Abstract
Background
This study aimed to evaluate short-term and long-term mortalities in a cohort of unselected hospitalized patients with serum sodium concentration ([Na+]) variations within and outside of reference range.
Methods
All adult patients admitted to the Mayo Clinic, Rochester, MN, USA from January 2011 to December 2013 (n = 147358) were retrospectively screened. Unique patients admitted during the study period were examined. The main exposure was serum [Na+] variation. Outcome measures were hospital and 1-year all-cause mortalities.
Results
A total of 60944 patients, mean age 63 ± 17 years, were studied. On admission, 17% (n = 10066) and 1.4% (n = 852) had hypo- and hypernatremia, respectively. During the hospital stay, 11044 and 4128 developed hypo- and hypernatremia, respectively, accounting for 52.3 and 82.9% of the total hypo- and hypernatremic patients. Serum [Na+] variations of ≥6 mEq/L occurred in 40.6% (n = 24 740) of the 60 944 patients and were significantly associated with hospital and 1-year mortalities after adjusting potential confounders (including demographics, comorbidities, estimated glomerular filtration rate, admission serum [Na+], number of [Na+] measurements and length of hospital stay). Adjusted odds ratios for hospital and 1-year mortalities increased with increasing [Na+] variations in a dose-dependent manner, from 1.47 to 5.48 (all 95% confidence intervals >1.0). Moreover, in fully adjusted models, [Na+] variations (≥6 mEq/L) within the reference range (135–145 mEq/L) or borderline hypo- or hypernatremia (133–137 and 143–147 mEq/L, respectively) compared with 138–142 mEq/L were associated with increased hospital and 1-year mortalities.
Conclusion
In hospitalized adults, [Na+] fluctuation (≥6 mEq/L) irrespective of admission [Na+] and borderline hypo- or hypernatremia are independent predictors of progressively increasing short- and long-term mortality burdens.
Keywords: borderline hypo- or hypernatremia, hypernatremia, hyponatremia, serum sodium ([Na+]) variation, short-term and long-term mortalities
INTRODUCTION
Multiple studies have shown that hypo- and hypernatremia, i.e. serum sodium concentration ([Na+]) outside the range of 135–145 mEq/L, are associated with increased mortality. Recent studies have also suggested that mild and even borderline hypo- or hypernatremia (133–137 and 143–145 mEq/L, respectively) can be independently associated with increased mortality [1–3]. It has been recognized that serum [Na+] in the range of 138–142 mEq/L, associated with the lowest mortality [3, 4], is the most physiological [5]. In practice, however, reference [Na+] has customarily been set between 135 and 145 mEq/L. Despite being in large part preventable, deviations from this reference range are often underappreciated [6–8].
In recent years, an association between serum [Na+] fluctuations (≥6 mEq/L) and increased short-term mortality was noted in two studies of critically ill surgical patients [9, 10]. Similar mortality impact was also observed in a subsequent study involving critically ill burn patients [11]. Whether such an association extends to general hospitalized patients is unknown.
This study aims to test the hypothesis that serum [Na+] variations within and outside reference ranges, including borderline hypo- or hypernatremia (133–137 and 143–145 mEq/L, respectively), are progressively associated with hospital (short-term) and 1-year (long-term) all-cause mortalities in a cohort of unselected hospitalized adults.
MATERIALS AND METHODS
Study population
The study was approved by the Mayo Clinic Institutional Review Board. All adult patients (≥18 years of age) admitted to the Mayo Clinic Rochester campus (total beds 2800) during the period January 2011–December 2013 were retrospectively screened. For patients with multiple admissions during the study period, the first hospital admission was examined. Patients with at least two serum [Na+] measurements during the index hospital stay were included (Supplementary data, Figure S1).
Data collection
Patient demographics, admission diagnosis and relevant laboratory test results were collected. The participants were stratified based on the difference between their lowest and highest [Na+] measurements during their hospital stay. The serum [Na+] variation during the hospital stay was defined as the absolute difference between the highest (peak) and the nadir [Na+] during the hospital stay. Participants were grouped based on the magnitude of serum [Na+] variation: 0–1, 2–3, 4–5, 6–7, 8–9, 10–11 and ≥12 mEq/L. Principal diagnoses were grouped based on the International Classification of Diseases, 9th Revision, detailed in a previous publication [12]. On admission, the Charlson comorbidity index (CCI) score was calculated to assess comorbidity [13]. The comorbid conditions were collected using a previously validated data abstraction algorithm [14]. The estimated glomerular filtration rate (eGFR) was derived using the Chronic Kidney Disease Epidemiology Collaboration equation [15].
Clinical outcomes
The primary outcome of the study was hospital all-cause mortality and the secondary outcome was 1-year all-cause mortality among hospital survivors. A sensitivity analysis of 1-year all-cause mortality for the entire cohort was undertaken to ensure that there was no survival bias.
