Abstract
Introduction
Sucroferric oxyhydroxide (PA21) is an efficacious, well-tolerated iron-based phosphate binder and a promising alternative to existing compounds. We compared the effects of PA21 with those of a conventional phosphate binder on renal function, mineral homeostasis and vascular calcification in a chronic kidney disease–mineral and bone disorder (CKD-MBD) rat model.
Methods
To induce stable renal failure, rats were administered a 0.25% adenine diet for 8 weeks. Concomitantly, rats were treated with vehicle, 2.5 g/kg/day PA21, 5.0 g/kg/day PA21 or 3.0 g/kg/day calcium carbonate (CaCO3). Renal function and calcium/phosphorus/iron metabolism were evaluated during the study course. Renal fibrosis, inflammation, vascular calcifications and bone histomorphometry were quantified.
Results
Rats treated with 2.5 or 5.0 g/kg/day PA21 showed significantly lower serum creatinine and phosphorus and higher ionized calcium levels after 8 weeks of treatment compared with vehicle-treated rats. The better preserved renal function with PA21 went along with less severe anaemia, which was not observed with CaCO3. Both PA21 doses, in contrast to CaCO3, prevented a dramatic increase in fibroblast growth factor (FGF)-23 and significantly reduced the vascular calcium content while both compounds ameliorated CKD-related hyperparathyroid bone.
Conclusions
PA21 treatment prevented an increase in serum FGF-23 and had, aside from its phosphate-lowering capacity, a beneficial impact on renal function decline (as assessed by the renal creatinine clearance) and related disorders. The protective effect of this iron-based phosphate binder on the kidney in rats, together with its low pill burden in humans, led us to investigate its use in patients with impaired renal function not yet on dialysis.
Keywords: chronic kidney disease, FGF-23, iron, phosphate binding, vascular calcification
KEY LEARNING POINTS
What is already known about this subject?
Iron-based phosphate binding is a relatively new treatment strategy to prevent hyperphosphataemia in chronic kidney disease (CKD) patients.
Sucroferric oxyhydroxide (PA21) is an efficacious, well-tolerated, iron-based phosphate binder in this population.
This study was initiated to investigate whether PA21, apart from its phosphate-binding capacity, might exert other not yet investigated/revealed beneficial effects in CKD.
What this study adds?
In a rat model of CKD–mineral and bone disorder, PA21 treatment, in addition to efficient phosphate binding, resulted in better preserved renal function, haematocrit and iron homeostasis, compared with vehicle and conventional (CaCO3) treatment.
Both PA21 and CaCO3 ameliorated the CKD-related hyperparathyroid bone.
PA21, in contrast to CaCO3, prevented a dramatic increase in fibroblast growth factor (FGF)-23 and significantly reduced the vascular calcium content.
What impact this may have on practice or policy?
PA21 should be considered an effective and safe treatment for CKD-MBD-related hyperphosphataemia, with a beneficial impact on renal function.
Since high circulating FGF-23 levels have been directly associated with cardiovascular events and mortality in haemodialysis patients and further investigation on the remarkable PA-21 induced reduction of circulating FGF-23 is of particular interest.
The results of this study point to a potential beneficial impact of PA21 on clinical care in CKD patients not yet on dialysis and demonstrate the necessity for further clinical investigation of the compound in this population.
INTRODUCTION
In patients with prolonged, progressive renal impairment, calcium, phosphorus, parathyroid hormone (PTH) and fibroblast growth factor (FGF)-23 are dysregulated, which ultimately leads to ectopic calcification in the arteries and bone abnormalities. These complications cover a broader syndrome defined as chronic kidney disease–mineral and bone disorder (CKD-MBD). In addition, these patients have iron deficiency, caused by impaired gastrointestinal iron absorption, blood losses and decreased iron release from body stores, which, along with less renal erythropoietin (EPO) production, results in anaemia [1].
Elevated serum phosphorus concentration and calcium × phosphorus product are identified as important risk factors for cardiovascular mortality in patients with end-stage renal disease [2–4]. To normalize serum phosphorus levels and prevent mineral disturbances dialysis patients are routinely treated with phosphate binders. Observational clinical studies have demonstrated that targeting hyperphosphataemia leads to improved survival in dialysis patients [5, 6]. Calcium carbonate (CaCO3) and calcium acetate have been extensively used to reduce dietary phosphate absorption, but their use is restricted in CKD patients with hyperkalaemia and low circulating PTH levels, because of the increased risk for adynamic bone disease and vascular calcification. Alternative non-calcium-containing agents such as sevelamer and lanthanum carbonate show an equally efficient capacity to lower serum phosphate as compared with calcium-containing phosphate binders. Clinical/pre-clinical studies suggest that treatment with non-calcium-containing phosphate binders might be preferred because of a lower vascular calcification progression rate [7–11] and consequently a survival benefit [12, 13].
Sucroferric oxyhydroxide (PA21) is a novel iron-based phosphate binder and promising alternative to existing compounds. Clinical trials have shown that PA21 is well-tolerated and efficacious in lowering serum phosphorus in dialysis patients, requiring a lower pill burden and hence better adherence compared with concurrent compounds, without evidence of iron accumulation [14, 15]. To investigate the effect of PA21 on the various aspects of the CKD-MBD syndrome, we compared the impacts of PA21 with those of CaCO3 treatment on renal function, iron and mineral metabolism, vascular calcification and bone status in an established rat model with adenine-induced CKD-MBD [16].
