient characteristics between studies; the results of our model concern adult patients diagnosed with temporal lobe epilepsy who continue to have seizures despite adequate trials of ≥2 appropriately selected antiseizure medications (ASM) (i.e., drug resistant). Due to the nature of the component studies on this topic, the model is optimally informative for patients with lesional disease receiving anterior temporal lobectomies. There is variation in the average age of adult patients included in the epilepsy surgery literature from which parameter estimates were extracted for the present study, but a base-case assumption of 35 years (SD 11 years) is reasonable.4
Analytic model
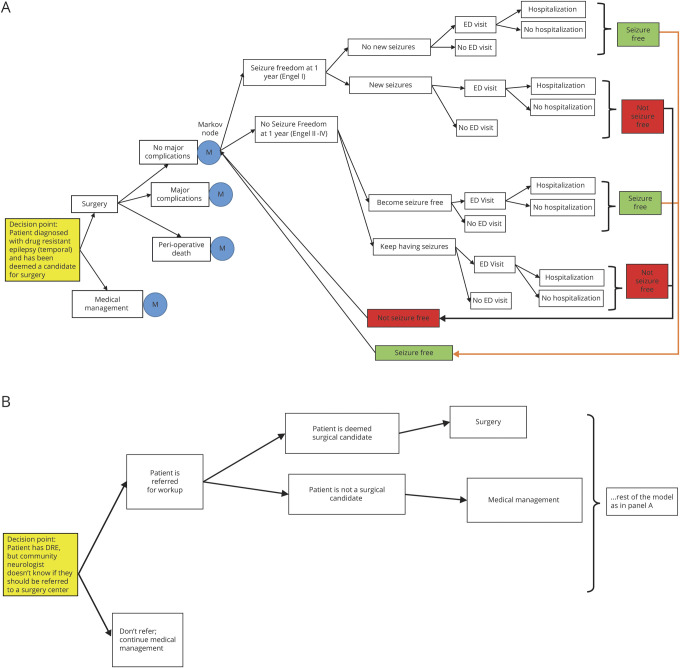
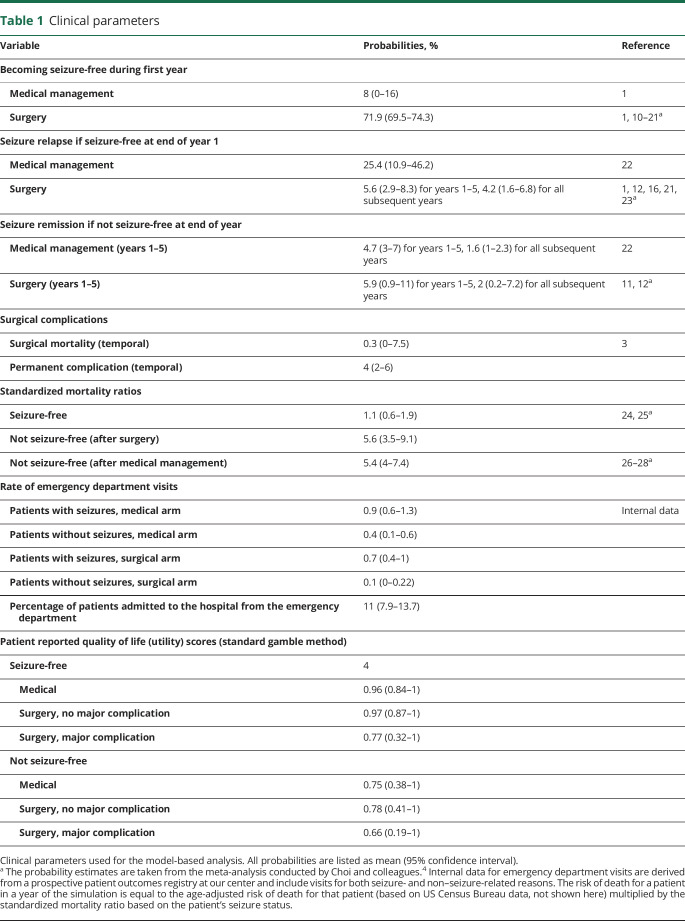
A model-based analysis was performed using a Markov decision-analytic model. For the primary analysis (model 1), we aimed to determine whether surgery was cost-effective compared to continued medical management in patients who had been deemed surgical candidates; thus, the baseline assumption was that a patient with DR-TLE had undergone surgical evaluation and had been deemed a candidate for surgery (figure 1A). For the secondary analysis (model 2), we aimed to determine whether it was cost-effective to evaluate patients with DR-TLE for surgery; thus, the baseline assumption was simply that a patient had been diagnosed with DR-TLE (figure 1B).
Figure 1. Diagram of Markov decision-analytic model used for primary analysis (model 1).
Illustration of the primary and secondary models developed to study cost-effectiveness. (A) Diagram shows the structure of the decision-analytic model used for cost-effectiveness analysis in patients with DR-TLE deemed to be surgical candidates. The M nodes represent Markov nodes that are built into the decision tree, allowing the creation of iterative loops (1-year cycles) during which patients can transition between health states. The 3 health states are seizure-free, having seizures, and dead (not shown). Each branch of the tree is annotated with an average estimate and a probability distribution, allowing probabilistic sensitivity analysis (Monte Carlo simulations). (B) Diagram of the decision-analytic model used for secondary analysis (model 2). The decision-analytic model was restructured as shown in this diagram in order to conduct the secondary analysis (evaluating whether referral for surgical evaluation is a cost-effective strategy). DRE = drug-resistant epilepsy; ED = emergency department.
