Abstract
Background
The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD; NCT03036150) trial was designed to assess the effect of the sodium–glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin on kidney and cardiovascular events in participants with CKD with and without type 2 diabetes (T2D). This analysis reports the baseline characteristics of those recruited, comparing them with those enrolled in other trials.
Methods
In DAPA-CKD, 4304 participants with a urinary albumin:creatinine ratio (UACR) ≥200 mg/g and estimated glomerular filtration rate (eGFR) between 25 and 75 mL/min/1.73 m2 were randomized to dapagliflozin 10 mg once daily or placebo. Mean eGFR was 43.1 mL/min/1.73 m2 and median UACR was 949 mg/g (108 mg/mmol).
Results
Overall, 2906 participants (68%) had a diagnosis of T2D and of these, 396 had CKD ascribed to a cause other than diabetes. The most common causes of CKD after diabetes (n = 2510) were ischaemic/hypertensive nephropathy (n = 687) and chronic glomerulonephritis (n = 695), of which immunoglobulin A nephropathy (n = 270) was the most common. A total of 4174 participants (97%) were receiving an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, 1882 (43.7%) diuretics, 229 (5.3%) mineralocorticoid receptor antagonists and 122 (2.8%) glucagon-like peptide 1 receptor agonists. In contrast to the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), the DAPA-CKD trial enrolled participants with CKD due to diabetes and to causes other than diabetes. The mean eGFR of participants in the DAPA-CKD trial was 13.1 mL/min/1.73 m2 lower than in CREDENCE, similar to that in the Finerenone in Reducing Kidney Failure and Disease Progression in DKD (FIDELIO-DKD) trial and the Study Of diabetic Nephropathy with AtRasentan (SONAR).
Conclusions
Participants with a wide range of underlying kidney diseases receiving renin–angiotensin system blocking therapy have been enrolled in the DAPA-CKD trial. The trial will examine the efficacy and safety of dapagliflozin in participants with CKD Stages 2–4 and increased albuminuria, with and without T2D.
Keywords: chronic kidney disease, dapagliflozin, randomized controlled clinical trial, sodium–glucose co-transporter-2 inhibitor
KEY LEARNING POINTS
What is already known about this subject?
In large cardiovascular (CV) outcome trials of patients with type 2 diabetes (T2D), sodium–glucose co-transporter 2 (SGLT2) inhibitors have demonstrated beneficial effects on CV outcomes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation demonstrated that the SGLT2 inhibitor canagliflozin reduced the risk of kidney failure and CV outcomes in patients with T2D and chronic kidney disease (CKD; estimated glomerular filtration rate 30–<90 mL/min/1.73 m2; urinary albumin:creatinine ratio >300–5000 mg/g) who were already receiving an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).
The kidney protective effects of SGLT2 inhibitors may involve not only improvements in metabolic parameters, but also favourable changes to glomerular haemodynamics, and so SGLT2 inhibitors may also be beneficial to patients with non-diabetic kidney disease.
What this study adds?
The Dapagliflozin and Prevention of Adverse outcomes in CKD (DAPA-CKD) trial tested the hypothesis that compared with placebo, the SGLT2 inhibitor dapagliflozin is superior in reducing the risk of kidney and CV events in a broad group of patients with CKD, the vast majority of whom are already receiving ACEis/ARBs.
The DAPA-CKD trial has enrolled a group of participants with a variety of kidney diseases and will allow assessment of SGLT2 inhibition in a broad cohort of patients with proteinuric CKD.
What impact this may have on practice or policy?
In the context of other trials in this field, the DAPA-CKD trial will provide unique insights into whether dapagliflozin confers kidney protection in patients with CKD and diverse kidney disease aetiologies.
INTRODUCTION
In large clinical trials recruiting participants with type 2 diabetes (T2D), sodium–glucose co-transporter 2 (SGLT2) inhibitors have demonstrated beneficial effects on cardiovascular (CV) and kidney outcomes that extend beyond glycaemic control [1–4]. One of these trials, and the first dedicated renal trial—the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE)—involved participants with T2D and chronic kidney disease (CKD). CREDENCE recruited individuals with an estimated glomerular filtration rate (eGFR) of 30–<90 mL/min/1.73 m2 and a urinary albumin:creatinine ratio (UACR) >300–5000 mg/g]. It demonstrated that the benefits of SGLT2 inhibitors on clinical outcomes extended to participants with T2D and CKD who were already receiving an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) as background renoprotective therapy [4].
An acute reduction in eGFR along with a reduction in albuminuria is observed on commencing SGLT2 inhibitors, even in participants with good glycaemic control [5]. This, in conjunction with strong experimental data [6], has led to speculation that the renoprotective effects of SGLT2 inhibitors may involve not only improvements in metabolic parameters, but also favourable changes to glomerular haemodynamics [7–9]. Thus, like ACEi and ARBs [10–12], SGLT2 inhibitors may also be beneficial to patients with non-diabetic kidney disease (DKD). The Dapagliflozin and Prevention of Adverse outcomes in CKD (DAPA-CKD) trial tested the hypothesis that compared with placebo, the SGLT2 inhibitor dapagliflozin is superior in reducing the risk of kidney and CV events in a broad group of participants with CKD, the vast majority of whom are already receiving ACEis/ARBs. Unlike CREDENCE, the DAPA-CKD trial included participants with CKD but without T2D, to explore whether the benefits of SGLT2 inhibitors extend to patients with non-DKD [13]. Here we describe the baseline characteristics of participants enrolled in the DAPA-CKD trial and compare these with characteristics of participants included in other recent trials involving participants with T2D and/or CKD from other causes reporting kidney outcomes as primary endpoints.
MATERIALS AND METHODS
Study design
The DAPA-CKD trial (NCT03036150) is a randomized, double-blind, parallel group, placebo-controlled trial that enrolled patients with CKD to evaluate the effects of dapagliflozin 10 mg once daily compared with placebo in patients receiving standard care, including either recommended doses or, if not, an individual maximum tolerated dose below the maximum recommended dose of an ACEi/ARB. The study design for the DAPA-CKD trial has been published [13].
Participants
Eligible participants had CKD with an eGFR ≥25–≤75 mL/min/1.73 m2 and a UACR ≥200–≤5000 mg/g (22.6–565 mg/mmol). Participants meeting these criteria could be enrolled whether or not their kidney disease was thought to be due to T2D. Participants with type 1 diabetes (T1D), autosomal dominant or recessive polycystic kidney disease, lupus nephritis or anti-neutrophilic cytoplasmic autoantibody-associated vasculitis were excluded. Participants who had received cytotoxic or immunosuppressive therapy for primary or secondary kidney disease in the 6 months prior to study enrolment, had a history of organ (including kidney) transplantation and were receiving therapy with an SGLT2 inhibitor within 8 weeks prior to enrolment were also excluded [13]. Randomization was capped so that a minimum of 30% of participants were assigned to either the diabetic or non-diabetic subgroups. Capping was also used to limit the proportion of participants with an eGFR 60–75 mL/min/1.73 m2 to <10%. Participants were maintained on a stable and individualized maximum tolerated dose of an ACEi or ARB for at least 4 weeks before screening, if not contraindicated. Participants with documented ACEi or ARB intolerance were allowed to participate in the study. Investigators were strongly encouraged to provide concordant care for all other health conditions in accordance with clinical practice guidelines.
