Abstract
There is an unmet need in multiple sclerosis (MS) therapy for treatments to stop progressive disability. The development of treatments may be accelerated if novel biomarkers are developed to overcome the limitations of traditional imaging outcomes revealed in early phase trials. In January 2019, the International Progressive MS Alliance convened a standing expert panel to consider potential tissue fluid biomarkers in MS in general and in progressive MS specifically. The panel focused their attention on neurofilament light chain (NfL) in serum or plasma, examining data from both relapsing and progressive MS. Here, we report the initial conclusions of the panel and its recommendations for further research. Serum NfL (sNfL) is a plausible marker of neurodegeneration that can be measured accurately, sensitively, and reproducibly, but standard procedures for sample processing and analysis should be established. Findings from relapsing and progressive cohorts concur and indicate that sNfL concentrations correlate with imaging and disability measures, predict the future course of the disease, and can predict response to treatment. Importantly, disease activity from active inflammation (i.e., new T2 and gadolinium-enhancing lesions) is a large contributor to sNfL, so teasing apart disease activity from the disease progression that drives insidious disability progression in progressive MS will be challenging. More data are required on the effects of age and comorbidities, as well as the relative contributions of inflammatory activity and other disease processes. The International Progressive MS Alliance is well positioned to advance these initiatives by connecting and supporting relevant stakeholders in progressive MS.
Treatment to prevent gradual progression of disability remains a major unmet medical need in multiple sclerosis (MS) and was a driving force for initiation of the International Progressive MS Alliance (“the Alliance”) in 2012. The specific mission of the Alliance is to accelerate the development of effective disease-modifying and symptom management therapies for persons with progressive forms of MS.1
The Alliance acknowledges that a central aspect to success of its mission is to accelerate proof-of-concept clinical trials of new therapies in progressive MS, thereby encouraging drug development2 and stimulating further investment from industry. Ideally, early-stage trials depend on treatment response biomarkers, which predict clinical benefits and allow treatment effects to be detected more quickly and with smaller sample sizes than trials using clinical measures as primary outcomes. For relapsing MS, this requirement has been met by lesion activity on conventional MRI.3
Numerous pathologic mechanisms are purported to contribute to the progression of disability in progressive forms of MS, but no dominant mechanism has been identified.2 Therefore, biomarkers of specific pathologic mechanisms are unlikely to be informative for proof-of-concept trials of therapies with varying modes of action. Instead, the focus has been to identify biomarkers of neurodegeneration, which integrates the end-stage consequences of combined pathologies. Sample size calculations from longitudinal studies have enabled the adoption of brain atrophy, measured using MRI, as a biomarker of progressive MS and used as the primary outcome in phase 2 trials.4–7
Despite its intuitive association with loss of neural tissue, brain atrophy has a number of limitations as a treatment response biomarker. Loss of volume is not pathologically specific, depends on many factors such as tissue hydration, and thus follows a complicated trajectory after starting treatment.8 Thus, additional biomarkers, in particular those related specifically to neuronal structure and function, are required to monitor progressive MS.
Body fluid biomarkers have the potential to be more pathologically specific than imaging biomarkers, may reflect ongoing pathology over the entire CNS, and may be more responsive to the effects of treatment. Through 2017, the Alliance considered a number of biomarkers and decided at its Future Strategies Meeting in Dublin, Ireland, in July 2018 to focus on neurofilament light chain (NfL) as a test candidate. This decision followed recent methodological developments that allow ultra-sensitive measurements of serum NfL (sNfL) concentrations, which avoids the need to sample CSF,9,10 thus offering more convenient testing and increased acceptability of sampling by patients. Modeling suggests that NfL as a biomarker may have comparable sensitivity to imaging outcomes for testing efficacy in phase 2 trials of relapsing MS.11
Consequently, the Alliance convened an expert panel to discuss the current state of research on NfL as a biomarker in MS in general and progressive MS specifically, with a view to mobilizing the MS community toward filling key gaps in knowledge and understanding. Because much of the initial NfL data were collected in relapsing MS and provided important insights regarding its use and relevance in MS, we have included it here as foundational studies. Here, we summarize the outcome of the group's meeting in Washington DC in January 2019 and its subsequent discussions. Based on published regulatory guidance,12 2 potential Contexts of Use were agreed upon as the basis for further work:
sNfL as a pharmacodynamic/treatment response biomarker, to be used as an end point/outcome monitor in clinical trials in progressive MS
sNfL as a prognostic biomarker that can predict disease progression, to be used for the selection of patients with progressive MS into trials
Plausibility and analytical validity
Neurofilaments are plausible biomarkers of neurodegeneration because they are cytoskeletal proteins confined to the neuroaxonal compartment.13 Their concentrations are elevated across a wide range of neurologic diseases, consistent with release on axonal damage from multiple causes.13 Although the most widely used monoclonal antibodies for NfL14 have been highly specific in NfL knock-out animal experiments,15 the extent to which intact NfL and its degradation products contribute to the immunoassay signal is unclear. This limits the interpretation of NfL kinetics in individual patients.