Statistical analysis
Continuous variables were reported as mean ± standard deviation and were compared among serum [Na+] variation groups using analysis of variance. Categorical variables were reported as number (%) and were compared among serum [Na+] variation groups using the chi-squared test. Serum [Na+] variation of 0–1 mEq/L was selected as the reference group for outcome comparison. Logistic regression was performed to evaluate the association between serum [Na+] variation during hospitalization and in-hospital mortalities. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Cox proportional hazards analysis was performed to evaluate the association between serum [Na+] variation during hospitalization and 1-year mortalities after hospital discharge among hospital survivors. Hazard ratios (HRs) with 95% CIs were reported. The proportional hazards assumption was checked by addition of an interaction between log (time) and serum [Na+] variation groups. A multivariable model was constructed to adjust for priori-defined variables. Model 1 was unadjusted; Model 2 was adjusted for age, sex, race, principal diagnosis, CCI score, history of coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease, cirrhosis, eGFR, number of serum [Na+] measurements and hospital length of stay; Model 3 was adjusted for all variables in Model 2, plus the admission [Na+]; Model 4 was adjusted for all variables in Model 2, plus the nadir [Na+] during hospitalization and Model 5 was adjusted for all variables in Model 2, plus the peak [Na+] during hospitalization. The trend test was conducted by treating the seven different serum [Na+] variation groups (0–1, 2–3, 4–5, 6–7, 8–9, 10–11 and ≥12 mEq/L) as a continuous variable when constructing the model to check the dose–response relationship between serum [Na+] variation and outcomes. The interaction between serum [Na+] variation and confounding variables on in-hospital mortality was tested. Sensitivity analysis was performed in patients with all serum [Na+] levels ranging between 135 and 145 mEq/L during hospitalization. A two-tailed P-value <0.05 was considered statistically significant. All analyses were performed using JMP statistical software (version 10; SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics and serum [Na+] distribution
The total number of patients with hypo- and hypernatremia (on admission and hospital acquired) was 26090 (42.8% of the entire cohort, N = 60944), of which 21110 patients experienced hyponatremia ([Na+] < 135 mEq/L) and 4980 had hypernatremia ([Na+] >145 mEq/L), 34.6 and 8.17%, respectively, of the entire cohort. On admission, 82% (n = 50 026) of the cohort had serum [Na+] within the range 135–145 mEq/L. The incidence of hyponatremia was 17% (n = 10066) and that of hypernatremia was 1.4% (n = 852) (Table 1). As serum [Na+] within the range 138–142 mEq/L is considered physiological and has been used as a criterion to define eunatremia and hypo- or hypernatremia [3, 4], we applied the 138–142 mEq/L [Na+] boundary to our cohort. With such a criterion, the incidence of hypo- and hypernatremia on admission was 41 and 9%, respectively, leaving only half of the patients having serum [Na+] within the range 138–142 mEq/L on admission. Hospital-acquired hyponatremia (<135 mEq/L) and hypernatremia (>145 mEq/L) were prevalent, found in 52.3% (n = 11 044) and 82.9% (n = 4128) of the total hypo- and hypernatremic patients, respectively.
Table 1.
Baseline clinical characteristics
| Variables | All | Change in serum [Na+] during hospitalization (mEq/L) |
||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | 4–5 | 6–7 | 8–9 | 10–11 | ≥12 | ||
| N | 60 944 | 7867 | 14 626 | 13 711 | 9362 | 6172 | 3757 | 5449 |
| Age (year), mean ± SD | 63 ± 17 | 61 ± 17 | 62 ± 17 | 63 ± 17 | 64 ± 17 | 64 ± 17 | 64 ± 16 | 63 ± 16 |
| Male, n (%) | 32 685 (54) | 4123 (52) | 7743 (53) | 7266 (53) | 5031 (54) | 3336 (54) | 2118 (56) | 3068 (56) |
