Abstract
Objective
The Systemic Synuclein Sampling Study (S4) measured α-synuclein in multiple tissues and biofluids within the same patients with Parkinson disease (PD) vs healthy controls (HCs).
Methods
S4 was a 6-site cross-sectional observational study of participants with early, moderate, or advanced PD and HCs. Motor and nonmotor measures and dopamine transporter SPECT were obtained. Biopsies of skin, colon, submandibular gland (SMG), CSF, saliva, and blood were collected. Tissue biopsy sections were stained with 5C12 monoclonal antibody against pathologic α-synuclein; digital images were interpreted by neuropathologists blinded to diagnosis. Biofluid total α-synuclein was quantified using ELISA.
Results
The final cohort included 59 patients with PD and 21 HCs. CSF α-synuclein was lower in patients with PD vs HCs; sensitivity/specificity of CSF α-synuclein for PD diagnosis was 87.0%/63.2%, respectively. Sensitivity of α-synuclein immunoreactivity for PD diagnosis was 56.1% for SMG and 24.1% for skin; specificity was 92.9% and 100%, respectively. There were no significant relationships between different measures of α-synuclein within participants.
Conclusions
S4 confirms lower total α-synuclein levels in CSF in patients with PD compared to HCs, but specificity is low. In contrast, α-synuclein immunoreactivity in skin and SMG is specific for PD but sensitivity is low. Relationships within participants across different tissues and biofluids could not be demonstrated. Measures of pathologic forms of α-synuclein with higher accuracy are critically needed.
Classification of evidence
This study provides Class III evidence that total CSF α-synuclein does not accurately distinguish patients with PD from HCs, and that monoclonal antibody staining for SMG and skin total α-synuclein is specific but not sensitive for PD diagnosis.
Parkinson disease (PD) accounts for a large proportion of the global burden of disease.1 Many clinical trials in PD have failed to identify disease-modifying therapies.2 To have a meaningful impact, intervention likely must occur in the earliest stages of pathology.3 Accurate PD biomarkers are needed to enable early diagnosis, test for target engagement, and serve as surrogate measures of disease in clinical trials.
α-Synuclein is a lead candidate PD biomarker based on its key role in PD pathophysiology. Studies show tremendous overlap between patients with PD and healthy controls (HCs) in total α-synuclein values in biofluids,4,5 likely due to various factors.6 There are, in addition, conflicting reports on occurrence of pathologic α-synuclein in peripheral tissue in PD,7–9 attributable to methodologic factors including specimen acquisition/processing, α-synuclein staining methods, and neuropathologist expertise and blinding.9 Studying the distribution of α-synuclein across the central and peripheral nervous system in vivo in patients with PD alongside HCs contributes to our understanding of PD pathophysiology, distribution of α-synuclein pathology, and disease progression.
The Systemic Synuclein Sampling Study (S4) assessed key gaps in knowledge by comparing interindividual and intraindividual total α-synuclein in CSF and peripheral (blood, saliva) fluid compartments, and the occurrence of immunohistochemically defined α-synuclein pathology in 3 peripheral tissues (colon, skin, and submandibular gland [SMG]) at different PD stages compared to HCs. Analyzing biofluids and tissue, we tested 2 main hypotheses: that (1) α-synuclein biomarkers in CSF and SMG have the highest sensitivity and specificity for PD diagnosis and (2) there are significant relationships among within-subject measures of α-synuclein.
Methods
The full S4 protocol is available at michaeljfox.org/files/S4_Clinical_Study_Protocol_Version_2.pdf.
The primary research questions addressed in this study were as follows:
1. What is the diagnostic accuracy of total α-synuclein in CSF, serum, plasma, whole blood, and saliva as an in vivo PD biomarker?
2. What is the prevalence of positive staining for pathologic α-synuclein in skin, SMG, and colon in patients with PD vs HCs and what is its diagnostic accuracy in each of these tissues as a PD biomarker?
3. What is the intraindividual, i.e., within-subject, relationship between α-synuclein in tissues and biofluids?
The design of this study provides Class III evidence for questions 1 and 2 and Class IV evidence for question 3.
Participants
S4, a cross-sectional observational study, enrolled participants from October 2015 to August 2017 at 6 sites in North America. As previously described,10–12 the study aimed to recruit 60 individuals with a diagnosis of idiopathic PD. Enrollment criteria were (1) age ≥40 years; (2) clinical diagnosis of PD, requiring presence of bradykinesia plus either rest tremor or rigidity; (3) decreased dopamine transporter (DAT) binding on SPECT (based on age-matched normative data); and (4) classification into 1 of 3 groups of disease severity: early PD (≤2 years since diagnosis, not treated with dopaminergic medication), moderate PD (2–5 years since diagnosis treated with dopaminergic medication but without motor fluctuations), and advanced PD (≥5 years since diagnosis, with motor fluctuations).
Twenty-one HCs, with normal DAT SPECT, were also recruited. In both groups, exclusion criteria were clinical diagnosis of dementia based on the site investigator's determination and comorbid medical conditions precluding specimen acquisition, as described.12
Standard protocol approvals, registrations, and patient consents
The study was performed in accordance with the Declaration of Helsinki. Written informed consent was provided by all participants. Institutional review board approval was obtained at each study site.
Assessments
Clinical assessments, imaging, and biospecimen acquisition occurred as follows:
Clinical/imaging assessments:
Demographics
Medical/neurologic history and medications
Motor assessments: Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III and IV and Hoehn & Yahr stage
Nonmotor assessments: neuropsychiatric symptoms were assessed with the MDS-UPDRS I and the Montreal Cognitive Assessment13; autonomic symptoms were assessed with the Scales for Outcomes in Parkinson's Disease–Autonomic14; presence of possible REM sleep behavior disorder (pRBD) was ascertained via review of study medical history and medication logs, which in turn reflected information obtained by the site investigator via patient interview. Participants were classified as having pRBD if they were listed as having RBD on the medical condition log or if RBD was listed as an indication for a medication on the medication log.