The model simulates several possible courses of treatment for the patients of interest. The patient could either receive surgery or continue medical management. If surgery is chosen, the patient could have either a permanent major complication (including hemiparesis or disability after surgery), perioperative death, or no major complication. After allocation into 1 of these 3 major categories, patients fall into 1 of 2 prespecified health states (free or not free of consciousness-impairing seizures) for a series of 1-year cycles until death. Patients could transition between these health states from year to year (Markov modeling). Patients could also die during each yearly cycle, with a risk of death calculated from the age-adjusted baseline mortality rate and their seizure status. On the basis of their course in the model, patients would also incur costs associated with their care, including 1-time fixed costs such as surgery, as well as iterative yearly costs such as annual outpatient visits. Each branch of the model is annotated with an estimated probability derived from the literature. The reportable outcomes from our model include life expectancy, quality-adjusted life expectancy, total costs, and ICER.
The outcomes from our model are based on parameter estimates; thus, it is important to quantify the variability in model predictions on the basis of these estimates. We conducted 1-way sensitivity analysis on all variables in the model. Furthermore, we used the Monte Carlo simulation method to conduct probabilistic sensitivity analysis, which allows us to quantify the variability in the model output produced by simultaneous variation in all model variables. To conduct this type of sensitivity analysis, we used a probability distribution (mean and 95% confidence interval) for each estimated probability in the model. We then simulated cohorts of 10,000 patients (i.e., 10,000 second-order Monte Carlo simulations). The reportable outcomes of this sensitivity analysis include acceptability curves, incremental cost-effectiveness scatters, and proportion of simulations favoring surgery.
Data
Clinical parameters
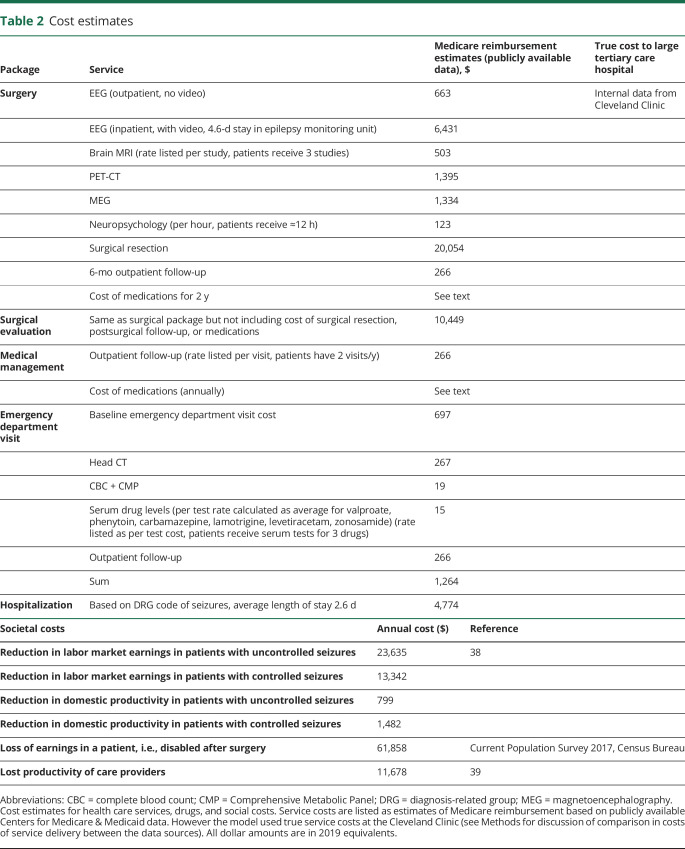
Clinical parameters used in the model are shown in table 1. A strong comparative effectiveness analysis on this topic has been published,4 and clinical parameters have been derived using extensive literature review and meta-analytic methods (random-effects model); we were able to use many of their clinical parameters for our model.1,12–30 Thus, the parameter estimates in our model represent the pooled and appropriately weighted averages of several component trials and case series. We were not aware of previously published reports on the frequency of emergency department visits and hospitalizations in this patient population and thus analyzed use data from the Cleveland Clinic for these parameters. The normal distribution was used to model clinical parameter estimates, except when doing so produced implausible probability estimates (i.e., probability <0% or >100%). In the latter cases, the beta distribution was used.
Table 1.
Clinical parameters
Mortality tables
We used the latest mortality tables available from the US Census Bureau. The risk of mortality for a patient in our model in a given year is equal to the age-adjusted risk of death multiplied by a standardized mortality ratio, which is determined by the patient's seizure status (table 1).
Preference-based utility (quality of life) scores
We use previously reported preference-based utility scores from patients surveyed at the Columbia University Medical Center using the standard gamble approach4 (table 1). Estimates were modeled with uniform distributions.