| Caucasian, n (%) | 56 706 (54) | 7342 (93) | 13 724 (94) | 12 802 (93) | 8681 (93) | 5689 (92) | 3478 (93) | 4990 (92) |
| Principal diagnosis, n (%) | ||||||||
| Cardiovascular | 15 141 (25) | 1409 (18) | 3015 (21) | 2937 (21) | 2251 (24) | 1926 (31) | 1484 (40) | 2119 (39) |
| Endocrine/metabolic | 1748 (3) | 169 (2) | 326 (2) | 412 (3) | 287 (3) | 197 (3) | 115 (3) | 242 (4) |
| Gastrointestinal | 6042 (10) | 658 (8) | 1398 (10) | 1516 (11) | 1026 (11) | 620 (10) | 337 (9) | 487 (9) |
| Hematology/oncology | 8830 (14) | 1111 (14) | 2103 (14) | 2140 (16) | 1506 (16) | 930 (15) | 434 (12) | 606 (11) |
| Infectious disease | 2276 (4) | 116 (1) | 317 (2) | 425 (3) | 451 (5) | 331 (5) | 227 (6) | 409 (8) |
| Respiratory | 2801 (4) | 276 (4) | 622 (4) | 655 (5) | 523 (6) | 301 (5) | 170 (5) | 254 (5) |
| Injury/poisoning | 9511 (16) | 1260 (16) | 2478 (17) | 2313 (17) | 1434 (15) | 857 (14) | 453 (12) | 716 (13) |
| Genitourinary | 1967 (3) | 193 (2) | 432 (3) | 475 (3) | 383 (4) | 230 (4) | 102 (3) | 152 (3) |
| Other | 12 628 (21) | 2675 (34) | 3935 (27) | 2838 (21) | 1501 (16) | 780 (13) | 435 (12) | 464 (9) |
| CCI score, mean ± SD | 1.9 ± 2.4 | 1.6 ± 2.2 | 1.8 ± 2.4 | 2.0 ± 2.5 | 2.2 ± 2.6 | 2.1 ± 2.5 | 2.0 ± 2.4 | 2.0 ± 2.3 |
| Comorbidity, n (%) | ||||||||
| CAD | 5042 (8) | 516 (7) | 1165 (8) | 1199 (9) | 820 (9) | 562 (9) | 298 (8) | 482 (9) |
| CHF | 4870 (8) | 339 (4) | 926 (6) | 1084 (8) | 915 (10) | 621 (10) | 364 (10) | 621 (11) |
| PAD | 2144 (4) | 159 (2) | 463 (3) | 473 (3) | 377 (4) | 262 (4) | 147 (4) | 263 (5) |
| Stroke | 4916 (8) | 529 (7) | 1078 (7) | 1177 (9) | 792 (8) | 564 (9) | 302 (8) | 474 (9) |
| DM | 13 094 (21) | 1339 (17) | 2842 (19) | 2942 (21) | 2206 (24) | 1519 (25) | 881 (23) | 1365 (25) |
| COPD | 5790 (10) | 536 (7) | 1240 (8) | 1282 (9) | 1004 (11) | 678 (11) | 413 (11) | 637 (12) |
| Cirrhosis | 1738 (3) | 165 (2) | 353 (2) | 373 (3) | 299 (3) | 200 (3) | 137 (4) | 211 (4) |
| eGFR (mL/min/1.73 m2), mean ± SD | 75 ± 29 | 82 ± 24 | 79 ± 27 | 75 ± 29 | 73 ± 31 | 71 ± 31 | 72 ± 30 | 69 ± 31 |
| Admission [Na+], mean ± SD | 138 ± 4 | 139 ± 3 | 138 ± 3 | 138 ± 3 | 137 ± 4 | 137 ± 5 | 137 ± 5 | 137 ± 7 |
| <135, n (%) | 10 066 (17) | 488 (6) | 1216 (8) | 2004 (15) | 2023 (22) | 1624 (26) | 1025 (27) | 1686 (31) |
| 135–145, n (%) | 50 026 (82) | 7360 (94) | 13 350 (91) | 11 606 (85) | 7214 (77) | 4429 (72) | 2634 (70) | 3433 (63) |
| 145, n (%) | 852 (1.4) | 19 (0.2) | 60 (0.4) | 101 (0.7) | 125 (1) | 119 (2) | 98 (3) | 330 (6) |
| Admission [Na+], n (%) | ||||||||
| <138 | 25 013 (41) | 2183 (28) | 4944 (34) | 5986 (44) | 4637 (50) | 3004 (49) | 1701 (45) | 2558 (47) |
| 138–142> | 30 702 (50) | 5286 (67) | 8762 (60) | 6671 (49) | 3916 (42) | 2506 (41) | 1592 (42) | 1969 (36) |
| 142 | 5229 (9) | 398 (5) | 920 (6) | 1054 (8) | 809 (9) | 662 (11) | 464 (12) | 922 (17) |
| Hospital-acquired hyponatremia (<135 mEq/L), n (%) | 11 044 (18) | 77 (1) | 521 (4) | 1273 (9) | 2019 (22) | 2321 (38) | 1836 (50) | 2937 (54) |
| Hospital-acquired hypernatremia (>145 mEq/L), n (%) | 4128 (7) | 6 (0.1) | 76 (0.5) | 271 (2) | 451 (5) | 514 (8) | 560 (15) | 2250 (41) |
| Number of serum [Na+] measurements, median (IQR) | 4 (3–8) | 2 (2–3) | 3 (2–4) | 4 (3–6) | 6 (4–9) | 9 (5–12) | 10 (8–15) | 16 (10–27) |
| Length of hospital stay, median (IQR) | 5 (3–8) | 3 (3–4) | 4 (3–5) | 5 (3–7) | 6 (4–8) | 7 (5–10) | 8 (5–12) | 11 (7–21) |
CAD, coronary artery disease; CHF, congestive heart failure; PAD, peripheral artery disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Variations of serum [Na+] during the hospital stay were common. About 40.6% (n = 24740) of the entire cohort experienced [Na+] variations of ≥6 mEq/L. A total of 31, 40 and 39% of patients with serum [Na+] variations of 8–9, 10–11 and ≥12 mEq/L, respectively, were admitted with a principal diagnosis of cardiovascular disease (Table 1).