Functional status: Modified Schwab and England Activities of Daily Living Scale and MDS-UPDRS part II
Olfaction was assessed with the University of Pennsylvania Smell Identification test: participants were classified as normosmic, hyposmic, or anosmic based on normative data accounting for age and sex15
DAT SPECT: mean striatal specific binding ratio (SBR) calculated as average of putamen and caudate SBR on right and left
Biofluid and peripheral tissue collection and α-synuclein detection
Procedures for collection of specimens and analysis for α-synuclein and hemoglobin are detailed in the S4 biologics manual16 and described in previous publications.10,12 Briefly, biofluids collected included CSF, blood, and saliva. Total α-synuclein was measured using a commercially available ELISA.10 Specimens with hemoglobin levels considered to be high enough to affect α-synuclein measures were excluded from analyses (200 ng/mL for CSF, saliva, and serum and 35 ng/mL for plasma4,17). To further account for possible influence of hemoglobin on α-synuclein levels in serum and plasma, we explored α-synuclein/hemoglobin ratio.4
Biopsies of skin, sigmoid colon, and SMG were obtained as described.10–12,16 Briefly, two 3-mm skin punch biopsies were obtained from the paravertebral posterior–inferior cervical area and 2 from the mid-thigh. Up to 8 colon biopsies were obtained via flexible sigmoidoscopy, and up to 5 SMG biopsies were obtained using a 16-gauge core biopsy instrument. Biopsies were processed as described.10,16 Four slides spaced equidistantly in each cassette were stained with hematoxylin & eosin to assess for the presence of adequate tissue, defined by ≥1 fragment(s) of a given tissue type containing a minimal aggregate amount (2 mm2) of target tissue. Target tissue was defined as glandular tissue (epithelial secretory acini and intervening stroma) for SMG, submucosa for colon, and dermis for skin.
Three slides from each cassette (each containing 2–3 biopsy fragments) were subjected to a protease, which removes nonpathologic forms of α-synuclein, then stained with the mouse monoclonal immunoglobulin 5C12 antibody as described.10,11 The immunohistochemical staining method applied in S4 was chosen after a rigorous, blinded comparison of multiple protocols available at the time of initiation of S4, using gold standard autopsy-confirmed peripheral tissue samples, as described.11 Images of stained slides were distributed to 3 independent neuropathologists blinded to diagnostic group. Neuropathologist ratings were used to classify each slide as positive or negative for α-synuclein pathology, as defined morphologically during training.11 For a given participant, up to 12 slides of colon, 6 slides of skin, and 6 slides of SMG could be stained and interpreted, but this varied based on presence of target tissue and staining adequacy.
Statistical analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Fisher exact, Wilcoxon rank-sum, and Kruskal-Wallis tests were used to compare demographics and clinical characteristics between the HC vs PD group and among PD subgroups.
Linear regression was used to compare biofluid α-synuclein measures across groups; all such models included age as a covariate and specified biofluid α-synuclein rank values as the outcome variable (with average ranks assigned in the event of tied values). Sensitivity and specificity of biofluid total α-synuclein levels, as a test for both PD diagnosis (PD vs HC) and disease severity (advanced vs early PD), were explored using cutoff values derived from receiver operating characteristic (ROC) curves. Optimal cutoffs were defined by the point on the ROC curve that yielded the highest Youden index (J = sensitivity + specificity − 1).18,19 ROC curves were based on univariate logistic regression models and estimates of the area under the ROC curve (AUC) were also computed.
Peripheral tissue α-synuclein measures were compared both at the participant and slide level. Subject-level differences were assessed using exact Cochran-Mantel-Haenszel tests of the association between group and α-synuclein positivity while adjusting for age tertile (<59 vs 59–65 vs >65 years); where applicable, group was defined as a nominal variable.
A participant was considered positive for pathologic α-synuclein for a given tissue when ≥1 slide was rated positive for α-synuclein by ≥2 neuropathologists. In sensitivity analyses, each slide examined was accounted for by using generalized estimating equations to assess the effect of group on slide positivity, while adjusting for age and correlation across slides collected from the same individual (based on an exchangeable correlation structure).
Exploratory analyses comparing patients with PD with positive vs negative tissue biopsies assessed categorical variables using odds ratios and continuous variables based on differences in medians, with 95% confidence intervals (CIs) derived using bootstrap resampling (n = 1,000 iterations).
Sample size justification
S4 was estimated to have 80% power to detect a difference of 40% or more in CSF total α-synuclein between patients with PD and HCs, with a sample size of 20 participants per group, and assuming a significance level of 0.05. This was based on data from the Parkinson's Progression Markers Initiative, in which mean baseline CSF levels of total α-synuclein were 1,845 ± 770 pg/mL.20 Due to the widely varying nature of tissue acquisition and interpretation for tissue α-synuclein in the literature, sample size calculations related to tissue were not considered feasible for the study here.
Statistical analysis was 2-sided and assessed for significance at the 5% level.
Data availability
De-identified data collected for this study will be available online at braincommons.org by December 31, 2020. Data requestors will need to sign a data access policy agreement via the BRAIN Commons website (braincommons.org) to gain access.
