Abstract
Early diagnosis and treatment significantly reduce sepsis mortality. Currently, no gold standard has been yet established to diagnose sepsis outside the ICU. The aim of the study was to evaluate the diagnostic accuracy of sepsis defined by SIRS Criteria of 1991, Second Consensus Conference Criteria of 2001, modified Second Consensus Conference Criteria of 2001 (obtaining SIRS Criteria and SOFA score), Third Consensus Conference of 2016, in addition to the dosage of Procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM). In this prospective study, 209 consecutive patients with clinical diagnosis of sepsis were enrolled (May 2014–June 2018) outside intensive care unit (ICU) setting. A diagnostic protocol could include SIRS criteria or qSOFA score evaluation, rapid testing of PCT and MR-proADM, and SOFA score calculation for organ failure definition. Using this approach outside the ICU, a rapid diagnostic and prognostic evaluation could be achieved, also in the case of negative SIRS, qSOFA or SOFA scores with high post-test probability to reduce mortality and improve outcomes.
Subject terms: Biomarkers, Health care, Medical research
Introduction
Sepsis is the first cause of death for infection accounting for 17% of intra-hospital mortality and reaching 26% in case of septic shock1 with estimated costs for over 24 billion of dollar per year2. In 2016, the Third international Consensus Conference (Sepsis-3) defined sepsis as a “life-threatening organ dysfunction caused by a dysregulated host response to infection” removing among diagnostic criteria the presence of the systemic inflammatory response syndrome (SIRS), previously used in the Sepsis-1 and Sepsis-2 Consensus3–6.
Septic shock was identified by the presence of at least one of persistent hypotension requiring vasopressor administration to maintain MAP ≥ 70 mmHg and serum lactate > 2 mmol/L (> 18 mg/dL) despite adequate blood volume expansion6. The Third Consensus Conference (Sepsis-3) established that, in presence of suspected or documented infection, an increase of Sequential Sepsis-related Organ Failure Assessment (SOFA) score in intensive care unit (ICU) ≥ 2 from baseline have to be considered diagnostic for sepsis6. An increase of the quick SOFA (qSOFA) score ≥ 2 from baseline may be suggestive of sepsis, mainly outside ICU6.
SOFA score ≥ 2 was associated with an intra-hospital mortality > 10% with values as high as 40% in case of septic shock6,7.
In the Third definition, SOFA and qSOFA replaced SIRS criteria of the 1991 definition (Sepsis-1)3 considered too much sensitive and not specific, causing overdiagnosis and inappropriate use of antibiotics. Patients admitted to the Emergency Department could present SIRS in many situations including metabolic and endocrine diseases, cancer, respiratory syndromes, infections, trauma and ischemia8. Factors as drugs or disease altering body temperature, cardiac or respiratory frequencies and leukocytes count can determine SIRS. Furthermore, the identification of the microbiological cause of sepsis is achieved in less than 50% of patients9 and only 30% of bacteremia are microbiologically documented10.
Since 2001, the Second Consensus Conference of Sepsis recommended adding the use of biomarkers to SIRS criteria to overcome these limits4,5,11. During infection, indeed, PCT has the ability to discriminate between infectious and not-infectious SIRS, and to guide antimicrobial therapy and follow-up12–15. However, PCT increase during infection by Gram-positive or fungal pathogens or antimicrobial treatment could be limited16–20.
Mid-regional pro-adrenomedullin (MR-proADM) has been recently proposed for sepsis diagnosis and prognosis, also providing etiological information12,21–24. Its levels significantly relate with septic patients’ outcomes showing good relation with prognosis and mortality rate25. Despite the availability of PCT and MR-proADM may be hampered mainly in the low income countries, they presented lower turnaround time and lower costs than other biomarkers of sepsis (interleukins, cytokines, and others biomarkers) that had similar diagnostic capability.
In sepsis and septic shock, MR-proADM compared to other well-known biomarkers or clinical scores, showed a prognostic accuracy higher than those of PCT, IL-6, CRP or clinical scores as Acute Physiology and Chronic Health (APACHE)23,24,26. Christ-Crain et al. analyzed MR-proADM levels in septic patients admitted to intensive care unit (ICU) showing a significantly higher correlation with sepsis severity than PCT and CRP26. Recently, Kim et al., reported a significant correlation between MR-proADM levels and septic shock, need for vasopressor, and 30-day mortality, suggesting its inclusion in the panel of biomarkers that may be useful for diagnosis and treatment management of critical patients in ICU27.
