Abstract
Sea turtles can detect airborne and waterborne odors, but whether they recognize scents from the same species and if so, how they affect their behavior remains unknown. The present study evaluated the behavioral effects of odorants on juvenile green sea turtles (Chelonia mydas). The odorants were derived from Rathke glands (external scent glands) of mature male green sea turtles, and from two types of food. The activity of the juveniles increased when exposed to food scents, and significantly decreased compared with controls when exposed to scents from Rathke glands. These findings indicated that scents from the same species affect behavior, and that chemical communication via olfaction has important outcomes for sea turtles.
Keywords: Chelonia mydas, ethology, odor, olfaction, Rathke gland
Green sea turtles (Chelonia mydas) are ocean-dwelling Testudines (family: Cheloniidae) that are distributed in tropical and sub-tropical oceans throughout the world [2]. After reaching maturity, they seasonally migrate between foraging and breeding areas [22], and adult males and females return to the sites of their birth (natal homing) to breed [7, 18, 28]. Although the mechanisms of breeding and natal homing behaviors of sea turtles remain moot, one possibility is the detection of chemical cues via olfaction [4, 6, 8, 9, 18], as recently found in the terrestrial painted turtle (Chrysemys picta) [12].
Some freshwater turtles, as well as loggerhead sea turtles (Caretta caretta) exhibit throat-pumping behavior (moving the throat slightly to transport water from the mouth to nostrils) to detect odors [19, 21, 26, 31]. This behavior was also evident in green sea turtles reared at the Ogasawara Marine Center (OMC) (Supplementary Video), especially when they encountered unfamiliar foods. We previously reported that sea turtles possess the morphologically-complicated nasal cavity [15, 16, 32] and that areas where water easily and hardly enters in the nasal cavity of green sea turtles are lined with different types of sensory epithelia [15], indicating that airborne and waterborne odors play important roles in their behaviors. In fact, sea turtles can detect airborne and waterborne odors [5, 20]. Due to the different properties such as diffusion in the air rapidly to a relatively long distance and diffusion in the water to a fairly close range, it is possible that odorants in the air and water are used properly depending on the situation. However, whether sea turtles can recognize odorants derived from the same species and if so, how their behaviors are affected and whether chemical communication proceeds via olfaction remain unknown.
Rathke glands are external scent glands in Testudines including some sea turtle species [3, 25, 27]. Green sea turtles normally have two pores in the axillary inframarginal region and one in the inguinal region [27]. The roles of powerful scents might include avoiding predators [3] and providing interspecific chemical cues [17], but the exact role remains unknown at 170 years after discovery. Here, we evaluated the behavioral effects of airborne odorants derived from mature male Rathke glands, and scents from two types of food on seven juvenile green sea turtles (age, ~ one year; straight carapace length, 20.1–27.2 cm) of unknown sex that were hatched and bred at OMC.
Rathke glands used in this study were sampled from both axillary inframarginal and inguinal regions of a dead mature male green sea turtle that was harvested for meals in Chichi-jima, the Ogasawara Islands in 2018. This harvesting is conducted strictly under the permission of the governor of Tokyo, and it is usually held during the mating season (February to May) of green sea turtles. The fat and muscle around the Rathke glands were carefully removed as much as possible, those sampled from axillary inframarginal and inguinal regions were stored frozen, and the mixture was used for the behavioral experiments described below. The present behavioral experiments proceeded at OMC during November 2018, and the Animal Care and Use Committee at Obihiro University of Agriculture and Veterinary Medicine approved the experimental protocol (Approval No: 19-239).
Turtles were exposed to odors from formula food (Kuroshio float EP-3, Higashimaru Co., Ltd., Kagoshima, Japan), shrimps and Rathke glands or distilled water (control) in a blindly random order. The formula food, shrimps and Rathke glands were dissolved in distilled water, and the intensity of scents was measured using an XP-329m odorant sensor with a minimum−maximum of 0–999 (New Cosmos Electric Co., Ltd., Osaka, Japan) immediately before the experiment. The mean (± SEM) average scent values to which the seven turtles were exposed were 111.5 ± 11.3 and the ranges of intensity of three stimuli were unified within ± 4.5 for the same individual. The experiments were based on a study by Endres et al. [5], and Fig. 1 shows the experimental system. Water was poured to a depth of 40 cm in a round container with a 48 cm diameter, and PVC pipe with a T-joint located at the center was inserted in the container. Air was then propelled via a PVC pipe into the area using a fan. Turtles were placed individually in the container which was covered with the Perspex sheet comprising two crossed strips of red tape, and each turtle was allowed five min to become acclimated to the environment. A camera was placed above the container to record the activities of each turtle, and a cup containing one of the three odors was placed on the T-joint. The experiment started when the first inhalation of the odor (namely, the first respiration after each presentation), and the activity for five min was evaluated, which is a same condition as Endres et al. [5]. Among this, the air inside the container was freely fall outside. All of seven turtles could move and breathe freely throughout the experiments. The cup was replaced with an empty cup and the lid was opened to ventilate the container for 10 min. This process was repeated for the next two odors. Two independent observers evaluated indexes related to the turtle activities as follows.
