Abstract
Background
Secondary hyperparathyroidism (sHPT), a common complication of chronic kidney disease, is characterized by elevated serum parathyroid hormone (PTH). Etelcalcetide is an intravenous calcimimetic that increases sensitivity of the calcium-sensing receptor to calcium and decreases PTH secretion. This open-label extension (OLE) trial evaluated the long-term effects of etelcalcetide for sHPT treatment in patients receiving hemodialysis.
Methods
This 52-week, multicenter, single-arm OLE enrolled patients from three parent trials: two randomized, double-blind, placebo-controlled trials and one open-label, single-arm, ‘switch’ study from cinacalcet to etelcalcetide. The primary endpoint was to investigate the nature, frequency, severity and relation to treatment of all adverse events (AEs) reported throughout the trial. Secondary endpoints included the proportion of patients with >30% reduction from baseline in PTH and the percentage change from baseline in PTH, albumin-corrected calcium (Ca), phosphate (P) and the calcium–phosphate product (Ca × P).
ClinicalTrials.gov identifier: NCT01785875; Amgen study: 20120231.
Results
Overall, 89.8% of the patients experienced one or more treatment-emergent AE. The most common were decreased blood Ca (43.3%), diarrhea (10.8%), vomiting (10.4%) and nausea (9.6%); symptomatic hypocalcemia occurred in 3.7% of the patients. Approximately 68% of patients achieved >30% reduction in PTH, and ∼56% achieved PTH ≤300 pg/mL. Mean percent changes from baseline ranged from −25.4% to −26.1% for PTH, −8.3% to −9.1% for Ca, −3.6% to −4.1% for P and −12.0% to −12.6% for Ca × P.
Conclusions
Etelcalcetide effectively lowered PTH and its effect was sustained, while no new safety concerns emerged over a 1-year treatment period.
Keywords: chronic kidney disease; etelcalcetide; open-label extension; safety, secondary hyperparathyroidism
INTRODUCTION
Secondary hyperparathyroidism (sHPT) is a common and clinically significant complication of advanced chronic kidney disease (CKD) [1–4]. sHPT is a component of CKD-mineral and bone disorder (MBD), which encompasses elevated serum parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) concentrations, reduced 1,25(OH)2 vitamin D concentrations and abnormal serum calcium (Ca) and phosphate (P) concentrations [1, 3, 5–7]. These abnormalities of CKD-MBD are associated with vascular calcification, cardiovascular morbidity and mortality, and bone fractures [3, 8, 9].
Multiple options for treating sHPT are available, including calcitriol and vitamin D analogs, calcimimetics, phosphate binders and subtotal parathyroidectomy [1–3, 10–13]. Although effective in lowering PTH concentrations, oral and intravenous (IV) vitamin D analogs can increase serum Ca, P and FGF-23 concentrations [14, 15]. Utilization of oral cinacalcet (Sensipar®/Mimpara®, Amgen Inc., Thousand Oaks, CA, USA) has been limited by reduced adherence [16, 17]. Oral cinacalcet had been the only available calcimimetic until the recent approval of the IV calcimimetic etelcalcetide (Parsabiv®, Amgen Inc.) [18, 19].
Etelcalcetide, an intravenously administered 8-amino acid allosteric activator of the calcium-sensing receptor (CaSR), increases the receptor’s sensitivity to Ca, thereby decreasing PTH secretion and thus reducing circulating PTH [20, 21]. The safety and efficacy of etelcalcetide administered three times weekly (TIW) at the completion of each hemodialysis session have been demonstrated in three separate 26-week Phase 3 trials. The study presented here was a 52-week, multicenter, single-arm, open-label extension (OLE) trial (NCT01785875) designed to characterize the long-term safety and efficacy of etelcalcetide in the treatment of sHPT in patients receiving hemodialysis.
MATERIALS AND METHODS
Patients
All patients from three parent trials were eligible: two were randomized, double-blind, placebo-controlled trials of etelcalcetide conducted in 1023 patients (NCT01785849, NCT01788046) [22], and the other was an open-label, single-arm, ‘switch’ study from cinacalcet to etelcalcetide in 158 patients (NCT01932970) [23]. This OLE trial was conducted in 17 countries in North America, Europe and Australia in accordance with the Declaration of Helsinki. All patients provided written informed consent via an institutional review board-approved form. Patients were eligible if they completed the treatment and follow-up period of (or had discontinued for rising PTH levels from) one of the etelcalcetide parent trials, had a prescribed dialysate Ca concentration ≥2.25 mEq/L and had not received cinacalcet between the last dose of investigational product in the parent trial and the start of dosing in the OLE trial. A complete list of inclusion and exclusion criteria is available in Supplementary data, Table S1.
Trial drug administration
After a 4-week washout period from the parent trial, patients initiated open-label treatment with etelcalcetide if albumin-corrected Ca was ≥8.3 mg/dL within 2 weeks prior to the visit. To maintain blinding from the parent trials, all patients underwent titration of their etelcalcetide dose from a starting dose of 5 mg per dialysis treatment, and investigators were blinded to central laboratory PTH results during the first 10 weeks of open-label treatment.
Patients were treated with etelcalcetide for 52 weeks. All patients initiated etelcalcetide at a starting dose of 5 mg IV bolus TIW immediately after hemodialysis, before or during rinse back or after rinse back through the venous needle. At least 150 mL of rinse-back volume was administered after etelcalcetide injection to ensure etelcalcetide reached the systemic circulation. If rinse back was completed without administration of etelcalcetide, then etelcalcetide was administered IV followed by a saline flush of at least 10 mL. During the first 10 weeks of dosing, etelcalcetide was titrated via an interactive voice/web response system by increments of 2.5 or 5 mg at Weeks 5 and 9 to achieve a PTH ≤300 pg/mL. During the maintenance phase (i.e. after Week 9), etelcalcetide titration by the investigator continued by 2.5 mg every 8 weeks up to a maximum dose of 15 mg per dialysis treatment, with the goal of achieving PTH ≤300 pg/mL. Etelcalcetide dosing was suspended after two consecutive PTH values <100 pg/mL or for serum Ca <7.5 mg/dL, then resumed once PTH returned to ≥150 pg/mL or serum Ca was ≥8.3 mg/dL, respectively.