Results
Cohort characteristics
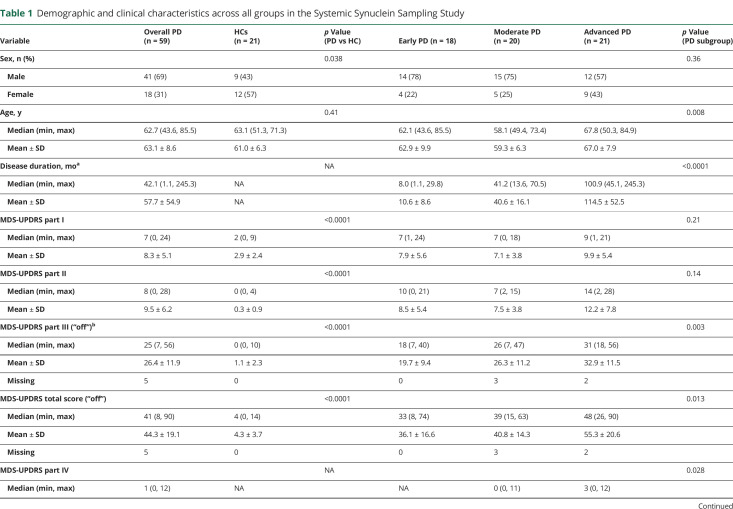
Eighty-two participants were enrolled. Eighty (59 PD, 21 HCs) contributed ≥2 tissue and ≥2 biofluid specimens and are included in this analysis. Cohort characteristics are shown in table 1. Male:female ratio was higher in PD vs HCs. The moderate PD subgroup was younger than the advanced PD subgroup.
Table 1.
Demographic and clinical characteristics across all groups in the Systemic Synuclein Sampling Study
As expected, the PD group had higher scores on all measures of motor and nonmotor manifestations of PD, and lower DAT binding, compared to HC. There was a gradient of increasing measures of motor severity with increasing disease duration when comparing the early vs moderate vs advanced PD subgroups (table 1). DAT binding was lowest in the advanced group, highest in the early group, and intermediate between the 2 in the moderate group.
α-Synuclein levels in biological fluids
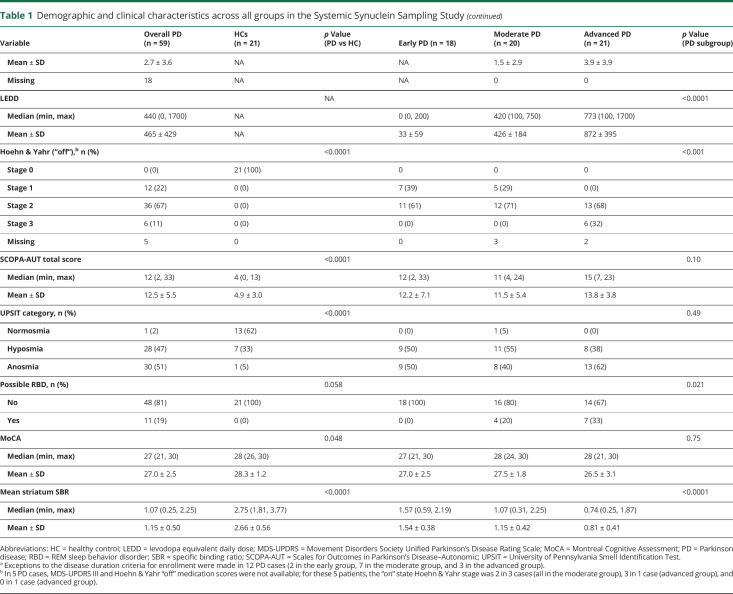
Overall, total α-synuclein levels in CSF were lower in PD vs HC (table 2 and figure 1). Sensitivity and specificity for PD diagnosis for CSF α-synuclein was 87.0% and 63.2%, respectively, and the AUC was 0.693 (95% CI 0.521–0.865). Total α-synuclein levels in the other biofluids were not significantly different among the PD and HC groups (table 2) and their sensitivity and specificity were therefore not considered.
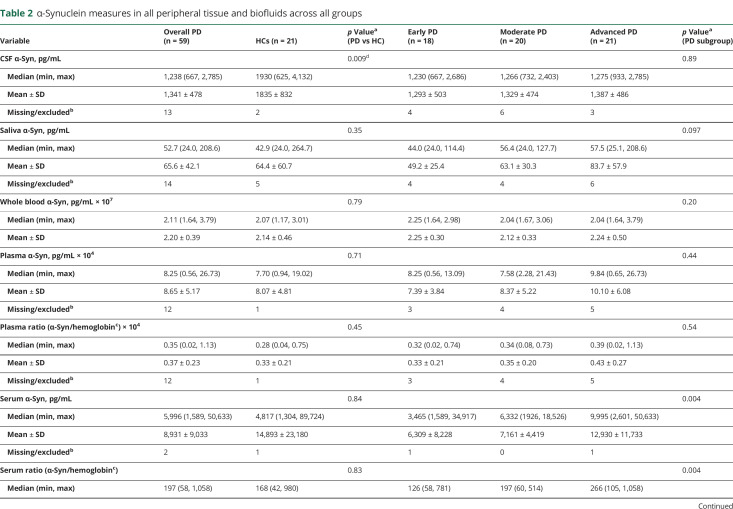
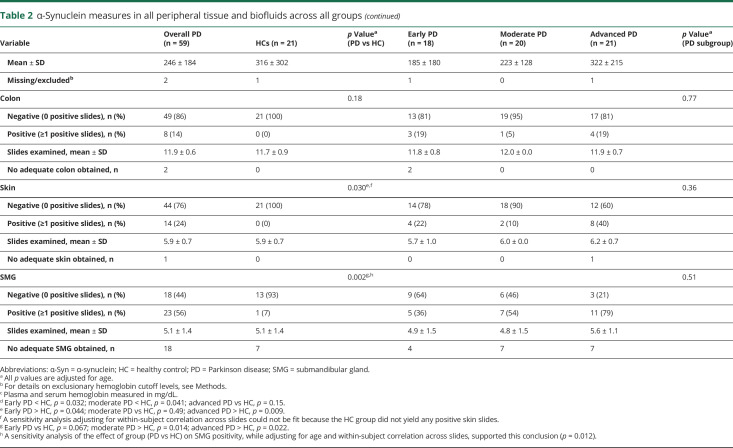
Table 2.