The combination of MR-proADM with other biomarkers, especially PCT, was proposed in previous studies21–23,28.
The combined measurement of PCT and MR-proADM significantly improved sepsis diagnosis, mainly in case of Gram-positive or fungal sepsis where PCT alone could present a lower positive predictive value (PPV)12.
Actually, the most significant approach to reduce sepsis-related mortality is based on early diagnosis by adequate microbiological cultures collection and administration of empirical antibiotic treatment within 3 h from clinical suspicion29. To reduce antimicrobial resistance, however, would be desirable to administer an appropriate antimicrobial treatment basing on the results of microbiological specimen even if these latter yield positive results just in less than 50% of cases9,10. Two recent systematic review and meta-analysis highlighted as an earlier than delayed antimicrobial treatment administration seemed not to reduce mortality in patients with septic shock, despite the effect of other specific treatment were not considered within the analysis30,31. These contrasting results is responsible for the lack of standardized treatment strategies.
The aim of the study was to evaluate the diagnostic accuracy of sepsis defined by SIRS Criteria of 1991, Second Consensus Conference Criteria of 2001, modified Second Consensus Conference Criteria of 2001 (obtaining SIRS Criteria and SOFA score), Third Consensus Conference of 2016, in addition to the dosage of Procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM).
Methods
Design and setting
This study was approved by the Ethical Committee of the University Hospital Campus Bio-Medico of Rome. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all patients prior enrollment in the study.
Patients selection and study design
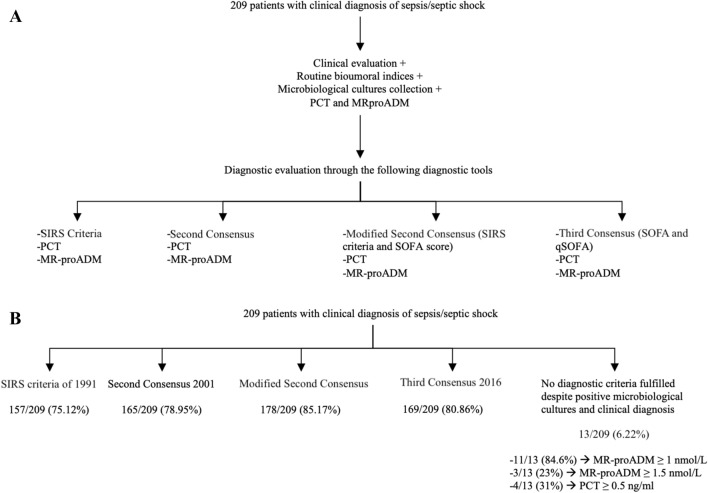
This prospective study was performed on consecutive enrolled patients with clinically suspected sepsis or septic shock admitted to the Diagnostic and Therapeutic Medicine Department and General Surgery of the University Hospital Campus Bio-Medico of Rome, between May 2014 and June 2018. Data were retrospectively evaluated comparing the diagnostic accuracy of sepsis as defined by SIRS criteria of 1991, Second Consensus Conference Criteria of 2001, modified Second Consensus Conference Criteria of 2001, Third Consensus Conference of 2016, in addition to the dosage of PCT and MR-proADM (Fig. 1A). Modified Second Consensus Conference Criteria of 2001 were obtained using SIRS Criteria of 1991 plus SOFA score of 20163–6.
Figure 1.
Algorithm used for sepsis clinical diagnosis (A) and diagnostic evaluation (B).
Inclusion criteria comprehended the clinical suspicion of sepsis and septic shock. Exclusion criteria were the lack of informed consent and pregnancy. At inclusion (Day 0), demographic characteristics such as age, gender, prior or current use of antibiotics, immunosuppressive treatments, immune status (active malignancy or other causes of an immunocompromised state), comorbidities and clinical presentation were recorded. For each patients a physical examination including cardiac, abdominal, respiratory and neurological evaluations was performed.
The real-world control group included fifty patients admitted to the Diagnostic and Therapeutic Medicine Department of Campus Bio-Medico of Rome for cardiac, kidney, liver, pulmonary and cancer diseases being responsible for a non-infectious related SIRS, qSOFA, or SOFA criteria positivity.
