Abstract
A coronary heart disease leads to increase in obesity and metabolic dysfunction. Protocatechuic acid (PCA), due to its antioxidant, anti-inflammatory, neuro protective activities was found efficient as cardio-protective in coronary heart disease. Our study investigated hypolipidemic and cardioprotective effects of protocatechuic acid in the coronary artery disease induced by high fat and fructose diet (HFD) rat models. A diet rich in fat and fructose was fed to male Wistar rats prior to the start of experimental procedures. Serum lipid levels and hepatic triglycerides (TG) and total cholesterol (TC) levels were examined and analyzed. Both in vitro an in vivo pancreatic lipase activity was determined as well. Histopathological examination was performed and their results were noted. Noteworthy reduction of serum lipid levels and hepatic TG and TC levels was seen in groups treated with simvastatin (SIM; 20 mg/kg) and PCA (50 and 100 mg/kg) in comparison to HFD groups. Pancreatic lipase activity was reduced in the SIM group and the group treated with doses of PCA (50 and 100 mg/kg). A marked increase in gain in body weight per week (P<0.05) was achieved in HFD group. Coronary risk index (CRI) and Atherogenic index of plasma (AIP) showed decreased index values after treatments with SIM and PCA (50 and 100 mg/kg), respectively. Our findings confirmed the efficacious cardio-protective and hypolipidaemic activities of protocatechuic acid in coronary artery disease induced in rats with fat and fructose rich diet.
Keywords: coronary artery disease, fructose, hypolipidemia, protocatechuic acid
Atherosclerosis and other disorders related to it, such as coronary artery disease (CAD), ischemic peripheral and peripheral vascular diseases show hyperlipidemic condition due to increased levels of lipids in the blood which also include fatty acids and fats, cholesterol, esters, triglycerides which are also responsible for the formation of plaques in atherosclerosis [12]. Various studies report the association of a hypercaloric diet with the occurrence of CAD which increases the percent of annual death due to cardiac diseases in obese people by 40 times more than non-obese people [8, 22, 31]. A study [35] reported that increased intake of a high-fat or fructose diet which usual contain cholesterol and fat causing hyperlipidemia, the lipids accumulation in the hepatic region, peroxidation of lipids, hepatotoxicity leads to increase in weight, obesity and eventually metabolic syndrome [7, 30]. Studies also report that such diets cause metabolic disorders due to cardiac alterations which are marked by significant increase in weight, hyperlipidemia leading to atherosclerosis [14].
Protocatechuic acid (PCA) is present in more than 450 plants as a chemical constituent that imparts various pharmacological properties mainly due to anti-oxidative activity. Many studies were conducted to identify plants or plant parts such as leaves, bark, stem, extracts, etc. that are generally used as cultural medicine which show various antioxidant, anti-inflammatory, anti-diabetic properties to prevent or treat obesity. PCA was found to show antioxidant and anti-radical property [28] upon careful study of its molecular structure. Esters of PCA showed greater antioxidant activity when compared to PCA as they contain high lipophilic character that is responsible for the inhibition of lipoperoxidation [25]. Various study models have depicted the neuroprotective property of PCA in Parkinsonism [34], murine cerebral cortex and hippocampal region by enhancing the endogenous antioxidant system to maintain the anti-oxidative homeostasis [29]. PCA shows its anti-inflammatory activity by targeting inflammatory regulators [2], cytokine production [1], inhibiting the generation NO [32], inhibiting TNFα-induced activation of NF-κB [16] and so on. PCA also shows anti-hyperglycaemic activity [27], pro-apoptotic activity specifically in cancer cells [13] and anti-microbial activity [20].
Therefore, our aim was to assess the potentiality of PCA by reducing the serum lipid concentrations in rat allowed to feed upon a diet rich in fat and fructose together with the anti-obesity property of PCA by decreasing the atherogenic index thereby offering protection to cardiac vascular system.
MATERIALS AND METHODS
Chemicals and drugs
Simvastatin and protocatechuic acid (98% purity) was procured from Sigma Aldrich (Beijing, China). The assay kits and reagents for biochemical estimations were obtained from Sigma Aldrich. Analytical grade chemicals and reagents were used for all practical purposes.
Animals and experimental design
Our study was conducted on male Wistar rats (110 to 150 g) obtained from Animal Research Centre, China. These rats were housed under controlled temperature (25°C) allowing access to food and water ad libitum. Prior to the animal experimentation, the study approval (IAEC/CA/PCA/0012/19_09) was obtained according to Animal Ethical guidelines for laboratory animals.
Rats were allowed to feed on regular diet for a week prior to the start of any treatment. Later they were grouped into six divisions with six rats in every group (n=6) namely Group-I-control; Group-II-HFD (High fat and fructose diet) group; Group-III-HFD + SIM (simvastatin) (20 mg/kg) group; Group-IV-HFD + PCA (50 mg/kg) and Group-V-HFD + PCA (100 mg/kg). The rats were daily checked for their weight until the end of the experiment.
Once experimentation was completed (10 weeks later), the rat models were weighed before sacrifice. Blood was obtained from the various groups of rats to separate serum through centrifugation (2,000 g, 4°C) for 20 min using pre-coated EDTA tubes. To perform biochemical estimation, the supernatants and organ such as liver were stored at −80°C. Liver tissue samples were homogenized and centrifuged for 20 min at 10,000 rpm. The supernatants were stored for biochemical analyses.