α-Synuclein measures in all peripheral tissue and biofluids across all groups
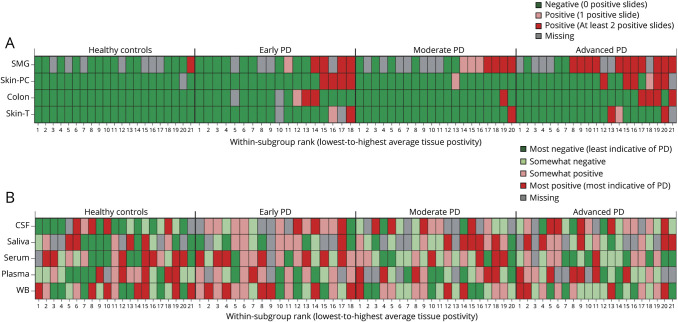
Figure 1. Heat map depicting, by subgroup, the relative degree of α-synuclein (α-Syn) positivity.
α-Syn positivity across (A) each tissue type, as defined by the number of positive slides; and (B) each biofluid, as defined using quartile scores. For CSF and serum α-Syn, rank values in the lower quartile (i.e., the lowest 25% of values) were defined as most indicative of Parkinson disease (PD), whereas for saliva, plasma, and whole blood (WB) α-Syn, rank values in the upper quartile (i.e., the highest 25% of values) were defined as most indicative of PD. For each panel, participants are sorted by subgroup in order from lowest-to-highest average tissue positivity; accordingly, each given column across the 2 panels corresponds to the same participant. PC = paravertebral cervical; SMG = submandibular gland; T = thigh.
Within the PD group, α-synuclein levels in serum were successively higher in progressive disease stages (table 2 and figures 1 and 2). In light of this finding, sensitivity, specificity, and the AUC of total serum α-synuclein for distinguishing between advanced vs early PD were explored and were 80.0%, 70.6%, and 0.776 (95% CI 0.619–0.934), respectively.
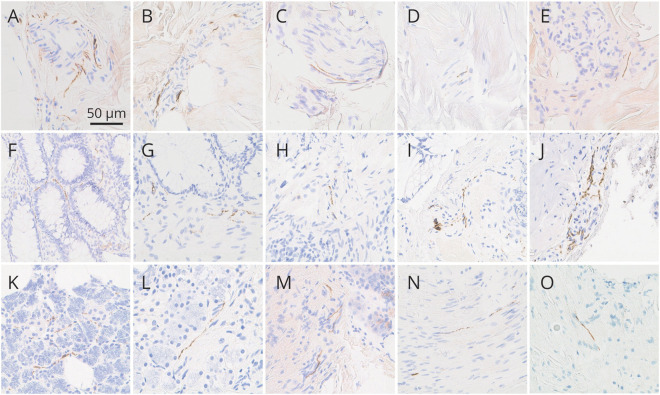
Figure 2. Photomicrographs of skin, colon, and submandibular gland immunohistochemically stained for pathologic α-synuclein with the 5C12 monoclonal antibody-based method.
Photomicrographs of skin (A–E), colon (F–J), and submandibular gland (K–O). All panels show immunoreactivity independently judged by at least 2 of 3 blinded neuropathologists to represent specific positive staining of nerve fibers. Specific positive staining in skin was most often seen in dermal periarteriolar locations (A, B) and within small intradermal nerve fascicles (C, D) and less often adjacent to sweat glands (E). Specific positive staining was present in the lamina propria of the mucosa (F, G) but more often in submucosal nerve fibers (H–J), sometimes in periarteriolar locations (J). Specific positive staining in submandibular gland was seen both in the glandular parenchyma (K, L) and stroma (M–O); in stroma, it was often localized to nerve fascicles (N, O). The calibration bar in (A) serves for all panels.
α-Synuclein in peripheral tissues
The mean number of slides per tissue type per diagnostic group examined by each neuropathologist is shown in table 2. Images of typical slides rated as positive by neuropathologists are shown in figure 2. Tissue biopsies for HCs were negative in all but one case. The number of patients with PD with positive staining for α-synuclein was 8/57 for colon, 14/58 for skin, and 23/41 for SMG; this was significantly greater in PD vs HCs in skin and SMG (table 2 and figure 1). The sensitivity of SMG α-synuclein staining for PD diagnosis (across disease stages) was 56.1% while specificity was 92.9%. The sensitivity of skin α-synuclein staining for PD diagnosis was 24.1% with a specificity of 100%. Skin was significantly more likely to be positive in early and advanced PD compared to HC, and SMG in moderate and advanced PD compared to HC. When restricting analyses to the advanced PD vs HC groups, sensitivity of SMG α-synuclein positivity was 78.6% and specificity was 92.9%; sensitivity of skin α-synuclein positivity was 40.0% and specificity was 100%.
Among the 39 patients with PD with all 3 tissues sites assessed, 14 had negative α-synuclein staining across all sites, 13 had 1 tissue positive, and 11 had 2 tissues positive, including 8 with positive SMG and skin vs 3 with positive SMG and colon. Only 1 patient, in the advanced PD group, had all 3 tissues positive; even in this case, only 1 of 2 skin sites was positive.
Fourteen patients with PD had a positive skin slide, of whom 13 had adequate specimens obtained from both the paravertebral cervical region and thigh. Of those 13, 3 had only thigh region positive, 7 had only paravertebral cervical region positive, and 3 (2 early PD and 1 advanced PD) had positivity in both regions.