[Na+] variations are associated with increased mortality
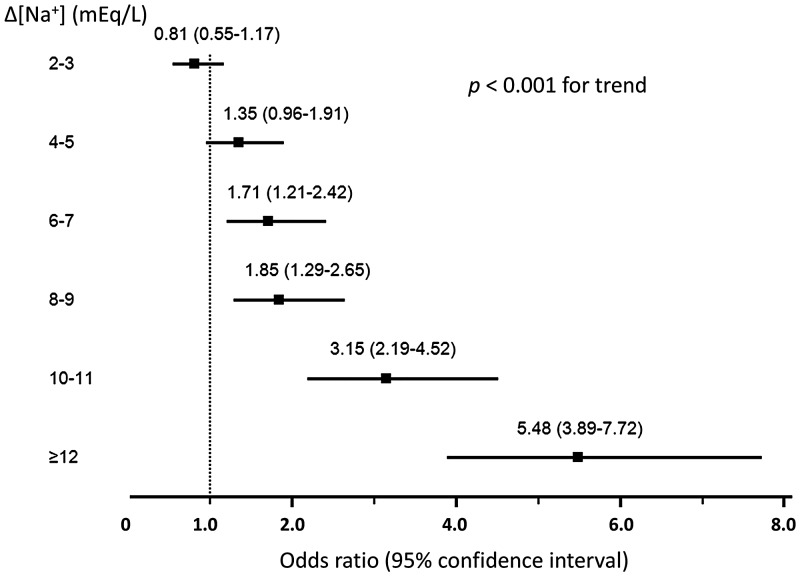
As shown in Table 2, [Na+] variations were associated with increased hospital mortality. Specifically, after adjustment of multiple potential confounders (including demographics, comorbidities, primary admission diagnoses, eGFR, number of [Na+] measurements and length of hospital stay) using a multivariate regression model (see ‘Materials and Methods’ section), the mortality increase remained significant (Model 2). Considering admission [Na+], as well as nadir and peak [Na+], can potentially affect the outcome (mortality) measure, we made some additional adjustments. After adjusting admission [Na+] (Model 3), hospital mortality remained significantly increased, with ORs of 1.71 (95% CI 1.21–2.42), 1.85 (1.29–2.65), 3.15 (2.19–4.52) and 5.48 (3.89–7.72) for [Na+] variations of 6–7, 8–9, 10–11 and ≥12 mEq/L, respectively (Figure 1). Similarly, a persistent increase in the hospital mortality with [Na+] variations ≥6 mEq/L was observed after adjustment of the nadir [Na+] and peak [Na+] (Models 4 and 5) (Table 2). Of note, effect modifications by age, principal admission diagnosis, eGFR and admission [Na+] were noted (Supplementary data, Table S1).
Table 2.
Clinical outcomes: hospital mortality (n = 60 944)
| Outcome | Change in serum [Na+] during hospitalization (mEq/L) |
P-value for trend | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | 4–5 | 6–7 | 8–9 | 10–11 | ≥12 | ||
| Hospital mortality | 43 (0.55) | 78 (0.53) | 141 (1.0) | 144 (1.5) | 109 (1.8) | 114 (3.0) | 414 (7.6) | |
| Mortality, OR (95% CI) | ||||||||
| Model 1: unadjusted | 1 (ref) | 0.98 (0.67–1.42) | 1.89 (1.34–2.66) | 2.84 (2.02–4.00) | 3.27 (2.29–4.66) | 5.69 (4.00–8.10) | 14.96 (10.91–20.52) | <0.001 |
| Model 2a | 1 (ref) | 0.81 (0.55–1.17) | 1.35 (0.96–1.91) | 1.71 (1.21–2.43) | 1.85 (1.29–2.65) | 3.15 (2.19–4.53) | 5.50 (3.91–7.73) | <0.001 |
| Model 3: Model 2 and admission serum [Na+] | 1 (ref) | 0.81 (0.55–1.17) | 1.35 (0.96–1.91) | 1.71 (1.21–2.42) | 1.85 (1.29–2.65) | 3.15 (2.19–4.52) | 5.48 (3.89–7.72) | <0.001 |
| Model 4: Model 2 and nadir serum [Na+] | 1 (ref) | 0.83 (0.57–1.21) | 1.46 (1.03–2.06) | 1.91 (1.35–2.72) | 2.14 (1.48–3.10) | 3.79 (2.61–5.51) | 7.01 (4.89–10.06) | <0.001 |
| Model 4: Model 2 and peak serum [Na+] | 1 (ref) | 0.78 (0.53–1.13) | 1.26 (0.89–1.79) | 1.55 (1.09–2.20) | 1.62 (1.13–2.33) | 2.67 (1.86–3.85) | 3.95 (2.77–5.64) | <0.001 |
ref, reference.
Adjusted for age; sex; race; principal diagnosis; CCI score; history of coronary artery disease, congestive heart failure, peripheral artery disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease and cirrhosis; eGFR; number of serum [Na+] measurements during hospital stay and length of hospital stay.
FIGURE 1.