Clinical and laboratory parameters, blood gas analysis, blood and microbiological cultures
The following clinical and laboratory parameters have been collected: body temperature, blood pressure, heart and respiratory rate, complete blood counts (CBC), PCT, MR-proADM, bilirubin, creatinine, lactate, PaO2/FIO2, and blood and microbiological cultures at the diagnosis and when clinically necessary.
PCT and MR-proADM plasma measurement
PCT and MR-proADM plasma concentrations were measured by an automated Kryptor analyzer, using a time-resolved amplified cryptate emission (TRACE) technology assay (Kryptor PCT; Brahms AG; Hennigsdorf, Germany), with commercially available immunoluminometric assays (Brahms)21–24.
Blood and microbiological cultures
Blood specimens from patients were collected in BACTEC bottles containing anaerobic or aerobic broth and resins. Blood culture bottles (BC) were incubated in BACTEC FX instrument (Becton Dickinson, Meylan, France) until they resulted positive for bacterial growth or for a maximum of 5 days. Positive BC samples were cultivated in selective agar media. Growing colonies were identified by MALDI-TOF22. Selective and non selective media were used for microbiological cultures.
Sepsis diagnosis
Patients with suspected sepsis or septic shock included in the study were retrospectively evaluated by SIRS criteria of 1991, Second Consensus Conference Criteria of 2001, the modified Second Consensus Conference Criteria of 2001 (obtaining SIRS Criteria and SOFA score), Third Consensus Conference Criteria of 2016, in addition to the dosage of PCT and MR-proADM3–6. A SOFA or a qSOFA scores ≥ 2 from baseline has been considered diagnostic of sepsis.
Statistical analysis
Data were analysed using Med-Calc 11.6.1.0 statistical package (MedCalc Software, Mariakerke, Belgium). Plasma levels of PCT, MR-proADM, SOFA and qSOFA score values, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria and SIRS criteria in septic patients and real-life control patients were compared using the non-parametric Mann–Whitney’s test; p value < 0.05 were considered as significant. Receiver operating characteristic (ROC) analysis was performed among independent variables associated with sepsis to define the cutoff point for plasma PCT, MRproADM, lactate, SOFA and qSOFA score values, SIRS criteria of 1991, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria and to define their diagnostic accuracy for sepsis prediction. ROC curves and areas under the curve (AUCs) were calculated for all markers and compared in patients with sepsis or septic shock versus real-life control patients32.
χ2 for proportions test was used to compare the relative percentage of patients with positivity and/or negativity to SIRS criteria, SOFA score, qSOFA score, PCT and MR-proADM. p value < 0.05 were considered as significant.
Pretest odds, posttest odds, and the consequent posttest probability and χ2 test for proportions have been computed to investigate whether combination of PCT, MR-proADM, lactate, SOFA, qSOFA scores, SIRS criteria of 1991, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria improves post-test probability33.
Results
Patients characteristics
The demographic and clinical characteristics of the study group including 209 patients with sepsis and of the 50 real-world control group patients are reported in Table 1. The control group included patients with cardiac, kidney, liver, pulmonary and cancer diseases being responsible for a non-infectious related SIRS, qSOFA, or SOFA criteria positivity.
Table 1.
Demographic and clinical characteristics of study and control groups.
| Variables | Study group = 209 | Control group = 50 | p value* |
|---|---|---|---|
| Median age (IQR) | 72 (64–80) | 74 (66–82) | 0.06 |
| Sex male n (%) | 107 (51) | 25 (50) | 0.89 |
| Hypertension n (%) | 105 (50) | 26 (51) | 0.89 |
| Hyperlipidemia n (%) | 42 (20) | 12 (23.5) | 0.59 |
| Diabetes mellitus n (%) | 44 (21) | 10 (19.6) | 0.82 |
| Hypertensive cardiopathy n (%) | 28 (13.4) | 10 (19.6) | 0.26 |
| Ischemic cardiopathy n (%) | 26 (12.4) | 5 (9.8) | 0.61 |
| Degenerative cardiopathy n (%) | 15 (7.2) | 4 (7.8) | 0.88 |
| Chronic cardiac failure n (%) | 9 (4.3) | 10 (19.6) | 0.0002 |
| Acute kidney injury (AKI) n (%) | 28 (13.4) | 0 (0) | 0.0062 |
| Chronic kidney disease (CKD) n (%) | 34 (16) | 8 (15.7) | 0.95 |
| Chronic obstructive pulmonary disease (COPD) n (%) | 31 (14.8) | 9 (17.6) | 0.62 |
| Viral hepatitis n (%) | 13 (6) | 0 (0) | 0.08 |
| Cirrhosis n (%) | 7 (1.9) | 3 (5.8) | 0.12 |
| Solid neoplasia n (%) | 59 (28) | 15 (3) | 0.0002 |
| Hematologic neoplasia n (%) | 7 (3) | 3 (6) | 0.30 |
| Autoimmune disease/immunosuppressive therapy n (%) | 17 (8) | 7 (14) | 0.18 |
| Antimicrobial therapy on course | 162 (77.5) | n.a | – |
| Septic shock | 84 (40) | n.a | – |
| 30-days mortality | 38 (18) | n.a | – |
| 30-days mortality for sepsis | 10 (8) | n.a | – |
| 30-days mortality for septic shock | 28 (33) | n.a | – |
| 90-days mortality | 48 (23) | n.a | – |
| 90-days mortality for sepsis | 16 (13) | n.a | – |
| 90-days mortality for septic shock | 32 (38) | n.a | – |
Bold italics identify statistically significant p-values.
