Abstract
Objective
To determine whether blood-based biomarkers can differentiate older veterans with and without traumatic brain injury (TBI) and cognitive impairment (CogI).
Methods
We enrolled 155 veterans from 2 veterans' retirement homes: 90 without TBI and 65 with TBI history. Participants were further separated into CogI groups: controls (no TBI, no CogI), n = 60; no TBI with CogI, n = 30; TBI without CogI, n = 30; and TBI with CogI, n = 35. TBI was determined by the Ohio State University TBI Identification Method. CogI was defined as impaired cognitive testing, dementia diagnosis, or use of dementia medication. Blood specimens were enriched for CNS-derived exosomes. Proteins (neurofilament light [NfL], total tau, glial fibrillary acidic protein [GFAP], α-synuclein, β-amyloid 42 [Aβ42], and phosphorylated tau [p-tau]) and cytokines (tumor necrosis factor–α [TNF-α], interleukin-6 [IL-6], and interleukin-10) were measured using ultrasensitive immunoassays.
Results
Veterans were, on average, 79 years old. In participants with TBI history, 65% had mild TBI; average time from most recent TBI was 37 years. In adjusted analyses, the TBI and CogI groups differed on CNS-enriched exosome concentration of p-tau, NfL, IL-6, TNF-α (all p < 0.05), and GFAP (p = 0.06), but not on Aβ42 or other markers. Adjusted area under the curve (AUC) analyses found that all significantly associated biomarkers combined separated TBI with/without CogI (AUC, 0.85; 95% confidence interval [CI], 0.74–0.95) and CogI with/without TBI (AUC, 0.88; 95% CI, 0.77–0.99).
Conclusions
Increased levels of blood-based, CNS-enriched exosomal biomarkers associated with TBI and CogI can be detected even decades after TBI.
Classification of evidence
This study provides Class II evidence that in veterans with a history of TBI, CNS-enriched exosome concentration of p-tau, NfL, IL-6, and TNF-α are associated with CogI.
Military veterans are at high risk of traumatic brain injury (TBI) compared to the civilian population, a risk not limited to those exposed to combat.1 Most of these injuries are classified as mild TBI (mTBI).2,3 Moderate and severe TBI in early and midlife is associated with a 2 to 3 times increased risk of late-life dementia.4–8 More recently, studies suggest that mTBI, even without loss of consciousness, also increases the risk of cognitive impairment9 and dementia.10,11
It is unclear whether TBI leads to Alzheimer disease (AD) type pathology, or whether TBI-associated dementia is a distinct pathologic entity, such as chronic traumatic encephalopathy (CTE) or other pathologic entities not yet described. Limited case series suggest that TBI-associated dementia may involve multiple pathologic processes, even within individuals.12 This distinction is critical to design effective prevention and treatment strategies for veterans and civilians with TBI. Biomarker studies may aid greatly in this investigation.
Whereas the link between TBI and dementia and cognitive impairment (CogI) is increasingly established, there are little data on the biomarker features of TBI-associated CogI.13 The 2 best-validated blood biomarkers of axonal injury are increased plasma total tau and neurofilament light (NfL) polypeptide level. Both are elevated in acute TBI, but much less is known about chronic profiles after TBI.13–15 The difficulty of collecting CSF after TBI has prompted the development and validation of peripherally accessed blood-based biomarkers.16 However, whether these peripherally accessed biomarkers reflect CNS processes remains unclear.13
Recent work has focused on examining exosomal protein biomarkers. Exosomes are extracellular vesicles released from all mammalian cells, including CNS cells, and are thought to be important in cell-to-cell signaling. Exosomes cross the blood–brain barrier and are detectable in the peripheral circulation. Peripheral blood samples can be enriched for CNS-derived exosomes, including neuronally and astrocyte-derived exosomes. Using these techniques, one study recently reported that elevated exosomal tau, β-amyloid 42 [Aβ42], and interleukin-10 [IL-10] were associated with mTBI and chronic TBI symptoms in younger veterans over a year after injury.17 We recently reported on CNS-enriched exosomes in TBI, suggesting that remote TBI might be associated with elevated neurodegenerative proteins.18 Another study found elevated tau in veterans with TBI, over a decade past injury.19 However, little is known about which biomarkers may be elevated in CNS-enriched exosomes in participants with TBI and CogI.