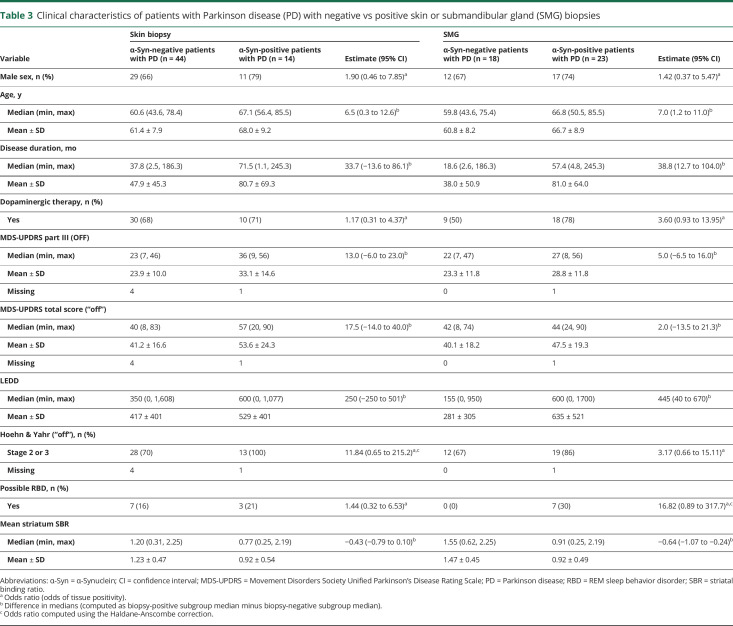
There were no differences among the 3 PD subgroups in the number of slides examined for any tissue type. The advanced PD group had the highest proportion of participants positive for skin and SMG, but these differences were not statistically significant (table 2). Participants with skin biopsy positive for α-synuclein (n = 14) were older compared to those without, as were those with positive SMG biopsy (table 3). There were no significant differences in motor and nonmotor measures in those with positive biopsy compared to those without. However, of note, all SMG biopsies obtained from patients with PD with pRBD yielded positive α-synuclein staining. A significant relationship was found between the odds of SMG positivity and mean striatal SBR, adjusting for age and disease duration (odds ratio 0.11 [95% CI 0.02–0.67]).
Table 3.
Clinical characteristics of patients with Parkinson disease (PD) with negative vs positive skin or submandibular gland (SMG) biopsies
Within-subject α-synuclein across tissue and biofluids
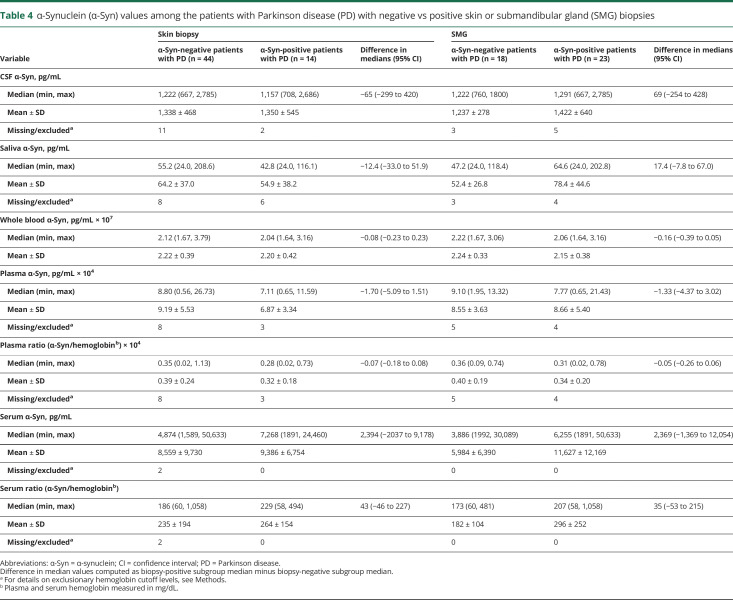
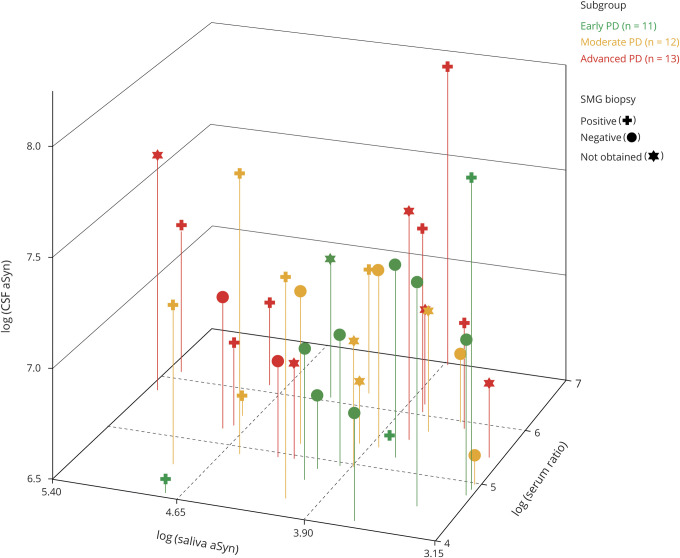
In the PD group, there were no significant differences in α-synuclein levels in any biofluid among those with vs without positive α-synuclein in skin or SMG tissue (table 4 and figure 3).
Table 4.
α-Synuclein (α-Syn) values among the patients with Parkinson disease (PD) with negative vs positive skin or submandibular gland (SMG) biopsies
Figure 3. Scatterplot of CSF α-synuclein (α-Syn) (pg/mL) vs saliva α-Syn (pg/mL) vs the ratio of serum α-Syn (pg/mL) to serum hemoglobin (mg/dL) among patients with Parkinson disease (PD) with adequate specimens obtained for all 3 biofluids.
All values were plotted on the log scale. Each point represents a single patient with PD, with different colors and symbols indicating disease stage and submandibular gland (SMG) biopsy status.
Discussion
S4 measured α-synuclein in multiple biofluids and peripheral tissues within patients with PD of different disease stages and compared to HC. The study was conducted with rigorous standard operating procedures (SOPs) and training of involved neurologists, gastroenterologists, otolaryngologists, and neuropathologists together with a robust infrastructure providing core study functions, including clinical site management, biorepository, statistical, and neuropathologic expertise. This allowed centralized and standardized specimen processing and analysis. We were not able to replicate several previously reported findings regarding high sensitivity of pathologic α-synuclein deposits in colon (up to 100%),21 skin (>90%),22 or SMG (up to 100%).23–27 Also, we did not find a correlation between intraindividual measures of total α-synuclein across the various biofluids and tissues as we had hypothesized. Despite that, several important insights regarding the distribution of α-synuclein have been garnered from this study: (1) total CSF α-synuclein was significantly lower in the PD compared to the HC group but the specificity of total CSF α-synuclein for PD diagnosis is low; (2) serum α-synuclein levels increase with disease severity, but do not distinguish patients with PD from HC; (3) tissue positivity was significantly more likely in PD vs HC in skin and SMG, and was highly specific for PD, though with low sensitivity in skin and SMG, at least in early PD23–25; (4) SMG tissue positivity had relatively high sensitivity and specificity in advanced PD and was related to DAT binding; and (5) positive tissue was seen across all PD disease stages (advanced > early).