Adjusted OR (95% CI) for hospital mortalities of the entire cohort of patients (n = 60 944) with serum [Na+] variations from 2 to ≥12 mEq/L. Adjusted for age; sex; race; principal diagnosis; CCI score; history of coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease and cirrhosis; eGFR; admission serum [Na+]; number of serum [Na+] measurements and length of hospital stay. x-axis: OR (95% CI) y-axis: Δ[Na+] (mEq/L) = peak serum [Na+] – nadir serum [Na+]
To determine whether more rapid [Na+] variations might play a role in the risk of mortality, we analyzed the occurrence of [Na+] variations within the first 48 h of the hospital admission. The [Na+] variations of ≥4 mEq/L were significantly associated with hospital mortality in a fully adjusted model (Supplementary data, Table S2 and Figure S2).
To determine the long-term impact of serum [Na+] variation, we investigated the 1-year survival rate in patients with serum [Na+] variations from 0 to ≥12. The survival rates worsened with a progressive increase in [Na+] variations in fully adjusted models (Table 3). Sensitivity analyses of 1-year mortality inclusive of the entire cohort (N = 60 944) showed a sustained significance of the association between [Na+] variations and mortality (Supplementary data, Table S3).
Table 3.
Clinical outcomes: 1-year mortality after discharge in hospital survivors (n = 59 901)
| Outcome | Change in serum [Na+] during hospitalization (mEq/L) |
P-value for trend | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | 4–5 | 6–7 | 8–9 | 10–11 | ≥12 | ||
| 1-year mortality (%) | 7.5 | 10.6 | 13.2 | 15.9 | 17.7 | 17.3 | 21.1 | |
| Mortality, HR (95% CI) | ||||||||
| Model 1: unadjusted | 1 (ref) | 1.43 (1.28–1.59) | 1.82 (1.64–2.02) | 2.23 (2.00–2.49) | 2.55 (2.27–2.86) | 2.46 (2.16–2.81) | 3.21 (2.87–3.61) | <0.001 |
| Model 2a | 1 (ref) | 1.25 (1.12–1.39) | 1.42 (1.28–1.58) | 1.54 (1.38–1.72) | 1.71 (1.52–1.93) | 1.69 (1.48–1.94) | 1.87 (1.64–2.13) | <0.001 |
| Model 3: Model 2 and admission serum [Na+] | 1 (ref) | 1.24 (1.11–1.38) | 1.38 (1.24–1.54) | 1.47 (1.31–1.64) | 1.60 (1.42–1.80) | 1.56 (1.36–1.78) | 1.65 (1.45–1.89) | <0.001 |
| Model 3: Model 2 and nadir serum [Na+] | 1 (ref) | 1.22 (1.09–1.36) | 1.34 (1.20–1.49) | 1.40 (1.25–1.57) | 1.50 (1.33–1.69) | 1.43 (1.25–1.65) | 1.49 (1.29–1.72) | <0.001 |
| Model 4: Model 2, and peak serum [Na+] | 1 (ref) | 1.28 (1.14–1.42) | 1.47 (1.32–1.64) | 1.62 (1.45–1.81) | 1.82 (1.62–2.05) | 1.82 (1.59–2.09) | 2.14 (1.87–2.45) | <0.001 |
ref, reference.
Adjusted for age; sex, race; principal diagnosis; CCI score; history of coronary artery disease, congestive heart failure, peripheral artery disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease and cirrhosis; eGFR; number of serum [Na+] measurements during hospital stay and length of hospital stay.
Serum [Na+] variations within 135–145 mEq/L are associated with increased mortality
To further define the mortality association with [Na+] fluctuations, we examined patients (n = 36 608) with serum [Na+] within the standard reference range (135–145 mEq/L) during their hospital stay. After adjustments of multiple potential confounders, including the number of serum [Na+] measurements and length of hospital stay (Supplementary data, Table S4a, Model 2), the hospital mortality remained significantly increased with [Na+] variations ≥6 mEq/L. The ORs were 1.95 (95% CI 1.22–3.12), 2.59 (1.46–4.58) and 4.33 (1.56–12.05) for [Na+] variations of 6–7, 8–9 and 10 mEq/L, respectively. The upward trajectory of the mortality risk persisted with further adjustment of the admission, nadir and peak serum [Na+] levels (Supplementary data, Table S4a, Models 3, 4 and 5 and Supplementary data, Figure S3).
Further examination of long-term survival showed that greater magnitudes of [Na+] variation within the range 135–145 mEq/L significantly and progressively worsened 1-year patient survival in fully adjusted models (Supplementary data, Table S4b). Note that the number of patients with a variation of 10 mEq/L was too small to be taken as meaningful (n = 178, <1.0% of 36608) and was excluded from the analysis.
[Na+] variations in ranges of borderline hypo- (133–137 mEq/L) and hypernatremia (143–147 mEq/L) are associated with increased mortality
To determine whether borderline [Na+] alterations might influence patient survival in this unselected cohort, we examined short-term (hospital) and long-term (1-year) mortalities among patients with serum [Na+] variations in the ranges of 133–137 mEq/L (borderline hyponatremia, n = 3473) and 143–147 mEq/L (borderline hypernatremia, n = 528). We compared the mortalities of these two groups with the mortality rate in patients with serum [Na+] in the range of 138–142 mEq/L [5] (n = 11531). After adjusting all potential confounders, the hospital mortalities were significantly higher in the groups of borderline hypo- and hypernatremia, with ORs of 1.89 (95% CI 1.16–3.08) for borderline hyponatremia and 2.66 (1.16–6.09) for borderline hypernatremia, relative to patients with serum [Na+] in the range of 138–142 mEq/L (Table 4). Of note, borderline hypernatremia had a higher OR for hospital mortality than borderline hyponatremia.