Our goal was to determine the blood-based biomarker profile within CNS-enriched exosomes among older veterans with and without a history of remote TBI and with and without CogI.
Methods
Study population
Participants were veterans residing at either the Armed Forces Retirement Home (Washington, DC) or the Veterans Home of California–Yountville. All participants were aged 50–95 years and could provide consent to participate in research. We excluded individuals with severe cognitive impairment (Mini-Mental State Examination [MMSE]20 <20), past penetrating head injury, those unable or unwilling to provide a blood sample, and those with medical conditions, hearing loss, or vision loss severe enough to preclude participating in the study. Residents were recruited for the study through flyers, social events, and word of mouth.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Human Research Committees at each site and all participants gave written informed consent.
Assessment of TBI history
Detailed TBI history was determined by the Ohio State University TBI Identification Method21 (OSU-TBI-ID), a structured clinical interview recommended by the National Institute of Neurologic Disorders and Stroke (NINDS) as a Common Data Element for the retrospective assessment of lifetime TBI in clinical research. TBI diagnosis was defined as head injury resulting in medical evaluation or hospitalization. No TBI was defined as no prior history of any head injury that produced neurologic symptoms or resulted in medical care. We reviewed the participants' retirement home medical charts and found confirmation of TBI history in 52% of the TBI participants and absence of TBI history in 100% of the non-TBI participants (n = 90).
Assessment of medical and psychiatric history
Past and current medical history (hypertension, stroke, and diabetes) was determined through a combination of chart review and self-report. Psychiatric history (depression, anxiety, post-traumatic stress disorder, and substance abuse) was collected in a similar manner.
Neuropsychological battery
The MMSE score was used as a measure of general cognition. Learning and memory were assessed via the Auditory Verbal Learning Task (AVLT),22,23 including AVLT Learning Trials (total trials 1–5) and AVLT Delayed Recall. To examine executive functioning and processing speed, participants completed the Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol Task.24
Given that norms are less developed for this group of very old veterans, we defined impairment using previously recommended standard definitions. A composite Z score was created from the AVLT learning score, AVLT delay score, MMSE, and WAIS Digit Symbol. The composite score was based on the Alzheimer's Disease Cooperative Study Preclinical Alzheimer Cognitive Composite score,25 a measure of early cognitive impairment. First, each participant's raw cognitive test scores were compared to demographically corrected normative data for each measure (Mayo's Older Americans Normative Studies age-corrected norms for AVLT Learning Trials26 and Delayed Recall27 and Alzheimer's Disease Centers' Uniform Data Set age-, sex-, and education-corrected norms28 for MMSE and WAIS-R Digit Symbol). Using these normative data, each individual's raw test scores were then converted into demographically corrected z scores, reflecting the extent to which an individual's test performance diverges from that of healthy, demographically similar peers. Finally, for each participant, the z scores for the separate test scores were combined to create a cognitive composite score.
CogI was defined as a cognitive composite score greater than 1 SD below normative values, medical record dementia diagnosis, or current dementia medication prescription recorded in medical records (donepezil, memantine, or rivastigmine).
Exosome isolation from human plasma
Blood samples were drawn via venipuncture, collected in ethylenediaminetetraacetic acid tubes, and centrifuged at 1,500 rpm for 15 minutes. The resulting plasma was isolated, aliquoted, and stored at −80°C until analysis.