Currently, the most widely used assay for measuring serum or plasma concentrations of NfL is the Quanterix platform, which uses single molecule array (Simoa) technology and is available commercially.16 Technical validation of this assay indicates good analytical accuracy. A recent multicenter study analyzing identical serum samples across different sites reported excellent interassay and intersite coefficients of variation (<10%).17 Further work is still required to establish interbatch and within-batch assay variability.18
A correlation between NfL levels in the serum or plasma and levels in the CSF has been demonstrated for various neurologic diseases and suggests that measures of ongoing neuroaxonal injury can be obtained from blood NfL levels without the need to obtain CSF by lumbar puncture.13 These results are consistent with small studies in progressive MS indicating a modest correlation between NfL concentrations in serum/plasma and CSF. For example, analysis of the progressive MS patient subset from Disanto et al.19 indicated an r of 0.7 (n = 18), although other studies showed a weaker correlation.20 Larger studies are needed to better understand the relationship between blood and CSF levels of NfL.
NfL concentrations are approximately 20% higher when measured in serum compared with plasma, which indicates that serum and plasma levels are not interchangeable within the same study. A few studies have assessed the stability of NfL. There appears to be minimal effect of freezing and thawing on NfL concentrations,21,22 and sNfL concentrations are stable in samples stored for 1 week at either room temperature or at 4°C21 (also Teunissen, unpublished). NfL appears to remain stable in samples stored under standard biobanking conditions over many years.19 Despite these encouraging findings, standard protocols are needed to define the acceptable parameters for type of collection tube, delay in processing, and processing methods.
Apart from Simoa, other high-sensitivity platforms apply similar reagents, such as the Olink proximity ligation protein analysis neuropanel. Novel automated systems include the Cobas Elecsys system by Roche and the ADVIA immunoassay system by Siemens. Although the increasing availability of multiple systems is likely to facilitate widespread implementation in research and clinical care, reference methods and materials are needed to ensure data comparability across different systems.
Clinical validity
NfL is a highly sensitive marker of neuronal injury, irrespective of the cause of that injury. However, NfL concentrations are typically far lower in MS than in many rapidly progressive primary neurodegenerative diseases, which show a faster rate of neuronal loss than MS.23 Average serum or plasma NfL concentrations are higher in relapsing and progressive MS than in controls,19,24,25 although the concentration ranges in MS overlap with controls to an extent that makes it difficult to define a pathologic cutoff at the individual patient level. This problem is further complicated by the fact that blood concentrations of NfL increase by an average 2.2% per year between ages 18 and 70 years in healthy controls.19,23,26 The reasons for this age-dependent increase are not well understood. The parallel increase in CSF and blood suggests that it is due mainly to physiologic age-dependent neuronal loss, but metabolic factors may also contribute, similar to the age-dependent increase of the CSF/serum albumin quotient.27 Hence, establishing reference values (e.g., a normative database) over a wide range of ages and evaluating the effects of comorbidities (i.e., cerebrovascular disease, diabetes, and smoking status) on serum concentrations are critical next steps for developing NfL as a tool for personalized medicine in MS, especially for patients with progressive MS.