Procedures
After randomization, face-to-face visits were scheduled after 2 weeks, at 2, 4 and 8 months and thereafter at 4-month intervals. At each follow-up visit, data about achieving endpoints, occurrence of adverse events, use of concomitant therapies and adherence to trial drug were collected. Additionally, vital signs were recorded and blood and urine were collected for laboratory analysis as previously described [13]. The statistical assumptions and approach to analysis has been described previously [13].
Outcomes
The primary composite endpoint of the DAPA-CKD trial is the worsening of kidney function, defined as sustained ≥50% decline in eGFR, occurrence of end-stage kidney disease or death due to kidney disease, or a CV death. Secondary and exploratory endpoints have been previously described [13].
Comparator trials
DKD studies
We compared the baseline characteristics of participants with T2D enrolled in the DAPA-CKD trial with those of participants enrolled in other contemporary Phase 3 trials enrolling patients with DKD and which assessed kidney outcomes as the primary endpoint. We identified three such studies: CREDENCE [4], the Finerenone in Reducing Kidney Failure and Disease Progression in DKD (FIDELIO-DKD) trial [14] and the Study Of diabetic Nephropathy with AtRasentan (SONAR) [15]. We also identified two other ongoing kidney outcomes studies—Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY; NCT03594110) [16] and the research study to see how semaglutide works compared with placebo in people with T2D and CKD (FLOW; NCT03819153) [17]—recruiting participants with T2D (participants with T1D are included in the EMPA-KIDNEY study) and CKD, for which baseline characteristics of the participants have not yet been published. For these studies, participants were compared with those recruited into the DAPA-CKD trial based on the eligibility criteria (Table 1).
Table 1.
Comparison of eligibility criteria for the DAPA-CKD trial and other contemporary Phase 3 trials recruiting participants with CKD and T2D (from clinicaltrials.gov)
| Parameter | DAPA-CKD | EMPA-KIDNEY | FLOW |
|---|---|---|---|
| Study drug | Dapagliflozin | Empagliflozin | Semaglutide |
| Comparator | Placebo | Placebo | Placebo |
| Recruitment | 4000 | 6000 | 3160 |
| Non-diabetic CKD included | Yes | Yes | No |
| T1D | Excluded | Included | Excluded |
| Background ACEi/ARB | Unless not tolerated | Unless not tolerated or not indicated | Unless not tolerated or contraindicated |
| Age (years) | ≥18 | ≥18 | ≥18 (Japan ≥20) |
| eGFR range | ≥25–≤75 mL/min/1.73 m² | ≥20–≤90 mL/min/1.73 m² | ≥25–≤75 mL/min/1.73 m² |
| UACR range | ≥200 mg/g to ≤5000 mg/g | ≥200 mg/g if eGFR ≥45 to <90 mL/min/1.73 m² |
≥300–≤5000 mg/g, ≥100 mg/g if eGFR ≤50 mL/min/1.73 m² |
Non-DKD trials
To compare the number of non-diabetic participants randomized in the DAPA-CKD trial to other relevant studies, we searched for Phase 3 randomized trials using therapeutic interventions in participants with non-DKDs reporting kidney outcomes as the primary endpoint. We found two completed trials that had recruited patients with immunoglobulin A (IgA) nephropathy (IgAN). The Therapeutic Evaluation of Steroids in IgAN Global trial (TESTING) [18] and the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgAN (STOP-IgAN) trial [19] both tested conventional immunosuppressive regimens against continued supportive care. Two ongoing trials examining the progression of CKD in participants with IgAN—A Study of the Effect and Safety of Sparsentan in the Treatment of Patients With IgAN (PROTECT) [20] and the Efficacy and Safety of Nefecon in Patients With Primary IgAN (NefIgArd) study [21]—were also identified.
RESULTS
Recruitment
The first participant was enrolled on 2 February 2017 and the first randomization occurred on 13 February 2017. Recruitment closed in the majority of participating countries on 6 July 2018. Recruitment in India, the USA and Canada was open until 19 October 2018, recruiting 4094 participants for randomization. Recruitment in China opened on 2 December 2019 and was ongoing until the trial end date of 3 April 2020, recruiting 210 participants from this country. Overall, 4304 were enrolled in the trial.
Patient characteristics
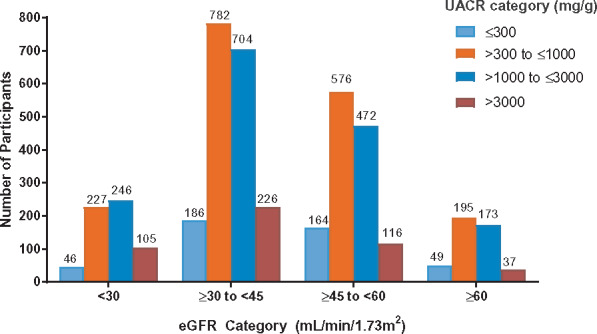
The mean age of the participants in the DAPA-CKD trial was 61.8 years and 66.9% were men (Table 2). There was a mix of races in the participants, with 53.2% White, 34.1% Asian and 4.4% Black. The mean systolic/diastolic blood pressure was 137/78 mmHg. The overall mean body mass index (BMI) was 29.5 kg/m2 and the mean haemoglobin A1c (HbA1c) was 7.1% (54 mmol/mol). Overall, the mean eGFR was 43.1 mL/min/1.73 m2. Among the 4304 participants enrolled, 624 (14.5%), 1898 (44.1%), 1328 (30.9%) and 454 (10.5%) had an eGFR <30, ≥30–<45, ≥45–<60 and ≥60 mL/min/1.73 m2, respectively. The median UACR was 949.3 mg/g (107.3 mg/mmol) with 1 (0.0%), 444 (10.3%) and 3859 (89.7%) participants having a UACR <30, 30–300 and >300 mg/g (<3.39, 3.39–33.9, >33.9 mg/mmol), respectively (Table 2). The proportion of participants in the eGFR categories <30, ≥30–<45, ≥45–<60 and ≥60 mL/min/1.73 m2 and UACR categories ≤300, >300–≤1000, >1000–≤3000 and >3000 mg/g are shown in Figure 1.
Table 2.