*χ2 for proportion: p value < 0.05 were considered statistically significant; n.a. not available.
Septic patients and control group were similar except for the presence of chronic cardiac failure that was significantly more represented in control population (p = 0.0002), acute kidney injury34 and solid cancer that were more prevalent in study group (p = 0.0062 and p = 0.0002, respectively). Septic shock was diagnosed in 82 out of 209 (39%) patients (Table 1). In 162/209 (77.5%) patients antimicrobial therapy was administered before sepsis diagnosis (Table 1). 30-day mortality was 8% in patients with sepsis, reaching values as high as 33% in case of septic shock, whereas 90-days mortality was 13% in sepsis and 38% in septic shock (Table 1).
Sepsis diagnosis
Sepsis was diagnosed in 157/209 (75.12%) patients by SIRS criteria of 1991, in 165/209 (78.95%) by Second Consensus Conference Criteria of 2001, in 178/209 (85.17%) by modified Second Consensus Conference Criteria of 2001, and in 169/209 (80.86%) by Third Consensus Conference Criteria of 2016 (Fig. 1B).
In 13/209 (6.22%) patients no criteria were fulfilled for sepsis diagnosis despite a blood culture positive for microbiological isolates. In particular, 3/13 (23%) were positive for bacterial endocarditis by Streptococcus sanguinis, Kytococcus schroeteri and Staphylococcus epidermidis; 5/13 (31%) presented urosepsis by Enterococcus faecium, Klebsiella pneumoniae, Staphylococcus aureus and Candida albicans; 1/13 (7.70%) had diagnosis of pneumonia by Enterococcus faecalis; 2/13 (15.40%) had diagnosis of osteomyelitis by Staphylococcus aureus and Staphylococcus hominis; 2/13 (15.4%) had diagnosis of catheter related bloodstream infection (CRBSI) by Raoultella ornithinolytica, Providencia stuartii, Proteus mirabilis and Klebsiella oxytoca. In these patients, 11/13 (84.62%) had positive bacteremia with MR-proADM values above the cut-off of ≥ 1 nmol/L and 3/13 (23%) above the values of > 1.5 nmol/L. PCT values was ≥ 0.5 ng/mL in 4/13 (31%) patients.
In 188/209 (90%) patients SIRS criteria, qSOFA, SOFA scores, or all of them were < 2. Particularly, 41/127 (32.33%) patients with sepsis had < 2 SIRS criteria, 32/125 (25.62%) had a SOFA score < 2, 35/125 (28%) had SIRS criteria and qSOFA score < 2, 30/125 (24%) had SOFA and qSOFA scores < 2, and 16/125 (12.81%) had SIRS criteria, qSOFA and SOFA scores < 2. Among patients with septic shock, 11/82 (13.43%) patients with septic shock had SIRS criteria < 2 and 10/82 (12.25%) SIRS criteria and qSOFA score < 2 (Table 2).
Table 2.