Total exosomes were isolated and neuronally enriched from 3 mL of plasma, using methods described in detail previously.17 Briefly, after sample thawing, thrombin was added to each sample, which was then centrifuged. Exoquick solution (System Biosciences, Inc., Mountainview, CA) was added to thrombin-treated plasma samples. Resulting solutions were incubated and then centrifuged. After centrifugation, the supernatant was aspirated and the exosome pellet was resuspended. Exosomes were enriched for CNS-derived extracellular vesicles containing CD171 marker L1 cell adhesion molecule (L1CAM) neural adhesion protein.
To lyse exosomes, each tube received equal amounts of M-PER mammalian protein extraction reagent (Thermo Scientific, Inc., Rockford, IL), containing 3 times the suggested concentrations of protease and phosphatase inhibitors. These suspensions were then assayed for biomarker concentrations using the Simoa, an ultrasensitive method to quantify protein concentrations.29
Protein quantification
All analyses were conducted using a site-specific Simoa HD-1 analyzer instrument using commercially available standard kits with established reliability. The instrument transferred 2 replicates from each well into sample cuvettes. The coefficient of variation of back-calculated concentrations was ≤15%. Data presented include measurements of the following proteins: neurofilament light (NfL), total tau (tau), glial fibrillary acidic protein (GFAP), α-synuclein (α-syn), Aβ42, and phosphorylated tau (p-tau) and cytokines (human tumor necrosis factor–α [TNF-α], interleukin-6 [IL-6], and IL-10). Each analyte was plated with 5 controls. The controls served as the reference and the detection curve was standardized to the controls.
Statistical analysis
The analysis focused on the 155 participants separated into groups by TBI and CogI: control (noTBI–noCogI; n = 60), noTBI–CogI (n = 30), TBI–noCogI (n = 30), and TBI–CogI (n = 35). We compared participant demographics and medical and psychiatric history between the groups, using Kruskal-Wallis test for continuous variables and χ2 test or Fisher exact test for categorical variables.
Because the distributions were not normally distributed (skewed to the right), the concentration of CNS-enriched exosomes was log-transformed. The concentration of proteins and cytokines in CNS-enriched exosomes was compared by groups using linear regression models. Post hoc tests (comparisons with control group) and contrasts (between noTBI–CogI and TBI–CogI group) were performed as necessary. To ensure that acute–subacute TBI was not driving findings, we performed a sensitivity analysis excluding all participants who sustained TBI within the past year (n = 5). We also conducted receiver operating characteristic (ROC) curve analysis to compare area under the curve (AUC) between participants in the 4 groups using all significantly associated exosome-derived markers. All statistical analyses were conducted using SAS, version 9.4 (Cary, NC).
Classification of evidence
The object of this study was to determine the blood-based biomarker profile within CNS-enriched exosomes among older veterans with and without a history of remote TBI and with and without cognitive impairment. This study provides Class II evidence that in veterans with a history of TBI, CNS-enriched exosome concentration of p-tau, NfL, IL-6, and TNF-α are associated with cognitive impairment.
Data availability
Data not provided in the article will be shared in anonymized form by request.