DAPA-CKD trial participant demographics and baseline clinical chemistry according to baseline diabetes status (full analysis set)
| Characteristic | Overall | With T2D | Without T2D |
|---|---|---|---|
| (N = 4304) | (n = 2906) | (n = 1398) | |
| Age (years), mean (SD) | 61.8 (12.1) | 64.4 (9.7) | 56.4 (14.6) |
| ≤65 years, n (%) | 2486 (57.8) | 1507 (51.9) | 979 (70.0) |
| >65 years, n (%) | 1818 (42.2) | 1399 (48.1) | 419 (30.0) |
| Gender, n (%) | |||
| Male | 2879 (66.9) | 1941 (66.8) | 938 (67.1) |
| Female | 1425 (33.1) | 965 (33.2) | 460 (32.9) |
| Race, n (%) | |||
| White | 2290 (53.2) | 1541 (53.0) | 749 (53.6) |
| Black | 191 (4.4) | 137 (4.7) | 54 (3.9) |
| Asian | 1467 (34.1) | 932 (32.1) | 535 (38.3) |
| American Indian/Alaska native | 136 (3.2) | 111 (3.8) | 25 (1.8) |
| Other | 220 (5.1) | 185 (6.4) | 35 (2.5) |
| Region, n (%) | |||
| Asia | 1346 (31.3) | 841 (28.9) | 505 (36.1) |
| Europe | 1233 (28.7) | 771 (26.5) | 462 (33.0) |
| North America | 813 (18.9) | 623 (21.4) | 190 (13.6) |
| Latin/South America | 912 (21.2) | 671 (23.1) | 241 (17.2) |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 137.1 (17.4) | 139.2 (17.3) | 132.6 (16.7) |
| Diastolic | 77.5 (10.5) | 76.5 (10.1) | 79.6 (10.9) |
| Systolic blood pressure categories, n (%) | |||
| >130 mmHg | 2762 (64.2) | 2033 (70.0) | 729 (52.1) |
| >140 mmHg | 1684 (39.1) | 1273 (43.8) | 411 (29.4) |
| Mean BMI (kg/m2) | n = 4296 | n = 2899 | n = 1397 |
| 29.5 | 30.3 | 27.9 | |
| HbA1c | n = 4284 | n = 2893 | n = 1391 |
| %, mean (SD) | 7.1 (1.7) | 7.8 (1.7) | 5.6 (0.4) |
| mmol/mol, mean (SD) | 54 (19) | 62 (19) | 38 (4) |
| Haemoglobin (g/L), mean (SD) | n = 4278 | n = 2892 | n = 1386 |
| 128.3 (18.1) | 125.9 (17.9) | 133.1 (17.6) | |
| Serum creatinine (mg/dL), mean (SD) | 1.7 (0.5) | 1.6 (0.5) | 1.8 (0.5) |
| eGFR (mL/min/1.73 m2), mean (SD) | 43.1 (12.4) | 43.8 (12.6) | 41.7 (11.7) |
| eGFR categories(mL/min/1.73 m2), n (%) | |||
| ≥60 | 454 (10.5) | 348 (12.0) | 106 (7.6) |
| 45–59 | 1328 (30.9) | 918 (31.6) | 410 (29.3) |
| 30–44 | 1898 (44.1) | 1239 (42.6) | 659 (47.1) |
| <30 | 624 (14.5) | 401 (13.8) | 223 (16.0) |
| Baseline UACR (mg/g), median | 949.3 | 1016.5 | 861.0 |
| Baseline median UACR categories, n (%) | |||
| <30 mg/g (Stage A1) | 1 (0.0) | 1 (0.0) | 0 (0.0) |
| 30–300 mg/g (Stage A2) | 444 (10.3) | 308 (10.6) | 136 (9.7) |
| >300 mg/g (Stage A3) | 3859 (89.7) | 2597 (89.4) | 1262 (90.3) |
FIGURE 1.
Proportion of participants in the eGFR and UACR categories.
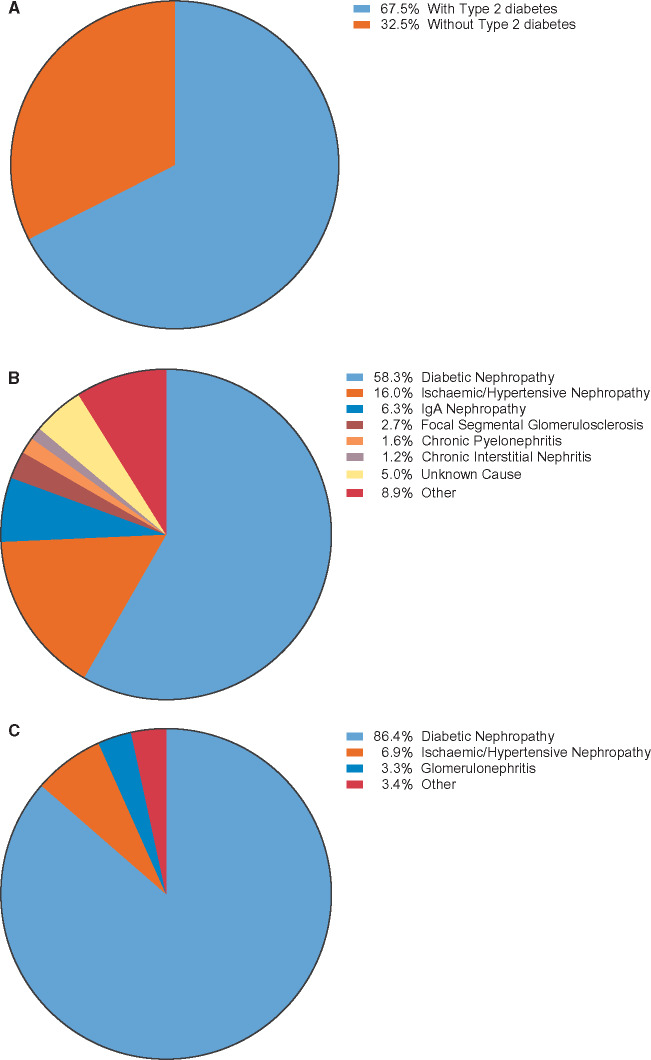
Of the 4304 randomized participants, 2888 had a history of T2D at the start of the trial. Of the participants with no history of diabetes, 19 had HbA1c ≥6.5% (48 mmol/mol) at Visit 1 (enrolment) and 2 (randomization) and thus fulfilled criteria for a diagnosis of diabetes. Therefore 2906 participants (67.5%) had a diagnosis of diabetes at baseline and were included in the diabetes subgroup in subsequent analyses (Figure 2a).
FIGURE 2.
Characteristics of study participants: (A) study participants by diabetes status, (B) investigator-reported causes of CKD in all study participants and (C) investigator-reported causes of CKD in study participants with T2D.
Causes of CKD
In participants without T2D, the most common investigator-reported cause of CKD was chronic glomerulonephritis (n = 598), followed by ischaemic/hypertensive nephropathy (n = 487) (Table 3 and Figure 2B). The cause of CKD was considered ‘unknown’ in 167 participants without diabetes. In 396 participants in the diabetic population, CKD was not attributed to diabetic nephropathy by the investigator (Figure 2C). The cause of CKD was based on kidney biopsy in 373 (12.8%) of the participants with T2D and 500 (35.8%) of the participants without T2D.
Table 3.