Estimation of biomarkers (serum and liver)
Estimation of serum lipid levels: The enzymatic colorimetric method was employed to estimate serum total cholesterol (TC) and triglycerides (TG) concentrations and high density lipoprotein cholesterol (HDL-c) using the colorimetric kits; Shandong Bio & Media Laboratories Co., Ltd. Shandong, China. Glycerol kinase and cholesterol oxidase assays were performed to measure serum TG and serum TC, respectively. Coloration intensities were evaluated at 505 nm and represented in terms of mmol/l. To calculate fractions of LDL-cholesterol and VLDL-cholesterol the below mentioned formula was used:
Low density lipoproteins = TC − HDL-c – (TG/2.2) [21].
Very low density lipoproteins = TG/2.2 [23].
To detect the presence of atherosclerosis or any cardiovascular dysfunction, the following formulae for Coronary risk index (CRI); Atherogenic index (AI) and Atherogenic index of plasma (AIP) were used:
CRI = TC/HDL [20].
Atherogenic index = Low density-c/High density lipoproteins-c [21].
Atherogenic Index of plasma = Log (Triglycerides/High density lipoproteins-c) [6].
Liver TC and TG levels
The colorimetric kits; Shandong Bio & Media Laboratories Co., Ltd., were used to determine the liver TG and TC concentrations. The findings were represented as µmol/g of hepatic tissue.
Evaluation of serum pancreatic lipase activity
The kinetic method was performed to determine the pancreatic lipase activity, using imported lipase assay kit (DLPS-100); Bioassay Systems (Hayward, CA, USA). Coloration intensities were evaluated at 550 nm and the result was represented as U/l. The following formula was used to calculate the serum lipase activity:
Lipase activity = (Δabs/min) Calicrator – (Δabs/min) Blank / (Δabs/min) Assay – (Δabs/min) Blank
where, (Δabs/min) Assay = Rate of change/min (sample)
(Δabs/min) Blank = Rate of charge/min (blank)
(Δabs/min) Calibrator = Rate of change/min for Lipase color calibrator.
Evaluation of in-vitro pancreatic lipase activity
The in-vitro modified procedure for pancreatic lipase activity was used to measure the inhibition activity of pancreatic lipase involving 4-methyl-umbelliferyl oleate as a substrate. Fifty µl of 4-methyl-umbelliferly oleate solution (0.1 mM) previously dissolved in a buffer (pH 8.2) solution containing 100 mM sodium chloride, 1.5 mM calcium chlori de and 15 mM Tris-HCl and added to 25 µl of sample solution in water. The mixture is stirred well on a microtiter plate. To this mixture, 30 µl lipase solution (0.75 mg/ml) was added to begin the enzymatic reaction at 37°C incubated for 10 min. The quantity of 4-methyl-umbelliferone released by the action of lipase was determined by fluorometric microplate reader at excitation (360 nm) and emission (470 nm) wavelength, respectively. Based on our preliminary laboratory studies, standard (simvastatin, 20 mg/kg) and PCA-50 mg/kg and 100 mg/kg test concentrations ranged from 0.25–0.75 mg/ml and 0.5–3 mg/ml, respectively (Table 3) for percent inhibition of in vitro pancreatic lipase activity were chosen. The plot of log of the standard and test concentrations was constructed against the percent activity (%) of pancreatic lipase to obtain standard and sample IC50 from the least-squares regression line.
Table 3. In vitro pancreatic lipase inhibition rate inhibitory concentrations (IC50) of Simvastatin and protocatechuic acid (PCA)-50 and 100 mg/kg.
| Inhibitor | Concentration (mg/ml) | Pancreatic lipase inhibition (%) | IC50 (mg/ml) |
|---|---|---|---|
| Simvastatin | 0.25 | 25.77 ± 3.23 | 0.45 |
| 0.5 | 62.13 ± 3.83 | ||
| 0.75 | 80.92 ± 4.04 | ||
| PCA (50 mg/kg) | 0.5 | 31 ± 3.04 | 1.51 |
| 1 | 41.86 ± 2.68 | ||
| 2 | 76.16 ± 3.22 | ||
| 3 | 100.1 ± 4.32 | ||
| PCA (100 mg/kg) | 0.5 | 26.08 ± 3.24 | 1.18 |
| 1 | 35.81 ± 2.59 | ||
| 2 | 64.78 ± 3.03 | ||
| 3 | 80.7 ± 2.69 |
Values are expressed as mean ± SEM (mean of three determinations). Simvastatin in vivo dose was 20 mg/kg whilst in vitro concentrations varied from 0.25 to 0.75 mg/ml. PCA50 mg/kg and PCA 100 mg/kg represents in vivo dose of protocatechuic acid tested at various concentrations in vitro between 0.5–3 mg/ml. The IC50 against the pancreatic lipase activity of PCA50 mg/kg and PCA100 mg/kg were 1.51 mg/ml and 1.18 mg/ml, respectively. The IC50 of PCA at both doses were higher than the simvastatin (IC50=0.45 mg/ml)
Histopathological examination
Liver tissue samples from various groups were firstly dissolved in Bouin solution for 24 hr, later in10% formalin solution and fixed in paraffin. These samples were sliced and stained using hematoxylin and eosin. These stained slices of liver tissues were used to investigate and assess the histological changes using a light microscope at 100 X (Olympus).