Laboratory measurements
Predialysis blood samples were drawn for hematology and chemistry laboratory measurements, including PTH, Ca and P (all three analyzed by a central laboratory), as well as anti-etelcalcetide antibody assessments. PTH was analyzed by the Advia Centaur Intact PTH Assay (assay range, 4.6–2200 pg/mL; serum reference range, 18.5–88.0 pg/mL; Siemens Healthcare Laboratory Diagnostics, Tarrytown, NY, USA). Ca was measured by the central laboratory every week in the first 10 weeks and approximately every 8 weeks thereafter. PTH was measured by the central laboratory every 2 weeks in the first 10 weeks and approximately every 8 weeks thereafter.
Endpoints
The primary endpoints were: the nature, frequency, severity and reported relation to treatment of all adverse events (AEs) reported throughout the trial; vital signs and changes in laboratory parameters, including clinical chemistry; and the evaluation of anti-etelcalcetide antibody formation. For secondary endpoints, only patients who completed ≥8 weeks of etelcalcetide treatment had an efficacy assessment phase (EAP), defined as the last 6 weeks before ending treatment. The EAP at 6 months (EAP6) was defined as the period from Weeks 20 to 26 (inclusive). The EAP at 12 months (EAP12) was defined as the period from Weeks 46 to 53 (inclusive). Secondary endpoints included: the proportion of patients with >30% reduction from baseline in PTH during the EAP and EAP12; the proportion of patients with PTH ≤300 pg/mL during the EAP and EAP12; and the percent change from baseline in intact PTH, Ca, P and the calcium–phosphate product (Ca×P) during the EAP and EAP12; tertiary endpoints comprised the same assessments during the EAP6.
Statistical analysis
AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA®) version 15.1 and were summarized by preferred term and system organ class by the treatment group assignment from the parent group and from the pooled group, regardless of the assigned treatment in the parent trial. We also estimated exposure-adjusted patient incidence rates (rate per 100 patient-years). Efficacy endpoints were reported as the proportion with >30% reduction of PTH from baseline, the proportion reaching an absolute value ≤300 mg/mL and the median and/or mean percentage change in PTH, Ca and P from baseline with 95% confidence intervals (CIs). Categorical variables (including demographics and baseline characteristics) were summarized using descriptive statistics. P-values were not specified for this analysis because it was a single-arm study, which lends itself to estimation (CIs) rather than hypothesis testing (P-values); 95% CIs were included for efficacy endpoints to support inferences regarding population estimates of the treatment effect.
Safety analyses included all patients who received one or more doses of etelcalcetide; efficacy analyses included all enrolled patients. We classified patients who completed etelcalcetide treatment and the 30-day safety follow-up period as completing the trial; patients who completed etelcalcetide treatment and subsequently rolled over to another OLE trial (study number 20130213; NCT02102204) were classified as discontinuing the trial because of protocol-specified criteria.
Data-sharing agreement
There is a plan to share data. This may include de-identified individual patient data for variables necessary to address the specific research question in an approved data-sharing request; also related data dictionaries, study protocol, statistical analysis plan, informed consent form and/or clinical study report. Data-sharing requests relating to data in this manuscript will be considered after the publication date and (i) this product and indication (or other new use) have been granted marketing authorization in both the USA and Europe or (ii) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data-sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of re-evaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors reviews requests. If not approved, requests may be further arbitrated by a Data-Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen’s sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data-sharing agreement. This may include anonymized patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at the following: https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
RESULTS
Patients
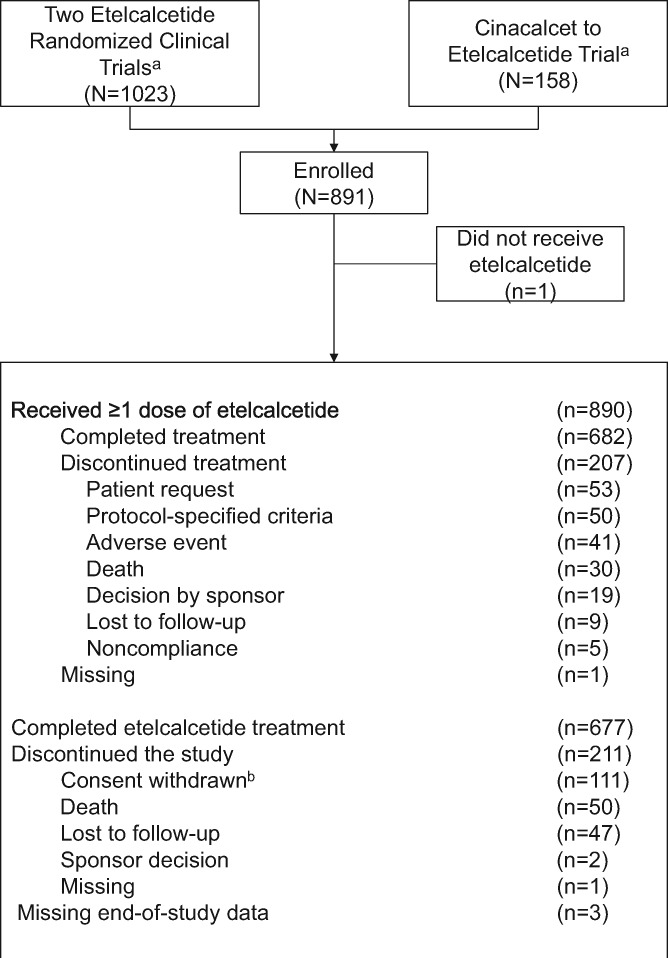
Between 31 July 2013 and 15 July 2015, 891 patients were enrolled in 205 centers in the USA, Canada, Europe, Israel, the Russian Federation and Australia. Of the 891 patients enrolled (efficacy analysis set), 890 (99.9%) received one or more doses of etelcalcetide (safety analysis set), and 682 (76.5%) completed the 52-week etelcalcetide treatment period. Overall, 207 patients (23.2%) discontinued etelcalcetide (Figure 1).