MATERIALS AND METHODS
Study design
Experimental procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals 85-23 (1996) and approved by the University of Antwerp Ethics Committee (2017-73). Animals were housed two per cage with free water and food access. Renal failure was induced in 64 male Wistar rats (225–250 g) by administration of an adenine-enriched diet with low vitamin K content [0.25% adenine, 0.2 mg/kg vitamin K, 1% calcium (Ca), 1% phosphorus (P), 1 IU/g vitamin D, 160 mg iron (Fe)/kg and 6% protein; ssniff Spezialdiäten, Soest, Germany] during 8 weeks, as described earlier [16]. Before the start of the study, animals were randomly assigned to four treatment groups: vehicle (n = 16), 5.0 g/kg/day (corresponding to 5% in the diet) PA21 (n = 16), 2.5 g/kg/day PA21 (n = 16) and 3.0 g/kg/day (corresponding to 3% in the diet) CaCO3 (n = 16). The 3.0 g/kg/day CaCO3 and 5.0 g/kg/day PA21 doses are considered equivalent in terms of active pharmaceutical moiety; 5 g/kg PA21 corresponds to 1 g Fe/kg and 3 g/kg CaCO3 corresponds to 1.2 g Ca/kg, in accordance with a previous study [17]. Animals were daily treated with vehicle (1% carboxymethylcellulose), 2.5 or 5.0 g/kg PA21 or 3.0 g/kg CaCO3 from the start of renal failure induction until the end of the study (8 weeks) by oral gavage. To achieve optimal phosphate binding, animals were gavaged with half of the indicated dose twice a day (during weekends they were treated with the indicated dose once a day). To enable dynamic bone parameter measurement, tetracycline (30 mg/kg) and demeclocycline (25 mg/kg) were administrated intravenously, 7 and 3 days before sacrifice, respectively. Before the induction of renal failure and every 2 weeks thereafter, animals were housed for 24 h in metabolic cages to collect urine samples, followed by blood sampling via the tail vein in a restrained, conscious condition. Ionized calcium, haematocrit and haemoglobin were measured in whole blood using the i-STAT 1 point of care analyser (Abbott, Berkshire, UK). After 8 weeks, animals were sacrificed by exsanguination through the retro-orbital plexus after anaesthesia through intraperitoneal administration of 60 mg/kg sodium pentobarbital (Nembutal, Ceva Santé Animale, Libourne, France).
Biochemistry
Creatinine was measured in serum/urine samples with the Jaffe method. Total serum/urine phosphorus levels were analysed with the Ecoline S Phosphate kit (Diasys, Holzheim, Germany) and serum/urinary calcium levels were determined with flame atomic absorption spectrometry (FAAS; Perkin-Elmer, Wellesley, MA, USA). Serum non-haem iron was measured by electrothermal atomic absorption spectrometry (ETAAS) after pre-treatment with hydrochloric acid (HCl)/trichloric acid [18]. Quality checks of this method included the acquiriing of the same results in haemolysed and non-haemolysed serum samples.
Serum intact PTH (Immutopics, San Clemente, CA, USA), (intact) FGF-23 (Kainos, Tokyo, Japan), 1,25-dihyrdoxyvitamin D [1,25(OH)2D; Cusabio, Houston, TX, USA], hepcidin (Cusabio) and ferritin (Kamiya Biomedical, Seattle, WA, USA) were measured using commercially available enzyme-linked immunosorbent assay kits. Serum alkaline phosphatase was measured by an auto-analyser at Antwerp University Hospital.
Iron measurement in liver and spleen
After weighing, parts of the liver/spleen were homogenized in HCl/trichloric acid. Subsequently the tissue homogenate was incubated 1.5 h at 95°C and centrifuged [10 min; 8200 rcf (10 000 g)]. Iron was measured by Zeeman-corrected ETAAS (Perkin Elmer) in the supernatant [18]. Liver and spleen iron concentrations are expressed per gram of wet weighed tissue. As a quality check, blood was added to tissue lysates, after which no increase in iron levels was seen.
Quantification of ectopic calcification
Ectopic calcification was quantified by measurement of total calcium content in the proximal abdominal aorta, the left carotid and femoral arteries and a transverse kidney slice. After digestion in 65% nitric acid(60°C, 6 h), the total calcium content was measured by FAAS. The calcium concentration is expressed per gram of wet weighed tissue.
Bone histomorphometry
After fixation in 70% ethanol overnight, the left tibia was processed for quantitative histomorphometric analysis [19] and dehydrated and embedded in 100% methyl methacrylate. Five-micrometre sections were Goldner stained for visualization/measurement of total bone area, mineralized bone area, osteoid area/width/perimeter, osteoblast perimeter, eroded perimeter, osteoclast perimeter, trabecular thickness and trabecular distance using the Axiovision image software (version 4.5, Carl Zeiss, Oberkochen, Germany). Ten-micrometre unstained tibial sections were used to measure the distance between and length of double tetracycline/demeclocycline labels by fluorescence microscopy. Dynamic bone parameters (bone formation rate, mineral apposition rate, adjusted apposition rate, mineralization lag time and osteoid maturation time) were calculated according to Dempster et al. [20].
Renal histological analysis
Immediately after isolation of the left kidney, a transverse slice was fixed in neutral buffered formalin for 4 h, rinsed with isopropanol and paraffin embedded.
Deparaffinized tissue sections (4 µm) were stained with periodic acid–Schiff/methyl green for morphological evaluation and quantitation of the tubulointerstitial area. Four random pictures (×200) of the renal cortex were obtained and tubulointerstitial area was quantified histomorphometrically using Fiji image analysis software [21].
Quantitative real-time polymerase chain reaction
Total messenger RNA (mRNA) of a snap-frozen section of the left kidney was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed to complementary DNA (cDNA) by the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Real-time polymerase chain reaction (PCR) with a QuantStudio 3 Real-Time PCR System (Applied Biosystems) was used for mRNA quantification. Taqmangene expression assays on demand were purchased from Applied Biosystems. The expression of each gene was analysed in triplicate and normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase. Gene expression was calculated with the comparative cycle threshold method.
Statistics
Results are expressed as mean ± standard error of the mean (SEM) and as individual data per animal (to not overload charts, individual data were provided for the data obtained at sacrifice only). Non-parametric statistical analyses were performed with Statistical Package for Social Sciences 24.0 (IBM, Armonk, NY, USA). Differences between multiple time points for each study group were determined by the Friedman test, followed by a Wilcoxon signed-rank test. Comparisons between the study groups for each time point were assessed using a Kruskal–Wallis test, followed by a Mann–Witney U-test when significant. Bonferroni correction was applied when appropriate. P-values <0.05 were considered significant.
Reference values of healthy Wistar rats with normal renal function (same age, gender and diet composition without adenine supplementation) were used for hepatic non-haem iron, ectopic calcifications, static/dynamic bone parameters and renal mRNA expression. These values were obtained in spare samples from recent studies performed in our laboratory. Samples had been stored under appropriate conditions and analysis was done concomitantly with sample analysis for this study. Reference values were not included in the statistical analyses.
RESULTS
Mortality
Mortality was limited; only 4 (1 of 16 in each group) animals (6.25%) died before planned sacrifice.