Table 4.
Hospital mortality in patients with borderline hypo- or hypernatremia compared with patients with [Na+] within the range 138–142 mEq/L
| Outcome | Serum [Na+] range during hospitalization (mEq/L) |
||
|---|---|---|---|
| 133–137 | 138–142 | 143–147 | |
| N | 3473 | 11 531 | 528 |
| Hospital mortality | 28 (0.8) | 44 (0.4) | 7 (1.3) |
| Unadjusted OR (95% CI) | 2.12 (1.32–3.41) | 1 (ref) | 3.51 (1.57–7.82) |
| a Adjusted OR (95% CI) | 1.89 (1.16–3.08) | 1 (ref) | 2.66 (1.16–6.09) |
ref, reference.
Adjusted for age; sex; race; principal diagnosis; CCI score; history of coronary artery disease, congestive heart failure, peripheral artery disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease, and cirrhosis and eGFR.
DISCUSSION
This retrospective study, comprised of unselected hospitalized adults, demonstrates a significant association between serum [Na+] variations (≥6 mEq/L, within or outside the reference [Na+] range) and progressively elevated hospital and 1-year mortalities. Moreover, borderline hypo- and hypernatremia ([Na+] ranges of 132–137 and 143–147 mEq/L, respectively) independently predicts hospital and 1-year mortalities.
Hypo- and hypernatremia have been associated with increased mortality in numerous studies. Moreover, even borderline (133–137 and 143–147 mEq/L) or mild hypo- and hypernatremia (130–135 and 145–150 mEq/L) can be highly associated with short-term mortality [2, 4, 16]. In the last few years, at least three studies reported an association of serum [Na+] variations with an elevated short-term (hospital or 28 day) morality in adult patients [9–11]. Sakr et al. [9], in a retrospective study of a large cohort (n = 10 923) of critically ill surgical patients, found serum [Na+] variations >6 mEq/L (percentage occurrence, unclear) to be independently associated with an increased risk for hospital and 28-day mortalities. More recently, Marshall et al. [10] reported an independent association of each 1-mmol [Na+] fluctuation and elevated 28-day mortality [adjusted OR 1.12 (95% CI 1.09–1.16)] in another cohort (n = 8600) of critically ill postsurgical patients. A third retrospective study, reported by Sen et al. [11], was conducted in a cohort (n = 212) of severe burn patients (≥15% total body surface). The authors show that the coefficient of [Na+] variations was significantly higher in nonsurvivors, 2.85 ± 1.1 versus 2.0 ± 0.7; large [Na+] variations independently predicted hospital with mortality. Whether the mortality association of [Na+] variations extends to the general hospitalized patients, to our knowledge, has not been previously reported.
In this cohort of unselected hospitalized adults, >40% of the patients experienced serum [Na+] abnormality, of which hyponatremia was dominant on admission while a large portion (>80%) of hypernatremia occurred during the hospital stay. These observations are similar to prior studies [3, 4] and, as this study involved general hospitalized adults and outcome measures included long-term mortality, it extended the findings by Oude Lansink-Hartgring et al. [17] in their 2016 report showing a doubling of hospital-acquired hypernatremia in recent decades in a cohort of critically ill patients. Furthermore, we found that serum [Na+] variations (≥6 mEq/L), even within the conventional reference range (135–145 mEq/L), and borderline hypo- and hypernatremia (133–137 and 143–148 mEq/L, respectively) were independently associated with an accelerated risk for both hospital and 1-year mortalities. We also found that [Na+] variation within the first 48 h of hospital admission was more strongly associated with mortality risk, with ≥4 mEq/L of [Na+] variation being a significant risk factor for hospital death. This observation is consistent with a less tolerable and potentially more damaging nature of relatively rapid (<48 h) serum [Na+] fluctuation.