Health care costs
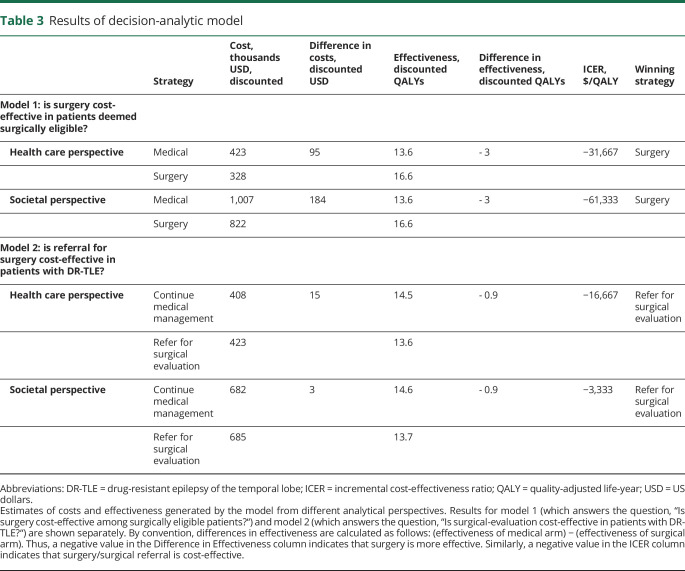
We used a bottom-up costing strategy to estimate costs of care whereby costs were modeled by creating standardized packages of care including all the component health care services that patients typically receive in their course. We developed care packages for surgical evaluation, surgery, medical management, emergency department visits, and hospitalization. The individual services included in each package are shown in table 2. There is poor standardization in cost-effectiveness literature for the question of which type of costs (hospital costs vs public payer reimbursement vs charges) should be used for analysis in the United States. Panel guidelines support the general recommendation of using costs that reflect the monetary cost to society, but a universal methodologic approach is not apparent.9 We recognize the merits of using public payer reimbursements, so we have included estimates based on Centers for Medicare & Medicaid data in table 2. These estimates were generated with the method described by Flowers et al.31 There are important limitations to using these data in the present study. First, only a subset of patients with epilepsy are Medicare recipients. Second, when we compared Medicare reimbursements to the true costs of services at our center, there were clear disparities. This observation is in line with the well-documented phenomenon that Medicare reimbursement often covers only a fraction of the cost of providing services at hospitals. For the model analysis, we used internal patient-level costs for each service provided. While these service costs cannot be published for proprietary reasons, they carry significant benefits over using publicly available reimbursement estimates. First, they are payer insensitive and represent the real cost to the hospital of providing a given service, as opposed to the charges assessed to a payer or the postnegotiation reimbursement. Second, this method allows a robust estimation of costs for a given service that does not vary with peculiarities of coding and billing methods across individual centers. Finally, the costs of services used in the model are considerably higher than the Centers for Medicare & Medicaid estimated reimbursements. Thus, our choice of using internal costs favors the medical treatment arm in the analysis. Costs are modeled with gamma distributions.
Table 2.
Cost estimates
Drug costs
There is no standard drug therapy for epilepsy, and patients with drug-resistant epilepsy are placed on an extremely wide range of drug regimens, which significantly complicates the task of modeling baseline drug costs. To overcome this issue, we manually reviewed electronic medical records of 100 randomly selected drug-resistant patients who received care at our center in the last decade (Appendix figure 1A, available in Dryad, doi.org/10.5061/dryad.kprr4xh1m). We use drug costs from the Federal Supply Schedule with appropriate adjustment (1.21 adjustment factor), as recommended by the Health Economics Resource Center.32 These amounts represent the cost of drugs to the health care system, not out-of-pocket costs to patients (which are highly variable). Analysis of drug costs of the medical regimens from our patient sample showed a significantly right-skewed distribution with a mean value of $21,772/y and SD of $19,144/y (Appendix figure 1B).
We recognize that the question of when to discontinue medications in seizure-free surgical patients is an ongoing area of debate, and no broadly adopted consensus guidelines are yet available.33–35 Previously published estimates of ASM use after epilepsy surgery show that at least 62% of patients have some kind of postoperative reduction in ASM use, and ≈21% have discontinuation.36 In our model, patients who are seizure-free after surgery continue to take medications for 2 years after surgery, after which they are transitioned to monotherapy (levetiracetam 1,000 mg) as long as they remain seizure-free. To ensure that the analysis was robust to variation at this point, we modeled probability of having to continue medications in seizure-free surgical patients as a variable with a wide range (0.2–0.8) and conducted 1-way sensitivity analysis on it (the model recommendations were not sensitive to variation within this range).
Societal costs
Following recommendations, we include a societal reference case including both health care costs and non–health care costs.9 This is appropriate because previous studies have suggested that the bulk of costs associated with epilepsy are indirect costs.37 We include 4 major societal costs in our analyses: reduction in labor market earnings in patients with controlled and uncontrolled seizures, reduction in domestic productivity in patients with controlled and uncontrolled seizures, lost productivity of care providers, and lost earnings of patients disabled after a permanent complication associated with surgery (table 2). Data for the first 3 of these categories are derived from the existing literature and are based on actuarial methods for estimating lost earnings attributable to epilepsy.38,39 The estimate of lost earnings in patients disabled after surgery is derived from the Current Population Survey (2017) assuming that patients were earning the median annual income before surgery. All patients are assumed to retire at the age of 65 years.