Results
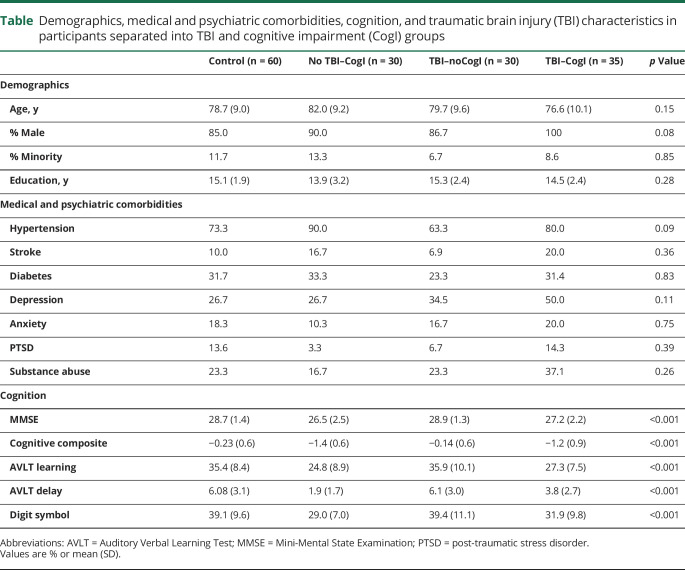
Veterans were, on average, 79 years old, had completed 15 years of education, and were mostly male. Average MMSE score was 28 out of 30. None of the demographic or medical or psychiatric history variables differed significantly when examined across all 4 groups (table; all p > 0.05). There were no significant differences in demographics or medical or psychiatric history in participants by CogI group in those without TBI (all p > 0.05). Participants with TBI only differed significantly on sex, with more men in the TBI–CogI group than the TBI–noCogI group (86.7% vs 100%, p = 0.04). The other comparisons were all nonsignificant (all p > 0.05). The cognitive composite score and the scores of the included tests are shown in the table. Participants without CogI performed better on all tests than participants with CogI (all p < 0.05, age- and sex-adjusted). The 2 CogI groups only differed significantly on the AVLT Delay score (p = 0.05, age- and sex-adjusted), with the noTBI–CogI group remembering fewer words after 30 minutes than the TBI–CogI group (1.9 vs 3.8 words).
Table.
Demographics, medical and psychiatric comorbidities, cognition, and traumatic brain injury (TBI) characteristics in participants separated into TBI and cognitive impairment (CogI) groups
Among the 65 participants with TBI, average time from first TBI to study visit was 51 years; average time from last TBI, 37 years. Eighty-eight percent of veterans had TBI >2 years before study participation. The majority (78%) of participants reported TBIs with loss of consciousness (LOC). Most participants had a history of mild TBI only (no LOC or LOC <30 minutes), but 35% had at least one previous moderate/severe TBI. In the TBI–noCogI group, 33% had a moderate/severe TBI compared to 37% in the TBI–CogI group. None of the TBI characteristics differed significantly between the 2 groups (all p > 0.10).
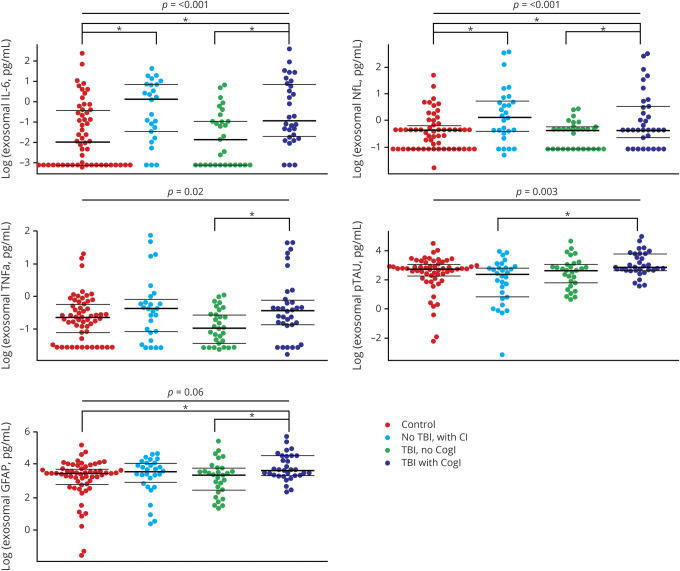
In analyses adjusted for age and sex, overall CNS-enriched exosome concentration among the 4 groups significantly differed for NfL, p-tau, IL-6, and TNF-α (all p < 0.05; figure 1), marginally differed for GFAP (p = 0.06), and did not differ for tau, α-syn, Aβ42, or IL-10 (all p > 0.10; data available from Dryad, see supplemental figure 1, doi.org/10.7272/Q6QF8R23). In the sensitivity analysis removing participants who sustained TBI in the past year, results and significance levels were nearly identical.