Statistical analysis
The results were expressed as mean ± SEM and analyzed using Graphpad Prism 8.0 version. One-way analysis of variance (ANOVA) followed by Dunnet’s t-test was employed to test the significance at P<0.05.
RESULTS
Protocatechuic acid (PCA) effects on serum lipid profiles
PCA effects on triglycerides (TG) levels: The levels of triglycerides (Table 1) were increased significantly by 96.95% in HFD group upon comparison to the control group. A remarkable reduction in the levels of triglycerides were noticed in treatment groups of HFD + SIM (20 mg/kg), HFD + PCA50 mg/kg and HFD + PCA100 mg/kg by 45.90%, 21.36% and 26.5% (P<0.05), respectively upon comparison to HFD group alone.
Table 1. Serum lipid profile in control and experimental groups of rats.
| Control | HFD | HFD + SIM (20 mg/kg) | HFD + PCA50 mg/kg | HFD + PCA100 mg/kg | |
|---|---|---|---|---|---|
| Triglycerides | 1.11 ± 0.10 | 2.20 ± 0.18 a) | 1.19 ± 0.03 b) | 1.73 ± 0.05 b) | 1.61 ± 0.05 b) |
| Total cholesterol | 1.11 ± 0.03 | 2.32 ± 0.10 a) | 1.20 ± 0.04 b) | 1.92 ± 0.05 b) | 1.62 ± 0.05 b) |
| High density lipoprotein cholesterol (HDL-c) | 0.55 ± 0.03 | 0.50 ± 0.04 | 0.86 ± .017 b) | 0.65 ± 0.03 b) | 0.71 ± 0.02 b) |
| LDL-c | 0.05 ± 0.02 | 0.82 ± 0.14 a) | 0.196 ± 0.07 b) | 0.49 ± 0.01 b) | 0.18 ± 0.03 b) |
| VLDL-c | 0.50 ± 0.04 | 0.99 ± 0.08 a) | 0.53 ± 0.01 b) | 0.78 ± 0.02 b) | 0.73 ± 0.02 b) |
| AIP | 0.30 ± 0.07 | 0.64 ± 0.07 a) | 0.14 ± 0.009 b) | 0.42 ± 0.01 b) | 0.35 ± 0.007 b) |
| AI | 0.10 ± 0.04 | 1.62 ± 0.23 a) | 0.22 ± 0.08 b) | 0.76 ± 0.05 b) | 0.25 ± 0.05 b) |
| CRI | 1.65 ± 0.55 | 4.66 ± 0.30 a) | 1.39 ± 0.07 b) | 2.96 ± 0.08 b) | 2.27 ± 0.07 b) |
Values are expressed as mean ± SEM (mean of six determinations). PCA: protocatechuic acid. Control group; HFD represents the group fed with high fat and fructose diet; SIM represents simvastatin; PCA50 mg/kg represents protocatechuic acid at 50 mg/kg and PCA100 mg/kg represents protocatechuic acid at 100 mg/kg; LDL-c: low-densitylipoprotein cholesterol; VLDL-c: very low-density lipoprotein cholesterol; AI: atherogenic index; AIP: atherogenic index of plasma; CRI: coronary artery risk index. a) and b) indicate significant differences compared to the values of Control and HFD groups, respectively at P<0.05.
PCA effects on total cholesterol (TC) levels: The levels of total cholesterol (Table 1) were increased significantly by 108.32% in HFD group upon comparison to the control group. The levels of total cholesterol were reduced markedly by 48.43%, 17.36%, 30.08% (P<0.05), respectively in HFD + SIM, HFD + PCA50 mg/kg and HFD + PCA100 mg/kg upon comparison to HFD group alone.
PCA effects on high-density lipoprotein cholesterol (HDL-c) levels: There were no significant (Table 1) changes were observed in HDL-c levels between HFD and control groups. However, the levels of high-density lipoprotein cholesterol were increased considerably (P<0.05) in treatment groups HFD + SIM (70.87%), HFD + PCA50 mg/kg (29.14%) and HFD + PCA100 mg/kg (41.72%) when upon comparison to HFD group alone.
PCA effects on low density lipoprotein cholesterol level (LDL-c): The levels of low-density lipoprotein cholesterol (Table 1) were increased significantly in HFD group by 93.13% upon comparison to the control group. The considerable decrease in the levels of low-density lipoprotein cholesterol were observed in treatment groups of HFD + SIM, HFD + PCA50 mg/kg and HFD + PCA100 mg/kg by 76.20%, 40.32% and 78.22% (P<0.05), respectively upon comparison to HFD group alone.
PCA effects on VLDL levels: The levels of very low density lipoproteins (VLDL; Table 1) were increased significantly by 96.70% in HFD group upon comparison to the control group. VLDL levels were decreased remarkably by 46.15%, 21.74%, 26.42% (P<0.05), respectively in HFD + SIM (20 mg/kg), HFD + PCA50 mg/kg and HFD + PCA100 mg/kg upon comparison to HFD group alone.
PCA effects on atherogenic index (AI)
The levels of AI (Table 1) were increased considerably (P<0.05) by 52.7% in HFD group upon comparison to the control group. The levels of atherogenic index (AI) were reduced remarkably in HFD + SIM (86.44%), HFD + PCA50 mg/kg (53.17%) and HFD + PCA100 mg/kg (84.59%) upon comparison to HFD group.