CKD diagnosis and other baseline comorbidities according to baseline diabetes status (full analysis set)
| Baseline comorbidities | Overall (N = 4304) |
With T2D (n = 2906) |
Without T2D (n= 1398) |
|---|---|---|---|
| Investigator reported aetiology of CKD | |||
| Diabetic nephropathy | 2510 (58.3) | 2510 (86.4) | 0 |
| Ischaemic/hypertensive nephropathy | 687 (16.0) | 200 (6.9) | 487 (34.8) |
| Chronic glomerulonephritis | 695 (16.1) | 97 (3.3) | 598 (42.8) |
| IgAN | 270 (6.3) | 38 (1.3) | 232 (16.6) |
| FSGS | 115 (2.7) | 22 (0.8) | 93 (6.7) |
| Membranous nephropathy | 43 (1.0) | 10 (0.3) | 33 (2.4) |
| Minimal change disease | 11 (0.3) | 2 (0.1) | 9 (0.6) |
| Other | 256 (5.9) | 25 (0.9) | 231 (16.5) |
| Chronic pyelonephritis (infectious) | 69 (1.6) | 12 (0.4) | 57 (4.1) |
| Chronic interstitial nephritis | 53 (1.2) | 13 (0.4) | 40 (2.9) |
| Obstructive nephropathy | 25 (0.6) | 5 (0.2) | 20 (1.4) |
| Renal artery stenosis | 10 (0.2) | 3 (0.1) | 7 (0.5) |
| Unknown | 214 (5.0) | 47 (1.6) | 167 (11.9) |
| Other | 41 (1.0) | 19 (0.7) | 22 (1.6) |
| Kidney biopsy performed | 873 (20.3) | 373 (12.8) | 500 (35.8) |
| Medical history and comorbidities | |||
| Obese (BMI ≥ 30 kg/m2) | 1917 (44.5) | 1437 (49.4) | 480 (34.3) |
| Hypertension | 4121 (95.7) | 2856 (98.3) | 1265 (90.5) |
| Duration of diabetes (years), mean | N/A | 15.0 | N/A |
| Any history of CV disease | 1610 (37.4) | 1281 (44.1) | 329 (23.5) |
| Heart failure | 468 (10.9) | 361 (12.4) | 107 (7.7) |
| Myocardial infarction | 392 (9.1) | 321 (11.0) | 71 (5.1) |
| Percutaneous coronary intervention | 294 (6.8) | 246 (8.5) | 48 (3.4) |
| Coronary artery bypass graft | 176 (4.1) | 163 (5.6) | 13 (0.9) |
| Stroke | 298 (6.9) | 230 (7.9) | 68 (4.9) |
| Foot ulcer | 152 (3.5) | 151 (5.2) | 1 (0.1) |
| Amputation | 181 (4.2) | 166 (5.7) | 15 (1.1) |
| Neuropathy | 955 (22.2) | 922 (31.7) | 33 (2.4) |
| Anaemia | |||
| Men | 1231 (28.6) | 955 (32.9) | 276 (19.7) |
| Women | 708 (6.4) | 527 (18.1) | 181 (12.9) |
Concomitant diseases
Overall, a history of CV disease was recorded in 37.4% of participants, 10.9% had a history of heart failure, 9.1% had a history of myocardial infarction and 6.9% had a history of stroke (Table 3). Among patients with T2D, the mean time since diabetes was confirmed was 15 years and 44.1% had a history of CV disease. A history of heart failure was recorded for 12.4%, myocardial infarction for 11.0% and stroke for 7.9% of participants (Table 3).
Blood pressure, renoprotective and lipid-lowering medications
Although the intention was to recruit patients taking the maximum target dose or, if not, at individually maximum tolerated dose of ACEi or ARB, patients could still be included if intolerant of these drugs. In the overall study population, 130 participants (3.0%) were not taking either medication at the baseline visit (Visit 2). More participants were being treated with ARBs (66.7%) than ACEis (31.5%; Table 4), while 229 (5.3%) were taking a mineralocorticoid receptor antagonist (MRA) and 3 participants (0.1%) were receiving a direct renin inhibitor. After ACEi/ARBs, calcium channel blockers were the most commonly prescribed blood pressure medication (50.7%), followed by diuretics (43.7%) and beta-blockers (39.0%). Statins were prescribed for 64.9% participants at baseline in the overall study population, with a higher proportion of participants with T2D (71.6%) receiving this class of medication than those without diabetes (50.9%).
Table 4.
Baseline medications according to baseline diabetes status (full analysis set)
| Baseline medication | Overall (N = 4304) |
With T2D (n = 2906) |
Without T2D (n = 1398) |
|---|---|---|---|
| RAS blockade | 4174 (97.0) | 2817 (96.9) | 1357 (97.1) |
| ACEi | 1354 (31.5) | 894 (30.8) | 460 (32.9) |
| ARB | 2870 (66.7) | 1958 (67.4) | 912 (65.2) |
| Direct renin inhibitor | 3 (0.1) | 0 | 3 (0.2) |
| ARNI | 3 (0.1) | 3 (0.1) | 0 |
| Diuretic medication | 1882 (43.7) | 1465 (50.4) | 417 (29.8) |
| Loop diuretic | 1056 (24.5) | 841 (28.9) | 215 (15.4) |
| Thiazide | 906 (21.1) | 715 (24.6) | 191 (13.7) |
| MRA | 229 (5.3) | 171 (5.9) | 58 (4.1) |
| Other diuretic | 27 (0.6) | 18 (0.6) | 9 (0.6) |
| Phosphate binder | 48 (1.1) | 39 (1.3) | 9 (0.6) |
| ESA | 89 (2.1) | 68 (2.3) | 21 (1.5) |
| Potassium binder | 117 (2.7) | 88 (3.0) | 29 (2.1) |
| Beta-blocking agent | 1680 (39.0) | 1267 (43.6) | 413 (29.5) |
| Calcium channel blocker | 2183 (50.7) | 1549 (53.3) | 634 (45.4) |
| Lipid-lowering medication | 2988 (69.4) | 2206 (75.9) | 782 (55.9) |
| Statin | 2794 (64.9) | 2082 (71.6) | 712 (50.9) |
| Other lipid-lowering medication | 645 (15.0) | 452 (15.6) | 193 (13.8) |
| Antithrombotic medication | 2042 (47.4) | 1649 (56.7) | 393 (28.1) |
| Antiplatelet agent | 1880 (43.7) | 1543 (53.1) | 337 (24.1) |
| Other antithrombotic medication | 225 (5.2) | 158 (5.4) | 67 (4.8) |
| Antihyperglycaemic medication | 2725 (63.3) | 2719 (93.6) | 6 (0.4) |
| Biguanide | 1250 (29.0) | 1244 (42.8) | 6 (0.4) |
| Sulphonylurea | 774 (18.0) | 774 (26.6) | 0 |
| DPP-4 inhibitor | 742 (17.2) | 742 (25.5) | 0 |
| Alpha-glucosidase inhibitor | 99 (2.3) | 99 (3.4) | 0 |
| GLP-1 receptor agonist | 122 (2.8) | 122 (4.2) | 0 |
| Insulin | 1598 (37.1) | 1598 (55.0) | 0 |
| Thiazolidinedione | 91 (2.1) | 91 (3.1) | 0 |
| Other antihyperglycaemic medication | 89 (2.1) | 89 (3.1) | 0 |
Values presented as n (%).
ARNI, angiotensin receptor-neprilysin inhibitor; DPP-4, dipeptidyl peptidase-4; ESA, erythropoiesis-stimulating agents.