For the use of NfL concentration as a biomarker in clinical trials, the effects of age and comorbidities can be controlled on relative grounds by both covariate adjustment and randomization, although these confounding variables may limit precision, interpretation, and strength of association with outcomes. Hence, a normative database of NfL is an indispensable tool to address this limitation. Such a database would also enable quantitative modeling of disease progression, which has been a valuable tool for parsing relevant covariates such as age that significantly influence relevant clinical trial outcomes. A normative database could help enable application of NfL measurement to individual patient monitoring and therapeutic decision making. Such models have been developed for other relevant outcome measures, reviewed by regulators, and made available for clinical trial optimization in diseases such as Alzheimer disease,28 Parkinson disease,29 and autosomal dominant polycystic kidney disease.30
Comparisons with imaging and disability measures
Numerous retrospective academic cohort studies19,25,31–33 and analyses of large phase 3 trials in relapsing MS24,34 suggest that the concentration of NfL in serum, plasma, and CSF is a promising biomarker in MS. Applications include (1) acute disease activity (including correlations with baseline T2 lesion volume and the number of enhancing T1 lesions) and (2) prediction of subsequent MRI lesion activity, brain volume loss, relapse rate, and worsening of disability. In patients with Alzheimer disease and amyotrophic lateral sclerosis, increasing mean serum concentrations occur months or years before the emergence of the first clinical manifestations.35,36 Similarly, an increased CSF NfL concentration in radiologically isolated syndrome is a risk factor for later transition to clinically definite MS.37,38
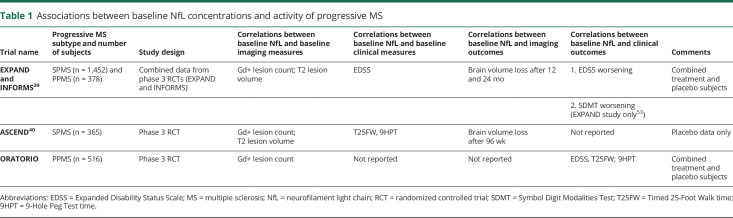
Recent results from clinical trials in progressive MS accord with those in relapsing-remitting disease (table 1) and suggest that the concentration of NfL is associated with concurrent disease activity and long-term disease outcome in all forms of MS. In placebo-controlled phase 3 trials of fingolimod and siponimod, baseline plasma NfL concentrations were higher in patients with Gd+ lesions at baseline compared with those without Gd+ lesions.39 In both trials, a high NfL concentration at baseline was associated with greater brain volume loss at 1–2 years and a higher likelihood of confirmed disability worsening. These associations were independent of treatment assignment or the presence of contrast-enhancing lesions at baseline. From these data, it was also estimated that a 1-year placebo-controlled trial would require a tentative sample size of 94 participants with secondary progressive MS per arm to detect a 20% reduction of NfL concentration with 80% power.39
Table 1.
Associations between baseline NfL concentrations and activity of progressive MS
Similarly, in a phase 3 trial of natalizumab, baseline sNfL concentration was associated with (1) baseline disease activity and disability measures, including the number of Gd+ lesions, T2 lesion volume, Timed 25-Foot Walk time (T25FW), and 9-Hole Peg Test time (9HPT), and (2) brain atrophy over 96 weeks. sNfL concentration at week 96 was also significantly higher in participants who progressed during the study (defined using the Expanded Disability Status Scale, T25FW, or 9HPT) compared with those who did not.40
When considered with the results in relapsing-remitting MS, these similar and more recent findings in progressive MS support the prognostic Context of Use for NfL defined earlier.41 However, these findings are at a group level and require deeper analysis of the existing trial data and further longitudinal studies to interpret NfL concentrations at an individual level and to build a disease model that might support trial enrichment. These studies should clarify (1) the relative contributions of neurodegeneration and acute inflammatory activity to longitudinal changes of clinical disability and NfL; (2) the extent and time course of the NfL trajectory following an acute inflammatory event (relapse or MRI lesion activity) because NfL concentrations are dynamic and are elevated for several months after acute neurologic events including clinical relapse23,42–44; and (3) the threshold level of change in the concentration of NfL, which equates to a threshold change of disability and, therefore, can be accepted as clinically meaningful.