Fig. 1.

Experimental system (A) and crossed lines on cover (B). Animals cannot see the investigators who are located behind a wall. Number of times the turtle head crossed the red lines were counted.
The number of times the head of the turtle crossed the red lines (Fig. 1B) within five mins of inhaling the odorants was established as a primary index (Fig. 2A). There were no significant differences between the order of presentation (Supplementary Fig. 1). One turtle that was hyperactive (>40 times) throughout all odor presentations including distilled water, was determined by Smirnov–Grubb tests to be an outlier among turtles exposed to Rathke gland scents. Thus, data from this individual were excluded. The activities of the juveniles increased when exposed to the formula food and shrimps, and decreased when exposed to that derived from Rathke glands compared with the control (Fig. 2A and 2B). Paired t-tests with Holm multiple comparison tests [11] showed that activities significantly differed between turtles exposed to Rathke gland and control scents (P<0.05, n=6), as well as shrimps (P<0.01, n=6; Fig. 2A). All data were statistically analyzed using R version 3.5 [24]. We then counted the number of water scratches with both forelimbs as a secondary index of activity (Fig. 2C). This index also showed decreased activity in turtles exposed to Rathke gland scent (Fig. 2C). These results indicated that scents derived from male mature Rathke glands suppress the activities of juvenile green sea turtles.
Fig. 2.
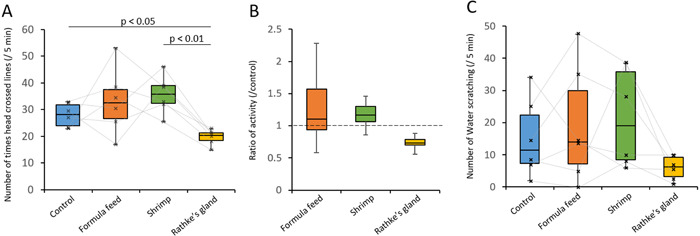
Effects of odorants derived from formula food, shrimps, and mature male Rathke glands on juvenile green sea turtles. (A) Box plots show number of times the head crossed lines within five minutes after initial inhalation of each odorant. Cross plots connected by lines indicate same individual. Significant differences are evident between Rathke gland and control (distilled water) as well as shrimp odors (P<0.05 and P<0.01, respectively; paired t-tests with Holm multiple comparison tests, n=6). (B) Box plots show prevalence of locomotion in response to each odor compared with control. (C) Box plots show number of water scratches using both forelimbs within five minutes of initially inhaling each odorant. Cross plots connected by lines indicate same individual.
The present findings showed that the odor derived from two types of foods activated green sea turtle behavior, as found in loggerhead sea turtles [5]. Sea turtles were fed with formula food and shrimps every day at OMC, and our findings indicated that they can use olfaction to search for food.
In contrast, the activity of the juvenile sea turtles decreased when exposed to the odor of male mature Rathke glands, which was similar to the freezing reaction of mice against predator [23, 29]. One assumption is that the juveniles might be simply confused by unknown odor resulted in inactivate reactions, because it was the first time to encounter the odor of mature male Rathke glands contrary to formula food and shrimp. However, it was indicated that some non-marine odors never exposed to sea turtles do not affect their activity [4], and thus the suppressive effects of Rathke gland odor on juvenile turtles shown herein were considered significant in their ecology.
Responses to odors seem to be determined by social relationships between the donors and recipients of chemicals. For example, subadult brown bears (Ursus arctos) investigate adult male odors more intensively than other odors [14], probably because subadults process such information to avoid adult males. Another example of odor response regarding to social relationships is reported in fathead minnows (Pimephales promelas) [1], and they use chemical disturbance cues as an antipredator signal to create group defenses such as shoaling, freezing and dashing [1]. Sea turtles spend most of their time alone in the wild and the biting behavior is observed under group feeding condition [10], thus the suppressive effects shown herein might be beneficial to maintain proper distance between individuals.