Percentage of septic (S) and septic shock (SS) patients negative for SIRS criteria (SIRS < 2) or negative for SIRS criteria (SIRS < 2) plus qSOFA score (qSOFA < 2) with positivity to other markers.
| S patients | SOFA ≥ 2 | qSOFA ≥ 2 | PCT ≥ 0.5 ng/mL | MR-proADM ≥ 1 nmol/L |
|---|---|---|---|---|
| SIRS < 2 (n = 41) | 22/41 (54%) | 4/41 (10%) | 21/41 (51%) | 32/41 (78%) |
| SIRS < 2 + qSOFA < 2 (n = 35) | 19/35 (54%) | – | 20/35 (57%) | 35,739 (89%) |
| SOFA < 2 (n = 32) | – | 1/32 (3%) | 9/32 (28%) | 17/32 (53%) |
| SOFA < 2 + qSOFA < 2 (n = 30) | – | – | 14/30 (%) | 28/30 (%) |
| SIRS < 2 + SOFA < 2 + qSOFA < 2 (n = 16) | – | – | 7/16 (%) | 13/16 (%) |
| SS patients | SOFA > 2 | qSOFA > 2 | PCT ≥ 0.5 ng/mL | MR-proADM ≥ 1 nmol/L |
|---|---|---|---|---|
| SIRS < 2 (n = 11) | 11/11 (100%) | 3/11 (27%) | 7/11 (64%) | 11/11 (100%) |
| SIRS < 2 + qSOFA < 2 (n = 10) | 8/10 (80%) | – | 6/10 (60%) | 10/10 (100%) |
PCT, MR-proADM, SIRS criteria, qSOFA and SOFA score values in study population
Median values, interquartile ranges (25th percentile and 75th percentile), and Mann–Whitney’s comparison of the different variables are reported in Table 3. In particular, the median number of SIRS criteria registered was two, the median qSOFA score was 1, the median SOFA score was 4, PCT and MR-proADM median levels were 1.16 ng/mL and 2.55 nmol/L, respectively. All variables resulted significantly higher in septic patients than control group (p < 0.0001) (Table 3).
Table 3.
Median values, interquartile ranges (25th percentile and 75th percentile), and Mann–Whitney’s comparison of the different variables registered in the study and control groups.
| Median values (IQR) | Septic patients = 209 | Control group = 50 | p value* |
|---|---|---|---|
| SIRS criteria | 2 (2–3) | 1 (0–1) | < 0.0001 |
| SOFA score | 4 (2–6) | 1 (0–2) | < 0.0001 |
| qSOFA score | 1 (0–2) | 0 (0–0) | < 0.0001 |
| PCT ng/mL | 1.16 (0.31–5.10) | 0.06 (0.05–0.15) | < 0.0001 |
| MR-proADM nmol/L | 2.55 (1.72–4.38) | 1.14 (0.8–1.51) | < 0.0001 |
*χ2 Mann–Whitney’s comparison. p value < 0.05 were considered statistically significant.
ROC curves analysis and areas under the curves (AUCs)
In septic patients, the AUCs values for SIRS criteria, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria, qSOFA and SOFA score are reported in Table 4.
Table 4.
ROC Curves analysis: Areas under the Curves (AUCs) values for SIRS criteria, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria, SOFA score and qSOFA score values in the study population.
| Variables | AUC value | Cut-off | Sens% | Spec% | LR + | p value |
|---|---|---|---|---|---|---|
| SIRS criteria | 0.85 | > 2 | 75.12 | 84.31 | 4.79 | < 0.0001 |
| Second Consensus Conference Criteria | 0.86 | > 4 | < 0.0001 | |||
| Modified Second Consensus Conference Criteria | 0.85 | > 4 | < 0.0001 | |||
| SOFA score | 0.82 | > 2 | 66.51 | 82.35 | 3.77 | < 0.0001 |
| qSOFA score | 0.77 | > 2 | < 0.0001 | |||
| PCT ng/mL | 0.93 | > 0.5 | 67.94 | 98.04 | 34.65 | < 0.0001 |
| MR-proADM nmol/L | 0.85 | > 1.5 | 83.0 | 76.47 | 3.53 | < 0.0001 |
ROC curves comparison between SIRS criteria, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria, qSOFA and SOFA score has been reported in Fig. 2. Any statistically significant difference has been highlighted. Adding PCT and MR-proADM to the ROC curve analysis, PCT AUC was significantly higher (p < 0.05) than all other variables (Fig. 3; Table 4).
Figure 2.

ROC curves comparison between SIRS, Second Consensus Conference, modified Second Consensus Conference Criteria, SOFA and qSOFA score values.
Figure 3.

ROC curves comparison between PCT, MR-proADM, SIRS, Second Consensus Conference, modified Second Consensus Conference Criteria, and SOFA score values.