Responsiveness to treatment
Results from a number of clinical trials in relapsing MS indicate that serum and plasma NfL concentrations respond consistently within 3–6 months of the start of anti-inflammatory therapies, that changes in NfL can be associated with changes in clinical and imaging outcomes, and that the response of NfL to higher-efficacy therapies such as alemtuzumab and fingolimod is larger than the response to interferon-beta.24,31
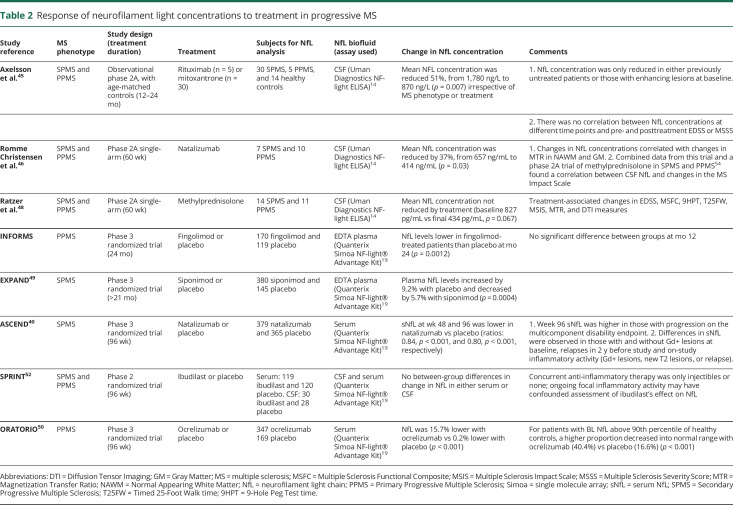
Previously, 2 small studies reported positive treatment effects on CSF concentrations of NfL in progressive MS (table 2).45,46 Lower concentrations were observed after treatment for 12–24 months with either mitoxantrone or rituximab in patients with primary progressive MS compared with baseline and with a small group of age-matched controls (table 2). The difference was most prominent in those patients with evidence of ongoing inflammatory activity.45 Treatment with natalizumab for 60 weeks was also associated with lower CSF NfL concentrations in a single-arm, open-label study in a progressive cohort.46 Furthermore, changes in CSF NfL correlated with clinical changes during treatment with natalizumab or monthly methylprednisolone.47,48
Table 2.
Response of neurofilament light concentrations to treatment in progressive MS
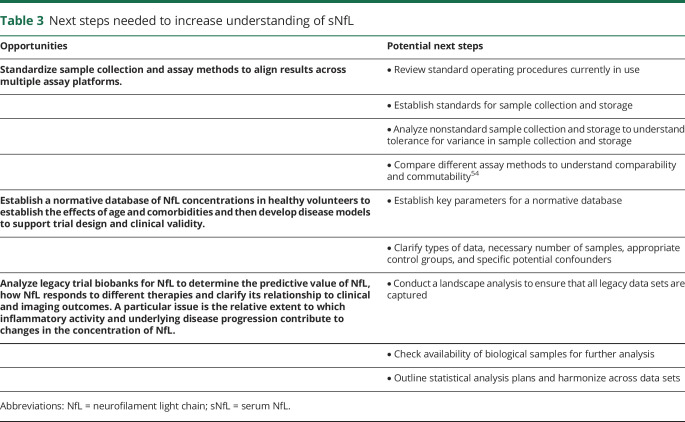
Table 3.
Next steps needed to increase understanding of sNfL
Recently, the initial analyses of peripheral blood NfL concentrations were communicated from the phase 3 trials of fingolimod and ocrelizumab in primary progressive MS (INFORMS and ORATORIO, respectively) and of siponimod and natalizumab in secondary progressive MS (EXPAND and ASCEND, respectively) (table 2).39,40,49,50 Treatment was associated with lower NfL concentrations compared with placebo in all 4 trials. A significant NfL response was apparent with or without the presence of observed inflammatory activity in both the siponimod and natalizumab trials, but the effect sizes were smaller in the progressive inactive subgroups.40,49 In the ASCEND and INFORMS trials, a robust reduction in NfL was observed despite an absence of clinical benefit.39,40 Ibudilast, which appears to act on noninflammatory processes to slow whole-brain atrophy without affecting relapse rate or lesion activity,51 has been reported on initial analysis of the SPRINT-MS phase 2 trial to have no effect on the concentration of NfL in either serum or CSF.52 Background immune modulating therapy in this trial was only injectable therapies (or none), and ongoing inflammatory activity may have obscured the ability to detect an ibudilast-related reduction in NfL. Further studies of NfL using legacy trial biobanks and ongoing trials will help to clarify the relationship of changes in NfL concentrations with disability measures, including the time course of NfL changes and their clinical meaningfulness. Importantly, clinical studies indicate that disease activity as measured by clinical relapses and MRI (either gadolinium-enhancing or new T2 lesions) is associated with increased NfL. Thus, measurement of NfL in progressive MS trials that target disease progression may be confounded by intercurrent disease activity, as may have occurred in the SPRINT-MS trial of ibudilast described above.