PCA effects on atherogenic index of plasma (AIP)
The significant increase in levels of AIP (Table 1) was noticed in HFD group by 93.42% upon comparison to the control group. The marked reduction (P<0.05) in the levels of atherogenic index of plasma (AIP) were observed in treatment groups of HFD + SIM (20 mg/kg), HFD + PCA50 mg/kg and HFD + PCA100 mg/kg by 78.01%, 33.43%, 44.48% respectively upon comparison to HFD group alone.
PCA effects on Coronary risk index (CRI)
The levels of CRI (Table 1) were increased significantly by 64.46% in HFD group upon comparison to the control group. The CRI levels were decreased considerably in treatment groups of HFD + SIM (70.19%), HFD + PCA50 mg/kg (36.37%) and HFD + PCA100 mg/kg (51.16%) upon comparison to HFD group alone.
PCA effects on hepatic biomarkers
PCA effects on hepatic triglycerides (TG): The significant increase in levels of hepatic lipid profile (triglycerides; Table 2) was observed in HFD group by 78.48% upon comparison to the control group. A marked (P<0.05) reduction in the levels of hepatic triglycerides were noticed in treatment groups of HFD + SIM (20 mg/kg), HFD + PCA50 mg/kg and HFD + PCA100 mg/kg by 78.48%, 28.52%, 36.39% upon comparison to HFD group alone.
Table 2. Hepatic lipid profile in control and experimental groups of rats.
| Control | HFD | HFD + SIM (20 mg/kg) | HFD + PCA50 mg/kg | HFD + PCA100 mg/kg | |
|---|---|---|---|---|---|
| Hepatic triglycerides | 4.24 3 ± 0.58 | 7.57 ± 0.23 a) | 4.02 ± 0.42 b) | 5.41 ± 0.40 b) | 4.81 ± 0.29 b) |
| Hepatic total cholesterol | 6.57 ± 0.69 | 16.37 ± 0.55 a) | 7.87 ± 0.68 b) | 12.56 ± 1.2 b) | 11.32 ± 0.49 b) |
Values are expressed as mean ± SEM (mean of six determinations). PCA: protocatechuic acid. Control group; HFD represents the group fed with high fat and fructose diet; SIM represents simvastatin; PCA50 mg/kg represents protocatechuic acid at 50 mg/kg and PCA100 mg/kg represents protocatechuic acid at 100 mg/kg. a) and b) indicate significant differences compared to the values of Control and HFD groups, respectively at P<0.05.
PCA effects on hepatic total cholesterol (TC): The levels of hepatic lipid profile (total cholesterol; Table 2) were increased significantly by 59.86% in HFD group upon comparison to the control group. A hepatic total cholesterol levels were reduced considerably (P<0.05) by 51.92%, 23.27%, 30.84% in HFD + SIM, HFD + PCA50 mg/kg and HFD + PCA100 mg/kg upon comparison to HFD group alone.
PCA effects on in-vivo and in vitro pancreatic lipase activity
The significant increase in levels of serum pancreatic lipase activity was observed in HFD group by 66.18% upon comparison to the control group. The levels of pancreatic lipase activity were reduced markedly (P<0.05) in treatment groups of HFD + SIM (28.56%), HFD + PCA50 mg/kg (20.16%) and HFD + PCA100 mg/kg (20.73%) upon comparison to HFD group alone.
Our in vitro pancreatic lipase activity results illustrated that PCA showed dose dependent and significant reduction in the lipase activity at 50 and 100 mg/kg respectively (Table 3). The IC50 values of PCA at 50 and 100 mg/kg for inhibiting pancreatic lipase activity were found to be 1.51 and 1.18 mg/ml respectively. The inhibitory concentrations (IC50) of PCA at both doses were higher than the simvastatin (IC50=0.45 mg/ml) in agreement with in vivo studies at different doses of simvastatin (20 mg/kg) and PCA (50 and 100 mg/kg), respectively.
PCA effects on final body weight (gram/rat)
A profound increase in the final body weight (Table 4) was noticed in HFD group by 24.31% upon comparison to the control group. The final body weight had decreased significantly by 20.05% in HFD + SIM group, 17.67% in HFD + PCA50 mg/kg group and 11.75% in HFD + PCA100 mg/kg group upon comparison to HFD group alone.
Table 4. Effects of different treatments on body weight gain.
| Control | HFD | HFD +SIM (20 mg/kg) | HFD + PCA50 mg/kg | HFD + PCA100 mg/kg | |
|---|---|---|---|---|---|
| Initial body weight (g/rat) | 148.3 ± 4.41 | 146.7 ± 3.52 | 155.7 ± 4.05 | 150.7 ± 3.18 | 152.7 ± 2.90 |
| Final body weight (g/rat) | 223.3 ± 3.75 | 277.3 ± 6.38 | 221.7 ± 4.91 | 228.3 ± 8.19 | 244.7 ± 6.69 |
| Body weight gain (g/rat/per week) | 10.68 ± 1.14 | 18.63 ± 1.43 a) | 9.427 ± 0.14 b) | 13.11 ± 0.56 b) | 11.06 ± 1.10 b) |
Values are expressed as mean ± SEM (mean of six determinations). PCA: protocatechuic acid. Control group; HFD represents the group fed with high fat and fructose diet; SIM represents simvastatin; PCA50 mg/kg represents protocatechuic acid at 50 mg/kg and PCA100 mg/kg represents protocatechuic acid at 100 mg/kg. a) and b) indicate significant differences compared to the values of Control and HFD groups respectively at P<0.05.