Renal function and anaemia
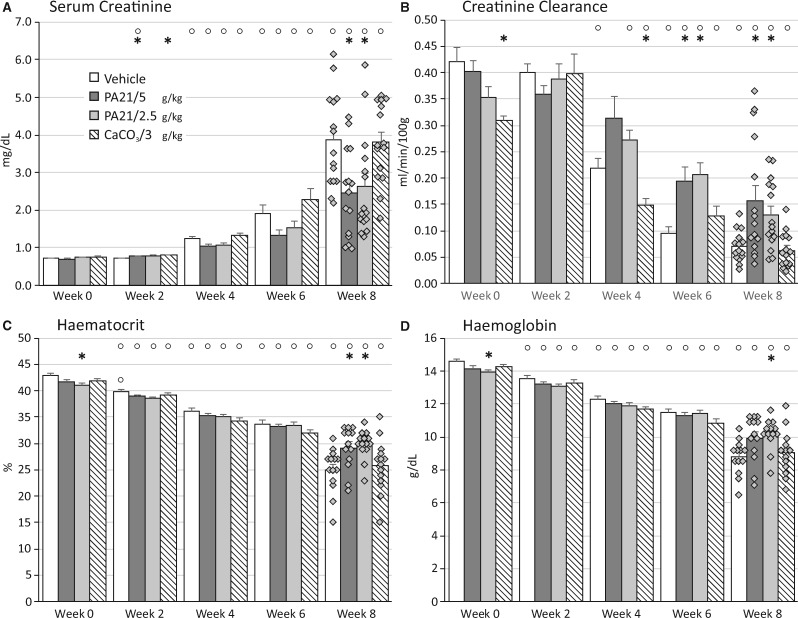
Adenine dosing resulted in severe, stable renal function decline. Serum creatinine levels rose to 6-fold baseline values (~4 mg/dL) in vehicle and CaCO3-treated animals (Figure 1A). Remarkably, PA21 treatment, at either the low or high dose, resulted in better preserved renal function (lower serum creatinine and higher creatinine clearance) from Week 4 onward, reaching significance at the end of the study (Figure 1A and B).
FIGURE 1.
(A) Serum creatinine, (B) creatinine clearance, (C) haematocrit and (D) haemoglobin in rats treated with either vehicle, PA21 or CaCO3 throughout the study course. *P < 0.05 versus vehicle at the same time point. °P < 0.05 versus Week 0 within the same study group.
Along with the renal function decline, haematocrit and haemoglobin levels significantly decreased (Figure 1C and D). The better preserved renal function of PA21-treated animals was reflected in haematocrit and haemoglobin values, which compared with those of vehicle and CaCO3, remained significantly higher.
Renal histology, inflammation, fibrosis and α-klotho expression
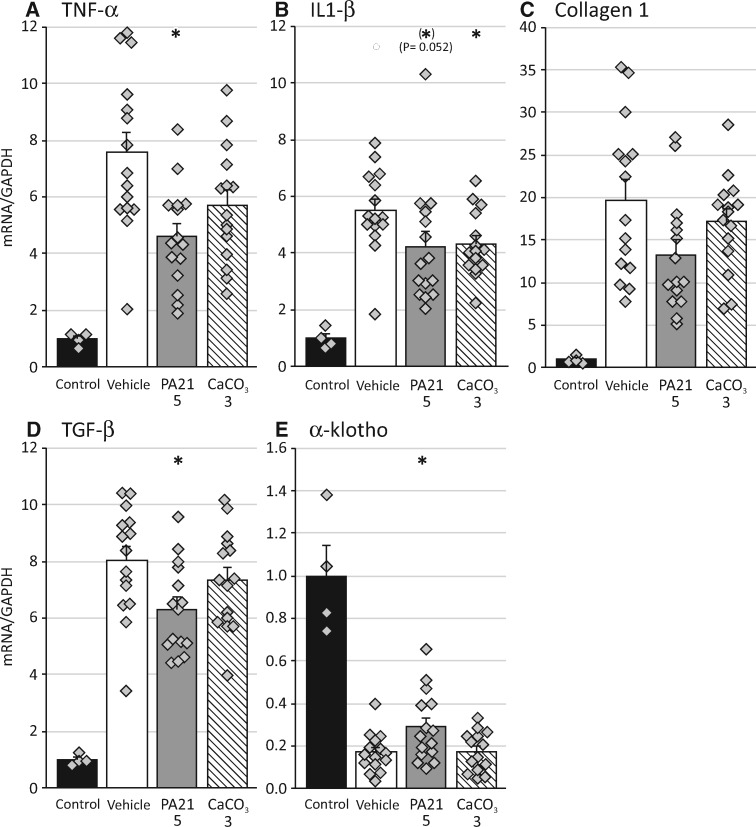
PA21 treatment significantly reduced renal expression of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and transforming growth factor (TGF)-β, while significantly better preserved renal α-klotho expression. CaCO3 treatment significantly reduced IL-1β expression (Figure 2A and B). Masson staining revealed the presence of excess extracellular matrix (ECM) in the expanded tubular interstitium in kidneys treated with adenine. Measurement of the tubulointerstitial area percentage showed that, despite a trend towards a lower tubulointerstitial area in the PA21-treated groups (47.0 ± 2.0 and 48.3 ± 1.8 in the high- and low-dose groups, respectively, versus 52.5 ± 1.6 and 50.7 ± 1.4 in the vehicle- and CaCO3-treated groups, respectively), no significant differences were found.
FIGURE 2.
mRNA expression of (A) TNF-α, (B) IL-1β, (C) collagen 1, (D) TGF-β and (E) α-klothoin the kidney of rats treated with either vehicle, PA21 or CaCO3 compared with control rats with normal renal function. *P < 0.05 versus rats treated with vehicle.