Although the retrospective design precluded any capacity to draw a causal relation, the observation of a significant increase in mortality risk across the study analyses in the general (this study) and in specialized (critically ill) patients in prior studies [9–11] suggests potential deleterious effects of serum [Na+] variations and borderline hypo- or hypernatremia [1–3]. [Na+] is the major effective osmole in the extracellular fluid (ECF). Under physiological conditions, serum [Na+] is tightly regulated by thirst and kidney mechanisms, involving arginine vasopressin (AVP) and tonicity-responsive element binding protein [18]. Fluctuation of serum [Na+] directly impacts ECF tonicity, evoking an osmotic water shift in (with [Na+] reduction) and out (with [Na+] elevation) of the cell, altering cell volume and threatening cell viability. To defend against such insults, the osmo-stressed cells activate adaptive regulatory volume increase (RVI) or regulatory volume decrease (RVD) to minimize the effects of [Na+] (tonicity) perturbation. Specifically, to counter the shrinkage force of [Na+] elevation, RVI triggers immediate influx of ionic osmolytes, followed by the accumulation of organic osmolytes through a transcriptional process that may take hours to days to accomplish [19]. During [Na+] reduction, RVD immediately signals cells to expel osmolytes (both ionic and organic) to minimize cell swelling. Ordaz et al. [20] show that a mere ∼3% drop in ECF osmolality can activate chloride current, leading to chloride efflux. Similarly, a minute (1.8 mOsm/min) reduction of osmolality can trigger immediate efflux of taurine [21], as opposed to its cellular accumulation (during [Na+] elevation) being a much slower transcriptional process [22]. The asymmetric time course of gaining and losing osmolytes due to [Na+] fluctuations may thus alter cellular osmolyte composition and abundance, thereby affecting molecular crowding, cell function and viability [23–27], which could also potentially be associated with osmotic demyelination or cerebral edema [28, 29]. Osmo-stress, in a variety of experimental models, has also been shown to interfere with cargo transport function of centriolar satellites, activate multiple apoptotic pathways, suppress anti-apoptotic gene expression and induce the generation of multiple cytokines and reactive oxygen species [30–35]. The exact mortality influence of osmo-stress and its related effects, however, has yet to be fully elucidated.
Several limitations of this study should not be overlooked. First, given this is a retrospective study, the observed associations cannot be construed as causal. Second, because the data were extracted from the institutional electronic database, detailed information of individual patients, especially the directions of [Na+] fluctuations and specific treatment that could have contributed to the [Na+] variation, is lacking. In general, hypo- or hypernatremia occurs as a result of disproportionate loss or gain of water or Na+ due to water regulatory defects (i.e. inappropriate AVP secretion and diabetes insipidus), illness-related factors (kidney failure, diarrhea and infection) and treatment-related factors (anisotonic fluid administration), often combined with inadequate thirst response, especially in elderly patients. We speculate these diverse factors have likely been involved in the genesis of hypo- or hypernatremia in this study. Fortunately, we focused on [Na+] variations documented in our electronic database and were able to obtain data of interest to achieve the study objectives. As well, 1-year mortality information was abstracted from our electronic records, which censors patient survival from a variety of sources, including the patient’s family and Social Security Death Index (http://www.genealogybank.com/gbnk/ssdi/). Nonetheless, we could have missed some patients who had expired and miscounted them as alive. Such an underestimation would, however, only undercut the elevated 1-year mortality, thus buttressing the results of increased 1-year mortality. Third, we did not factor hyperglycemia into the [Na+] variation, mainly because (i) only 2.87% of the study cohort was admitted for endocrine-/metabolic-related issues and (ii) hyperglycemia would have falsely lowered [Na+] levels, biasing the study results toward null. Moreover, prior studies have shown that in the setting of hyponatremia due to hyperglycemia, hyponatremia per se does not seem to predict mortality [9, 36]. Fourth, this is a single-center study and although the number of patients is large and patients were admitted for a variety of diagnoses, caution should be applied when generalizing the results to other geographic regions or to community patients. Despite these limitations, the consistency of the results across all models supports the notion that serum [Na+] variations within the conventional reference [Na+] range or minimally altered (borderline hypo- and hypernatremia) can be significantly associated with patient mortality.
In summary, we show a previously unrecognized role of [Na+] fluctuation in short-term and long-term mortalities in a large cohort of unselected hospitalized adults. We also provide affirmatory data showing an increased mortality in patients with borderline hypo- or hypernatremia irrespective of their admission diagnoses. Prospective controlled trials are required to establish causality.