Figure 1. Bee swarm plots for significant biomarkers showing the distribution of all 4 groups.
Overall p values (adjusted for age and sex) are displayed at the top of each plot. The brackets below show the significant (*p < 0.05) pairwise comparisons for each marker. CogI = cognitive impairment; GFAP = glial fibrillary acidic protein; IL-6 = interleukin-6; NfL = neurofilament light; p-tau = phosphorylated tau; TBI = traumatic brain injury; TNF-α = tumor necrosis factor–α;
Pairwise comparisons between controls and the other 3 groups on biomarkers that differed at least at p < 0.10 in the overall analysis showed that the control group and the TBI–noCogI group did not significantly differ on any of the biomarkers. However, both CogI groups differed from the control group in CNS-enriched exosomal concentration of several biomarkers. Compared to controls, noTBI–CogI had higher concentrations of NfL and IL-6 (both p < 0.05 in age- and sex-adjusted analyses). Compared to controls, TBI–CogI had higher concentrations of IL-6, NfL, and GFAP (p < 0.05 in age- and sex-adjusted analyses). Comparing the 2 CogI groups (noTBI–CogI and TBI–CogI), we found that only p-tau was higher in the TBI–CogI group than the noTBI–CogI group (p > 0.001, age- and sex-adjusted). Comparing the 2 TBI groups (TBI–noCogI and TBI–CogI), several markers were significantly higher in the TBI–CogI group than in the TBI–noCogI group in age- and sex-adjusted analyses: IL-6 (p < 0.001), NfL (p < 0.01), TNF-α (p < 0.01), and GFAP (p < 0.05).
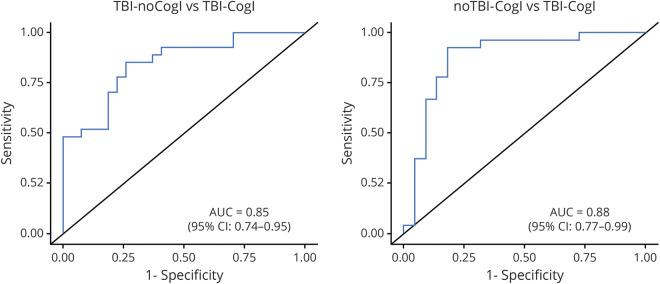
To assess the ability of the biomarkers to separate the groups of participants, we examined the ROC curve for the biomarkers that differed (p < 0.10) in the overall comparison between groups (NfL, p-tau, IL-6, TNF-α, and GFAP) (figure 2; to see the individual ROC curves for each biomarker, see supplemental figure 2 from Dryad, doi.org/10.7272/Q6QF8R23). AUC analysis demonstrated that this combination of biomarkers could separate the 2 TBI groups (TBI–noCogI and TBI–CogI) from each other (adjusted AUC = 0.85, 95% confidence interval [CI], 0.74–0.95; p = 0.01). The combined biomarkers were also able to distinguish the 2 CogI groups (noTBI–CogI and TBI–CogI; adjusted AUC = 0.88, 95% CI, 0.77–0.99, p = 0.01).
Figure 2. Receiver operating characteristic curves and areas under the curve (AUCs) (adjusted for age and sex) for all biomarkers significantly associated with the traumatic brain injury (TBI) and cognitive impairment groups combined (phosphorylated tau, neurofilament light, glial fibrillary acidic protein, interleukin-6, and tumor necrosis factor–α).
CI = confidence interval; CogI = cognitive impairment.
For this study we also examined Simoa measurements of all biomarkers in regular plasma samples (not enriched for CNS-derived exosomes). Given the very low concentrations for most proteins and the relatively modest sample size, it was difficult to make any conclusions, but the overall patterns looked similar.