Mineral homeostasis
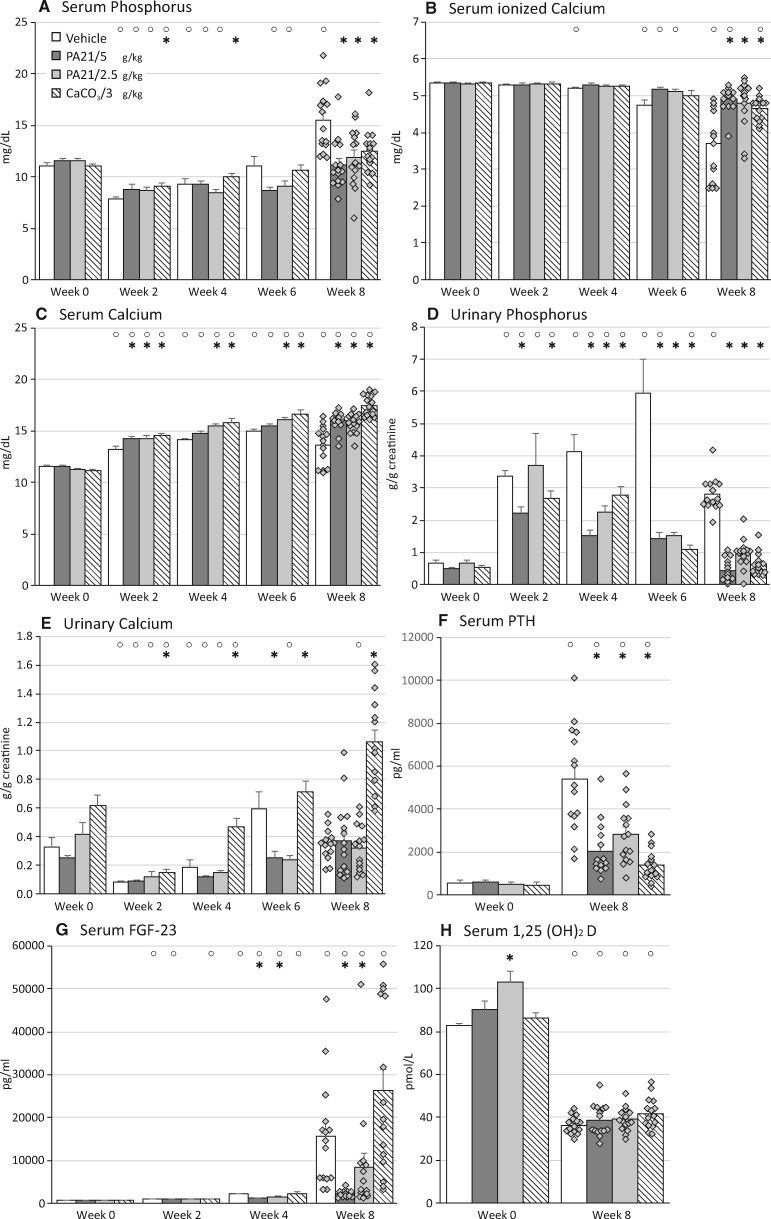
Along with the development of renal function impairment, rats developed hyperphosphataemia and hypocalcaemia (Figure 3A and B). Compared with vehicle, PA21 and CaCO3 treatment prevented an increase in serum phosphorus levels and a decrease in ionized calcium levels.
FIGURE 3.
(A) Serum phosphorus, (B) serum ionized calcium, (C) serum calcium, (D) urinary phosphorus, (E) urinary calcium, (F) serum PTH, (G) serum FGF-23and (H) serum 1,25(OH)2D in rats treated with either vehicle, PA21 or CaCO3 throughout the study course. *P < 0.05 versus vehicle at the same time point. °P < 0.05 versus Week 0 within the same study group.
Serum total calcium levels were significantly higher in PA21- and CaCO3-treated rats as compared with vehicle-treated rats (Figure 3C), with the highest levels in CaCO3-treated rats. PA21 and CaCO3 treatment significantly decreased urinary phosphorus excretion (Figure 3D) as compared with vehicle treatment. Together with lower serum phosphorus levels, this indicates efficient intestinal phosphate binding, which was as efficient as in the highest PA21 and the CaCO3 groups. Urinary calcium levels did not differ between vehicle- and PA21-treated rats (Figure 3E). In contrast, CaCO3 treatment significantly increased urinary calcium excretion, reflecting increased calcium load in these animals.
Inherent to renal function decline, a significant increase in serum PTH levels was seen in the vehicle group (Figure 3F). Serum PTH values were significantly lower in all groups receiving phosphate-binding treatment, which were most pronounced in the CaCO3 group (Figure 3F). As renal function declined, FGF-23 levels dramatically increased in vehicle-treated animals (Figure 3G), which was partially, but significantly prevented in rats treated with both PA21 doses, in contrast to CaCO3, which could not control FGF-23 levels. Renal insufficiency also led to decreased serum 1,25(OH)2D levels, with no difference between study groups (Figure 3H).
Iron homeostasis
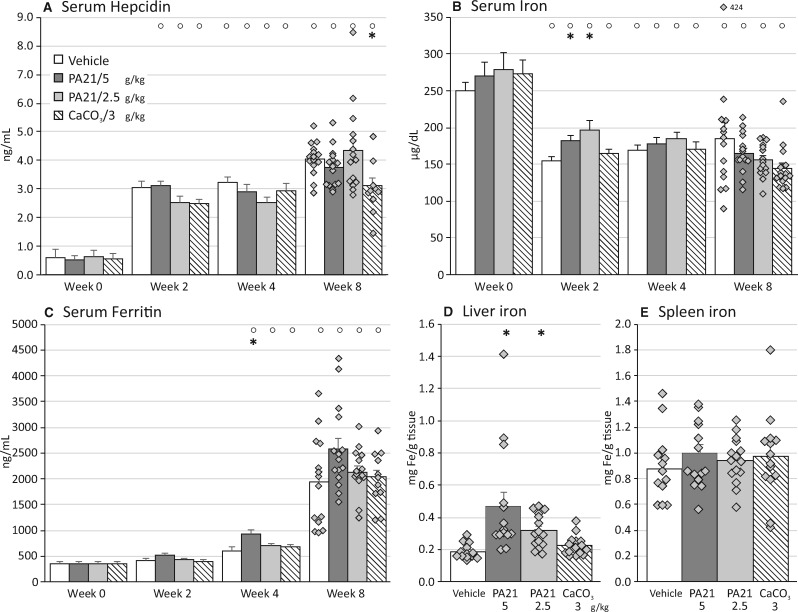
After 2 weeks of adenine supplementation to the diet, serum hepcidin levels were significantly elevated, whereas serum iron was significantly decreased in all study groups compared with baseline levels (Figure 4A and B). Eight-week CaCO3 treatment resulted in significantly lower circulating hepcidin levels as compared with vehicle treatment (Figure 4A). PA21 treatment significantly increased circulating iron levels in rats (Figure 4B), after 2 weeks of treatment, compared with vehicles. Serum ferritin levels (Figure 4C) increased with study duration in all groups and tended to be higher in the highest PA21 dose group.
FIGURE 4.
(A) Serum hepcidin, (B) serum iron,(C) serum ferritin, (D) hepatic ironand (E) spleen ironlevels in rats treated with either vehicle, PA21 or CaCO3. °P < 0.05 versus Week 0 within the same study group. *P < 0.05 versus vehicle at the same time point.