As far as we can ascertain, this is the first study to determine the behavioral effects of odorants derived from adult males on the species of juvenile sea turtles, indicating that green sea turtles use chemical signals in various situations via olfaction. Importantly, the suppressive effects of airborne scents from male mature Rathke glands on the activities of juveniles does not rule out the notion that odorants from the same species are associated with breeding behavior in sea turtles. In fact, responses to chemical cues derived from the same species, even for the same odorants, depend on sex and maturity status from lampreys [30] to mammals [13]. Therefore, further studies using various combinations of scent recipients and donors are required to understand the overall nature of chemical communications via olfaction in green sea turtles.
Supplementary
Acknowledgments
This study was supported by the Okinawa Churashima Foundation (grant number: 205). We are grateful to Ms. Yuki Hamada, Mr. Hideaki Tanaka and Mr. Kosuke Enomoto for cooperation with this study, and Ms. Norma Foster for critical reading of the manuscript.
REFERENCES
- 1.Bairos-Novak K. R., Ferrari M. C. O., Chivers D. P.2019. A novel alarm signal in aquatic prey: Familiar minnows coordinate group defences against predators through chemical disturbance cues. J. Anim. Ecol. 88: 1281–1290. doi: 10.1111/1365-2656.12986 [DOI] [PubMed] [Google Scholar]
- 2.Bowen B. W., Karl S. A.1997. Population genetics, phylogeography, and molecular evolution. pp. 29–50. In: The Biology of Sea Turtles. (Lutz, P. L. and Musick, J. A. eds.), CRC Press, Washington, D.C. [Google Scholar]
- 3.Ehrenfeld J. G., Ehrenfeld E. W.1973. Externally secreting glands of freshwater and sea turtles. Copeia 1973: 305–314. doi: 10.2307/1442969 [DOI] [Google Scholar]
- 4.Endres C. S., Lohmann C. M.2013. Detection of coastal mud odors by loggerhead sea turtles: a possible mechanism for sensing nearby land. Mar. Biol. 160: 2951–2956. doi: 10.1007/s00227-013-2285-6 [DOI] [Google Scholar]
- 5.Endres C. S., Putman N. F., Lohmann K. J.2009. Perception of airborne odors by loggerhead sea turtles. J. Exp. Biol. 212: 3823–3827. doi: 10.1242/jeb.033068 [DOI] [PubMed] [Google Scholar]
- 6.Endres C. S., Putman N. F., Ernst D. A., Kurth J. A., Lohmann C. M. F., Lohmann K. J.2016. Multi-model homing in sea turtles: modeling dual use of geomagnetic and chemical cues in island-finding. Front. Behav. Neurosci. 10: 19. doi: 10.3389/fnbeh.2016.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FitzSimmons N. N., Limpus C. J., Norman J. A., Goldizen A. R., Miller J. D., Moritz C.1997. Philopatry of male marine turtles inferred from mitochondrial DNA markers. Proc. Natl. Acad. Sci. USA 94: 8912–8917. doi: 10.1073/pnas.94.16.8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassman M. A., Owens D. W., McVey J. P., M R. M.1984. Olfactory-based orientation in artificially imprinted sea turtles. Science 224: 83–84. doi: 10.1126/science.224.4644.83 [DOI] [PubMed] [Google Scholar]
- 9.Grassman M. A., Owens D. W.1987. Chemosensory imprinting in juvenile green sea turtles, Chelonia mydas. Anim. Behav. 35: 929–931. doi: 10.1016/S0003-3472(87)80133-1 [DOI] [Google Scholar]
- 10.Higgins B. M.2003. Sea turtle husbandry. pp. 411–440. In: The Biology of Sea Turtles II (Lutz, P. L., Musick, J. A., and Wyneken, J. eds.), CRC Press, Washington, D.C. [Google Scholar]
- 11.Holm S.1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6: 65–70. [Google Scholar]
- 12.Iverson J. B., Klondaris H., Angell C. S., Tori W. P.2016. Olfaction as a cue for nest-site choice in turtles. Chelonian Conserv. Biol. 15: 206–213. doi: 10.2744/CCB-1199.1 [DOI] [Google Scholar]
- 13.Johnston R. E.2003. Chemical communication in rodents: from pheromones to individual recognition. J. Mammal. 84: 1141–1162. doi: 10.1644/BLe-010 [DOI] [Google Scholar]