Discussion
We found that blood-based, CNS-enriched exosomal protein differences could be detected in older veterans more than 5 decades, on average, after a TBI. We found that markers differed with both TBI and CogI status. Specifically, p-tau, NfL, GFAP, and the inflammatory cytokines IL-6 and TNF-α differed significantly among the 4 TBI and CogI groups. By combining the biomarkers significantly associated with the 4 groups (p-tau, NfL, GFAP, IL-6, and TNF-α), we were able to distinguish the 2 TBI groups (TBI–noCogI and TBI–CogI) and the 2 CogI groups (noTBI–CogI and TBI–CogI) with high accuracy (AUC = 0.88 for noTBI–CogI vs TBI–CogI; AUC = 0.85 for TBI–noCogI and TBI–CogI). These findings have substantial implications for biomarker-supported diagnosis of TBI and TBI-associated CogI in aging cohorts.
It has been hypothesized that TBI–CogI is pathologically similar to AD, which is characterized by Aβ plaques and tau-associated tangles, with an acceleration of Aβ and tau deposits. Notably, within hours after a fatal severe TBI, a buildup of amyloid precursor protein that results in the formation of diffuse Aβ plaques has been reported.30–32 However, more recent research has focused on CTE, a neuropathology with prominent accumulation of p-tau in the form of neurofibrillary tangles and neuropil threads in a unique and pathognomonic perivascular and depths-of-sulci cortical distribution.33,34 CTE has been described predominantly among individuals who have had repetitive mild TBIs such as athletes or veterans with multiple blast exposure.
Our results do not point to an obvious single pathology underlying TBI–CogI. We found differences in the TBI and CogI groups in GFAP, NfL, and p-tau and have confirmed our ability to use combined CNS-enriched exosome proteins to distinguish TBI–CogI from TBI–noCogI. We previously reported on a subset of participants from one site, in an independent laboratory, with different assays measuring neurodegenerative proteins, and also found elevations with TBI history.18 Others have reported that GFAP, NfL, and p-tau are increased after acute TBI,35,36 and some studies reported that these markers are associated with short-term outcomes.13,37–40 However, few studies have examined biomarkers in remote TBI.19 It may be that these proteins remain elevated chronically, especially in those who develop CogI. Or it may be that the proteins increase later, possibly with CogI and accompanying neurodegeneration.
In this study, we did not find a higher level of Aβ42 in the TBI–CogI group. Studies of patients with AD have found that Aβ42 is higher in patients with AD compared to controls41 and predicts conversion to dementia.42 Blood levels of Aβ42 have been found to be higher in studies of TBI17,43 up to 3 years after injury, but were not seen in more remote TBI.19 One possibility for the lack of an observed elevated Aβ42 is that the neuropathologic basis of CogI after TBI in this study is not AD. In fact, our previous work in older veterans found that those with TBI had CogI, showing impairment in executive functioning and processing speed, but not memory.9,44 A better understanding of the temporal changes in Aβ42 after remote TBI is warranted. We also did not find higher Aβ42 in our noTBI–CogI group. In order to capture CogI broadly across elderly populations with and without TBI, we did not require memory impairment, therefore, it is likely that many of these veterans did not have AD, but rather mild, mixed type of dementia.
Many of the biomarkers elevated after acute TBI are also elevated in people with AD and may be nonspecific markers of neurodegeneration. GFAP and p-tau41 are both increased in neuronally derived exosomes in AD and have been shown to predict conversion from mild cognitive impairment to dementia.42 NfL is also increased in AD45 as well as other neurodegenerative diseases. It is likely that these proteins are markers of neuronal degradation or loss, rather than markers of mechanisms specific to TBI–CogI or AD.14
In addition to abnormal protein accumulation, inflammation plays a critical role in both the acute and chronic phases of TBI and CogI. Increased activity of inflammatory cytokines, particularly interleukin, in the acute period after TBI have been associated with worse outcomes.46 Little is known about the association between TBI-associated cognitive and behavioral symptoms and chronic neuroinflammation, but recent evidence suggests there may be a link.17 Chronic neuroinflammation is also characteristic of other neurodegenerative disorders, including AD and CTE, and may contribute to the accumulation of toxic proteins. In this study, we found that levels of TNF-α and IL-6 were increased in CogI and TBI.