Compared with vehicle-treated animals, significantly higher hepatic iron levels were seen in both PA21 groups (Figure 4D) but not in the CaCO3 group. Hepatic iron levels of all rats with impaired renal function were higher compared with hepatic iron levels in rats with normal renal function (i.e. 0.15 ± 0.3 mg/g tissue). The iron content of the spleen was similar in the different treatment groups (Figure 4E).
Ectopic calcification
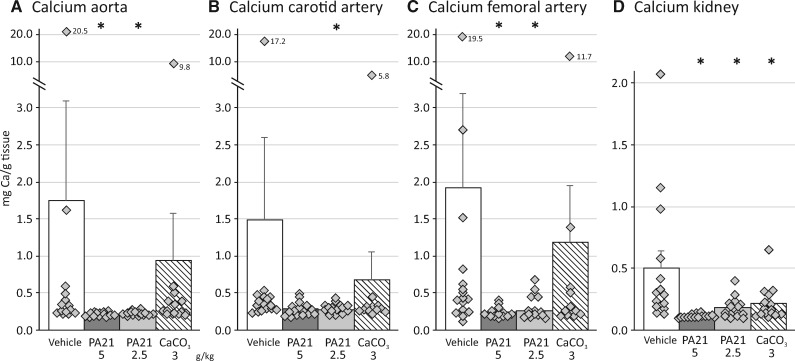
Adenine-induced renal failure resulted in an increased calcium content in the aorta, carotid and femoral arteries and the kidney. Aortic renal calcium content was 1.75 ± 1.33 and 0.50 ± 0.14 mg/g of wet tissue in vehicle-treated rats with impaired renal function versus 0.13 ± 0.03 and 0.08 ± 0.01 mg/g of wet tissue in rats with normal renal function. Treatment with both PA21 doses significantly reduced the calcium concentration in the vessels/kidney (Figure 5A-D). CaCO3-treated rats showed calcium concentrations in the vessels similar to those of vehicle-treated rats, while renal calcium concentration was significantly lower in CaCO3-treated versus vehicle-treated animals (Figure 5A-D).
FIGURE 5.
Calcium content in the (A) aorta, (B) carotid artery, (C) femoral arteryand (D) kidney of rats treated with either vehicle, PA21 or CaCO3. *P < 0.05 versus vehicle.
Bone histomorphometry
To evaluate the effect of the different phosphate binders on bone metabolism, static/dynamic bone parameters were determined (Table 1). Renal function impairment led to high bone turnover disease, characterized by relatively high amounts of osteoid, osteoblast/osteoclast numbers and bone formation rates (compared with reference values of normal renal function rats).
Table 1.
Static and dynamic bone parameters and serum alkaline phosphatase values of CKD rats treated with either vehicle, PA21 or CaCO3.
| Parameters | NRF | CKD + vehicle | CKD + PA21 5 g/kg | CKD + PA21 2.5 g/kg | CKD + CaCO3 3 g/kg |
|---|---|---|---|---|---|
| Bone area/tissue area (%) | 23.5 ± 1.5 | 28.0 ± 3.6 | 16.9 ± 1.9 | 17.6 ± 2.5 | 22.7 ± 2.0 |
| Mineralized area/tissue area (%) | 23.4 ± 1.5 | 26.6 ± 3.4 | 16.2 ± 1.8* | 16.6 ± 2.3* | 21.2 ± 1.8* |
| Osteoid area/bone area (%) | 0.65 ± 0.28 | 4.8 ± 0.4 | 4.2 ± 0.7 | 5.3 ± 1.1 | 6.1 ± 1.5 |
| Eroded perimeter/total perimeter (%) | 12.7 ± 1.2 | 20.9 ± 1.7 | 10.2 ± 0.8* | 13.7 ± 1.2* | 8.3 ± 1.2* |
| Osteoblast perimeter/osteoid perimeter (%) | 20.1 ± 8.8 | 80.6 ± 1.6 | 59.7 ± 4.5* | 63.7 ± 4.1* | 53.95 ± 4.0* |
| Osteoclast perimeter/eroded perimeter (%) | 5.0 ± 1.8 | 32.2 ± 1.8 | 31.0 ± 1.5 | 31.6 ± 1.8 | 31.9 ± 1.4 |
| Bone formation rate (µm2/mm2/day) | 1436 ± 112 | 6554 ± 787 | 3042 ± 496* | 3853 ± 634* | 2721 ± 457* |
| Mineralization lag time (days) | 0.74 ± 0.21 | 2.0 ± 0.2 | 2.5 ± 0.6 | 2.2 ± 0.3 | 19.0 ± 11.8 |
| Adjusted apposition rate (µm/day) | 11.9 ± 4.1 | 3.3 ± 0.2 | 2.9 ± 0.4 | 2.6 ± 0.2 | 2.0 ± 0.4* |
| Osteoid maturation time (days) | 2.1 ± 0.4 | 1.0 ± 0.1 | 1.3 ± 0.1* | 1.1 ± 0.1 | 1.9 ± 0.41 |
| Serum alkaline phosphatase levels (U/L) | ND | 90.3 ± 4.9 | 66.7 ± 2.4* | 70.0 ± 4.4* | 72.0 ± 3.9* |
Results are expressed as mean ± SEM.
*P < 0.05 versus vehicle.
NRF, normal renal function; ND, not determined.