Medications for treatment of diabetes
Almost all participants with T2D (93.16%) were receiving glucose-lowering medications, with more than half (55.0%) receiving insulin (Table 4). Of the oral glucose-lowering agents, biguanides predominated (42.8%), followed by sulphonylureas (26.6%) and dipeptidyl peptidase-4 inhibitors (25.5%). Only 4.2% of participants with T2D were receiving a glucagon-like peptide-1 receptor agonist (GLP1-RA) and 3.4% were receiving thiazolidinediones.
Comparisons of participants with and without T2D
Participants with T2D tended to be older than those without diabetes (mean age 64 versus 56 years) and the proportion ≥65 years was greater (48% versus 30%), but both subgroups had a similar racial distribution (Table 2). Patients with diabetes had a higher BMI than those without diabetes (mean BMI 30.3 versus 27.9 kg/m2), were more likely to be hypertensive (98.3% versus 90.5%) and were more likely to be anaemic whether male (32.9% versus 19.7%) or female (18.1% versus 12.9%). The mean HbA1c in patients with and without diabetes was 7.8% (62 mmol/mol) and 5.6% (38 mmol/mol), respectively. Those with T2D had a slightly higher mean eGFR compared with those without diabetes (43.8 versus 41.7 mL/min/1.73 m2) and a higher median level of albuminuria [1016.5 mg/g versus 861.0 mg/g (114.9 mg/mmol versus 97.3 mg/mmol)]. Participants with T2D were more than twice as likely to have had a prior myocardial infarction (11.0% versus 5.1%) or history of heart failure (12.4 versus 7.7%; Table 3).
Comparison of the baseline characteristics of study participants of the DAPA-CKD trial with those of participants recruited into other contemporary diabetic nephropathy and other CKD trials
One other trial, CREDENCE [4], assessed the clinical benefits and safety of an SGLT2 inhibitor in patients with T2D and CKD on a background of ACEi/ARB therapy; however, patients with non-DKD were not enrolled in this study (Table 5). The DAPA-CKD trial participants with diabetes were racially more diverse and had a lower BMI compared with those enrolled in CREDENCE. The mean eGFR of the overall population of patients recruited to the DAPA-CKD trial was 13.1 mL/min/1.73 m2 lower than in CREDENCE (43.1 versus 56.2 mL/min/1.73 m2, respectively). Overall, the UACR was similar to that in CREDENCE [949 (107) versus 927 mg/g (105 mg/mmol)] despite the lower level for inclusion in the DAPA-CKD trial (200 mg/g) compared with CREDENCE (300 mg/g). Comparing the populations with T2D, the median UACR value for DAPA-CKD [1017 mg/g (115 mg/mmol)] was similar to that for CREDENCE [927 mg/g (105 mg/mmol)]. The UACR in the DAPA-CKD, FIDELIO-DKD and SONAR studies was similar (949 versus 851 and 802 mg/g [107 versus 96 and 91 mg/mmol]) [14, 15]. The mean eGFR of the DAPA-CKD trial participants with T2D (43.8 mL/min/1.73 m2) was similar to that reported for two other completed trials, FIDELIO-DKD (44.3 mL/min/1.73 m2) [14] and SONAR (43.8 mL/min/1.73 m2) [15].
Table 5.
Comparison of the DAPA-CKD diabetic subpopulation with populations recruited to other contemporary trials of DKD in patients already receiving RAS blockade
| Characteristic | DAPA-CKD | CREDENCE [4] | FIDELIO-DKD [14] | SONAR [31] |
|---|---|---|---|---|
| Patients with CKD and diabetes | 2906 | 4401 | 5674 | 2648a |
| Patients with DKD, % | 86.4 | 100 | 100 | 100 |
| Other aetiology, % | 13.6 | 0 | 0 | 0 |
| Study drug | Dapagliflozin | Canagliflozin | Finerenone | Atrasentan |
| Age (years), mean | 64.4 | 63.0 | 65.6 | 64.8 |
| Gender, % | ||||
| Male | 66.8 | 66.1 | 70.2 | 74.2 |
| Female | 33.2 | 33.9 | 29.8 | 25.8 |
| Race, % | ||||
| White | 53.0 | 66.6 | 65.9 | 56.5 |
| Black | 4.7 | 5.1 | 4.7 | 5.6 |
| Asian | 32.1 | 19.9 | 25.3 | 34.0 |
| Other | 10.2 | 8.4 | 3.8 | 3.8 |
| Duration of diabetes (years), mean (SD) | Median 15.0 | 15.8 (8.6) | 16.6 (8.8) | 16.7 (9.1) |
| History of CV disease, % | 44.1 | 50.4 | 45.9 | – |
| History of heart failure, % | 12.4 | 14.8 | 7.5 | – |
| History of myocardial infarction, % | 11.0 | – | 13.5 | 6.0 |
| History of stroke, % | 7.9 | – | 12.1 | – |
| Blood pressure (mmHg), mean (SD) | ||||
| Systolic | 139 (17) | 140 (16) | 138 (14) | 136 (15) |
| Diastolic | 77 (10) | 78 (9) | 76 (10) | 75 (10) |
| BMI (kg/m2), mean (SD) | Median 29.0 | 31.3 (6.2) | 31.1 (6.0) | – |
| HbA1c (%), mean (SD) | 7.8 (1.7) | 8.3 (1.3) | 7.7 (1.3) | 7.8 (1.5) |
| HbA1c (mmol/mol), mean (SD) | 62 (19) | 67 (14) | 61 (14) | 62 (16) |
| eGFR, (mL/min/1.73 m2), mean (SD) | 43.8 (12.6) | 56.2 (18.2) | 44.3 (12.6) | 43.8 (13.7) |
| eGFR categories (mL/min/1.73 m2), % | ||||
| ≥60 | 12.0 | 40.2 | 11.6 | – |
| ≥45–<60 | 31.6 | 28.8 | 33.5 | – |
| ≥30–<45 | 42.6 | 27.1 | 52.5b | – |
| <30 | 13.8 | 3.9 | – | – |
| <25 | – | – | 2.4 | |
| UACR (mg/g), median (25–75 percentile) | 1017 | 927 (463–1833) | 851 (446–1634) | 802 (450–1469) |
| UACR categories (mg/g), % | ||||
| <30 | 0 | 0.7c | 0.4 | – |
| 30–300 | 10.6 | 11.3c | 12.1d | – |
| >300 | 89.4 | 88.0c | 87.4d | – |
| Antihyperglycaemic medications, % | ||||
| Biguanide | 42.8 | 57.8 | 43.8 | 38.1 |
| Sulphonylurea | 26.6 | 28.8 | 23.4 | 28.4 |
| DPP-4 inhibitor | 25.5 | 17.1 | 26.8 | – |
| Alpha-glucosidase inhibitor | 3.4 | – | – | – |
| GLP-1 receptor agonist | 4.2 | 4.2 | 7.0 | – |
| Insulin | 55.0 | 65.5 | 64.1 | 62.5 |
| Thiazolidinedione | 3.1 | – | – | – |
| CV medications, % | ||||
| RAS blockade | 96.9 | 99.9e | 99.9 | 98.0 |
| Diuretic | 50.4 | 46.7 | 56.6 | 82.6 |
| Antithrombotic | 56.7 | 59.6 | 56.8 | – |
| Beta-blocker | 43.6 | 40.2 | 52.2 | 41.7 |
| Statin | 71.6 | 69.0 | 74.3 | 78.4 |
| Calcium channel blocker | 53.3 | – | 63.2 | 57.6 |
Number of participants who were classed as ‘responders’ and enrolled into UACR responder stratum of the study.
eGFR range 25–<45 mL/min/1.73 m2.