FIGURE 1.
Patient disposition. aTwo of the trials were randomized, double-blind, placebo-controlled trials conducted in 1023 patients [22]; the other trial was an open-label, single-arm, ‘switch’ from cinacalcet to etelcalcetide in 158 patients [23]. bSixteen patients from one site withdrew consent on 20 or 21 November 2014, subsequent to the investigator’s decisions to withdraw from the trial. Additionally, three patients from the same site had missing end-of-study data.
Patient demographics and baseline clinical characteristics are shown in Table 1. Mean (SD) baseline PTH was 770 (574) pg/mL, and mean (SD) baseline Ca was 9.67 (0.68) mg/dL after a 4-week washout period from the parent trials. Furthermore, 405 patients (45.5%) had baseline PTH <600 pg/mL, 221 patients (24.8%) had PTH 600–1000 pg/mL and 228 patients (25.6%) had PTH >1000 pg/mL. Most had dialysate Ca that was ≥2.5 mEq/L at baseline.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | Etelcalcetide (n = 891) |
|---|---|
| Sex, n (%) | |
| Male | 550 (61.7) |
| Female | 341 (38.3) |
| Race, n (%) | |
| White | 567 (63.6) |
| Black | 270 (30.3) |
| Asian | 29 (3.3) |
| Other | 25 (2.7) |
| Age, mean (SD), years | 58.3 (14.4) |
| <65, n (%) | 577 (64.8) |
| ≥65, n (%) | 314 (35.2) |
| ≥75, n (%) | 125 (14.0) |
| Dialysate calcium, n (%) | |
| <2.5 mEq/L | 63 (7.1) |
| ≥2.5 mEq/L | 828 (92.9) |
| Baseline PTH, n (%) | |
| <600 pg/mL | 405 (45.5) |
| 600–1000 pg/mL | 221 (24.8) |
| >1000 pg/mL | 228 (25.6) |
| Missing | 37 (4.2) |
| Laboratory values, mean (SD) | |
| PTH, pg/mL | 770 (574) |
| Ca, mg/dL | 9.7 (0.7) |
| P, mg/dL | 5.6 (1.8) |
| Ca × P, mg2/dL2 | 54.4 (17.2) |
Safety
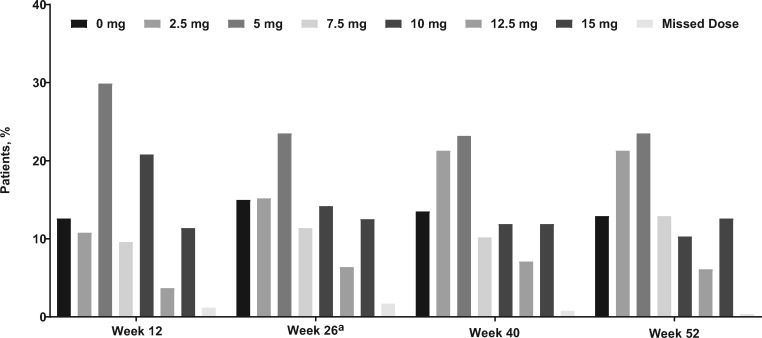
Median (range) duration of etelcalcetide exposure was 362 (1–383) days. Mean (SD) average weekly dose was 21.3 (12.3) mg/week during EAP6 and 20.0 (12.8) mg/week during EAP12 (Figure 2). Most patients [89.8% (n = 799)] experienced one or more treatment-emergent AEs during the trial with an exposure-adjusted rate of 356.9/100 patient-years; the exposure-adjusted rate was 712.6/100 patient-years for the placebo-controlled trials. Serious AEs were reported for 40.0% (n = 356) of patients with an exposure-adjusted rate of 55.4/100 patient-years; the exposure-adjusted rate was 56.5/100 patient-years for the placebo-controlled trials. In all, 51 patients (5.7%) died during the study, with an exposure-adjusted rate of 6.1/100 patient-years; the exposure-adjusted rate was 4.1/100 patient-years for the placebo-controlled trials. A total of 13 patients (1.5%; Table 2) had serious AEs that were deemed treatment related (hypocalcemia, n = 2; arthralgia and hypophosphatemia, aspartate aminotransferase increased, bone pain, hemorrhagic gastritis, purpura, hemodialysis-induced symptom, Henoch–Schönlein purpura, radius fracture, sarcoma, seizure, sinus node dysfunction, n = 1 each). The 50 deaths referred to in Figure 1 include only those patients who discontinued the study because of a fatality; the 51 deaths in the safety analysis included an additional patient who died during 30 days of follow-up after trial completion. The most commonly reported AEs are shown in Table 2.
FIGURE 2.
Proportion of patients receiving each dose level of etelcalcetide (mg/session) at selected visits. aOne patient received 17.5 mg of etelcalcetide in Week 26, although the maximum dose per protocol was 15 mg.
Table 2.