Further analyses of clinical trial data sets with particular attention to disease activity will help clarify the appropriate use and utility of NfL in clinical trials.
Limitations of NfL
In addition to the technical challenges mentioned earlier, there are several limitations in the application of NfL to individual patients with MS and the evaluation of MS therapies. NfL is a cytoskeletal protein that can be released as a result of almost any type of brain injury. NfL is not specific to MS, and thus, any neurologic disease or injury can confound efforts to use NfL to characterize MS and response to MS therapies. NfL release can arise from infiltrative inflammation seen in relapsing MS (and less frequently in progressive MS), but also the various different pathologies associated with progressive MS. This confounding may limit the ability of NfL to measure the neurodegenerative aspects of progressive MS and potential impact of putative neuroprotective therapies. Understanding the impact of different comorbid conditions such as cerebrovascular disease, diabetes, and smoking status on serum concentrations is a critical next step in developing NfL as a tool for personalized medicine in MS, especially for patients with progressive MS. The utility of NfL monitoring for individual patient management is not yet defined and might require integrating more clinical, biological, and imaging features in the future.
Ongoing studies
There are many ongoing studies that will further characterize NfL and understand its relevance to MS in general and in progressive MS specifically. Many clinical trials that biobanked serum or plasma samples are analyzing NfL. A US NIH-funded study (1U01NS111678-01A1) funded in 2020 will evaluate NfL as a prognostic and monitoring biomarker in over 5,700 individuals with MS. A study funded by the US National MS Society will evaluate sNfL from the US National Health and Nutrition Examination Survey of healthy adults to assess the effects of demographics, lifestyle factors, and comorbidities on sNfL levels and establish demographic-specific reference ranges of sNfL. A study funded by the Swiss National Science Foundation will investigate the relationship between sNfL and MRI characteristics, treatment response, and quality of life and characterize NfL turnover in the blood. NfL is being studied in other neurologic diseases too. The Biomarkers Consortium of the Foundation of the NIH aims to establish whether NfL in blood provides a prognostic marker that can accelerate the development of disease-modifying therapeutics in familial frontotemporal dementia.
Other initiatives focus on the standardization of measurements to prepare for the use of blood biomarkers in both clinical trials and routine clinical practice. For example, the International Federation of Clinical Chemistry Working Group performs a round robin/commutability study of NfL in plasma and serum to study the correlation between the different assays and identify candidate Reference Materials. Future Certified Reference Materials can be used to align measurements across analytical platforms. The Standardization of Alzheimer Blood Based Biomarkers Working Group of the Alzheimer Association Global Biomarkers Standardization Consortium develops standard operating procedures for blood collection and processing for a broad range of potential markers, including NfL. These and other efforts will help further our understanding of the role of NfL in identifying new therapies and managing the disease in people living with MS.
Conclusions
Our review of existing data suggests that sNfL may provide a plausible biomarker of progressive MS, addressing some of the limitations of current imaging biomarkers to accelerate drug development through the proposed Contexts of Use. However, significant gaps remain in our understanding of NfL and must be addressed before NfL can be accepted as a biomarker by the progressive MS community and, potentially, by regulatory agencies. These gaps include the following:
Sample collection and assay methods should be standardized to align results across current (and future) assay platforms, which will support analytical validity across the globe.
A normative database of sNfL concentrations in healthy volunteers is required. This database should include the effects of age and comorbidities, which will allow the development of disease models to support trial design and clinical validation.
A deeper analysis of legacy clinical trial data sets will help clarify the predictive value of baseline concentrations of sNfL, define how sNfL responds to different types of therapies, and clarify the relationship between NfL levels and clinical and imaging outcomes. A particular issue is the relative extent to which inflammatory activity including activated microglia and other disease processes contribute to changes in NfL.
Acknowledgment
Marco Salvetti, Catherine Lubetzki, and Susan Kohlhass provided critical review of the manuscript on behalf of the International Progressive MS Alliance. Their contributions are appreciated.