Normoalbuminuria/microalbuminuria/macroalbuminuria.
<30/30–<300/≥300 mg/g.
Renin–angiotensin–aldosterone system blockade.
Unlike CREDENCE, patients intolerant of ACEi/ARB were eligible for the DAPA-CKD trial, although only 3% of participants were not recorded as receiving these medications at the time of recruitment as compared 0.1% in CREDENCE [4]. This requirement for the use of prior renin–angiotensin system (RAS) blockade agents was also applied in the FIDELIO-DKD trial (99.9%) [14], but was less stringent in the SONAR trial [15], with 2% of patients not receiving these agents at baseline [22]. Compared with these other studies, a similar proportion of participants with diabetes recruited into the DAPA-CKD trial were prescribed statins (72% in DAPA-CKD versus 69% in CREDENCE, 74% in FIDELIO-DKD and 78% in SONAR) and GLP1-RA (4.2% in DAPA-CKD and CREDENCE, 7.0% in FIDELO-DKD). Finally, the FLOW study of semaglutide [17] is recruiting participants with T2D with an eGFR range similar to that of the DAPA-CKD trial.
After DKD, chronic glomerulonephritis was the most common diagnosis of kidney disease in the DAPA-CKD trial, of which the largest group of participants had a diagnosis of IgAN. Figure 3 shows that the size of the IgAN cohort in the DAPA-CKD trial was larger than that of other recently completed IgAN trials.
FIGURE 3.
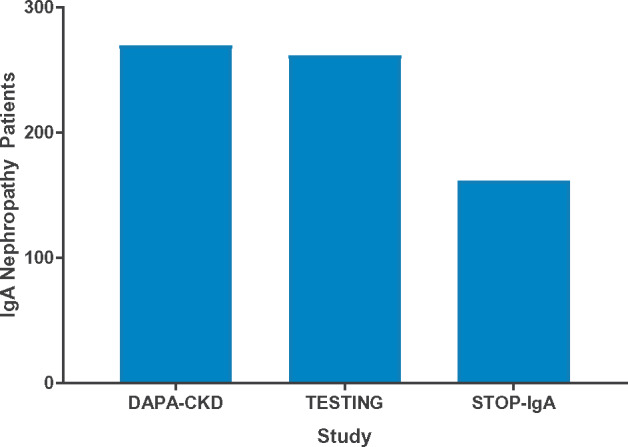
Number of participants with IgAN in clinical trials [18, 19].
DISCUSSION
The DAPA-CKD trial has enrolled participants with albuminuria and a wide range of kidney diseases, the majority already receiving RAS blockade therapy. It is one of three large outcome trials (with CREDENCE and EMPA-KIDNEY) assessing the effects of SGLT2 inhibitors on the progression of CKD with kidney endpoints as the primary outcome. Notably, the study was stopped early following routine review by the Independent Data Monitoring Committee, who cited overwhelming efficacy [23].
Unlike CREDENCE, the only SGLT2 inhibitor trial to have reported results to date [4], the DAPA-CKD trial recruited patients with CKD both with and without T2D and will be the first SGLT2 inhibitor trial to report clinical outcome data in non-diabetic CKD patients. Inclusion of non-diabetic patients with CKD will help to determine whether the renoprotective effects of SGLT2 inhibitors demonstrated in CREDENCE [4] also extend to these individuals. Of the 2906 patients with T2D included, the mean duration of diabetes is broadly similar to other DKD studies, reflecting the fact that patients generally develop kidney disease several years after the diagnosis of T2D. All but 3% of participants were receiving ACEi/ARB at the time of enrolment.
The DAPA-CKD trial recruited participants with a mean baseline eGFR of 43.1 mL/min/1.73 m2, 13.1 mL/min/1.73 m2 lower than in CREDENCE, allowing assessment of renoprotection in a group of patients with more severely impaired kidney function than previously studied. The DAPA-CKD trial also included 624 patients (14.5%) with a baseline eGFR <30 mL/min/1.73 m2. Although such participants were theoretically excluded from CREDENCE on the basis of blood results at screening visits, 174 (4%) CREDENCE participants had an eGFR ≤30 mL/min/1.73 m2 on the day of randomization (G. Bakris, submitted for publication). The only kidney outcome trial in participants with T2D and CKD to have recruited patients with a lower mean eGFR than DAPA-CKD was Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) (41 mL/min/1.73 m2) [24]. In comparison, the mean eGFR values of the SONAR (43.8 mL/min/1.73 m2) [15, 22] and FIDELIO-DKD (44.3 mL/min/1.73 m2) [14] populations are similar to the DAPA-CKD trial. These differences are likely to reflect the eGFR inclusion criteria for these trials, with CREDENCE having an upper cut-off of 90 mL/min/1.73 m2 [25] as compared with 75 mL/min/1.73 m2 for the DAPA-CKD, FIDELIO-DKD and SONAR [13–15] trials. In addition, the lower eGFR cut-off for CREDENCE was higher, at 30 mL/min/1.73 m2, than for the DAPA-CKD, SONAR and FIDELIO-DKD trials (25 mL/min/1.73 m2). Despite the inclusion criteria of the DAPA-CKD trial allowing recruitment of patients with a lower UACR than CREDENCE [200 mg/g (22.6 mg/mmol) compared with 300 mg/g (33.9 mg/mmol)], the median UACR values in the DAPA-CKD diabetic population were higher than in CREDENCE.
Even among patients with T2D, other causes of CKD are often identifiable. Of the 2906 participants with diabetes recruited into the DAPA-CKD trial, 2510 (86%) had been given a diagnosis of ‘diabetic nephropathy’ at baseline by the investigator, with 396 diagnosed with an alternative kidney disease. Among all participants with diabetes, 373 had undergone a kidney biopsy. These data are consistent with prior observational studies indicating that a high proportion of participants with T2D and CKD have kidney pathologies other than DKD when systematically subjected to kidney biopsy [26]. It seems highly likely that other studies enrolling participants with T2D and CKD, including CREDENCE, have recruited participants with non-DKD because histological diagnosis based on prior kidney biopsy has not been an inclusion criterion. Because these participants have not been clearly identified in prior studies, subgroup analysis assessing the impact of interventions on progression of non-DKD has not been possible.
Taking the DAPA-CKD trial participants overall, the most common non-diabetic cause of CKD was ‘chronic glomerulonephritis’ (predominantly IgAN) followed by ‘ischaemic/hypertensive nephropathy’. In total, there were 270 participants with a diagnosis of IgAN, including 38 participants with diabetes, making the DAPA-CKD trial one of the largest trials to date involving participants with confirmed IgAN (Figure 3). Larger recruitment targets are proposed for forthcoming trials in IgAN, including the PROTECT [27] and NefigArd studies [21].