All cost data are adjusted to 2019 dollar equivalents using the Consumer Price Index for health care from the Bureau of Labor Statistics. We use a standard half-cycle correction for our Markov process. We modeled global discount rate as a variable with a normal distribution (with a mean of 3%) and included this variable in sensitivity analyses.
Data availability
As detailed above, we have used data from published literature to populate the decision-analytic model; the parameter estimates can thus be replicated by referring to the cited literature.
Results
Model validation
While there is no firm consensus on how to assess the validity of cost-effectiveness models,40 panel guidelines recommend internal validation (i.e., the model is responsive to chosen parameters) and external validation (i.e., model predictions are comparable to outside observed data that were not used in model construction).9
Internal validation
We compared the life expectancy and quality-adjusted life expectancy estimates generated by our model to published estimates4 that used similar clinical parameters. Our results are very similar, suggesting that our model is responsive to the parameters we have chosen (Appendix figure 2, A and B, doi.org/10.5061/dryad.kprr4xh1m).
External validation
We compared the survival curves of a simulated cohort in our model with the observed survival curves in a longitudinal study.41 Comparison shows that the modeled survival curves are virtually identical to observed survival curves in the surgical arm (Appendix figure 3, A and B, doi.org/10.5061/dryad.kprr4xh1m). In the medical arm, however, the survival curves from our model overstate seizure-free survival associated with medical management. This is apparent from the modeled survival curve of medically managed patients wherein the proportion of seizure free patients in the cohort actually increases over the first 8 years of the simulation before decreasing toward zero (Appendix figure 3B). In reality, the proportion of seizure-free patients decreases as early as the second year of observation.41 For further verification, we compared our model-generated 5-year and 10-year seizure-free survival estimates after surgery with those in the published literature. Our model estimates 62% and 53% seizure-free survival at 5 and 10 years, respectively. These estimates are well within the ranges expected from the published literature: 5-year seizure-free survival between 50% and 75% and 10-year seizure-free survival between 41% and 66%.42
Primary analysis
Model results
The results of the cost-effectiveness analysis for both models are tabulated in table 3. In interpreting these data, it is important to underscore that the 2 models are informative for 2 conceptually distinct questions. While model 1 answers the question of whether surgery is cost-effective in patients who have already been found to be surgical candidates after extensive evaluation, model 2 answers the question of whether referral for surgical evaluation (with the possibility of subsequent surgery) is cost-effective among patients with a diagnosis of DR-TLE. These data suggest that, among patients who have already been found to be eligible for surgery, surgery is the cost-effective strategy from both the health care and societal perspectives. Among patients diagnosed with DR-TLE who may or may not be surgically eligible, referral for surgical evaluation is the cost-effective strategy from both the health care and societal perspectives. In fact, over the lifetime time horizon for the simulated cohort, these strategies actually become cost-saving in addition to being more clinically effective.
Table 3.
Results of decision-analytic model
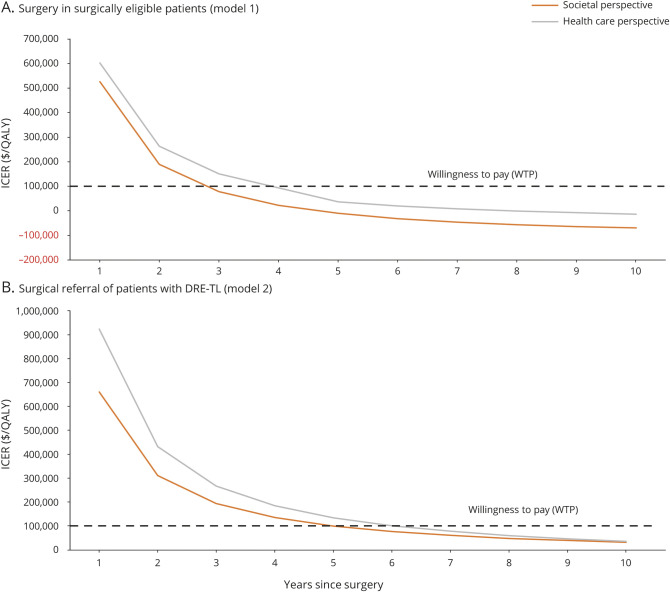
Figure 2 shows how the ICER changes over time for both models. This method of data representation allows us to answer the question of how long a given strategy would have to be adapted before it became cost-effective for the simulated cohort. For patients who are surgically eligible, surgery becomes cost effective within 3 to 4 years (depending on whether we consider the societal or health care perspective.) For patients diagnosed with DR-TLE, referral for surgical evaluation becomes a cost-effective strategy for the cohort within 5 to 7 years. Notably, both these strategies become cost-effective more rapidly when we adopt a societal perspective, which is consistent with the significant societal costs associated with uncontrolled seizures.
Figure 2. ICER vs time plot for surgery and referral for surgery.