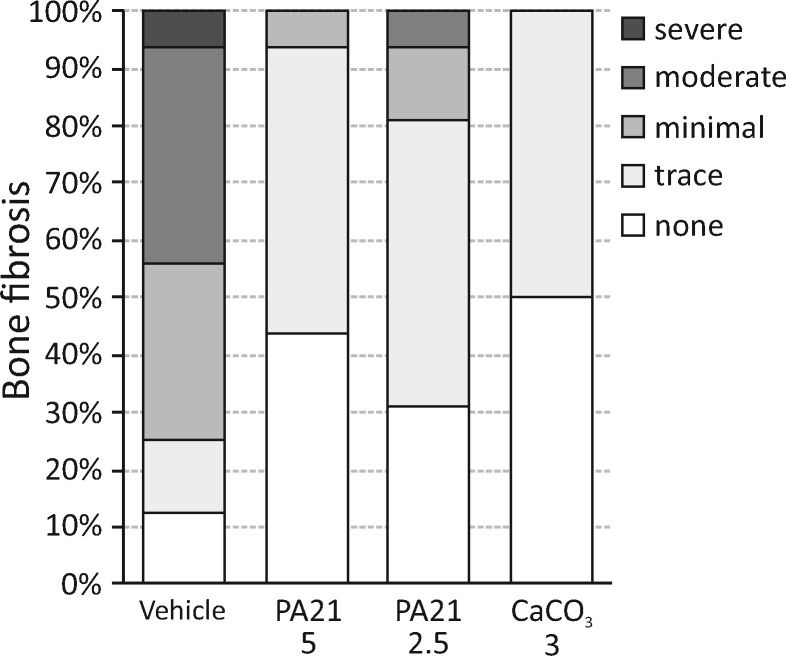
Static bone parameters revealed a trend towards decreased bone area in the animals receiving phosphate binder versus vehicle, mainly due to the significantly decreased mineralized bone area, as the amount of osteoid did not differ significantly between treatment groups. These observations, together with the decreased eroded/osteoblast perimeters, point towards normalization of bone turnover, which is fully in line with the significantly decreased bone formation rate in the phosphate binder treatment groups. In the PA21 groups, the mineralization lag time (time interval between osteoid deposition and mineralization) and adjusted apposition rate (the rate at which osteoid is mineralized and in the absence of a mineralization defect equals the osteoid apposition rate) do not significantly differ from the vehicles. In the CaCO3-treated animals, however, the significantly decreased adjusted apposition rate in combination with a tendency towards an increased mineralization lag time and osteoid maturation time (i.e. time interval between the osteoid deposition and the initiation of mineralization) may point towards less efficient mineralization. In the vehicle group, the increased bone turnover was accompanied by the presence of trace to severe bone marrow fibrosis, which was dramatically reduced by phosphate binder treatment and closely relates to the decreased bone formation rate (Figure 6).
FIGURE 6.
Degree of bone fibrosis in rats treated with either vehicle, PA21 or CaCO3.
After 8 weeks, rats receiving either PA21 or CaCO3 had lower circulating alkaline phosphatase levels compared with vehicles (Table 1), which is in line with the decreased number of osteoblasts/bone formation rate.
DISCUSSION
This study aimed to compare the effect of PA21 with CaCO3, a conventional phosphate binder, on mineral and iron homeostasis, ectopic/vascular calcification and bone turnover/mineralization using an (adenine-induced) CKD-MBD rat model. This model of continuous (0.25%) adenine administration closely mimics the clinical situation as evidenced by severely impaired renal function (assessed by renal creatinine clearance), hyperphosphataemia, hypocalcaemia, moderate–severe secondary hyperparathyroidism, increased FGF-23, reduced 1,25(OH)2D levels and mild ectopic calcification. Rats with impaired renal function also developed anaemia (reduced haematocrit and haemoglobin levels). Intriguingly, PA21 treatment, at either high or low dose, partially protected against renal function decline. Lower serum creatinine levels and higher creatinine clearance in PA21-treated rats clearly show a beneficial impact on renal function. In addition, we observed significantly less expression of renal inflammation/fibrosis markers in PA21-treated rats and better preserved α-klotho expression compared with vehicle-treated rats. The trend towards a decreased tubulointerstitial area in PA21-treated rats suggests less cellular infiltration/deposition of ECM in these animals. In contrast, treatment with CaCO3 did not modulate renal impairment throughout the study. In line with the better preserved renal function, anaemia was less severe in PA21-treated rats compared with rats receiving vehicle or CaCO3.
In support of our findings, Nemoto et al. [22] reported that PA21 reduced glomerulosclerosis/tubulointerstitial injury as well as renal inflammation/fibrosis in the remnant kidney rat model, despite no effect on renal function . A beneficial renal effect was also reported with ferric citrate in this rat model. Remnant kidney rats treated with ferric citrate had lower serum creatinine and blood urea nitrogen levels, higher creatinine clearance and haemoglobin levels, as well as higher serum iron levels, as compared with vehicle-treated animals [23]. Furthermore, ferric citrate–treated rats showed less inflammation, oxidative stress and fibrosis and higher iron levels in the remnant kidney, less cardiac hypertrophy and fibrosis and a more beneficial gut microbiome compared with vehicle-treated rats [23–25]. This, together with our current findings, using a model with different renal disease characteristics, is of particular interest, as the potential protective effect of iron-based phosphate binders on the kidneys of patients with impaired renal function but not yet on dialysis needs to be investigated.
Reduced serum/urinary phosphorus concentrations and increased serum ionized calcium levels point towards efficient phosphate binding of PA21 and CaCO3. Both doses of PA21 as well as CaCO3 in particular also strongly inhibited the pronounced rise in PTH levels. Of particular interest, the highest and to a lesser extent the lowest PA21 dose also significantly prevented the dramatic FGF-23 increase, whereas CaCO3 did not.
The mechanisms by which iron-based phosphate binders may halt renal function decline remain speculative. Do small increases in iron absorption directly protect against renal damage or does an inhibitory effect on FGF-23 production indirectly exert a protective effect on the kidney? Recent studies have shown that excessive FGF-23 in CKD patients might affect different organs, among which is the kidney [26]. FGF-23 has been shown to augment pro-fibrotic signalling in injury-primed renal fibroblasts [27] and to promote renal interstitial fibrosis in unilateral ureteral obstruction [28]. Hence PA21, by inhibiting the increase in FGF-23 levels, might retard the development/progression of renal fibrosis, resulting in better conserved kidney function. Further studies are required to unravel underlying mechanisms and (multiple) pathways by which PA21 treatment modulates renal function. Renal α-klotho expression was better preserved in the PA21-treated (compared with vehicle and CaCO3) rats, which strongly suggests that the beneficial effect of PA21 on FGF-23, at least partially, occurred indirectly via the more preserved renal function. Evidence for a possible direct effect deserves further investigation. In this context, results from a recent study evaluating the efficacy of PA21 in haemodialysis patients are of interest since they also reported a decreased serum FGF-23 level and improved anaemia [29]. Likewise, ferric citrate treatment reduced circulating FGF-23 and improved EPO responsiveness in haemodialysis patients [30]. In line with our findings, experimental studies of Phan et al. [17, 31] also revealed a decline in FGF-23 levels in PA21-treated adenine rats, while no effect was seen for other phosphate binders. In this context, it is worth mentioning that serum FGF-23 and iron levels are strongly linked [32]. Low serum iron levels caused by inflammation and the concomitant high hepcidin levels are associated with high circulating FGF-23 levels [32]. As such, the strong effect of PA21 on FGF-23 may possibly be explained by increased iron availability in the PA21-treated rats, which is supported by the slightly, but significantly increased serum iron levels after 2 weeks of treatment and the increased hepatic iron content. Interestingly, the higher serum iron levels after 2 weeks of PA21 treatment also preceded the lower serum FGF-23 levels after 4 weeks of treatment. While the FGF-23 lowering effect due to iron is one potential explanation, as evidenced above, it is not supported by data from a large clinical study in which no difference was found with regard to FGF-23 reduction between PA21 and sevelamer carbonate treatment [33]. In addition, the impact of slight differences in serum phosphorus levels on FGF-23 levels remains unclear.