The second-largest non-diabetic nephropathy group were participants reported to have had hypertensive/ischaemic nephropathy, a diagnosis often made without kidney biopsy. A further 115 participants had a diagnosis of focal segmental glomerulosclerosis (FSGS), more than the number studied in the randomized, double-blind, active-control, dose-escalation (DUET) Phase 2 trial, which compared the effect of the dual endothelin type A and angiotensin 2 type 1 receptor antagonist sparsentan versus irbesartan on albuminuria in patients with a diagnosis of primary FSGS [28]. The ongoing Phase 3 DUPLEX trial has been designed to assess the impact of sparsentan on eGFR slope and proteinuria in 300 participants with biopsy-proven FSGS [29].
As expected in participants with proteinuric CKD, many of the DAPA-CKD participants had a prior history of CV diseases, including heart failure. Participants with T2D were about twice as likely to have had a prior history of coronary artery disease, including myocardial infarction, percutaneous coronary intervention and coronary artery bypass, than those with CKD but without diabetes. A similar pattern was seen with heart failure (12.4% of participants with T2D but only 7.7% of participants without diabetes). The higher prevalence of both atherosclerotic CV disease and heart failure in the diabetic population is important, as it may impact treatment effects and result in a differentiated secondary outcome between the diabetic and non-diabetic population in the DAPA-CKD trial.
More than 50% of the diabetic cohort were receiving insulin, reflecting the long duration of diabetes in many of these participants. Biguanides were the most commonly prescribed oral hypoglycaemic agents, bearing in mind that only 15% of the diabetic subgroup had an eGFR <30 mL/min/1.73 m2 (the level at which most guidelines preclude the prescription of metformin due to the enhanced risk of lactic acidosis) [30].
Two other classes of drug, namely MRAs and GLP1-RA, are of particular interest because their impact on kidney outcomes is currently being assessed in clinical trials in patients with diabetes and CKD. The FIDELIO-DKD trial is assessing the clinical efficacy and safety of finerenone on kidney outcomes in a similar cohort of participants with diabetes and kidney disease (Table 5). According to a recent press release, the FIDELIO-DKD study met its primary and secondary endpoints [32]. The FLOW trial is examining the efficacy and safety of semaglutide, a GLP1-RA, added to an ACEi/ARB in a similar population (Table 1). With 5.9% of the population with diabetes in the DAPA-CKD trial prescribed MRAs and 4.2% GLP1-RAs, the study will be limited in providing a robust assessment of the effect of dapagliflozin on kidney and CV endpoints when added to these medications.
In conclusion, the DAPA-CKD trial has enrolled a group of participants with a variety of kidney diseases and will allow assessment of SGLT2 inhibition in a broad cohort of patients with proteinuric kidney disease. While small mechanistic studies have demonstrated that SGLT2 inhibitors exert physiological effects in people with non-DKD, including a characteristic haemodynamic ‘dip’ in GFR [9], the DAPA-CKD trial is the first to capture the effects on clinical kidney outcomes. In the context of other trials in this field, the DAPA-CKD trial will provide unique insights into how to treat patients with kidney disease due to diverse aetiologies.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Parita Sheth and Nicola Truss, inScience Communications, London, UK, for assistance in editing and preparation of figures. This support was funded by AstraZeneca.
Members of the DAPA-CKD Executive Committee
Hiddo J.L. Heerspink (Co-chair; University Medical Centre Groningen, Groningen, The Netherlands), David C. Wheeler (Co-chair; University College London, London, UK), Glenn Chertow (Stanford University School of Medicine, Palo Alto, CA, USA), Ricardo Correa-Rotter (National Medical Science and Nutrition Institute Salvador Zubirán, Mexico City, Mexico), Tom Greene (University of Utah Health Sciences, Salt Lake City, UT, USA), Fan Fan Hou (Southern Medical University, Guangzhou, China), John McMurray (University of Glasgow, Glasgow, UK), Peter Rossing (Steno Diabetes Centre, Gentofte, Denmark), Robert Toto (UT Southwestern Medical Center, Dallas, TX, USA), Bergur Stefansson (AstraZeneca, Gothenburg, Sweden) and Anna Maria Langkilde (AstraZeneca, Gothenburg, Sweden).
Members of the DAPA-CKD Independent Data Monitoring Committee
Marc A. Pfeffer (Chair; Brigham and Women’s Hospital, Boston, USA), Stuart Pocock (London School of Hygiene and Tropical Medicine, London, UK), Karl Swedberg (University of Gothenburg, Göteborg, Sweden), Jean L. Rouleau (Montréal Heart Institute, Montreal, Québec, Canada), Nishi Chaturvedi (University College London, London, UK), Peter Ivanovich (Northwestern University, Chicago, USA), Andrew S. Levey (Tufts Medical School, Boston, USA) and Heidi Christ-Schmidt (Statistics Collaborative, Washington, DC, USA).
Members of the DAPA-CKD Event Adjudication Committee
Claes Held, Professor (Chair; Uppsala Clinical Research Center, Sweden), Christina Christersson (Co-Chair; Uppsala Clinical Research Center, Sweden), Johannes Mann (Co-chair; University of Erlangen-Numberg, Munich, Germany) and Christoph Varenhorst (Co-chair; Uppsala Clinical Research Center, Sweden).
FUNDING
This study was funded by AstraZeneca.