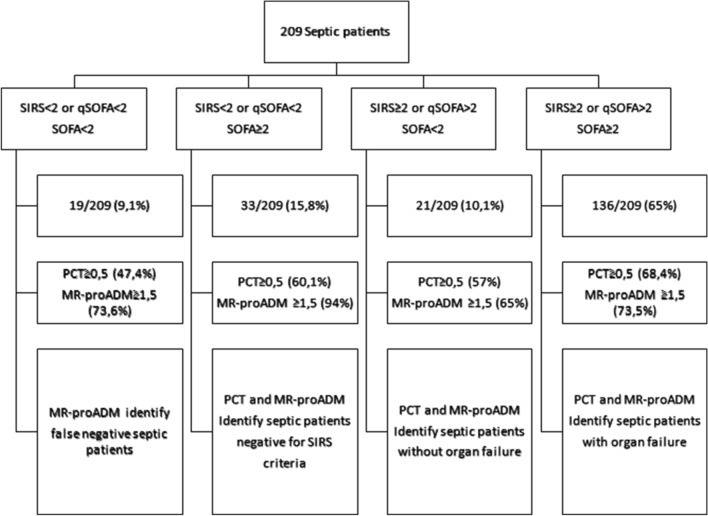
Based on SIRS criteria of 1991, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria, qSOFA and SOFA score ROC curve overlapping, septic patients were stratified using SIRS criteria of 1991 or qSOFA score, easy and rapid to calculate, and SOFA score, the actual diagnostic tool. PCT and MR-proADM biomarkers evaluation in septic patients stratified by SIRS, qSOFA and SOFA score were reported in Fig. 4. In particular, 19/209 (9.11%) patients presented SIRS criteria < 2, qSOFA score < 2 and SOFA score < 2. Among these patients, 9/19 (47.47%) had PCT levels ≥ 0.5 ng/mL, 14/19 (73.63%) MR-proADM > 1.5 nmol/L and 15/19 (78.92%) positive blood culture with documented microbiological isolates. In 33/209 (15.78%) patients the fulfilled SIRS criteria was < 2, qSOFA score < 2 but SOFA score value ≥ 2; within this group, 20/33 (60%) of patients showed PCT ≥ 0.5 ng/mL and 31/33 (94%) MR-proADM > 1.5 nmol/L. In 21/209 (10%) patients SIRS criteria were ≥ 2, qSOFA score ≥ 2 but SOFA score values < 2. Among these, 12/21 (57%) had PCT levels ≥ 0.5 ng/mL and 14/21 (65%) MR-proADM > 1.5 nmol/L. 136/209 (65%) patients had SIRS criteria ≥ 2, qSOFA score ≥ 2 and SOFA score ≥ 2. In these patients, 93/136 (68.46%) had PCT levels ≥ 0.5 ng/mL and 100/136 (73.56%) MR-proADM > 1.5 nmol/L.
Figure 4.
PCT and MR-proADM biomarkers evaluation in septic patients stratified by SIRS, qSOFA and SOFA score.
Globally, in 45/209 (21.56%) septic patients, SIRS criteria and qSOFA score were < 2. In these patients some confounding factors potentially influencing SIRS criteria and qSOFA evaluation were present. In particular, 41/45 (92%) of patients were receiving drugs with negative chronotropic effect such as beta-blockers, calcium antagonists or other antiarrhythmic drugs with impact on cardiac rate. In 6/45 (13.51%) a pacemaker DDD for bradyarrhythmia was present, affecting cardiac rate. In 36/45 (81%) antimicrobial therapy and in 4/45 (8.01%) paracetamol were administered affecting both body temperature or heart rate increase. Regarding influence on respiratory rate, 6/45 (13.52%) were receiving benzodiazepine treatment and 3/45 (6.70%) chronic oxygen therapy.
χ2 test for proportions in patients with sepsis and septic shock in case of negativity for SIRS criteria: SOFA score, qSOFA score, PCT and MR-proADM comparison
Forty-one patients with sepsis and 11 patients with septic shock presented SIRS criteria < 2. In Table 5, the percentage of patients with sepsis and septic shock with SIRS criteria < 2 has been stratified by SOFA score, qSOFA score, PCT and MR-proADM. In case of sepsis, 56% of patients presented SOFA score ≥ 2, 10% qSOFA ≥ 2, 54% PCT ≥ 0.5 ng/mL, and 82% MR-proADM ≥ 1 nmol/L. In septic shock, 100% of patients presented SOFA score ≥ 2, 27% qSOFA ≥ 2, 64% PCT ≥ 0.5 ng/mL, and 100% MR-proADM ≥ 1 nmol/L (Table 2).