(A) Curves show how the incremental cost-effectiveness ratio (ICER) varies with time from surgery in patients found eligible for surgery. (B) Curves show how the ICER varies with time from surgical referral (and possible subsequent surgery) in patients with drug-resistance temporal lobe epilepsy (DR-TLE). Results of the analysis from the societal (orange) and health care (gray) perspective are shown. Negative values of ICER occur when surgery becomes cost-saving compared to medical management. QALY = quality-adjusted life-year.
Probabilistic sensitivity analysis
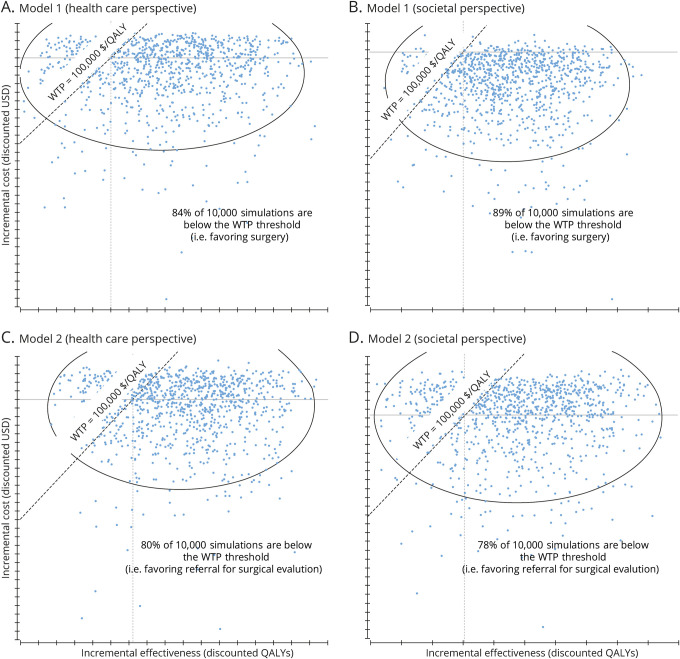
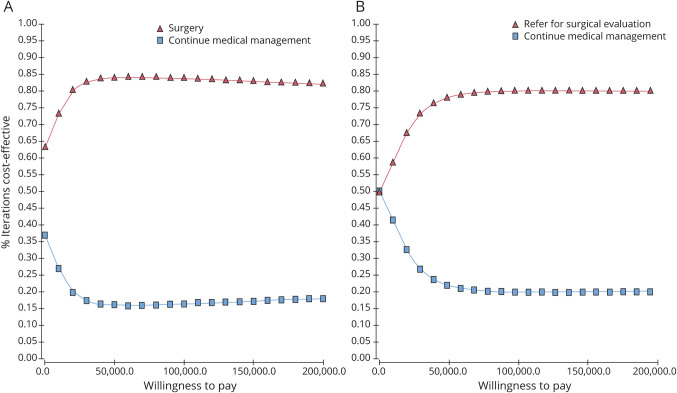
Figure 3 shows the incremental cost-effectiveness scatters produced when 10,000 second-order Monte Carlo simulations of the models were generated from societal and health care perspectives. Each dot in the scatter represents a separate simulation; whenever a dot falls to the right of (i.e., below) the WTP line, the model recommends surgery. When the simulations are run until cohort death (i.e., lifetime time horizon), 84% of simulations recommend surgery among surgically eligible patients from the health care perspective, and 89% of simulations recommend surgery from the societal perspective. Among patients with DR-TLE who have not yet received surgical evaluation, 80% of simulations favored referral from the health care perspective, and 78% favored referral from the societal perspective. Figure 4 shows acceptability curves that demonstrate variation in the proportion of simulations that recommend surgery at different thresholds of WTP. These data demonstrate that surgery and referral for surgical evaluation would be cost-effective strategies across a wide range of WTP thresholds.
Figure 3. Results of probabilistic sensitivity analysis.
Scatterplots (A–D) show the results of 10,000 Monte Carlo simulations (probabilistic sensitivity analysis) of the decision-analytic models. Each blue dot represents the results of an individual simulation. Whenever a dot falls to the right of (below) the willingness to pay (WTP) line, the model would find surgery (model 1) or referral for surgical evaluation (model 2) to be cost-effective. QALY = quality-adjusted life-year; USD = US dollars.
Figure 4. Acceptability curves for surgery and referral for surgery strategies.
Acceptability curves are generated by running a set of 10,000 simulations for a range of willingness to pay (WTP) and charting the proportion of simulations at each threshold that favor a surgical or medical strategy. For any 1 value of WTP on the horizontal axis, the proportion of simulation recommending surgery and medical management adds up to 1. (A) Acceptability curves for surgery in surgically eligible patients. (B) Acceptability curves for surgical evaluation in patients with drug-resistant epilepsy of the temporal lobe in general.