AEs
| AEs, n (%) [rate per 100 patient-years] | OLE trial Etelcalcetide (n = 890) | Placebo-controlled trials Etelcalcetide (n = 503) |
|---|---|---|
| All treatment-emergent AEs | 799 (89.8) [356.9] | 461 (91.7) [712.6] |
| SAEs | 356 (40.0) [55.4] | 130 (25.8) [56.5] |
| Treatment-related SAE | 13 (1.5) [1.6] | 8 (1.6) [3.0] |
| AE leading to discontinuation of etelcalcetide | 41 (4.6) [4.9] | 9 (1.8) [3.4] |
| Fatal AEs | 51 (5.7) [6.1] | 11 (2.2) [4.1] |
| Common AEs (patient incidence ≥5% in either group) | ||
| Blood calcium decreased (asymptomatic)a | 385 (43.3) [69.1] | 321 (63.8) [240.3] |
| Diarrhea | 96 (10.8) [12.2] | 54 (10.7) [21.6] |
| Vomiting | 93 (10.4) [11.8] | 45 (8.9) [17.8] |
| Nausea | 85 (9.6) [10.7] | 54 (10.7) [21.6] |
| Muscle spasms | 79 (8.9) [9.9] | 58 (11.5) [23.5] |
| Hypotension | 75 (8.4) [9.3] | 30 (6.0) [11.5] |
| AV fistula site complication | 68 (7.6) [8.5] | 29 (5.8) [11.2] |
| Hypertension | 65 (7.3) [8.1] | 31 (6.2) [12.0] |
| Hyperkalemia | 56 (6.3) [6.9] | 22 (4.4) [8.4] |
| Upper respiratory tract infection | 56 (6.3) [6.9] | 21 (4.2) [8.0] |
| Cough | 55 (6.2) [6.8] | 22 (4.4) [8.4] |
| Headache | 53 (6.0) [6.5] | 38 (7.6) [14.9] |
| Back pain | 50 (5.6) [6.1] | 22 (4.4) [8.4] |
| Dyspnea | 50 (5.6) [6.1] | 24 (4.8) [9.2] |
| Arthralgia | 49 (5.5) [6.0] | 21 (4.2) [8.0] |
| Pain in extremity | 47 (5.3) [5.8] | 24 (4.8) [9.2] |
| Fall | 45 (5.1) [5.5] | 15 (3.0) [5.7] |
| Hypocalcemia (symptomatic)b | 33 (3.7) [4.0] | 35 (7.0) [13.7] |
AV, arteriovenous; SAE, serious AE.
Defined as an asymptomatic decrease in blood calcium from baseline or to <8.3 mg/dL.
Defined as a symptomatic decrease in blood calcium or decrease in Ca to <7.5 mg/dL.
A total of 4.6% (n = 41) of patients, 4.9/100 patient-years, had AEs that led to discontinuation of etelcalcetide; the exposure-adjusted rate was 3.4/100 patient-years for the placebo-controlled trials. The most commonly reported AEs leading to discontinuation of etelcalcetide in more than one patient were cardiac arrest (n = 4 patients; 0.5/100 patient-years) and nausea, vomiting, cellulitis, hypocalcemia (defined as a symptomatic decrease in blood Ca or decrease in Ca <7.5 mg/dL) and seizure (n = 2 patients each; 0.2/100 patient-years). One patient had etelcalcetide withheld because of low Ca levels (7.8 mg/dL) and subsequently experienced seizure and discontinued from the trial. The investigator reported that although hypoglycemia was the main reason for the seizure, low Ca could have contributed to the event by decreasing the seizure threshold.
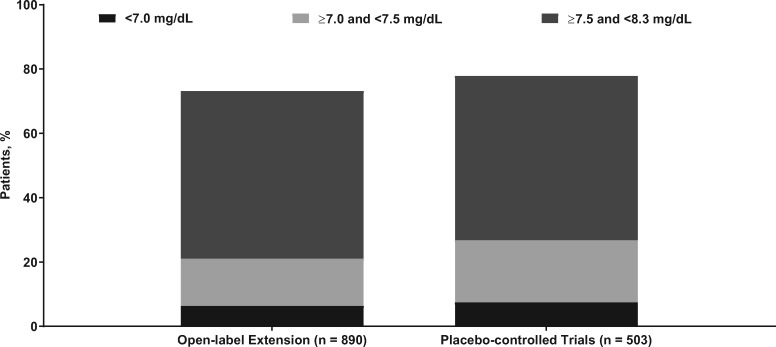
Serum Ca concentrations were similar between the placebo-controlled trials (n = 503) and the OLE (n = 890; Figure 3). The proportion of patients with their lowest Ca values <7.0 mg/dL was 6.4% (n = 57); between 7.0 and <7.5 mg/dL was 14.7% (n = 131); and between 7.5 and <8.3 mg/dL was 52.1% (n = 464). The proportions in the etelcalcetide arms from the 6-month, Phase 3, placebo-controlled studies were 7.6%, 19.3% and 51.1%, respectively. An AE of symptomatic hypocalcemia (i.e. a symptomatic decrease in blood Ca or decrease in Ca to <7.5 mg/dL) was reported in 3.7% of patients (n = 33; 4.0/100 patient-years), and two patients (0.2/100 patient-years) were classified as having a serious AE of hypocalcemia; the exposure-adjusted rates for the placebo-controlled trials were 13.7 and 0/100 patient-years, respectively. Most occurrences of hypocalcemia occurred in the first 5 months of the study (Supplementary data, Figure S1), and among the patients who developed hypocalcemia, only one patient did so after an etelcalcetide dose increase (from 5 to 10 mg). Bone fractures occurred in 4.0% (n = 36) of patients. No patient had an event in the category of adynamic bone.
FIGURE 3.
Proportion of patients with low Ca values during the trial for the current OLE study (black) versus the active treatment arm of the placebo-controlled trials [22] (gray). Ca, albumin-corrected calcium.