Table 5.
χ2 for proportions in patients with sepsis (S) and septic shock (SS) and negative for SIRS criteria (SIRS < 2): SOFA, qSOFA, PCT and MR-proADM comparison.
| Patients with sepsis and SIRS < 2 (n = 41) | SOFA ≥ 2 (56%) | qSOFA ≥ 2 (10%) | PCT ≥ 0.5 ng/mL (54%) | MR-proADM ≥ 1 nmol/L (82%) |
|---|---|---|---|---|
| MR-proADM ≥ 1 nmol/L (82%) | p = 0.0014 | p < 0.0001 | p = 0.0084 | – |
| PCT ≥ 0.5 ng/mL (54%) | p = 0.96 | p < 0.0001 | – | p = 0.0084 |
| SOFA ≥ 2 (56%) | – | p < 0.0001 | p = 0.91 | p = 0.0014 |
| qSOFA ≥ 2 (10%) | p < 0.0001 | – | p < 0.0001 | p < 0.0001 |
| Patients with septic shock and SIRS < 2 (n = 11) | SOFA ≥ 2 (100%) | qSOFA ≥ 2 (27%) | PCT ≥ 0.5 ng/mL (64%) | MR-proADM ≥ 1 nmol/L (100%) |
|---|---|---|---|---|
| MR-proADM ≥ 1 nmol/L (100%) | p = 0.15 | p = 0.001 | p = 0.006 | – |
| PCT ≥ 0.5 ng/mL (54%) | p = 0.09 | p = 0.11 | – | p = 0.006 |
| SOFA ≥ 2 (85%) | – | p = 0.002 | p = 0.09 | p = 0.15 |
| qSOFA ≥ 2 (23%) | p = 0.002 | – | p = 0.11 | p = 0.001 |
Bold identify statistically significant p-values.
χ2 test for proportions analysis showed that in septic patients with SIRS criteria < 2, MR-proADM is significantly superior to SOFA score (p = 0.0014), qSOFA score (p < 0.0001) and PCT (p = 0.0084) (Table 5). SOFA score and PCT are both significantly superior to qSOFA score (p < 0.0001) (Table 5). In septic shock patients, MR-proADM is significantly superior to PCT (p = 0.006) and qSOFA score (p = 0.0001) but it is comparable to SOFA score (p = 0.15) (Table 5). SOFA score is significantly superior to qSOFA score (p = 0.002) (Table 5).
χ2 test for proportions in patients with sepsis and septic shock in case of negativity for SIRS criteria plus qSOFA score: SOFA, PCT and MR-proADM comparison
In 35 patients with sepsis and 10 patients with septic shock SIRS criteria and qSOFA score were < 2. Stratifying septic patients for SOFA score, PCT and MR-proADM values (Table 2), 54% presented SOFA score ≥ 2, 57% PCT ≥ 0.5 ng/mL, and 89% MR-proADM ≥ 1 nmol/L. In case of septic shock, SOFA score was ≥ 2 in 80%, PCT was ≥ 0.5 ng/mL in 60% and MR-proADM was ≥ 1 nmol/L in 100% (Table 2).
χ2 test for proportions analysis showed that in septic patients with SIRS criteria and qSOFA score < 2, MR-pro ADM was superior to SOFA score (p = 0.001) and PCT (p = 0.002) (Table 6). In septic shock, MR-proADM was comparable to SOFA score (p = 0.34) but superior to PCT (p = 0.03) (Table 6).
Table 6.