One-way sensitivity analysis
All variables included in the model were subjected to sensitivity analysis. The model was sensitive to 1-way variation for 5 variables. Surgery would not be cost-effective if any of these 5 conditions were met:
The true probability of initial seizure freedom after surgery was <21% (data-derived estimate used in model is 71.9%)
The true probability of relapse after initial surgical success was >27% (data-derived estimate used in model is 5.6%)
The patient's age was >97 years
The cost of surgery was >5 times our estimated cost
The true proportion of patients who were found to be eligible for surgery after initial surgical referral was <5% (at our center, the proportion of referred patients who are found to be surgically eligible is between 20% and 40%)
Notably, the sensitivity analysis did not show that the model recommendation was sensitive to 1-way variation in the probability of having to continue medications in seizure-free surgical patients. Even if only 20% of seizure-free surgical patients were able to discontinue medications (with no other reductions in ASM use in postsurgical patients), the ICER was below societal WTP.
Discussion
Our reference case results suggest that surgery is both cost-saving and more effective in the long run for surgically eligible patients and that referral for surgical evaluation is cost-effective for patients with a diagnosis of DR-TLE. The recommendation for surgery can be made from both the health care perspective and the societal perspective, although it is notable that surgery becomes cost-effective at a faster rate when viewed from a societal perspective. Referral for surgical evaluation is likely to be cost-effective even if there is only a small (≈5%) probability that the patient is a surgical candidate. A key clinical implication of our study is that a strong cost-effectiveness argument favors referral of patients with DR-TLE for surgical evaluation. It is important to recognize that this is the same recommendation made by previously reported decision-analytic tools that made recommendations on the basis of clinical effectiveness alone.43 Taken together, these studies show a concordant recommendation for referral based on both clinical and cost-effectiveness grounds.
Previous work has repeatedly demonstrated that epilepsy surgery is likely underused across the world, including in developed nations,44–46 and this trend has been slow to change despite consensus recommendations and guidelines.47 There are multiple reasons that community practitioners may be hesitant to refer patients for surgical evaluation, including a lack of familiarity with the safety profile of the procedure and the chances of postoperative success and a misunderstanding of the evidence-based definition of drug resistance.48 The vast majority of neurologists (>75%) also cite a lack of health care resources as a major barrier for surgical evaluation.48 It is not uncommon, in our experience, for practitioners to express concern about the potential futility of a costly surgical evaluation for patients with epilepsy who are not offered surgery, expressing regret at the “wasted” health care resources and the inconvenience of extensive workup for the patient. In direct response to this notion, our work suggests that rather than being hesitant to offer surgical evaluation to drug-resistant patients due to substantial upfront cost, we should recognize that a referral-based strategy is the cost-effective one; the upfront cost is absolutely reasonable in patients with even a low chance of being surgical candidates and is likely cost-saving for patients with high probability of being surgically eligible.
Only a small number of cost-effectiveness studies have been conducted on the topic of epilepsy surgery, only 1 of which, to the best of our knowledge, was in the US health care context. Langfitt6 published a study in 1997, far before the majority of outcomes studies in this context had been reported. Langfitt noted at the time that the paucity of data resulted in an inability to model transitions between health states and noted that the use of Markov modeling (as we did here) would allow a better determination of cost-effectiveness in this context. Furthermore, those analyses were limited to a health care perspective, and there was some ambiguity around which health care costs were finally included in the analysis. The result of that analysis was that evaluation for surgery was likely to be both more costly and more effective from the provider perspective. Langfitt noted that “only under the most optimistic assumptions of the model was evaluation associated with long term cost savings.”6 Outside of the US context, we are aware of cost-effectiveness studies for epilepsy surgery from France7 and Canada,8 both of which have nationalized health care systems. It is important to underscore how dramatically different the cost structures are between these earlier studies and the current study (focusing on the US context.) The French study estimated an annual cost of ASM of around $3,400/y, which is ≈88% less than the average cost of drugs estimated in the current study. Similarly, the French study estimated that the total cost of surgery (including full workup, surgical resection, and postoperative care) was around $14,000. This figure would be insufficient to cover even the surgical evaluation in the US context. Nonetheless, it is worthwhile to note that both the French and Canadian studies found that surgery was a cost-effective strategy; the French study found that surgery became cost-effective after 10 years, and the Canadian study reported (in the pediatric context) that surgery had an ICER of $369 per percent reduction in seizures.
We made several assumptions in constructing our model. Perhaps most notably, we assume that resective surgery and continued medical management are the only therapies available for patients with drug-resistant epilepsy. This is not the case; a significant subset of patients may benefit from novel therapeutic modalities such as responsive neuromodulation and laser ablations. The clinical effectiveness of these technologies is still being established, and long-term outcomes data are not yet available and thus were not included in the present work. We also assume that patients can transition between health states (i.e., having seizures vs being seizure-free) only on an annual basis. This is a simplification of the true clinical picture; patients may in reality transition between these health states more frequently. In the same vein, we assume that the annual rate of relapse is consistent over the first 5 years after surgery and then takes on a new value for the rest of the patient's lifespan. This is again a simplification of the true clinical picture; time-dependent variation in relapse rates is likely to be more complex in real patient cohorts. These simplifying assumptions reflect the metrics used in the limited longitudinal studies available. Our model validation analyses indicate that the outputs of the model closely match the outcomes observed in real clinical cohorts, suggesting that the assumptions do not limit the usefulness of model outputs.