The incidence of binding anti-etelcalcetide antibodies was 7.6% (n = 67 with on-trial results). Of these 67 patients, 59.7% (n = 40) presented with pre-existing antibodies at baseline (defined as Day 1 of the parent trial for those previously treated with etelcalcetide in the parent trial or Day 1 of the OLE for those who received placebo in the parent trial). Of the remaining 27 patients, 40.7% (n = 11) exhibited transient antibodies (i.e. patients were negative for anti-etelcalcetide antibody results at the last available time point, after being positive at least once).
Efficacy
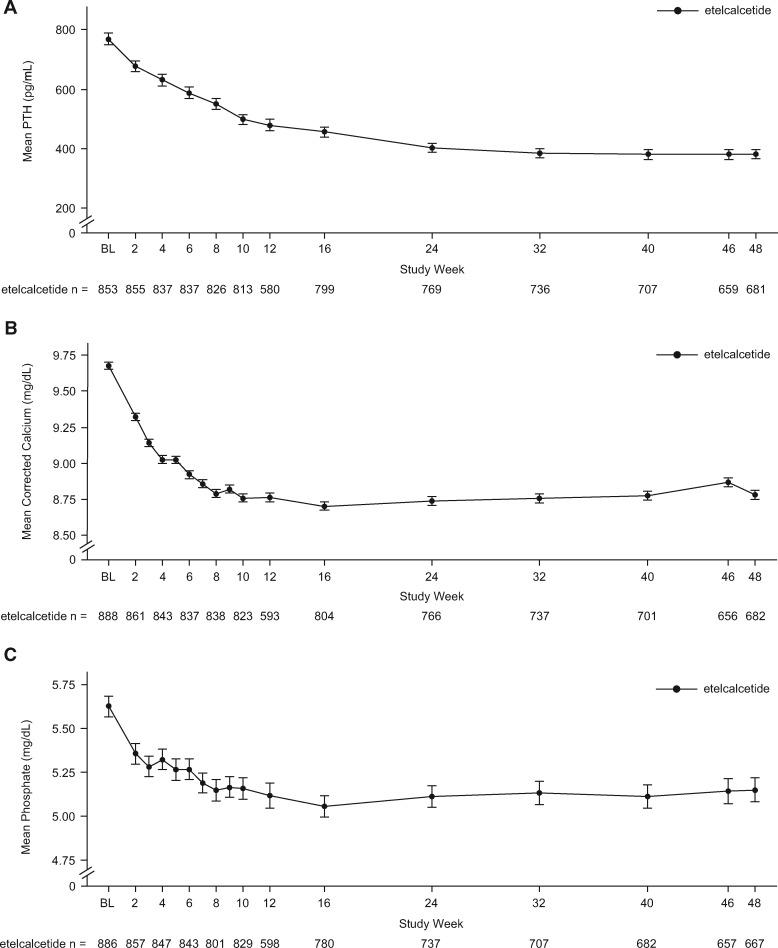
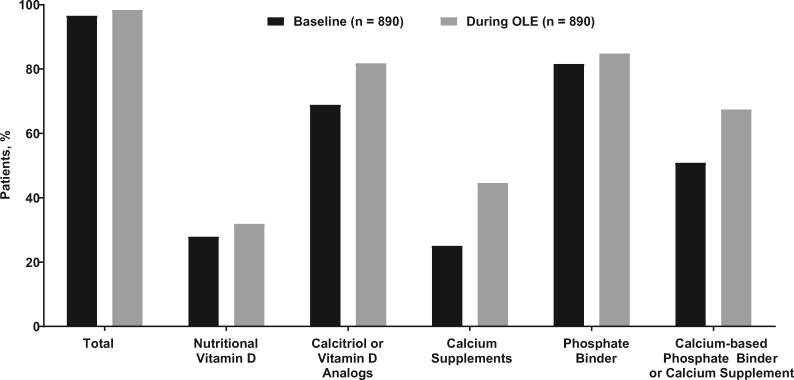
Regardless of the period examined, the proportion of patients with >30% PTH reduction was approximately 68% [mean (95% CI) ranged from 67.5% (63.8–71.0%) to 68.1% (64.6–71.4%)], the proportion with PTH ≤300 pg/mL was ∼56% [mean (95% CI) ranged from 55.5% (52.0–59.1%) to 57.3% (53.8–60.7%)] and the mean (95% CI) percent change from baseline in PTH was −26.1% (−34.0%, −18.1%), −25.4% (−37.2%, −13.5%) and −25.6% (−34.6%, −16.6%) for the EAP, EAP6 and EAP12 periods, respectively (Table 3). The mean percent changes for Ca, P and Ca × P across the EAP periods are shown in Table 3. After an initial period of reduction, predialysis serum concentrations of PTH, Ca and P were maintained at these lower concentrations over the course of the trial (Figure 4). During the study, 81.8% used calcitriol or vitamin D analogs and 67.5% used calcium supplements or a calcium-based phosphate binder; 73.9 and 59.7% in the placebo-controlled studies used those respective medications (Figure 5).
Table 3.