Similar to other studies,4,5 we found lower total CSF α-synuclein in the PD group compared to the HC group. However, total α-synuclein in the other biofluids was not different in patients with PD vs HCs. The results are similar to another recent multicenter study, using the same SOP and analytical kits.8 This most likely relates to the “total” nature of α-synuclein being quantified with currently available assays and antibodies. While several assays for total α-synuclein have been developed and proven to be robust in several independent cohorts and one large round robin study,7 they differ in the exact epitope bound by the antibodies. No method ensures that full-length α-synuclein is actually present, as opposed to N- or C-terminal-truncated versions. These assays also have no specificity for PD-specific pathologic forms of α-synuclein. Another challenge in measurement of α-synuclein in biofluids, especially whole blood and its compartments, is the presence of massive amounts of α-synuclein in red blood cells and the influence of hemolysis on α-synuclein levels. It is likely because of these limitations that total α-synuclein in CSF performs only moderately well as a PD diagnostic biomarker, especially in smaller cohorts. Additional work, using assays that are more sensitive, detect disease-specific forms of α-synuclein, and/or different strains and aggregation propensity are needed.28–31 For example, the measurement of soluble oligomeric forms of α-synuclein may offer the advantage of detecting the earliest pathologic changes occurring in α-synuclein.32 “Seeding assays,” such as the real‐time quaking‐induced conversion technique or protein misfolding cyclic amplification that both capitalize on the prion-like aggregation properties of α-synuclein also hold great promise, as several studies show, independently and robustly across different laboratories, high sensitivity and specificity for PD diagnosis.33 In turn, more disease-specific α-synuclein measures in biofluids may better correlate with tissue α-synuclein, especially if combined with application of seeding assays to peripheral tissue.34 This approach will be tested in remaining S4 samples. As for differences in biofluid α-synuclein in relationship to PD disease severity, we found significant stage-wise differences in total α-synuclein in serum, raising the possibility that serum α-synuclein may have the potential as a marker of PD severity. However, serum total α-synuclein did not distinguish the overall PD group from HCs, indicating that more accurate assays are needed in this regard.
Regarding the utility of peripheral tissue α-synuclein immunostaining as a PD biomarker, the sensitivity of α-synuclein for PD diagnosis in the literature, using various methods, has been variably estimated at 36%–100% in colon,21 80%35 to >90%22 in skin, and 74%–100%23–27 in SMG. In the small studies done to date, specificity of α-synuclein has generally been high (>90%) for SMG and skin but variable for colon.9,21,36 In S4, we found high specificity of both SMG and skin α-synuclein positivity for PD diagnosis, confirming some previous results.23,24,37 The prevalence of colon positivity was exceedingly low, though.
Among all the tissues studied, SMG α-synuclein had the highest sensitivity, though it remained only moderate at 56%. This improved when restricting analyses to the advanced PD group. The relatively low sensitivities for skin and SMG at earlier disease stages could reflect inclusion of misdiagnosed patients with parkinsonism due to the higher ratio, in S4, of earlier PD disease stages relative to prior studies.23 While the use of DAT SPECT makes misdiagnosis less likely,38 it does not eliminate it especially among neurodegenerative parkinsonian syndromes.39
The infrequent occurrence of α-synuclein positivity in colon, despite examination of twice the number of slides compared to other tissues, suggests that, using current immunohistologic methods, colon is not an attractive biomarker site for PD. This is in contrast to a study showing high sensitivity of colon α-synuclein21; those results may have stemmed from false-positive nonspecific staining, risk of which is mitigated by improved staining protocols and rigorous pathologist training, as used in a multicenter blinded-panel study and by S4.9,11,40 Another possibility is that the S4 colon biopsies did not sample sufficient nervous structures within the superficial colon submucosa. While only biopsies confirmed to contain colonic submucosa were analyzed in S4, neuron-specific staining of colon tissue to confirm presence of neuronal tissue elements was not conducted. Deeper and thereby more invasive biopsies, attempting to obtain greater amounts of colonic submucosa, may increase yield, but with higher risk. Postbiopsy microdissection techniques warrant further study as a means of improving sensitivity.41 Another point regards the distal location of gastrointestinal biopsy chosen in S4: the sigmoid colon was chosen because sigmoidoscopy is safe and requires minimal preparation. However, there is a known rostral-caudal gradient of α-synuclein immunoreactivity across the gastrointestinal (GI) tract, with higher α-synuclein load in more proximal/oral areas.21,42,43 Recent data point to the promising application of seeding assays also to biopsies in more rostral GI regions, including the stomach, as well as other rostral areas of the environment–brain interface, such as the olfactory mucosa,34,44 to identify sensitive PD biomarkers. Future multicenter studies that follow similar rigorous methodology to S4 will be needed to replicate these promising preliminary results.