Supplementary Material
ACKNOWLEDGEMENTS
The authors appreciate the input from the Mayo Clinic Biostatistics Shared Resources, including the Center for Translational Science Activities, on the statistical analyses of this study.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Sajadieh A, Binici Z, Mouridsen MR. et al. Mild hyponatremia carries a poor prognosis in community subjects. Am J Med 2009; 122: 679–686 [DOI] [PubMed] [Google Scholar]
- 2. Darmon M, Diconne E, Souweine B. et al. Prognostic consequences of borderline dysnatremia: pay attention to minimal serum sodium change. Crit Care 2013; 17: R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsipotis E, Price LL, Jaber BL. et al. Hospital-associated hypernatremia spectrum and clinical outcomes in an unselected cohort. Am J Med 2018; 131: 72–82.e1 [DOI] [PubMed] [Google Scholar]
- 4. Wald R, Jaber BL, Price LL. et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010; 170: 294–302 [DOI] [PubMed] [Google Scholar]
- 5. Kumar S, Berl T.. Sodium. Lancet 1998; 352: 220–228 [DOI] [PubMed] [Google Scholar]
- 6. Snyder NA, Feigal DW, Arieff AI.. Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med 1987; 107: 309–319 [DOI] [PubMed] [Google Scholar]
- 7. Palevsky PM, Bhagrath R, Greenberg A.. Hypernatremia in hospitalized patients. Ann Intern Med 1996; 124: 197–203 [DOI] [PubMed] [Google Scholar]
- 8. Alshayeb HM, Showkat A, Babar F. et al. Severe hypernatremia correction rate and mortality in hospitalized patients. Am J Med Sci 2011; 341: 356–360 [DOI] [PubMed] [Google Scholar]
- 9. Sakr Y, Rother S, Ferreira AM. et al. Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit Care Med 2013; 41: 133–142 [DOI] [PubMed] [Google Scholar]
- 10. Marshall DC, Salciccioli JD, Goodson RJ. et al. The association between sodium fluctuations and mortality in surgical patients requiring intensive care. J Crit Care 2017; 40: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sen S, Tran N, Chan B. et al. Sodium variability is associated with increased mortality in severe burn injury. Burns Trauma 2017; 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thongprayoon C, Cheungpasitporn W, Cheng Z, Qian Q.. Chloride alterations in hospitalized patients: prevalence and outcome significance. PLoS One 2017; 12: e0174430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlson M, Szatrowski TP, Peterson J, Gold J.. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 14. Singh B, Singh A, Ahmed A. et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc 2012; 87: 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilbride HS, Stevens PE, Eaglestone G. et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013; 61: 57–66 [DOI] [PubMed] [Google Scholar]
- 16. Girardeau Y, Jannot AS, Chatellier G. et al. Association between borderline dysnatremia and mortality insight into a new data mining approach. BMC Med Inform Decis Mak 2017; 17: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oude Lansink-Hartgring A, Hessels L, Weigel J. et al. Long-term changes in dysnatremia incidence in the ICU: a shift from hyponatremia to hypernatremia. Ann Intensive Care 2016; 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qian Q. Salt, water and nephron: mechanisms of action and link to hypertension and chronic kidney disease. Nephrology (Carlton) 2018; 23: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeon US, Kim JA, Sheen MR. et al. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol 2006; 187: 241–247 [DOI] [PubMed] [Google Scholar]
- 20. Ordaz B, Tuz K, Ochoa LD. et al. Osmolytes and mechanisms involved in regulatory volume decrease under conditions of sudden or gradual osmolarity decrease. Neurochem Res 2004; 29: 65–72 [DOI] [PubMed] [Google Scholar]
- 21. Tuz K, Ordaz B, Vaca L. et al. Isovolumetric regulation mechanisms in cultured cerebellar granule neurons. J Neurochem 2001; 79: 143–151 [DOI] [PubMed] [Google Scholar]
- 22. Ito T, Fujio Y, Uozumi Y. et al. TauT gene expression is regulated by TonEBP and plays a role in cell survival. Adv Exp Med Biol 2006; 583: 91–98 [DOI] [PubMed] [Google Scholar]
- 23. Dai G, Yu H, Kruse M. et al. Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P2 levels. Proc Natl Acad Sci USA 2016; 113: E3290–E3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan LJ. Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Animal Model Exp Med 2018; 1: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimizu T, Numata T, Okada Y.. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci USA 2004; 101: 6770–6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heimlich G, Cidlowski JA.. Selective role of intracellular chloride in the regulation of the intrinsic but not extrinsic pathway of apoptosis in Jurkat T-cells. J Biol Chem 2006; 281: 2232–2241 [DOI] [PubMed] [Google Scholar]
- 27. Lourenco R, Camilo ME.. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp 2002; 17: 262–270 [PubMed] [Google Scholar]
- 28. Burg MB, Ferraris JD, Dmitrieva NI.. Cellular response to hyperosmotic stresses. Physiol Rev 2007; 87: 1441–1474 [DOI] [PubMed] [Google Scholar]
- 29. Gankam Kengne F, Decaux G.. Hyponatremia and the brain. Kidney Int Rep 2018; 3: 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reinehr R, Graf D, Fischer R. et al. Hyperosmolarity triggers CD95 membrane trafficking and sensitizes rat hepatocytes toward CD95L-induced apoptosis. Hepatology 2002; 36: 602–614 [DOI] [PubMed] [Google Scholar]
- 31. Kagan VE, Tyurin VA, Jiang J. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 2005; 1: 223–232 [DOI] [PubMed] [Google Scholar]
- 32. Shapiro L, Dinarello CA.. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA 1995; 92: 12230–12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopez-Rodriguez C, Aramburu J, Jin L. et al. Bridging the NFAT and NF-κB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 2001; 15: 47–58 [DOI] [PubMed] [Google Scholar]
- 34. Zhang WC, Zheng XJ, Du LJ. et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015; 25: 893–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tellechea M, Buxade M, Tejedor S. et al. NFAT5-regulated macrophage polarization supports the proinflammatory function of macrophages and T lymphocytes. J Immunol 2018; 200: 305–315 [DOI] [PubMed] [Google Scholar]
- 36. Milo-Cotter O, Cotter G, Weatherley BD. et al. Hyponatraemia in acute heart failure is a marker of increased mortality but not when associated with hyperglycaemia. Eur J Heart Fail 2008; 10: 196–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.