Effects of etelcalcetide on PTH, Ca, P and Ca × P during EAP, EAP6 and EAP12
| OLE trial Etelcalcetide (n = 891) |
Placebo-controlled trialsa Etelcalcetide (n = 509) | |||
|---|---|---|---|---|
| EAP | EAP6 | EAP12 | EAP6 | |
| >30% reduction in PTH, % (n/N1) | 67.7 (527/779) | 68.1 (505/742) | 67.5 (456/676) | 74.7 (380/509) |
| PTH ≤300 pg/mL, % (n/N1) | 57.3 (467/815) | 55.5 (431/776) | 56.4 (399/708) | 51.5 (262/509) |
| Median percentage change in PTH, % (n) | −52.2 (779) | −51.6 (742) | −52.9 (676) | −63.6 (456) |
| Mean percentage change in PTH, % (n) (95% CI) | −26.1 (779) | −25.4 (742) | −25.6 (676) | −56.3 (456) |
| (−34.0, −18.1) | (−37.2, −13.5) | (−34.6, −16.6) | (−58.9, −53.6) | |
| Mean percentage change in Ca, % (n) (95% CI) | −8.4 (807) | −9.1 (774) | −8.3 (704) | −7.0 (456) |
| (−9.07, −7.75) | (–9.8, –8.4) | (–8.9, –7.6) | (–7.8, –6.2) | |
| Mean percentage change in P, % (n) (95% CI) | −3.6 (796) | −4.1 (743) | −3.6 (703) | −8.7 (450) |
| (−6.0, −1.3) | (−6.6, −1.5) | (−6.0, −1.2) | (−11.3, −6.0) | |
| Mean percentage change in Ca × P, % (n) (95% CI) | −12.0 (786) | −12.6 (737) | −12.0 (701) | −15.1 (450) |
| (−14.3, −9.8) | (−15.1, −10.0) | (−14.2, −9.7) | (−17.6, −12.5) | |
Phase 3 results are for the EAP between Weeks 20 and 27, inclusive.
EAP = efficacy assessment phase (the last 6 weeks prior to ending treatment in patients completing ≥8 weeks); EAP6 = EAP Weeks 20–26 inclusive; EAP12 = EAP Weeks 46–53 inclusive; N1=number of evaluable patients at that time point.
FIGURE 4.
Mean (SE) predialysis PTH (A), Ca (B) and P (C) concentrations over time. BL, baseline; Ca, albumin-corrected calcium; P, phosphate.
FIGURE 5.
Concomitant medication use.
DISCUSSION
In this 1-year, multicenter, single-arm OLE trial, we observed sustained reductions of PTH following etelcalcetide treatment for 1 year with an acceptable safety profile, similar to previous trials of shorter duration. The safety results observed in the OLE mirrored those reported from pooled data from the Phase 3 trials [22]. Approximately 90% of patients in the OLE had a treatment-emergent AE. As was seen with cinacalcet in the active-controlled study [24], the most frequently reported AEs on trial (occurring in ≥10% of patients) were decreased serum Ca and gastrointestinal symptoms commonly seen in dialysis patients, events that were typically mild to moderate in severity. Less than 5% of patients had AEs that led to discontinuation of etelcalcetide treatment. The crude incidences of serious AEs (40.0% versus 25.8%), AEs leading to etelcalcetide discontinuation (4.6% versus 1.8%) and fatalities (5.7% versus 2.2%) were numerically higher in the OLE than in the shorter placebo-controlled Phase 3 trials [25]; however, after adjusting for exposure, the incidences of these events were more similar between the OLE and the placebo-controlled Phase 3 trials (serious AEs, 55.4 versus 56.5/100 patient-years; AEs leading to etelcalcetide discontinuation, 4.9 versus 3.4/100 patient-years; fatalities, 6.1 versus 4.1/100 patient-years). The safety results observed in this trial were also comparable to those seen in a previous 52-week, open-label trial of etelcalcetide [26] although the rates of severe AEs and fatalities in that trial were lower than those observed here.
The use of calcimimetics has been shown to increase the sensitivity of CaSR, resulting in a decrease in both PTH secretion and serum Ca [2, 18, 27] potentially resulting in hypocalcemia [18, 27]. The proportion of patients in the OLE with the lowest Ca values between 7.5 and <8.3 mg/dL or <7.5 mg/dL was similar to the proportion in the shorter placebo-controlled studies, suggesting longer term treatment with etelcalcetide does not increase the risk of hypocalcemia, or at a minimum that the risk can be mitigated through healthcare providers’ best judgment for clinical management. Symptomatic hypocalcemia was reported in 33 of 890 (3.7%) patients, with two patients experiencing a serious AE of symptomatic hypocalcemia. Most cases of symptomatic hypocalcemia or asymptomatic decreased blood Ca reported were transient and mild to moderate in severity. Incidence of AEs potentially associated with hypocalcemia, such as cardiac events (e.g. QTc prolongation, ventricular arrhythmias), neuromuscular irritability and seizures, were low, occurring in ≤1% of patients treated with etelcalcetide. There was no evidence to suggest an association between hypocalcemia and cardiac events. With respect to the Ca-lowering effects of etelcalcetide, data from previous studies indicate that cardiac repolarization and effects on potassium ion channel currents are mediated by decreases in blood Ca and not caused by the etelcalcetide compound directly [28]. Thus, monitoring of Ca can mitigate the potential for hypocalcemia-induced dysrhythmia. Recently published results from a post hoc analysis of the EValuation Of Cinacalcet Hydrochloride (HCl) Therapy to Lower CardioVascular Events (EVOLVE) trial [29] suggest that mild hypocalcemia may be tolerated in patients receiving hemodialysis and treated with calcimimetics to avoid excess Ca loading. Furthermore, hypocalcemia associated with etelcalcetide use is manageable and generally without long-term clinical consequence for most patients.
Vascular calcification is a known risk of CKD and sHPT [30], and use of calcium–phosphate binders has been associated with the progression of vascular calcification [31]. Although the present study did not evaluate vascular calcification, a large proportion of patients received calcium supplements or calcium-based phosphate binders; however, after an initial decline in serum Ca concentration with etelcalcetide treatment, levels were well maintained and few patients experienced hypercalcemia (n = 17; 1.9%). Previous studies have shown that calcimimetics can exhibit cardiovascular protective effects, including the prevention of vascular calcification and arterial hardening [32, 33].