The study has several strengths, including that participants completed a highly detailed clinical evaluation with a comprehensive neuropsychological battery to determine cognitive impairment. Also, the study used the OSU-TBI-ID, recommended by the NINDS, for TBI determination and corroboration of TBI history with chart review whenever possible. Our findings are also strengthened by the analysis of multiple groups to consider both the TBI and CogI variables. In addition, we only enrolled veterans residing in the same retirement communities for all groups, so that the groups were as homogeneous as possible and limiting unaccounted confounders.
The results of this study need to be interpreted with a few considerations in mind. We used a highly regarded clinical interview to determine TBI history; however, much of the TBI history reported by participants was remote. This may have led to inaccurate reporting of details of very distant TBIs. The number of participants in each group was relatively modest; larger group sizes may have allowed for stronger and more significant findings. Due to the cross-sectional design of the study, we are unable to determine whether the CogI present was chronic or progressive. In addition, while we went to great lengths to do a comprehensive assessment of each participant including medical chart review, cognitive battery, and behavioral and functional assessments, we did not have recent neuroimaging for most participants. Therefore, it is difficult to describe etiology of cognitive impairment in the noTBI–CogI group and the TBI–CogI group. Due to the age and high levels of comorbidities in the veteran sample, we speculate that the CogI in the no TBI group likely has vascular as well as AD etiology. The final limitation was a homogeneous population of predominantly elderly white men, which, while representative of the veteran population residing at the study sites, may not be representative of the overall veteran or civilian elderly population and makes our results less generalizable to female or ethnically diverse elderly populations.
Overall our study found that increased levels of blood-based, centrally derived exosomal protein biomarkers associated with TBI–CogI can be detected even decades after TBI. The combined markers differentiated TBI and CogI status and consisted of neurodegenerative proteins and inflammatory cytokines. Understanding the etiology of TBI–CogI is essential for the development of targeted therapies.
Acknowledgment
This project was supported by the Department of Defense (W81XWH-14-2-0137). The authors thank their research participants; the Armed Forces Retirement Home in Washington, DC; the Veterans Home of California in Yountville; their dedicated study staff, including Kim Kelley, Dan Freimer, and Cora Davis; and Feng Xia, MPH, for data analysis.
Glossary
- α-syn
α-synuclein
- Aβ42
β-amyloid 42
- AD
Alzheimer disease
- AUC
area under the curve
- AVLT
Auditory Verbal Learning Test
- CI
confidence interval
- CogI
cognitive impairment
- CTE
chronic traumatic encephalopathy
- GFAP
glial fibrillary acidic protein
- IL-6
interleukin-6
- IL-10
interleukin-10
- LOC
loss of consciousness
- MMSE
Mini-Mental State Examination
- mTBI
mild traumatic brain injury
- NfL
neurofilament light
- NINDS
National Institute of Neurologic Disorders and Stroke
- OSU-TBI-ID
Ohio State University TBI Identification Method
- p-tau
phosphorylated tau
- ROC
receiver operating characteristic
- TBI
traumatic brain injury
- TNF-α
tumor necrosis factor–α
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This project was supported by the Department of Defense (W81XWH-14-2-0137). The views expressed in this publication are those of the authors and do not represent official views or policy of the Department of Defense, Department of Veterans Affairs, or the United States Government.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352:2043–2047. [DOI] [PubMed] [Google Scholar]
- 2.Cameron KL, Marshall SW, Sturdivant RX, Lincoln AE. Trends in the incidence of physician-diagnosed mild traumatic brain injury among active duty U.S. military personnel between 1997 and 2007. J Neurotrauma 2012;29:1313–1321. [DOI] [PubMed] [Google Scholar]
- 3.Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil 2006;21:398–402. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology 2014;83:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 2003;74:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 2014;71:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology 2000;54:1316–1323. [DOI] [PubMed] [Google Scholar]
- 8.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 2000;55:1158–1166. [DOI] [PubMed] [Google Scholar]