It is important to recognize that our model has been deliberately designed to have a bias toward continued medical management and that the final recommendation for surgery is made despite this bias. The bias toward medical management exists because (1) we include a higher surgical mortality rate than that seen in the vast majority of centers, (2) the survival curves for medically managed patients in our model show that simulated patients are likely to have longer seizure-free survival than patients in real cohorts, and (3) we assume that patients who suffer major complications and are disabled from surgery were fully employed and earning the median income before surgery (thus inflating the societal costs of surgery).
We have performed both probabilistic and deterministic sensitivity analyses on our model. The probabilistic analyses allow for simultaneous variation in all variables in the model and thus provide a more robust indication of the variation in the model as a whole. Depending on the perspective adopted, 78% to 90% of simulations favored surgery or referral for surgical evaluation. Our deterministic (1-way) sensitivity analysis revealed that extreme variation for 5 variables would meaningfully alter the suggested strategy, including if the initial surgical success rate was <21%, the rate of surgical eligibility was <5%, and the true cost of the surgical package was >5 times our estimated value. The extreme conditions identified in this sensitivity analysis are highly improbable in real patient cohorts.
As with any cost-effectiveness analysis, the predictions made by our model are informative only for the patient population that was studied for estimation of clinical parameters. In this case, the clinical parameters are based on a heterogeneous group of patients who have been diagnosed with pharmacoresistant temporal epilepsy. This includes patients with mesial temporal sclerosis, malformations of cortical development, MRI-negative disease, and others. Our model is based on average clinical parameters for patients with temporal disease and is not able to refine recommendations according to individual patient parameters (lesional vs nonlesional disease, unifocal vs multifocal disease, etc). In terms of scope, we note that our model deals only with patients who do not require invasive EEG monitoring as part of presurgical workup. An increasingly large subset of patients requires invasive investigation before surgery,49 and it is possible that the cost-effectiveness of surgical referral and subsequent surgery may be substantively different among those patients. As data on the long-term marginal utility of invasive EEG to guide resection emerge, future studies that specifically look at the cost-effectiveness of these strategies will be particularly meaningful.
In the United States, temporal-lobe epilepsy surgery is cost-effective in patients who have been deemed surgical candidates, and surgical evaluation is cost-effective in pharmacoresistant patients even if the probability of being a surgical candidate is low (≈5%). An important future direction of research should be to develop cost-effectiveness prediction models that can be tailored to individual patient characteristics.
Acknowledgment
Shehryar Sheikh gratefully acknowledges scholarship support from the American Academy of Neurology.
Glossary
- ASM
antiseizure medications
- DR-TLE
drug-resistant temporal lobe epilepsy
- ICER
incremental cost-effectiveness ratio
- QALY
quality-adjusted life-year
- WTP
willingness to pay
Appendix. Authors
Footnotes
Editorial, page 417
Podcast: NPub.org/wtj0x0
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Epilepsia 2003;44:741–751. [DOI] [PubMed] [Google Scholar]
- 4.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA 2008;300:2497–2505. [DOI] [PubMed] [Google Scholar]
- 5.Langfitt J, Holloway R, McDermott M, et al. Health care costs decline after successful epilepsy surgery. Neurology 2007;68:1290–1298. [DOI] [PubMed] [Google Scholar]
- 6.Langfitt JT. Cost-effectiveness of anterotemporal lobectomy in medically intractable complex partial epilepsy. Epilepsia 1997;38:154–163. [DOI] [PubMed] [Google Scholar]
- 7.Picot MC, Jaussent A, Neveu D, et al. Cost-effectiveness analysis of epilepsy surgery in a controlled cohort of adult patients with intractable partial epilepsy: a 5-year follow-up study. Epilepsia 2016;57:1669–1679. [DOI] [PubMed] [Google Scholar]
- 8.Widjaja E, Li B, Schinkel CD, et al. Cost-effectiveness of pediatric epilepsy surgery compared to medical treatment in children with intractable epilepsy. Epilepsy Res 2011;94:61–68. [DOI] [PubMed] [Google Scholar]
- 9.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 10.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 11.Bridges JF, Onukwugha E, Mullins CD. Healthcare rationing by proxy. Pharmacoeconomics 2010;28:175–184. [DOI] [PubMed] [Google Scholar]
- 12.Spencer SS, Berg A, Vickrey B, et al. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology 2003;61:1680–1685. [DOI] [PubMed] [Google Scholar]
- 13.Kelley K, Theodore WH. Prognosis 30 years after temporal lobectomy. Neurology 2005;64:1974–1976. [DOI] [PubMed] [Google Scholar]
- 14.Foldvary N, Nashold B, Mascha E, et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology 2000;54:630. [DOI] [PubMed] [Google Scholar]
- 15.Di Gennaro G, Quarato PP, Sebastiano F, et al. Postoperative EEG and seizure outcome in temporal lobe epilepsy surgery. Clin Neurophysiol 2004;115:1212–1219. [DOI] [PubMed] [Google Scholar]