PCA effects on body weight gain per week (gram/rat)
The percentage of body weight gain per week (Table 4) had increased significantly in HFD group by 74.43% when comparison to the control group. The percent of body weight gain per week were reduced significantly in treatment groups of HFD + SIM (49.39%), HFD + PCA50 mg/kg (29.62%) and HFD + PCA100 mg/kg, (40.63%) upon comparison to HFD group alone.
Histological examination
The biochemical findings observed were further demonstrated by histopathological studies. It was observed that an accumulation of lipids was observed in the hepatocytes of liver tissues as vacuoles as shown in Fig. 2B as compared to control group (Fig. 2A), where no such vacuoles are found expect the normal architecture of the liver tissues with sinusoids with intact arteries. Upon treatment with simvastatin (20 mg/kg) and PCA50 mg/kg and PCA100 mg/kg respectively, significant changes in the liver architecture was noticed, thus restoring to normal with decrease number of lipid filled vacuoles (Fig. 2C–E). These reductions in the lipid filled vacuoles were observed as dose dependent with PCA50 mg/kg and 100 mg/kg, respectively.
Fig. 2.
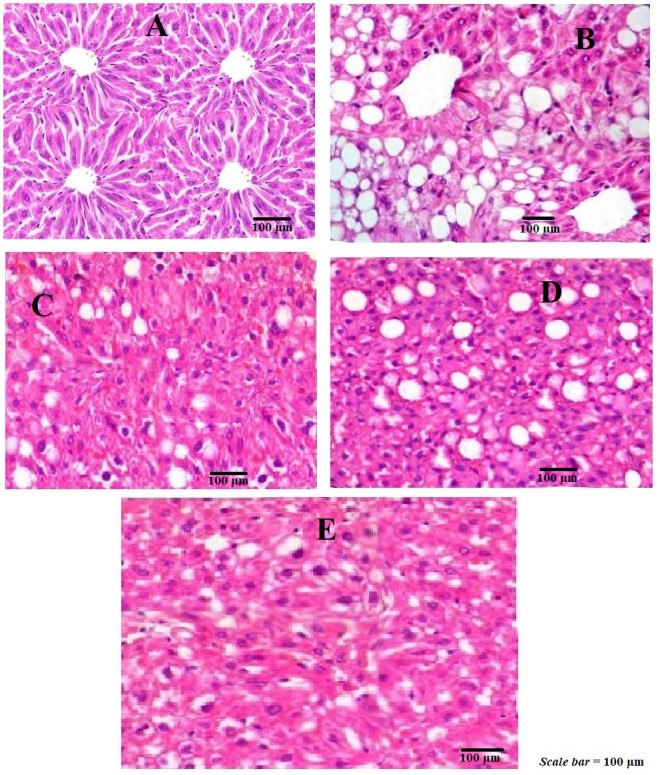
Histopathology of liver tissue (×100) of control and experimental animals. A represents control; B = HFD represents group fed with high fat and fructose diet; C=represents simvastatin; D=represents HFD+PCA50 mg/kg and E=represents HFD+PCA100 mg/kg.
These findings suggested that PCA was able to inhibit the infiltration of lipids vacuoles into the liver sinusoids, thus reducing the development of lipid filled droplets.
DISCUSSION
PCA has been found to depict its effective role against the peroxidation of lipids and oxidative stress [5, 9] by increasing the superoxide dismutase activity [21]. Many studies support the effect of PCA against anti-obesity due to its strong anti-microbial activity [6, 20, 23]. Hence, application of PCA represents a potential strategy in counteracting obesity and its related pathological conditions namely coronary artery disease.
This study was conducted on rats treated with a diet rich in fat and fructose (HFD). After 10 weeks the HFD group rats showed increase in their body weight, lipase activity in the pancreas, hepatic lipid profiles on comparison to the rats fed a regular diet i.e. control group. These HFD group rats also showed marked rise in the serum levels of TG and TC, hepatic TG and TC profiles and elevated levels of coronary risk index (CRI), depicting that hyperlipidemic model was successfully developed mimicking the coronary artery disease. The HFD rats showed significant reduction in their body weight, pancreatic lipase activity, hepatic lipid profiles, serum TG and TC levels, hepatic TG and TC profile and elevated levels of coronary risk index (CRI) when treated with either PCA50 mg/kg or PCA100 mg/kg or simvastatin. Thus, suggesting role of PCA in the reduction of these serum and hepatic biomarkers associated to coronary artery disease.
Our results showed that the serum and hepatic triglycerides (TG) and total cholesterol (TC) levels were markedly (P<0.01) reduced in the HFD + PCA50 mg/kg or PCA100 mg/kg, respectively and SIM groups when compared to the control group. This showed that PCA exhibited the capability of reducing the lipids in a dose dependent manner in both serum and hepatic tissue with intake of diet rich in fat and fructose for a longer period of time. These findings further support that PCA play an effective role as a cardio-protectant similar to that of simvastatin. High density lipoprotein cholesterol levels increased significantly when treated with SIM and PCA- 50 and 100 mg/kg in agreement with few other studies [17, 18]. These increased levels of high-density lipoprotein cholesterol are required for the transfer of cholesterol into the liver from serum for its breakdown and excretion to decrease the coronary artery disease risk [33]. In addition, LDL-c and VLDL levels were reduced significantly in the SIM and PCA50 mg/kg or PCA100 mg/kg group when compared HFD group. Our findings support that the LDL-cholesterol receptors are decreased in the HFD rats due to a rise in their LDL-cholesterol level by the fats provided in their diet leading to hyperlipidemic condition [24].