Glossary
- MS
multiple sclerosis
- NfL
neurofilament light chain
- Simoa
single molecule array
- sNfL
serum NfL
- T25FW
Timed 25-Foot Walk time
- 9HPT
9-Hole Peg Test time
Appendix. Authors
Study funding
Support for this manuscript was provided by the International Progressive MS Alliance, which is funded by its society, trust, foundation, and industry members. See progressivemsalliance.org for additional details.
Disclosure
R. Kapoor, K.E. Smith, M. Allegretta, D.L. Arnold, W. Carroll, M. Comabella, R. Furlan, C. Harp, J. Kuhle, and D. Leppert report no disclosures related to this manuscript. T. Plavina is currently employed by Quanterix, which is a diagnostic company with patient interests in NfL testing. F. Sellebjerg, C. Sincock, C.E. Teunissen, I. Topalli, F. von Raison, E. Walker, and R.J Fox report no disclosures related to this manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler 2012;18:1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvetti M, Landsman D, Schwarz-Lam P, Comi G, Thompson AJ, Fox RJ. Progressive MS: from pathophysiology to drug discovery. Mult Scler 2015;21:1376–1384. [DOI] [PubMed] [Google Scholar]
- 3.Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 2013;12:669–676. [DOI] [PubMed] [Google Scholar]
- 4.Altmann DR, Jasperse B, Barkhof F, et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology 2009;72:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoor R, Furby J, Hayton T, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 2010;9:681–688. [DOI] [PubMed] [Google Scholar]
- 6.Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 2014;383:2213–2221. [DOI] [PubMed] [Google Scholar]
- 7.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016;50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannoni G, Cutter G, Pia-Sormani M, et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord 2017;12:70–78. [DOI] [PubMed] [Google Scholar]
- 9.Gisslen M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016;3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 11.Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann Clin Transl Neur 2019;6:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA-NIH Biomarker Working Group. BEST (Biomarkers E, and Other Tools) Resource. Silver Spring: Food and Drug Administration (US); 2016. Available from: www.ncbi.nlm.nih.gov/books/NBK326791/. Co-published by National Institutes of Health (US), Bethesda (MD). [Google Scholar]
- 13.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 14.Norgren N, Karlsson JE, Rosengren L, Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybridoma Hybridom 2002;21:53–59. [DOI] [PubMed] [Google Scholar]
- 15.Bacioglu M, Maia LF, Preische O, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016;91:494–496. [DOI] [PubMed] [Google Scholar]
- 16.Summary and Explanation of the NF-Light® Test. Available at: www.quanterix.com/sites/default/files/assays/Simoa_NF-light_Data_Sheet_HD-1.pdf. Accessed April3, 2018. [Google Scholar]
- 17.Kuhle J, Barro C, Hrusovsky K, et al. International multi-site analytical validation of the Simoa NF-light assay in human serum samples from multiple sclerosis patients. Mult Scler J 2018;24:249–251. [Google Scholar]
- 18.Sharma A, Petrillo M, Zhao G, et al. Strategic platform selection and validation of biomarker assays to measure serum neurofilament light and heavy chain in multiple sclerosis. Mult Scler J 2018;24:660–661. [Google Scholar]
- 19.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harp CT, Ma XY, Hendricks R, et al. NfL levels in CSF, serum, and plasma of RRMS and patients in a cross-sectional UCSF cohort. Neurology 2019;92(15S);P2.2–082. [Google Scholar]
- 21.Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLos One 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhle J, Plattner K, Bestwick JP, et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler J 2013;19:1597–1603. [DOI] [PubMed] [Google Scholar]
- 23.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019;76:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019;92:E1007–E1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018;141:2382–2391. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with alzheimer disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrado-Fernández C, Blennow K, Hansson M, Leoni V, Cedazo-Minguez A, Björkhem I. Evidence for sex difference in the CSF/plasma albumin ratio in ∼20 000 patients and 335 healthy volunteers. J Cell Mol Med 2018;22:5151–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero K, Ito K, Rogers JA, et al. The future is now: model-based clinical trial design for Alzheimer's disease. Clin Pharmacol Ther 2015;97:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrado DJ, Nicholas T, Tsai K, et al. Dopamine transporter neuroimaging as an enrichment biomarker in early Parkinson's disease clinical trials: a disease progression modeling analysis. Clin Transl Sci 2018;11:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone RD, Mouksassi MS, Romero K, et al. A drug development tool for trial enrichment in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep 2017;2:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canto E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019;76:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler J 2018;24:1046–1054. [DOI] [PubMed] [Google Scholar]