Hyperphosphatemia has been associated with mortality risk in patients with CKD [34]. In this trial, long-term administration of etelcalcetide in conjunction with calcium supplements and phosphate binders was associated with reduction and maintenance of serum P toward levels consistent with recommendations in recent practice guidelines [1], and may thereby contribute to better overall patient management.
The majority of patients with anti-etelcalcetide binding antibodies were determined to have pre-existing antibodies at baseline [35]; these antibodies were likely not induced by exposure to etelcalcetide [36]. Previous studies have found no evidence that pre-existing or developing anti-etelcalcetide antibodies altered the pharmacokinetic profile, clinical response or safety profile of etelcalcetide [18, 19]. Although there is the potential for antibodies to occur, to date, they have not been clinically significant [35].
The recently updated Kidney Disease: Improving Global Outcomes guidelines on CKD-MBD do not identify a specific serum PTH level for patients with CKD receiving hemodialysis, but instead recommend maintaining PTH levels in the range of two to nine times the upper limit of normal for the assay and state that trending elevations in PTH should be addressed prior to reaching the threshold of nine times the upper limit of normal [1]. The data from the current trial demonstrate that etelcalcetide can clinically reduce and maintain serum PTH levels with long-term treatment. Moreover, dose appeared relatively stable over time, suggesting the absence of tachyphylaxis.
This extension trial is the longest analysis of the use of etelcalcetide in patients receiving dialysis to date. The exposure-adjusted rates of serious AEs in this trial, as well as the incidence of hypocalcemia, suggest that the long-term risks associated with etelcalcetide treatment are similar to those observed in the prior shorter term studies. Overall, these results indicate that long-term administration of etelcalcetide exhibits a reasonable safety profile with sustained reductions in PTH, Ca and P.
Supplementary Material
ACKNOWLEDGEMENTS
All authors met International Committee of Medical Journal Editors criteria for authorship and approved the final draft for submission. Holly Tomlin (past employee and stockholder of Amgen Inc.), William W. Stark, Jr, PhD (Amgen employee and stockholder), and Martha Mutomba and Jonathan Plumb, PhD (on behalf of Amgen Inc.), as well as Meghan Johnson, PhD, and James Balwit, MS (Complete Healthcare Communications, LLC, [CHC] North Wales, PA, a CHC Group company), whose work was funded by Amgen Inc., provided medical writing assistance including editing and journal formatting for submission.
FUNDING
This study was funded by Amgen Inc.
AUTHORS’ CONTRIBUTIONS
D.A.B., G.M.C., S.C. and G.A.B. were involved with the conception and design of the study. D.A.B., G.M.C., S.C., N.K., K.J.M., A.R., P.U-T., M.V. and G.A.B. were involved with patient data collection and acquisition. All authors were involved with the analysis and interpretation of data and participated in the writing and revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
D.A.B. reports being a consultant for Amgen during the conduct of the study; personal fees from and being a consultant for Tricida, Vifor/Relypsa and Sanofi/Genzyme outside the submitted work; and stock in Amgen as well as stock and stock options in Tricida. G.M.C. reports a grant from Amgen for support to staff members for analysis of data from EVOLVE; personal fees from Akebia, AMAG Pharmaceuticals, Inc., Ardelyx, AstraZeneca, Gilead and Keryx; stock options for Ardelyx, Cloudcath, Cricket, Durect, DxNow, Outset Medical and Physiowave; and is on the Board of Directors for Satellite Healthcare. S.C. is an employee of Amgen. H.D. was an employee and stockholder of Amgen at the time the manuscript was accepted. N.K. reports employment by Northeast Clinical Research Center, which was a clinical site in the study and received payment from Amgen; and serving on a speakers’ bureau for Amgen. K.J.M. reports being a consultant for Amgen. A.R. reports a research grant, travel support and an honorarium from Amgen; grants, personal fees and serving as a speaker for Cubist; personal fees from and serving on a speakers' bureau for Fresenius Medical Care; personal fees from Medscape; grants, personal fees and travel support from and serving on a speakers’ bureau and advisory board for Relypsa; grants, research support and personal fees from and serving on a speakers' bureau for Sanofi; grants and clinical trial support from and serving on a speakers’ bureau and as a consultant for Kadmon; grants and research support for clinical trials from AMAG Pharmaceuticals, Inc.; grants and research support for clinical trials from and serving as a speaker for Otsuka; grants and research support for clinical trials from AstraZeneca; grants and research support for clinical trials from Bayer; grants and research support for clinical trials from Genzyme; grants and research support for clinical trials from GlaxoSmithKline; grants and research support for clinical trials from Omerus; grants and research support for clinical trials from Otsuka; grants and research support for clinical trials from Overture; grants and research support for clinical trials from Quesctor; grants and research support for clinical trials from Sandoz; grants and research support for clinical trials from VPI; grants and research support for the Systolic Blood Pressure Intervention Trial; serving as a speaker for Janssen; grants and research support for clinical trials from Reata; and serving as a speaker for Ironwood. P.U-T. reports receiving grants from Amgen and Hemotech; receiving personal fees from Amgen, Vifor Pharma FMC and Medice; being a consultant for Medice; and participating in clinical trials for AbbVie, Amgen and Astellas. M.V. reports receiving grants from FMC, Amgen and Abbvie; receiving personal fees from Vifor Fresenius Medical Care Renal Pharma, Amgen, Ostuka, Medice, Baxter and B Braun Medical; has served on advisory boards for Otsuka and Medice; and has served as a speaker for Baxter and B Braun Medical. G.A.B. reports being a consultant for Amgen, Kirin Corporation, Daiichi-Sankyo, Keryx, Ardelyx and Tricida; has received speaker’s honoraria from Kirin Corporation, OPKO, ONO Pharmaceuticals and Cara; has served on steering committees for Amgen and Akebia; has served on an advisory board for Reata; has received travel support from Amgen, OPKO, ONO Pharmaceuticals and Keryx; has received an investigator grant from Keryx; and has equity ownership in Ardelyx.