To detect the presence of atherosclerosis, as an indicator for coronary artery disease, the indices namely AI and AIP were calculated. Our findings showed that HFD + PCA50 mg/kg or PCA100 mg/kg and SIM treated groups decreased indices values suggesting the presence of atherosclerosis induced in rats fed with diet rich in fat and fructose and was lowered significantly after treatments with simvastatin and PCA in dose-dependent manner. Consequently, it was observed that the risk of coronary artery disease was least observed after treatments with SIM (20 mg/kg) and PCA50 mg/kg or PCA100 mg/kg treated groups as they were able to lower values of coronary risk index (CRI).
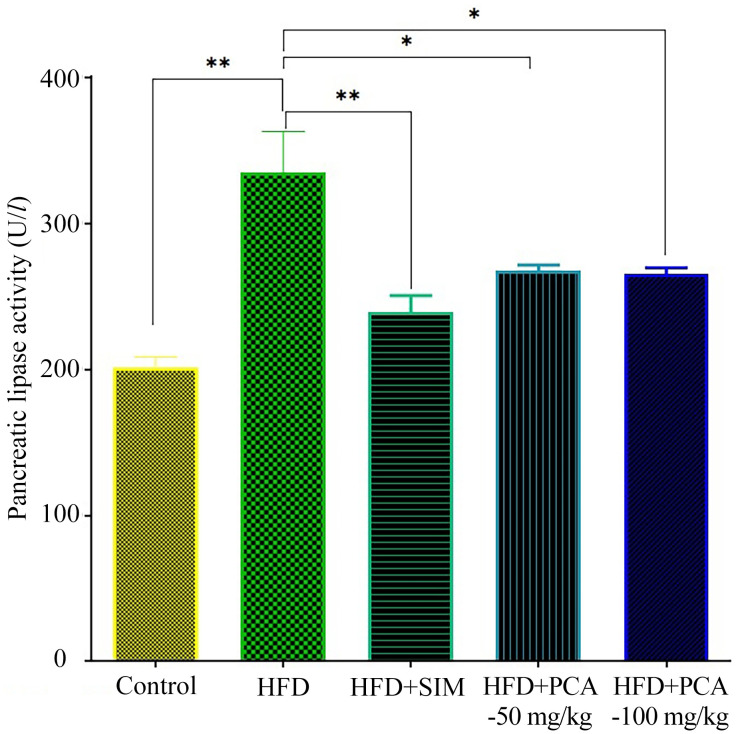
Pancreatic lipase inhibitors are considered as potential agents to treat hyperlipidemia and atherosclerosis. To absorb triglycerides from the gastrointestinal tract [15], pancreatic lipase plays an essential role as it hydrolyses the fats present in the diet [3]. Simvastatin and PCA-50 and 100 mg/kg groups showed remarkable decline in the activity of pancreatic lipase on comparison to the HFD group (Fig. 1).
Fig. 1.
Levels of pancreatic lipase activity (U/l) in control and experimental groups of rats. Control group; HFD represents the group fed with high fat and fructose diet; SIM represents simvastatin; PCA50 mg/kg represents protocatechuic acid (PCA) at 50 mg/kg and PCA100 mg/kg represents protocatechuic acid at 100 mg/kg, respectively. Data are expressed at mean ± SEM of six rats in each group. *P<0.05; **P<0.01 indicate significant differences compared to the values of control and HFD, respectively.
We also studied in vitro pancreatic lipase activity, which indicated that PCA exhibited dose-dependent lipase activity at 50 mg and 100 mg/kg doses. Our findings agree with various other studies as well [7, 18], indicating that PCA exhibits hypolipidemic and lipid absorption inhibiting properties which help in the prevention of coronary artery disease [4].
In support to our findings other than pancreatic lipase inhibition, a study [10] found that PCA present in the preparation of onions improved enzymatic activity through antioxidant mechanism in ceacal and faecal microbiota. PCA showed a strong antioxidant effect, increasing through serum anticardiolipin (P=0.033), and decreasing hepatic glutathione disulfide (P=0.070). In another study in a randomized clinical trial, PCA found that the intake of virgin olive oil containing polyphenols enhanced the cardioprotective effect corroborating the theory of the function of gut microbiota with substantially increased populations of gut bifidobacteria and associated microbial metabolite [22].
In another research [11], phenolic compounds such as PCA have been shown to restore reduced antioxidant enzymatic defense systems (glutathione peroxidase, superoxide dismutase catalase) in HFD rat models and elevated levels of oxidative stress biomarkers such as lipid peroxides, reduced glutathione, protein fragmentation and oxidation in high fructose diet-induced metabolic syndrome.
Authors of a study [19] stated that in rats fed with a Trans Fatty Acid Diet (TFA), the aggregation of lipids in the liver along with the activities and mRNA expressions of lipogenic factors had decreased following PCA therapy. It was also found that with PCA treatment the elevated levels of pro-inflammatory cytokines in the liver as a consequence of TFA were reduced dramatically.