- 16.Markand ON, Salanova V, Whelihan E, Emsley CL. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia 2000;41:749–759. [DOI] [PubMed] [Google Scholar]
- 17.Uijl SG, Leijten FS, Arends JB, Parra J, Van Huffelen AC, Moons KG. Prognosis after temporal lobe epilepsy surgery: the value of combining predictors. Epilepsia 2008;49:1317–1323. [DOI] [PubMed] [Google Scholar]
- 18.Kelemen A, Barsi P, Erőss L, et al. Long-term outcome after temporal lobe surgery: prediction of late worsening of seizure control. Seizure 2006;15:49–55. [DOI] [PubMed] [Google Scholar]
- 19.Salanova V, Markand O, Worth R. Longitudinal follow-up in 145 patients with medically refractory temporal lobe epilepsy treated surgically between 1984 and 1995. Epilepsia 1999;40:1417–1423. [DOI] [PubMed] [Google Scholar]
- 20.Burneo JG, Villanueva V, Knowlton RC, Faught RE, Kuzniecky RI. Kaplan–Meier analysis on seizure outcome after epilepsy surgery: do gender and race influence it? Seizure 2008;17:314–319. [DOI] [PubMed] [Google Scholar]
- 21.Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 2005;46:1273–1279. [DOI] [PubMed] [Google Scholar]
- 22.Jeha L, Najm I, Bingaman W, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006;66:1938–1940. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004;127:2018–2030. [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Heiman G, Pandis D, et al. Seizure remission and relapse in adults with intractable epilepsy: a cohort study. Epilepsia 2008;49:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz TH, Jeha L, Tanner A, Bingaman W, Sperling MR. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia 2006;47:567–573. [DOI] [PubMed] [Google Scholar]
- 26.Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia 2002;43:170–174. [DOI] [PubMed] [Google Scholar]
- 27.Sperling MR, Feldman H, Kinman J, Liporace JD, O'Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol 1999;46:45–50. [DOI] [PubMed] [Google Scholar]
- 28.Annegers J, Coan SP, Hauser W, Leestma J. Epilepsy, vagal nerve stimulation by the NCP system, all-cause mortality, and sudden, unexpected, unexplained death. Epilepsia 2000;41:549–553. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia 2003;44:575–581. [DOI] [PubMed] [Google Scholar]
- 30.Nashef L, Fish D, Sander J, Shorvon S. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry 1995;58:462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost–effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 2005;5:153–162. [DOI] [PubMed] [Google Scholar]
- 32.Health Economics Resource Center, US Department of Veterans Affairs. Determining the cost of pharmaceuticals for a cost-effectiveness analysis [online]. Available at: www.herc.research.va.gov/include/page.asp?id=pharmaceutical-costs.
- 33.Schmidt D, Sillanpaa M. Stopping epilepsy treatment in seizure remission: good or bad or both? Seizure 2017;44:157–161. [DOI] [PubMed] [Google Scholar]
- 34.Braun KP, Schmidt D. Stopping antiepileptic drugs in seizure-free patients. Curr Opin Neurol 2014;27:219–226. [DOI] [PubMed] [Google Scholar]
- 35.Jehi L. Medication management after epilepsy surgery: opinions versus facts. Epilepsy Curr 2013;13:166–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yardi R, Irwin A, Kayyali H, et al. Reducing versus stopping antiepileptic medications after temporal lobe surgery. Ann Clin Transl Neurol 2014;1:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer HM, Spottke A, Aletsee C, et al. Direct and indirect costs of refractory epilepsy in a tertiary epilepsy center in Germany. Epilepsia 2006;47:2165–2172. [DOI] [PubMed] [Google Scholar]
- 38.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 39.Murray MI, Halpern MT, Leppik IE. Cost of refractory epilepsy in adults in the USA. Epilepsy Res 1996;23:139–148. [DOI] [PubMed] [Google Scholar]
- 40.McCabe C, Dixon S. Testing the validity of cost-effectiveness models. Pharmacoeconomics 2000;17:501–513. [DOI] [PubMed] [Google Scholar]
- 41.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol 2007;62:382–389. [DOI] [PubMed] [Google Scholar]
- 42.Jehi L, Martinez-Gonzalez J, Bingaman W. Outcome and complications of epilepsy surgery. In: Wyllie's Treatment of Epilepsy: Principles and Practice, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 43.Jette N, Quan H, Tellez-Zenteno JF, et al. Development of an online tool to determine appropriateness for an epilepsy surgery evaluation. Neurology 2012;79:1084–1093. [DOI] [PubMed] [Google Scholar]
- 44.Jetté N, Sander JW, Keezer MR. Surgical treatment for epilepsy: the potential gap between evidence and practice. Lancet Neurol 2016;15:982–994. [DOI] [PubMed] [Google Scholar]
- 45.De Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol 2010;17:619–625. [DOI] [PubMed] [Google Scholar]
- 46.Benbadis SR, Heriaud L, Tatum WO IV, Vale FL. Epilepsy surgery, delays and referral patterns: are all your epilepsy patients controlled? Seizure 2003;12:167–170. [DOI] [PubMed] [Google Scholar]
- 47.Haneef Z, Stern J, Dewar S, Engel J. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts JI, Hrazdil C, Wiebe S, et al. Neurologists' knowledge of and attitudes toward epilepsy surgery: a national survey. Neurology 2015;84:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jehi L, Friedman D, Carlson C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia 2015;56:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As detailed above, we have used data from published literature to populate the decision-analytic model; the parameter estimates can thus be replicated by referring to the cited literature.