With regard to the improvement of anaemia in the PA21 groups, the question arises as to whether the higher haemoglobin/haematocrit levels reflect improved renal function or whether other factors are also involved. Although increased iron bioavailability could possibly improve anaemia, FGF-23 signalling has also been reported to significantly impact anaemia. Blocking FGF-23 signalling in 5/6 nephrectomized mice stimulates erythropoiesis and EPO secretion and abolishes iron-deficient anaemia [34]. As such, it is plausible that, in this study, the beneficial effect on FGF-23 levels positively impacts anaemia.
The induction of renal failure (reduced renal creatinine clearance) in this study went along with significantly increased serum hepcidin values that led to reduced iron absorption and decreased iron release from body stores, resulting in significantly decreased serum iron levels, reflecting the clinical situation. Further evidence for this comes from the distinct increase in serum ferritin levels of rats with renal failure (decreased creatinine clearance) compared with baseline values before the induction of renal function decline, which also points to increased body iron stores. Compared with the vehicle- and CaCO3-treated animals, both PA21-treated groups showed higher serum iron levels after 2 weeks of treatment. Although serum iron levels at this time point were still dramatically lower than baseline values (normal renal function), and thus far from being increased above normal levels, one may suggest that minimal iron absorption had occurred in these groups. This is in line with the fact that gastrointestinal iron absorption from PA21 treatment is considered to be minimal [35]. A recent randomized Phase 3 study also observed initial increases in iron-related parameters such as transferrin saturation and serum ferritin in PA21-treated dialysis patients [36]. Among PA21-treated patients, a trend towards a reduced use of anti-anaemic agents was found compared with sevelamer carbonate-treated patients [36]. As such, patients with decreased renal function, who suffer from iron deficiency, could likely benefit from low systemic iron absorption by PA21 treatment, as the need for iron supplementation might be reduced or even be avoided.
With regard to the development of vascular calcification, PA21 significantly decreased the calcium content in the vessels, whereas CaCO3 did not, this despite the fact that in this study only mild calcification had developed. As may reasonably be expected, the calcium load with CaCO3 treatment will be higher compared with PA21, which in this study is supported by the higher urinary calcium excretion and serum total calcium levels, and this might abolish the beneficial effect of phosphate lowering on vascular calcification. In addition, the better preserved renal function and the significantly lower serum FGF-23 levels [37, 38] could also have contributed to less calcification in both PA21-treated groups. These findings are in accordance with the findings of Phan et al. [17, 31], who also observed a protective effect of PA21 on the vasculature.
Quantitative bone histomorphometry revealed adenine-induced renal failure to go along with a dramatically increased bone formation rate, which in combination with the presence of moderate–severe fibrosis and distinctly increased serum PTH levels indicates the development of a pronounced hyperparathyroid bone disease. These features of high bone turnover disease were clearly reversed by treatment with phosphate binders: decreased bone resorption (evidenced by reduced eroded perimeter), reversal of bone marrow fibrosis, decreased bone formation rate, number of osteoblasts and circulating alkaline phosphatase levels. These findings were also reported by Yaguchi et al. [39], who demonstrated that PA21 treatment suppressed osteoid formation, fibrosis and porosity in rats with CKD. In the PA21-treated rats, the mineralization lag time and adjusted apposition rate did not substantially differ from those of the vehicle-treated group. While in the low PA21 dose group the osteoid maturation time did not differ from the vehicle group, a minimal but statistically significant increase was seen in the highest PA21 dose group (which should be attributed to the low biological variation as compared with the relatively high biological variation in the CaCO3 group). The significantly decreased adjusted apposition rate in combination with a tendency towards an increased mineralization lag time/osteoid maturation time points towards less efficient mineralization in the CaCO3-treated animals.
In conclusion, PA21 should be considered an effective and safe treatment for CKD-MBD-related hyperphosphataemia, with a beneficial impact on renal function (renal creatinine clearance). Furthermore, since high circulating FGF-23 has been associated with cardiovascular events and mortality among haemodialysis patients [40, 41], the remarkable reduction of circulating FGF-23 with PA21 treatment might have benefits for both the vasculature and the bone. All this, together with its relatively low tablet count for human use [15, 42–44] (associated with improved adherence compared with other therapies), favours investigating the use of this iron-based phosphate binder in patients with reduced renal function who are not yet on dialysis.
ACKNOWLEDGEMENTS
We especially thank Geert Dams, Hilde Geryl, Ludwig Lamberts, Rita Marynissen and Simonne Dauwe for their excellent technical assistance and Dirk De Weerdt for his help with the graphics.
FUNDING
E.N. was a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO, grant number 12B5916N).
AUTHORS’ CONTRIBUTIONS
E.N. contributed to the conception and design of the study; acquisition, analysis and interpretation of data and drafting of the manuscript. R.C., B.V. and G.B. contributed to the acquisition and analysis of data. F.F., S.W. and P.C.D. contributed to the conception and design of the study, interpretation of data and critical revision of the manuscript. A.V. contributed to the conception and design of the study; acquisition, analysis and interpretation of data and critical revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
F.F. and S.W. are employees of Vifor (International) Ltd., Glattbrugg, Switzerland. Vifor provided financial support for this study. E.N., R.C., B.V., G.B, P.C.D. and A.V. have no conflicts to disclose.