With respect to skin, we found a lower prevalence of positive skin biopsies in the S4 PD cohort as compared to other studies.35,37,45–47 The high specificity of the S4 immunohistochemical method,11 the neuropathologist training and blinding, and consensus-based decision-making could have resulted in fewer false-positives. The staining protocol in S4 included pretreatment with a protease prior to 5C12 antibody application. Extensive prestudy testing evidenced this method optimized specificity and reduced false-positive staining for nonpathologic forms of α-synuclein. While the 5C12 protocol chosen for S4 was also the most sensitive among multiple tested protocols, it is possible that some methods used by non-S4 groups may be more sensitive. A double-immunostaining protocol for α-synuclein phosphorylated at serine-129 had a 70%–100% sensitivity and 100% specificity for PD diagnosis in small studies,35 and preliminary work to replicate across sites has demonstrated high reproducibility though lower sensitivity than in the single-center studies.47 Application of that and other antibodies with specific affinity for toxic/pathogenic forms of α-synuclein, such as truncated or aggregated forms, should be tested, and S4 provides a robust biorepository for such work in the future. Another area of promise that warrants investigation is the application of seeding assays to tissue.34
S4 results suggest that tissue α-synuclein positivity (in SMG and skin) is seen across all PD stages. We also noted a relationship between presence of SMG α-synuclein and lower DAT binding, a measure of striatonigral denervation. However, the small sample sizes across disease stages limits interpretation and studies in a larger number of subjects and longitudinal studies are needed to further explore this observation. The higher prevalence of positivity in the advanced PD group argues against the idea that with long-standing PD, loss of peripheral nerve fibers reduces the chances of detection of α-synuclein,48 at least in the regions biopsied in S4. Our results do not support the hypothesis that peripheral pathology begins before the affection of the CNS in PD,49 but our study was not designed to test this hypothesis: we did not include prodromal/at-risk subjects and our early PD cohort was small. It is also possible that the 5C12 monoclonal antibody is less sensitive to the species of pathologic α-synuclein seen in early PD. This needs to be tested in a larger and longitudinal cohort that includes participants along a spectrum of increased risk for PD, and again using assays that more accurately detect pathologic forms of α-synuclein, especially in early PD.
As for the within-subject distribution of α-synuclein, while the 2 tissue types most often demonstrating positivity together were skin and SMG, only 31% of the PD cohort had 2 or more positive tissues. These findings suggest that in the periphery, α-synuclein distribution is patchy rather than diffuse. This will be an important consideration in interpreting future studies of peripheral tissue α-synuclein. Regarding the relationship between biofluid and tissue α-synuclein, saliva α-synuclein was higher in those with positive SMG, but this did not reach significance. Differences in the type of α-synuclein detected in biofluids vs tissue may account for the lack of correlations of α-synuclein biofluid levels–tissue positivity within subjects.
S4 is a multicenter study with rigorous methods across all stages of specimen acquisition, processing, and interpretation, which is a major strength. However, several study limitations warrant mention. The small sample size in the PD subgroups limits power to detect differences and interpret the results. As discussed above, the assays used for biofluid α-synuclein detect total α-synuclein, and not necessarily its pathologic forms. In tissue, the usage of protease pretreatment successfully removes nonpathologic forms of α-synuclein and also improves epitope exposure in paraffin-embedded tissue. Without protease pretreatment, antibodies against total or unmodified α-synuclein stain normal peripheral nervous tissue, making it difficult or impossible to definitively identify α-synuclein pathology. However, overly aggressive protease treatment may impact staining sensitivity. Future work to identify more sensitive tissue α-synuclein stains that also preserve high specificity is needed.
Several conclusions can be drawn from S4. The safety and feasibility of multisite, multicenter tissue and biofluid sampling in PD has been demonstrated. Our biofluids work emphasizes the need for α-synuclein assays that measure PD-specific forms of α-synuclein. Even in CSF, where significant differences in total α-synuclein between PD and HC were found, there was still overlap among values (even after eliminating high hemoglobin samples) in the 2 groups and suboptimal sensitivity and specificity. While we found high specificity for PD diagnosis for α-synuclein staining with the 5C12 antibody in skin and SMG, S4 fails to replicate the high sensitivity reported in several small, single-center studies of mostly advanced PD cases in colon, skin, and SMG α-synuclein. Instead, S4 indicates that, with the method used, α-synuclein in these tissues is not a sensitive biomarker for PD diagnosis, at least in the early stages of PD, where accurate diagnostic biomarkers are most critically needed. As for the utility of tissue α-synuclein as a marker of disease severity, we found that tissue positivity occurs in all disease stages, and we report a novel finding of SMG positivity being more likely with greater striatal denervation as measured by DAT binding. This may indicate that SMG positivity could be a marker of more severe disease, but this requires additional studies and replication. With the limitations of current biofluid assays and tissue staining procedures, we also show a diffuse distribution of α-synuclein across the body and across disease stages, though without the hypothesized relationship across sites and fluids or within individual participants, at least in the small numbers of participants examined here. Importantly, S4 also provides the research community with samples of fluids and tissues (accessible via michaeljfox.org), with which to assess promising new assays and stains. Ultimately, understanding the distribution of α-synuclein in biofluids and tissues will help advance development of PD biomarkers and our understanding of PD pathology including its progression.
Acknowledgment
DaTscan doses were provided in-kind by GE Healthcare.