- 9.Peltz CB, Gardner RC, Kenney K, Diaz-Arrastia R, Kramer JH, Yaffe K. Neurobehavioral characteristics of older veterans with remote traumatic brain injury. J Head Trauma Rehabil 2017;32:E8–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 2018;75:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 2013;8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenney K, Iacono D, Edlow BL, et al. Dementia after moderate-severe traumatic brain injury: coexistence of multiple proteinopathies. J Neuropathol Exp Neurol 2018;77:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol 2016;12:563–574. [DOI] [PubMed] [Google Scholar]
- 14.Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev 2016;68:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neselius S, Zetterberg H, Blennow K, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj 2013;27:425–433. [DOI] [PubMed] [Google Scholar]
- 16.Andreasson U, Blennow K, Zetterberg H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimers Dement 2016;3:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill J, Mustapic M, Diaz-Arrastia R, et al. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj 2018;32:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetzl EJ, Peltz CB, Mustapic M, Kapogiannis D, Yaffe K. Neuron-derived plasma exosome proteins after remote traumatic brain injury. J Neurotrauma 2019;27:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenney K, Qu BX, Lai C, et al. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj 2018;32:1276–1284. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI identification method. J Head Trauma Rehabil 2007;22:318–329. [DOI] [PubMed] [Google Scholar]
- 22.Rey AL. L'Examen Clinique en Psychologie. Paris: Presses, Universitaires de France; 1964. [Google Scholar]
- 23.Taylor EM. Psychological Appraisal of Children with Cerebral Deficits. Cambridge: Harvard University Press; 1959. [Google Scholar]
- 24.Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation, Harcourt Brace & Company; 1997. [Google Scholar]
- 25.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014;71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psychol Assess 1990;2:304. [Google Scholar]
- 27.Ivnik RJ, Malec J, Smith GE, et al. Mayo's older Americans normative studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychologist 1992;6:83–104. [Google Scholar]
- 28.Shirk SD, Mitchell MB, Shaughnessy LW, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther 2011;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rissin DM, Fournier DR, Piech T, et al. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem 2011;83:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012;76:886–899. [DOI] [PubMed] [Google Scholar]
- 31.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol 2013;9:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013;9:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma 2014;31:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogousslavsky T, Wilson D, Chen Y, et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid beta up to 90 days after traumatic brain injury. J Neurotrauma 2017;34:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vos PE, Jacobs B, Andriessen TM, et al. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 2010;75:1786–1793. [DOI] [PubMed] [Google Scholar]
- 39.Wiesmann M, Steinmeier E, Magerkurth O, Linn J, Gottmann D, Missler U. Outcome prediction in traumatic brain injury: comparison of neurological status, CT findings, and blood levels of S100B and GFAP. Acta Neurol Scand 2010;121:178–185. [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein R, Chang B, Yue JK, et al. Comparing plasma phospho tau, total tau, and phospho tau-total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol 2017;74:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 2015;11:600–607.e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement 2016;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetzl EJ, Elahi FM, Mustapic M, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J 2019;33:5082–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaup AR, Peltz C, Kenney K, Kramer JH, Diaz-Arrastia R, Yaffe K. Neuropsychological profile of lifetime traumatic brain injury in older veterans. J Int Neuropsychol Soc 2017;23:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer's Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinson HE, Rowell S, Schreiber M. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg 2015;78:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article will be shared in anonymized form by request.