REFERENCES
- 1. Nissenson AR, Strobos J.. Iron deficiency in patients with renal failure. Kidney Int 1999; 55(Suppl 69): S18–S21 [DOI] [PubMed] [Google Scholar]
- 2. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 3. Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium×phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 4. Ganesh SK, Stack AG, Levin NW. et al. Association of elevated serum PO(4), Ca×PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–2138 [DOI] [PubMed] [Google Scholar]
- 5. Isakova T, Gutierrez OM, Chang Y. et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 2009; 20: 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannata-Andia JB, Fernandez-Martin JL, Locatelli F. et al. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int 2013; 84: 998–1008 [DOI] [PubMed] [Google Scholar]
- 7. Cozzolino M, Staniforth ME, Liapis H. et al. Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int 2003; 64: 1653–1661 [DOI] [PubMed] [Google Scholar]
- 8. Russo D, Miranda I, Ruocco C. et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 2007; 72: 1255–1261 [DOI] [PubMed] [Google Scholar]
- 9. Chertow GM, Burke SK, Raggi P.. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62: 245–252 [DOI] [PubMed] [Google Scholar]
- 10. Asmus HG, Braun J, Krause R. et al. Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant 2005; 20: 1653–1661 [DOI] [PubMed] [Google Scholar]
- 11. Block GA, Spiegel DM, Ehrlich J. et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68: 1815–1824 [DOI] [PubMed] [Google Scholar]
- 12. Block GA, Raggi P, Bellasi A. et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 2007; 71: 438–441 [DOI] [PubMed] [Google Scholar]
- 13. Di Iorio B, Molony D, Bell C. et al. Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis 2013; 62: 771–778 [DOI] [PubMed] [Google Scholar]
- 14. Floege J, Covic AC, Ketteler M. et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Floege J, Covic AC, Ketteler M. et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 2015; 30: 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neven E, Bashir-Dar R, Dams G. et al. Disturbances in bone largely predict aortic calcification in an alternative rat model developed to study both vascular and bone pathology in chronic kidney disease. J Bone Miner Res 2015; 30: 2313–2324 [DOI] [PubMed] [Google Scholar]
- 17. Phan O, Maillard M, Peregaux C. et al. PA21, a new iron-based noncalcium phosphate binder, prevents vascular calcification in chronic renal failure rats. J Pharmacol Exp Ther 2013; 346: 281–289 [DOI] [PubMed] [Google Scholar]
- 18. Liang L, D'Haese PC, Lamberts LV. et al. Direct determination of iron in urine and serum using graphite furnace atomic absorption spectrometry. Analyst 1989; 114: 143–147 [DOI] [PubMed] [Google Scholar]
- 19. Evenepoel P, Behets GJS, Laurent MR. et al. Update on the role of bone biopsy in the management of patients with CKD-MBD. J Nephrol 2017; 30: 645–652 [DOI] [PubMed] [Google Scholar]
- 20. Dempster DW, Compston JE, Drezner MK. et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2013; 28: 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schindelin J, Rganda-Carreras I, Frise E. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemoto Y, Kumagai T, Ishizawa K. et al. Phosphate binding by sucroferric oxyhydroxide ameliorates renal injury in the remnant kidney model. Sci Rep 2019; 9: 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jing W, Nunes ACF, Farzaneh T. et al. Phosphate binder, ferric citrate, attenuates anemia, renal dysfunction, oxidative stress, inflammation, and fibrosis in 5/6 nephrectomized CKD rats. J Pharmacol Exp Ther 2018; 367: 129–137 [DOI] [PubMed] [Google Scholar]
- 24. Goto M, Suematsu Y, Nunes ACF. et al. Ferric citrate attenuates cardiac hypertrophy and fibrosis in a rat model of chronic kidney disease. Iran J Kidney Dis 2019; 13: 98–104 [PubMed] [Google Scholar]
- 25. Lau WL, Vaziri ND, Nunes ACF. et al. The phosphate binder ferric citrate alters the gut microbiome in rats with chronic kidney disease. J Pharmacol Exp Ther 2018; 367: 452–460 [DOI] [PubMed] [Google Scholar]
- 26. Richter B, Faul C.. FGF23 actions on target tissues—with and without Klotho. Front Endocrinol (Lausanne) 2018; 9: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith ER, Holt SG, Hewitson TD.. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int J Biochem Cell Biol 2017; 92: 63–78 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Q, Zeng DK, Li FQ.. FGF23 promotes renal interstitial fibrosis by activating β-catenin. Eur Rev Med Pharmacol Sci 2018; 22: 174–183 [DOI] [PubMed] [Google Scholar]
- 29. Shima H, Miya K, Okada K. et al. Sucroferric oxyhydroxide decreases serum phosphorus level and fibroblast growth factor 23 and improves renal anemia in hemodialysis patients. BMC Res Notes 2018; 11: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maruyama N, Otsuki T, Yoshida YN. et al. Ferric citrate decreases fibroblast growth factor 23 and improves erythropoietin responsiveness in hemodialysis patients. Am J Nephrol 2018; 47: 406–414 [DOI] [PubMed] [Google Scholar]
- 31. Phan O, Maillard M, Malluche HH. et al. Effects of sucroferric oxyhydroxide compared to lanthanum carbonate and sevelamer carbonate on phosphate homeostasis and vascular calcifications in a rat model of chronic kidney failure. Biomed Res Int 2015; 2015:515606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eisenga MF, van Londen M, Leaf DE. et al. C-terminal fibroblast growth factor 23, iron deficiency, and mortality in renal transplant recipients. J Am Soc Nephrol 2017; 28: 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ketteler M, Sprague SM, Covic AC. et al. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol Dial Transplant 2019; 34: 1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agoro R, Montagna A, Goetz R. et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J 2018; 32: 3752–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cozzolino M, Funk F, Rakov V. et al. Preclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxide. Curr Drug Metab 2015; 15: 953–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Covic AC, Floege J, Ketteler M. et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant 2017; 32: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada S, Giachelli CM.. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone 2017; 100: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jimbo R, Kawakami-Mori F, Mu S. et al. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int 2014; 85: 1103–1111 [DOI] [PubMed] [Google Scholar]
- 39. Yaguchi A, Tatemichi S, Takeda H. et al. PA21, a novel phosphate binder, improves renal osteodystrophy in rats with chronic renal failure. PLoS One 2017; 12: e0180430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jimbo R, Shimosawa T.. Cardiovascular risk factors and chronic kidney disease—FGF23: a key molecule in the cardiovascular disease. Int J Hypertens 2014; 2014:381082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghimire S, Castelino RL, Lioufas NM. et al. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One 2015; 10: e0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ix JH, Isakova T, Larive B. et al. Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: the COMBINE trial. J Am Soc Nephrol 2019; 30: 1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gray K, Ficociello LH, Hunt AE. et al. Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis 2019; 12: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]