On the other hand, PCA was shown to combat the atherogenic action of oxLDL in murine macrophages [26] by its antiapoptotic action, thereby preventing damage to arterial walls by stimulating p53-dependent apoptosis. It prevents apotosis by counteracting oxidant distress and activation of caspase. Such findings have significant consequences for managing atherosclerosis by affecting macrophages in lesioned vessels in order to prevent the initiation and emergence of atherosclerotic plague.
In conclusion, we evaluated the properties of PCA in the reduction of weight gain representing its role as anti-hyperlipidemic agent, decrease in the levels of cholesterol, serum and hepatic tissue lipid levels in the rats fed with a diet rich in fat and fructose. This suggests the role of PCA a strong cardioprotective agent in the prevention of atherosclerosis and coronary artery disease related to hyperlipidemia.
REFERENCES
- 1.Amin H. P., Czank C., Raheem S., Zhang Q., Botting N. P., Cassidy A., Kay C. D.2015. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 59: 1095–1106. doi: 10.1002/mnfr.201400803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee N., Dua T. K., Khanra R., Joardar S., Nandy A., Saha A., De Feo V., Dewanjee S.2017. Protocatechuic acid, a phenolic from Sansevieriaroxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front. Pharmacol. 8: 251. doi: 10.3389/fphar.2017.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birari R. B., Bhutani K. K.2007. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov. Today 12: 879–889. doi: 10.1016/j.drudis.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 4.Brunzell J. D., Zambon A., Deeb S. S.2012. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim. Biophys. Acta 1821: 365–372. doi: 10.1016/j.bbalip.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou T. H., Ding H. Y., Lin R. J., Liang J. Y., Liang C. H.2010. Inhibition of melanogenesis and oxidation by protocatechuic acid from Origanum vulgare (oregano). J. Nat. Prod. 73: 1767–1774. doi: 10.1021/np100281g [DOI] [PubMed] [Google Scholar]
- 6.Dao M. C., Clément K.2018. Gut microbiota and obesity: Concepts relevant to clinical care. Eur. J. Intern. Med. 48: 18–24. doi: 10.1016/j.ejim.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 7.George V., Tremblay A., Després J. P., Leblanc C., Bouchard C.1990. Effect of dietary fat content on total and regional adiposity in men and women. Int. J. Obes. 14: 1085–1094. [PubMed] [Google Scholar]
- 8.Getz G. S., Reardon C. A.2007. Nutrition and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 27: 2499–2506. doi: 10.1161/ATVBAHA.107.155853 [DOI] [PubMed] [Google Scholar]
- 9.Giovannini C., Scazzocchio B., Matarrese P., Varì R., D’Archivio M., Di Benedetto R., Casciani S., Dessì M. R., Straface E., Malorni W., Masella R.2008. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: protective effects of phenolic compounds. J. Nutr. Biochem. 19: 118–128. doi: 10.1016/j.jnutbio.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Grzelak-Błaszczyk K., Milala J., Kołodziejczyk K., Sójka M., Czarnecki A., Kosmala M., Klewicki R., Fotschki B., Jurgoński A., Juśkiewicz J.2020.Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. 11: 3585–3597. doi: 10.1039/C9FO02633A [DOI] [PubMed] [Google Scholar]
- 11.Ibitoye O. B., Ajiboye T. O.2018. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 124: 410–417. doi: 10.1080/13813455.2017.1415938 [DOI] [PubMed] [Google Scholar]
- 12.Jain K. S., Kathiravan M. K., Somani R. S., Shishoo C. J.2007. The biology and chemistry of hyperlipidemia. Bioorg. Med. Chem. 15: 4674–4699. doi: 10.1016/j.bmc.2007.04.031 [DOI] [PubMed] [Google Scholar]
- 13.Jeong M., Kim H. M., Kim H. J., Choi J. H., Jang D. S.2017. Kudsuphilactone B, a nortriterpenoid isolated from Schisandrachinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells. Arch. Pharm. Res. 40: 500–508. doi: 10.1007/s12272-017-0902-5 [DOI] [PubMed] [Google Scholar]
- 14.Kasim-Karakas S. E., Vriend H., Almario R., Chow L. C., Goodman M. N.1996. Effects of dietary carbohydrates on glucose and lipid metabolism in golden Syrian hamsters. J. Lab. Clin. Med. 128: 208–213. doi: 10.1016/S0022-2143(96)90013-X [DOI] [PubMed] [Google Scholar]
- 15.Kim T. H., Kim J. K.,, Ito H., Jo C.2011. Enhancement of pancreatic lipase inhibitory activity of curcumin by radiolytic transformation. Bioorg. Med. Chem. Lett. 21: 1512–1514. [DOI] [PubMed] [Google Scholar]
- 16.Krga I., Monfoulet L. E., Konic-Ristic A., Mercier S., Glibetic M., Morand C., Milenkovic D.2016. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 599: 51–59. doi: 10.1016/j.abb.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 17.Ktari N., Mnafgui K., Nasri R., Hamden K., Bkhairia I., Ben Hadj A., Boudaouara T., Elfeki A., Nasri M.2013. Hypoglycemic and hypolipidemic effects of protein hydrolysates from zebra blenny (Salariabasilisca) in alloxan-induced diabetic rats. Food Funct. 4: 1691–1699. doi: 10.1039/c3fo60264h [DOI] [PubMed] [Google Scholar]