- 14.Jojola S. M., Rosell F., Warrington I., Swenson J. E., Zedrossera A.2012. Subadult brown bears (Ursus arctos) discriminate between unfamiliar adult male and female anal gland secretion. Mamm. Biol. 77: 363–368. doi: 10.1016/j.mambio.2012.05.003 [DOI] [Google Scholar]
- 15.Kondoh D., Kitayama C., Yamaguchi Y., Yanagawa M., Kawai Y. K., Suzuki C., Itakura R., Fujimoto A., Satow T., Kondo S., Sato T.2019. Nasal cavity of green sea turtles contains 3 independent sensory epithelia. Chem. Senses 44: 427–434. doi: 10.1093/chemse/bjz033 [DOI] [PubMed] [Google Scholar]
- 16.Kondoh D., Kitayama C., Aiko Y., Yamaguchi Y.2020. Main airway throughout the nasal cavity of green sea turtles is lined by keratinized stratified squamous epithelium. Tissue Cell 65: 101370. doi: 10.1016/j.tice.2020.101370 [DOI] [PubMed] [Google Scholar]
- 17.Lewis C. H., Molloy S. F., Chambers R. M., Davenport J.2007. Response of common musk turtles (Sternotherus odoratus) to intraspecific chemical cues. J. Herpetol. 41: 349–353. doi: 10.1670/0022-1511(2007)41[349:ROCMTS]2.0.CO;2 [DOI] [Google Scholar]
- 18.Lohmann K. J., Lohmann C. M. F., Brothers J. R., Putman N. F.2013. Natal homing and imprinting in sea turtles. pp. 59–77. In: The Biology of Sea Turtles III (Wyneken, J., Lohmann, K. J. and Musick, J. A. eds.), CRC Press, Washington, D.C. [Google Scholar]
- 19.Manton M. L.1979. Olfaction and behavior. pp. 289–301. In: Turtles: Perspectives and Research (Harmless, M. and Morlock, H. eds.), John Wiley & Sons, New York. [Google Scholar]
- 20.Manton M. L., Karr A., Ehrenfeld D. W.1972. Chemoreception in the migratory sea turtle, Chelonia mydas. Biol. Bull. 143: 184–195. doi: 10.2307/1540338 [DOI] [PubMed] [Google Scholar]
- 21.McCutcheon F. H.1943. The respiratory mechanism in turtles. Physiol. Zool. 16: 255–269. doi: 10.1086/physzool.16.3.30151698 [DOI] [Google Scholar]
- 22.Musick J. A., Limpus C. J.1997. Habitat utilization and migration in juvenile sea turtles. pp. 137–164. In: The Biology of Sea Turtles (Lutz, P. L. and Musick, J. A. eds.), CRC Press, Washington, D.C. [Google Scholar]
- 23.Otsuka S.2017. Predator odor-induced freezing test for mice. Bio Protoc. 7: e2534. doi: 10.21769/BioProtoc.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 25.Rathke H.1848. About the Development of the Turtles. Friedrich Vieweg und Sohn, Braunschweig (in German). [Google Scholar]
- 26.Root R. W.1949. Aquatic respiration in the musk turtle. Physiol. Zool. 22: 172–178. doi: 10.1086/physzool.22.2.30152040 [DOI] [PubMed] [Google Scholar]
- 27.Rostal D. C., Williams J. A., Weldon P. J.1991. Rathke’s gland secretion by loggerhead (Caretta caretta) and Kemp’s ridley (Lepidochelys kempii) sea turtles. Copeia 1991: 1129–1132. doi: 10.2307/1446113 [DOI] [Google Scholar]
- 28.Shamblin B. M., Bjorndal K. A., Bolten A. B., Nairn C. J.2012. Natal homing by an adult male green turtle at Tortuguero, Costa Rica. Mar. Turtle Newsl. 134: 21–22. [Google Scholar]
- 29.Takahashi L. K., Nakashima B. R., Hong H., Watanabe K.2005. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 29: 1157–1167. doi: 10.1016/j.neubiorev.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Walaszczyk E. J., Goheen B. B., Steibel J. P., Li W.2016. Differential effects of sex pheromone compounds on adult female sea lamprey (Petromyzon marinus) locomotor patterns. J. Biol. Rhythms 31: 289–298. doi: 10.1177/0748730416629248 [DOI] [PubMed] [Google Scholar]
- 31.Walker W. F.1959. Closure of the nostril in the Atlantic loggerhead and other sea turtles. Copeia 1959: 257–259. doi: 10.2307/1440406 [DOI] [Google Scholar]
- 32.Yamaguchi Y., Kitayama C., Tanaka S., Kondo S., Miyazaki A., Okamoto K., Yanagawa M., Kondoh D.2020. Computed tomographic analysis of internal structures within the nasal cavities of green, loggerhead and leatherback sea turtles. Anat. Rec. (Hoboken). doi: 10.1002/ar.24469 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