The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.KDIGO Workgroup. KDIGO 2017 Clinical Practice Guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham J, Locatelli F, Rodriguez M.. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011; 6: 913–921 [DOI] [PubMed] [Google Scholar]
- 3. Goodman WG, Quarles LD.. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int 2008; 74: 276–288 [DOI] [PubMed] [Google Scholar]
- 4. Thomas R, Kanso A, Sedor JR.. Chronic kidney disease and its complications. Prim Care 2008; 35: 329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Boer IH, Gorodetskaya I, Young B. et al. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 2002; 13: 2762–2769 [DOI] [PubMed] [Google Scholar]
- 6. Levin A, Bakris GL, Molitch M. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31–38 [DOI] [PubMed] [Google Scholar]
- 7. Fliser D, Kollerits B, Neyer U. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007; 18: 2600–2608 [DOI] [PubMed] [Google Scholar]
- 8. Gal-Moscovici A, Sprague SM.. Bone health in chronic kidney disease-mineral and bone disease. Adv Chronic Kidney Dis 2007; 14: 27–36 [DOI] [PubMed] [Google Scholar]
- 9. Moe S, Drueke T, Cunningham J. et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945–1953 [DOI] [PubMed] [Google Scholar]
- 10. Fishbane S, Shapiro WB, Corry DB. et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008; 3: 1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moe SM, Chertow GM, Coburn JW. et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 2005; 67: 760–771 [DOI] [PubMed] [Google Scholar]
- 12. Wetmore JB, Liu S, Krebill R. et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 2010; 5: 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wetmore JB, Quarles LD.. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clin Pract Nephrol 2009; 5: 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bushinsky DA, Messa P.. Efficacy of early treatment with calcimimetics in combination with reduced doses of vitamin D sterols in dialysis patients. NDT Plus 2008; 1: i18–i23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parikh C, Gutgarts V, Eisenberg E. et al. Vitamin D and clinical outcomes in dialysis. Semin Dial 2015; 28: 604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Francisco AL, Gillespie IA, Gioni I. et al. Anti-parathyroid treatment effectiveness and persistence in incident haemodialysis patients with secondary hyperparathyroidism. Nefrologia 2016; 36: 164–175 [DOI] [PubMed] [Google Scholar]
- 17. Reams BD, Dluzniewski PJ, Do TP. et al. Dynamics of cinacalcet use and biochemical control in hemodialysis patients: a retrospective new-user cohort design. BMC Nephrol 2015; 16: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsabiv™ (etelcalcetide). Full Prescribing Information. Thousand Oaks, CA: Amgen Inc, 2017 [Google Scholar]
- 19.Parsabiv™ (etelcalcetide). Summary of Product Characteristics. Breda, The Netherlands: Amgen Europe B.V, 2017 [Google Scholar]
- 20. Alexander ST, Hunter T, Walter S. et al. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol 2015; 88: 853–865 [DOI] [PubMed] [Google Scholar]
- 21. Ma JN, Owens M, Gustafsson M. et al. Characterization of highly efficacious allosteric agonists of the human calcium-sensing receptor. J Pharmacol Exp Ther 2011; 337: 275–284 [DOI] [PubMed] [Google Scholar]
- 22. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]
- 23. Liss K, Block GA, Chertow GM. et al. Initiation of AMG 416 after discontinuation of cinacalcet. J Am Soc Nephrol 2015; 26: 204A [abstr TH-PO516] [Google Scholar]
- 24. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 25. Block G, Chertow G, Sullivan J. et al. An integrated analysis of safety and tolerability of etelcalcetide in adult patients on hemodialysis with secondary hyperparathyroidism. Presented at: American Society of Nephrology Annual Meeting, New Orleans, LA, USA, 31 October–5 November 2017
- 26. Shigematsu T, Fukagawa M, Yokoyama K. et al. Long-term effects of etelcalcetide as intravenous calcimimetic therapy in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 2018; 22: 426–436 [DOI] [PubMed] [Google Scholar]
- 27.Sensipar® (cinacalcet). Full Prescribing Information. Thousand Oaks, CA: Amgen Inc, 2017 [Google Scholar]
- 28. Fielden MR, Dean C Jr, Black K. et al. Nonclinical safety profile of etelcalcetide, a novel peptide calcimimetic for the treatment of secondary hyperparathyroidism. Int J Toxicol 2016; 35: 294–308 [DOI] [PubMed] [Google Scholar]
- 29. Floege J, Tsirtsonis K, Iles J. et al. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with cinacalcet in the EVOLVE trial. Kidney Int 2018; 93: 1475–1482 [DOI] [PubMed] [Google Scholar]
- 30. Isakova T, Nickolas TL, Denburg M. et al. KDOQI US commentary on the 2017 KDIGO Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 2017; 70: 737–751 [DOI] [PubMed] [Google Scholar]
- 31. Block GA, Wheeler DC, Persky MS. et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012; 23: 1407–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim K, Hamano T, Thadhani R.. Vitamin D and calcimimetics in cardiovascular disease. Semin Nephrol 2018; 38: 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raggi P, Chertow GM, Torres PU. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 34. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 35. Kroenke MA, Weeraratne D, Deng H. et al. Clinical immunogenicity of the D-amino acid peptide therapeutic etelcalcetide: method development challenges and anti-drug antibody clinical impact assessments. J Immunol Methods 2017; 445: 37–44 [DOI] [PubMed] [Google Scholar]
- 36. Gorovits B, Clements-Egan A, Birchler M. et al. Pre-existing antibody: biotherapeutic modality-based review. AAPS J 2016; 18: 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.