CONFLICT OF INTEREST STATEMENT
The DAPA-CKD trial is sponsored by AstraZeneca. The sponsor was involved in the trial design, the writing of the report and the decision to submit the article for publication. H.J.L.H. is a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Gilead, Janssen, Merck, Mundi Pharma, Mitsubishi Tanabe, Novo Nordisk and Retrophin. He received research support from Abbvie, AstraZeneca, Boehringer Ingelheim and Janssen. D.C.W. has received honoraria from AstraZeneca, Amgen, Astellas, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, Sanofi, Tricida and Vifor Fresenius. B.V.S., M.L., C.D.S. and A.M.L. are employees of and own stock in AstraZeneca. M.B. has received honoraria from AstraZeneca and Bayer and has received operational funding for clinical trials from AstraZeneca. O.B. has received personal fees from AbbVie, AstraZeneca, Menarini Group, Merck Sharp & Dohme, Sanofi, Synevo and the World Health Organization and non-financial support from Sanofi and Merck Sharp & Dohme. D.Z.I.C has received honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, Bristol Myers Squibb and Novo Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca and Novo Nordisk. G.M.C. has received fees from AstraZeneca for the DAPA-CKD trial steering committee, research grants from National Institute of Diabetes and Digestive and Kidney Diseases and Amgen; he is on the board of directors for Satellite Healthcare, has received fees for advisory boards for Baxter, Cricket, DiaMedica and Reata; has received fees for advisory boards and holds stock options in Ardelyx, CloudCath, Durect, DxNow and Outset; has received fees from Akebia, Sanifit and Vertex for trial steering committees and has received fees for Data and Safety Monitoring Board service from Angion, Bayer and ReCor. W.D. has received honoraria for educational and research activities from Astra Zeneca, Raffo, GlaxoSmithKline, Ultragenyx and Gador. J.P.D. has received fees from AstraZeneca for the conduct of this study; has received fees from Sanofi-Avensis and CSL Behring as part of a steering committee; has received fees from Novo Nordisk for outcome adjudication for a trial; has received fees from Goldfinch Bio, Birdrock Bio and Boeringer-Ingleheim for study design and received personal fees from Bayer. E.E. has nothing to disclose. R.P.F. has received honoraria paid to his employer for consultancy and educational activities from AstraZeneca, Novo Nordisk, Akebia and Retrophin and research support from Fresenius Medical Care. H.F. has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk. J.L.G. has received honoraria from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Mundipharma, Novartis, Vifor Fresenius and Novo Nordisk. T.G. has received grants for statistical consulting from AstraZeneca, CSL Behring and Boehringer Ingelheim and has received personal fees from Janssen Pharmaceuticals, DURECT and Pfizer for statistical consulting. H.H. has received lecture fees from Bayer, Alexion, Boehringer Ingelheim and AstraZeneca. F.F.H. has received honoraria from AbbVie and AstraZeneca. S.W.K. reports no conflicts of interest. R.I. reports no conflicts of interest. D.K. has served on the advisory boards of AstraZeneca, Novartis, Sanofi, Boehringer and Biocon. P.B.M. had received honoraria from Pfizer, AstraZeneca, Novartis, Bristol Myers Squibb, Napp, Vifor and Pharmacosmos and research support from Boehringer Ingelheim. J.J.V.M. has received payments to his employer, Glasgow University, for work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cardurion, Cytokinetics, GlaxoSmithKline, Novartis, Pfizer and Theracos; personal lecture fees from the Corpus, Abbott, Hickma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology and Servier and is Director at Global Clinical Trial Partners. N.K. is a consultant for AstraZeneca, Gilead, Kyowa-Kirin and GlaxoSmithKline and has received honoraria from AstraZeneca, Astellas, Boehringer Ingelheim, GlaxoSmithKline, Takeda, Daiichi-Sankyo, Otsuka and Kyowa-Kirin. M.N. has received honoraria from Boehringer Ingelheim, Swixx Biopharma, Roche, Sanofi Genzyme and Omeros. F.P. has served as a consultant on advisory boards or as an educator for AstraZeneca, Novo Nordisk, Sanofi, Mundipharma, MSD, Boehringer Ingelheim, Novartis and Amgen and has received research grants to his institution from Novo Nordisk, Amgen and AstraZeneca. R.C.R. is consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Medtronic and Novo Nordisk and has received research support from AstraZeneca, GlaxoSmithKline and Novo Nordisk. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor and research support from AstraZeneca and Novo Nordisk. R.D.T. is a consultant for AstraZeneca, Bayer, Boerhinger Ingelheim, Otsuka, Reata and Relypsa. K.U. has received research funding and consulting fees from AstraZeneca and has received consulting fees from Novo Nordisk. P.V.B. has received research funding and consulting fees from AstraZeneca. I.W. has received honoraria from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Merck Sharp & Dohme and Sanofi.
REFERENCES
- 1. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 5. Heerspink HJ, Perkins BA, Fitchett DH. et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772 [DOI] [PubMed] [Google Scholar]
- 6. Thomson SC, Rieg T, Miracle C. et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 2012; 302: R75–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherney DZ, Perkins BA, Soleymanlou N. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597 [DOI] [PubMed] [Google Scholar]
- 8. van Bommel EJM, Muskiet MHA, van Baar MJB. et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 2020; 97: 202–212 [DOI] [PubMed] [Google Scholar]
- 9. Cherney DZI, Dekkers CCJ, Barbour SJ. et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 2020; 8: 582–593 [DOI] [PubMed] [Google Scholar]
- 10. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349: 1857–1863 [PubMed] [Google Scholar]
- 11. Maschio G, Alberti D, Janin G. et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med 1996; 334: 939–945 [DOI] [PubMed] [Google Scholar]
- 12. Hou FF, Zhang X, Zhang GH. et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 2006; 354: 131–140 [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Stefansson BV, Chertow GM. et al. Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020; 35: 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bakris GL, Agarwal R, Anker SD. et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol 2019; 50: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heerspink HJL, Andress DL, Bakris G. et al. Rationale and protocol of the study of diabetic nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab 2018; 20: 1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov. A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW) ( NCT03819153) https://clinicaltrials.gov/ct2/show/NCT03819153
- 18. Lv J, Zhang H, Wong MG. et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 2017; 318: 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rauen T, Eitner F, Fitzner C. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 20. Barratt J, Rovin B, Diva U. et al. Implementing the kidney health initiative surrogate efficacy endpoint in patients with IgA nephropathy (the PROTECT trial). Kidney Int Rep 2019; 4: 1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov. Efficacy and Safety of Nefecon in Patients with Primary IgA (Immunoglobulin A) Nephropathy (Nefigard) ( NCT03643965) 2020; https://clinicaltrials.gov/ct2/show/NCT03643965 (accessed July 2020)
- 22. Heerspink HJL, Parving HH, Andress DL. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937–1947 [DOI] [PubMed] [Google Scholar]
- 23.AstraZeneca. Farxiga Phase III DAPA-CKD Trial Will Be Stopped Early After Overwhelming Efficacy in Patients with Chronic Kidney Disease 2020; https://www.astrazeneca.com/media-centre/press-releases/2020/farxiga-phase-iii-dapa-ckd-trial-will-be-stopped-early-after-overwhelming-efficacy-in-patients-with-chronic-kidney-disease.html (accessed July 2020)
- 24. Lambers Heerspink HJ, Weldegiorgis M, Inker LA. et al. Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am J Kidney Dis 2014; 63: 244–250 [DOI] [PubMed] [Google Scholar]
- 25. Jardine MJ, Mahaffey KW, Neal B. et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017; 46: 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma SG, Bomback AS, Radhakrishnan J. et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 2013; 8: 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov. A Study of the Effect and Safety of Sparsentan in the Treatment of Patients With IgA Nephropathy (PROTECT) ( NCT03762850) 2020; https://clinicaltrials.gov/ct2/show/NCT03762850 (accessed July 2020)
- 28. Trachtman H, Nelson P, Adler S. et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol 2018; 29: 2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov. Study of Sparsentan in Patients with Primary Focal Segmental Glomerulosclerosis (FSGS) (DUPLEX) 2020; https://clinicaltrials.gov/ct2/show/NCT03493685 (accessed July 2020)
- 30. Lazarus B, Wu A, Shin JI. et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178: 903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heerspink HJL, Andress DL, Bakris G. et al. Baseline characteristics and enrichment results from the SONAR trial. Diabetes Obes Metab 2018; 20: 1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bayer’s Finerenone Meets Primary Endpoint in Phase III FIDELIO-DKD Renal Outcomes Study in Patients with Chronic Kidney Disease and Type 2 Diabetes 2020; https://www.drugs.com/clinical_trials/bayer-s-finerenone-meets-primary-endpoint-phase-iii-fidelio-dkd-renal-outcomes-study-patients-18718.html (accessed July 2020)