Glossary
- CI
confidence interval
- DAT
dopamine transporter
- GI
gastrointestinal
- HC
healthy control
- MDS-UPDRS
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- pRBD
possible REM sleep behavior disorder
- PD
Parkinson disease
- S4
Systemic Synuclein Sampling Study
- SBR
specific binding ratio
- SMG
submandibular gland
- SOP
standard operating procedure
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Editorial, page 373
Class of Evidence: NPub.org/coe
Study funding
Michael J. Fox Foundation for Parkinson's Disease Research.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 2.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol 2015;11:25–40. [DOI] [PubMed] [Google Scholar]
- 3.Lang AE, Espay AJ. Disease modification in Parkinson's disease: current approaches, challenges, and future considerations. Mov Disord 2018;33:660–677. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Mollenhauer B, Coffey CS, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson's disease: the Parkinson's progression markers initiative study. Acta Neuropathol 2016;131:935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. Alpha-synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 2011;10:230–240. [DOI] [PubMed] [Google Scholar]
- 6.Mollenhauer B, Batrla R, El-Agnaf O, et al. A user's guide for alpha-synuclein biomarker studies in biological fluids: perianalytical considerations. Mov Disord 2017;32:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollenhauer B, DuBois BF, Drake D, et al. Antibody-based methods for the measurement of alpha-synuclein concentration in human cerebrospinal fluid: method comparison and round robin study. J Neurochem 2019;149:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman JG, Andrews H, Amara A, et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: relationships among biomarkers and Parkinson's disease features. Mov Disord 2018;33:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JM, Derkinderen P, Kordower JH, et al. The search for a peripheral biopsy indicator of alpha-synuclein pathology for Parkinson disease. J Neuropathol Exp Neurol 2017;76:2–15. [DOI] [PubMed] [Google Scholar]
- 10.Chahine LM, Beach TG, Seedorff N, et al. Feasibility and safety of multicenter tissue and biofluid sampling for α-synuclein in Parkinson's disease: the systemic synuclein sampling study. J Parkinsons Dis 2018;8:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach TG, Serrano GE, Kremer T, et al. Immunohistochemical method and histopathology judging for the Systemic Synuclein Sampling Study (S4). J Neuropathol Exp Neurol 2018;77:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visanji NP, Mollenhauer B, Beach TG, et al. The systemic synuclein sampling study: toward a biomarker for Parkinson's disease. Biomark Med 2017;11:359–368. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA-AUT. Mov Disord 2004;19:1306–1312. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 16.Systemic Synuclein Sampling Study (S4). S4 Biologics Manual [online]. Available at: michaeljfox.org/files/S4_Biologics_Manual_Version_2.pdf. Accessed August 14, 2018. [Google Scholar]
- 17.Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 2010;133:713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 20.Marek K, Chowdhury S, Siderowf A, et al. Establishing a Parkinson's disease biomarker cohort. Ann Clin Transl Neurol 2018;5:1460–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung SJ, Kim J, Lee HJ, et al. Alpha-synuclein in gastric and colonic mucosa in Parkinson's disease: limited role as a biomarker. Mov Disord 2016;31:241–249. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology 2016;87:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler CH, Dugger BN, Hinni ML, et al. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 2014;82:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler CH, Dugger BN, Hentz JG, et al. Peripheral synucleinopathy in early Parkinson's disease: submandibular gland needle biopsy findings. Mov Disord 2016;31:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campo F, Carletti R, Fusconi M, et al. Alpha-synuclein in salivary gland as biomarker for Parkinson's disease. Rev Neurosci 2019;30:455–462. [DOI] [PubMed] [Google Scholar]
- 26.Vilas D, Iranzo A, Tolosa E, et al. Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol 2016;15:708–718. [DOI] [PubMed] [Google Scholar]
- 27.Shin J, Park SH, Shin C, et al. Submandibular gland is a suitable site for alpha synuclein pathology in Parkinson disease. Parkinsonism Relat Disord 2019;58:35–39. [DOI] [PubMed] [Google Scholar]
- 28.Shahnawaz M, Tokuda T, Waragai M, et al. Development of a biochemical diagnosis of Parkinson disease by detection of alpha-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74:163–172. [DOI] [PubMed] [Google Scholar]
- 29.Groveman BR, Orru CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol Commun 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang W, Chen W, Yang Q, et al. Salivary total alpha-synuclein, oligomeric alpha-synuclein and SNCA variants in Parkinson's disease patients. Sci Rep 2016;6:28143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 2016;3:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Steenoven I, Majbour NK, Vaikath NN, et al. Alpha-synuclein species as potential cerebrospinal fluid biomarkers for dementia with Lewy bodies. Mov Disord 2018;33:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang UJ, Boehme AK, Fairfoul G, et al. Comparative study of cerebrospinal fluid alpha-synuclein seeding aggregation assays for diagnosis of Parkinson's disease. Mov Disord 2019;34:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Luca CMG, Elia AE, Portaleone SM, et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson's disease and multiple system atrophy. Transl Neurodegener 2019;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol 2017;133:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visanji NP, Marras C, Kern DS, et al. Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 2015;84:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donadio V, Incensi A, Leta V, et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 2014;82:1362–1369. [DOI] [PubMed] [Google Scholar]
- 38.Massa J, Chahine LM. Revision of diagnosis in early parkinsonism with abnormal dopamine transporter imaging. J Parkinsons Dis 2019;9:327–334. [DOI] [PubMed] [Google Scholar]
- 39.Beach TG, Adler CH. Importance of low diagnostic accuracy for early Parkinson's disease. Mov Disord 2018;33:1551–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beach TG, Corbille AG, Letournel F, et al. Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson's disease and control subjects. J Parkinsons Dis 2016;6:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebouvier T, Neunlist M, Bruley des Varannes S, et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One 2010;5:e12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- 43.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenyi A, Leclair-Visonneau L, Clairembault T, et al. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol Dis 2019;129:38–43. [DOI] [PubMed] [Google Scholar]
- 45.Doppler K, Volkmann J, Sommer C. Skin biopsies in the differential diagnosis of parkinsonism: are we ready for simplified protocols? Brain 2016;139:e5. [DOI] [PubMed] [Google Scholar]
- 46.Donadio V, Incensi A, Piccinini C, et al. Skin nerve misfolded alpha-synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 2016;79:306–316. [DOI] [PubMed] [Google Scholar]
- 47.Donadio V, Doppler K, Incensi A, et al. Abnormal alpha-synuclein deposits in skin nerves: intra- and inter-laboratory reproducibility. Eur J Neurol 2019;26:1245–1251. [DOI] [PubMed] [Google Scholar]
- 48.Orimo S, Takahashi A, Uchihara T, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson's disease. Brain Pathol 2007;17:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol 2018;17:618–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data collected for this study will be available online at braincommons.org by December 31, 2020. Data requestors will need to sign a data access policy agreement via the BRAIN Commons website (braincommons.org) to gain access.