- 33.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabresi P, Kuhle J, Arnold DL, et al. Serum neurofilament light (NfL) for disease prognosis and treatment monitoring in multiple sclerosis patients: is it ready for implementation into clinical care? Mult Scler J 2018;24:59–60. [Google Scholar]
- 35.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med 2019;25:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol 2018;84:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matute-Blanch C, Villar LM, Alvarez-Cermeno JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018;141:1085–1093. [DOI] [PubMed] [Google Scholar]
- 38.Thouvenot E, Demattei C, Uygunoglu U, et al. Neurofilament-light chain levels are predictive of on-going disease activity in radiologically isolated syndrome. Neurology 2019;92(15S):S37.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhle J, Kropshofer H, Haring DA, et al. Neurofilament light levels in the blood of patients with secondary progressive MS are higher than in primary progressive MS and may predict brain atrophy in both MS subtypes. Mult Scler J 2018;24:111. [Google Scholar]
- 40.Kapoor R, Sellebjerg F, Hartung HP, et al. Natalizumab reduces serum concentrations of neurofilament light chain in secondary progressive multiple sclerosis patients from the phase 3 ASCEND study. Neurology 2019;92. [Google Scholar]
- 41.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US). 2016- Co-published by National Institutes of Health (US), Bethesda (MD). Available at: http://www.ncbi.nlm.nih.gov/books/NBK326791/. [PubMed]
- 42.Thelin EP, Zeiler FA, Ercole A, et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front Neurol 2017;8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003;61:1720–1725. [DOI] [PubMed] [Google Scholar]
- 44.Modvig S, Degn M, Horwitz H, et al. Relationship between cerebrospinal fluid biomarkers for inflammation, demyelination and neurodegeneration in acute optic neuritis. PLoS One 2013;8:e77163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Axelsson M, Malmestrom C, Gunnarsson M, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult Scler J 2014;20:43–50. [DOI] [PubMed] [Google Scholar]
- 46.Romme Christensen J, Ratzer R, Bornsen L, et al. Natalizumab in progressive MS: results of an open-label, phase 2A, proof-of-concept trial. Neurology 2014;82:1499–1507. [DOI] [PubMed] [Google Scholar]
- 47.Christensen JR, Komori M, von Essen MR, et al. CSF inflammatory biomarkers responsive to treatment in progressive multiple sclerosis capture residual inflammation associated with axonal damage. Mult Scler J 2019;25:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratzer R, Iversen P, Bornsen L, et al. Monthly oral methylprednisolone pulse treatment in progressive multiple sclerosis. Mult Scler J 2016;22:926–934. [DOI] [PubMed] [Google Scholar]
- 49.Kuhle J, Kropshofer H, Barro C, et al. Siponimod reduces neurofilament light chain blood levels in secondary progressive multiple sclerosis patients. Neurology 2018;90(15S):S8.006. [Google Scholar]
- 50.Bar-Or A, Thanei GA, Harp CT, et al. Blood neurofilament light levels are lowered to a healthy donor range in patients with RMS and PPMS following ocrelizumab treatment. Mult Scler J 2019;25:52. [Google Scholar]
- 51.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med 2018;379:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox R, Karafa M, Konig V, et al. Effect of ibudilast on neurofilament-light chain in progressive MS: analysis from a phase II trial. Neurology 2019;92. [Google Scholar]
- 53.Kuhle J, Kropshofer H, Barro C, et al. The predictive value of neurofilament light chain levels in blood for cognitive impairment in patients with secondary progressive multiple sclerosis. Neurology 2019;92(15S):S8.006. [Google Scholar]
- 54.Andreasson U, Kuhlmann J, Pannee J, et al. Commutability of the certified reference materials for the standardization of beta-amyloid 1-42 assay in human cerebrospinal fluid: lessons for tau and beta-amyloid 1-40 measurements. Clin Chem Lab Med 2018;56:2058–2066. [DOI] [PubMed] [Google Scholar]