- 18.Lassoued I., Trigui M., Ghlissi Z., Nasri R., Jamoussi K., Kessis M., Sahnoun Z., Rebai T., Boualga A., Lamri-Senhadji M., Nasri M., Barkia A.2014. Evaluation of hypocholesterolemic effect and antioxidant activity of Boopsboops proteins in cholesterol-fed rats. Food Funct. 5: 1224–1231. doi: 10.1039/C3FO60705D [DOI] [PubMed] [Google Scholar]
- 19.Liu W. H., Lin C. C., Wang Z. H., Mong M. C., Yin M. C.2010. Effects of protocatechuic acid on trans fat induced hepatic steatosis in mice. J. Agric. Food Chem. 58: 10247–10252. doi: 10.1021/jf102379n [DOI] [PubMed] [Google Scholar]
- 20.Martín-Peláez S., Mosele J. I., Pizarro N., Farràs M., de la Torre R., Subirana I., Pérez-Cano F. J., Castañer O., Solà R., Fernandez-Castillejo S., Heredia S., Farré M., Motilva M. J., Fitó M.2017. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. Eur. J. Nutr. 56: 119–131. doi: 10.1007/s00394-015-1063-2 [DOI] [PubMed] [Google Scholar]
- 21.Masella R., Varì R., D’Archivio M., Di Benedetto R., Matarrese P., Malorni W., Scazzocchio B., Giovannini C.2004. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr. 134: 785–791. doi: 10.1093/jn/134.4.785 [DOI] [PubMed] [Google Scholar]
- 22.Messerli M. L., Parmley T., Woodruff J. D., Lilienfeld A. M., Bevilacqua L., Rosenshein N. B.1987. Inter- and intra-pathologist variability in the diagnosis of gestational trophoblastic neoplasia. Obstet. Gynecol. 69: 622–626. [PubMed] [Google Scholar]
- 23.Moreno N. J. M., Serino M., Blasco B. V., Azalbert V., Barton R. H., Cardellini M., Jèssica L., Francisco O., Mònica S. M., Rémy B., Marc E. D., Wifredo R., Massimo F., José M. F. R.2018. Gut microbiota interacts withmarkers of adipose tissue browning, insulin action and plasma acetatein morbid obesity. Mol. Nutr. Food Res. 62. [DOI] [PubMed] [Google Scholar]
- 24.Mustad V. A., Etherton T. D., Cooper A. D., Mastro A. M., Pearson T. A., Jonnalagadda S. S., Kris-Etherton P. M.1997. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J. Lipid Res. 38: 459–468. [PubMed] [Google Scholar]
- 25.Reis B., Martins M., Barreto B., Milhazes N., Garrido E. M., Silva P., Garrido J., Borges F.2010. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food Chem. 58: 6986–6993. doi: 10.1021/jf100569j [DOI] [PubMed] [Google Scholar]
- 26.Rosaria V., Beatrice S., Carmela S., Carmelina F., Fabio G.2015Protocatechuic acid prevents oxLDL-induced apoptosis by activating JNK/Nrf2 survival signals in macrophages. Oxid. Med. Cell. Longev. 2015: 351827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semaming Y., Kukongviriyapan U., Kongyingyoes B., Thukhammee W., Pannangpetch P.2016. Protocatechuic Acid Restores Vascular Responses in Rats With Chronic Diabetes Induced by Streptozotocin. Phytother. Res. 30: 227–233. doi: 10.1002/ptr.5520 [DOI] [PubMed] [Google Scholar]
- 28.Sroka Z., Cisowski W.2003. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 41: 753–758. doi: 10.1016/S0278-6915(02)00329-0 [DOI] [PubMed] [Google Scholar]
- 29.Thakare V. N., Dhakane V. D., Patel B. M.2017. Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab. Brain Dis. 32: 401–413. doi: 10.1007/s11011-016-9922-y [DOI] [PubMed] [Google Scholar]
- 30.Tucker L. A., Kano M. J.1992. Dietary fat and body fat: a multivariate study of 205 adult females. Am. J. Clin. Nutr. 56: 616–622. doi: 10.1093/ajcn/56.4.616 [DOI] [PubMed] [Google Scholar]
- 31.Urquiaga I., Guasch V., Marshall G., San Martín A., Castillo O., Rozowski J., Leighton F.2004. Effect of Mediterranean and Occidental diets, and red wine, on plasma fatty acids in humans. An intervention study. Biol. Res. 37: 253–261. doi: 10.4067/S0716-97602004000200012 [DOI] [PubMed] [Google Scholar]
- 32.Winter A. N., Brenner M. C., Punessen N., Snodgrass M., Byars C., Arora Y., Daniel A. L.2017. Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuicacid and 4-hydroxybenzoic acid. Oxid. Med. Cell. Longev. 2017: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young C. E., Karas R. H., Kuvin J. T.2004. High-density lipoprotein cholesterol and coronary heart disease. Cardiol. Rev. 12: 107–119. doi: 10.1097/01.crd.0000097140.29929.8a [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Li G., Szeto S. S. W., Chong C. M., Quan Q., Huang C., Cui W., Guo B., Wang Y., Han Y., Michael Siu K. W., Yuen Lee S. M., Chu I. K.2015. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 84: 331–343. doi: 10.1016/j.freeradbiomed.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 35.Zou Y., Li J., Lu C., Wang J., Ge J., Huang Y., Zhang L., Wang Y.2006. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 79: 1100–1107. doi: 10.1016/j.lfs.2006.03.021 [DOI] [PubMed] [Google Scholar]