χ2 for proportions in patients with sepsis and septic shock and negative at SIRS criteria (SIRS < 2) and qSOFA < 2: SOFA, PCT and MR-proADM comparison.
| Patients with sepsis SIRS < 2 + qSOFA < 2 (n = 35) | SOFA ≥ 2 (54%) | PCT ≥ 0.5 ng/mL (57%) | MR-proADM ≥ 1 nmol/L (89%) |
|---|---|---|---|
| SOFA ≥ 2 (54%) | – | p = 0.80 | p = 0.001 |
| PCT ≥ 0.5 ng/mL (57%) | p = 0.80 | – | p = 0.002 |
| MR-proADM ≥ 1 nmol/L (89%) | p = 0.001 | p = 0.002 | – |
| Patients with septic shock SIRS < 2 + qSOFA < 2 (n = 10) | SOFA > 2 (80%) | PCT ≥ 0.5 ng/mL(60%) | MR-proADM ≥ 1 nmol/L (100%) |
|---|---|---|---|
| SOFA ≥ 2 (80%) | – | p = 0.34 | p = 0.14 |
| PCT ≥ 0.5 ng/mL (60%) | p = 0.40 | – | p = 0.03 |
| MR-proADM ≥ 1 nmol/L (100%) | p = 0.34 | p = 0.03 | – |
Bold identify statistically significant p-values.
Combined PCT, MR-proADM, SIRS criteria, qSOFA and SOFA scores measurement in sepsis diagnosis: the post-test probability
Post-test probability analysis was performed to define the diagnostic value derived from the use of the single clinical score or criteria or of the single biomarker as well as from the combination of all the clinical parameters and laboratory markers. The results of the post-test probability are reported in Table 7. The association between PCT measurement and SIRS criteria or PCT and qSOFA score reached a diagnostic accuracy of 99.9%.
Table 7.
Post-test probability analysis used to define the diagnostic value derived from the combined use of PCT, MR-proADM, SOFA score and SIRS criteria in patients with sepsis or septic shock.
| Diagnostic test | LR + | Post-test probability |
|---|---|---|
| PCT | 34.65 | 0.990 |
| SIRS criteria | 9.79 | 0.970 |
| qSOFA score | 5.93 | 0.960 |
| MR-proADM | 3.48 | 0.940 |
| SOFA score | 3.77 | 0.940 |
| Test combination | Post-test probability | |
|---|---|---|
| SIRS criteria + PCT | 0.999 | |
| qSOFA + PCT | 0.999 | |
| PCT + MR-proADM | 0.997 | |
| SOFA score + PCT | 0.997 | |
| SOFA score + PCT + MR-proADM | 0.999 | |
| SIRS criteria + PCT + MR-proADM | 0.999 | |
| qSOFA score + PCT + MR-proADM | 0.999 |
The combination of PCT, SIRS or qSOFA and MR-proADM provide a diagnostic and prognostic evaluation in 99.9% of patients with a turnaround time of about 45 min, whereas the combination of PCT, SOFA score and MR-proADM reaching comparable accuracy (99.9%) requires a turnaround time of about 90 min.
Discussion
The physiopathology of sepsis highlights the need of unambiguous diagnostic criteria for a rapid patients identification and adequate therapy administration, within one hour from symptoms presentation35. Sepsis definition and diagnostic criteria proposed from 1991 until now still lack of specificity36–39.
Confounding factors influencing body temperature, heart and respiratory rates and white blood cell count included in SIRS comprehended beta-blockers, calcium-antagonists and other antiarrhythmic drugs or pace-maker DDD (heart rate), paracetamol, anti-inflammatory drugs and antimicrobials (body temperature); benzodiazepine, sedative and chronic oxygen administration (respiratory rate); immunosuppressive drugs and antimicrobials (white blood cell count). The presence of these factors could have a significant impact on clinical criteria positivity40.
Ideally, the best criteria should be as rapid as practically reliable for an early diagnosis and treatment of sepsis. In this prospective study, sepsis was diagnosed according to SIRS Criteria of 1991, Second Consensus Conference Criteria, modified Second Consensus Conference Criteria, Third Consensus Conference Criteria, in comparison with PCT and MR-proADM measurement. ROC curve analysis used to evaluate the diagnostic accuracy of the different criteria showed complete overlapping of the curves. On this basis, it should be convenient to prefer using bedside SIRS criteria or qSOFA in non-ICU setting rather than SOFA score requiring laboratory screening, Glasgow coma scale determination and knowledge of patients’ comorbidities or previous organ failures. Second Consensus Criteria of 2001 require the measurement of multiple clinical as well as bioumoral parameters needing long determination time. In this study, SIRS criteria and qSOFA allowed a diagnosis in 97% and 96% of patients, respectively, in case of suspicion of sepsis outside ICU. In the last years, plasma biomarkers have been proposed as tools for a rapid diagnosis and good indicator of prognosis. Among these, PCT and MR-proADM showed the best diagnostic and prognostic accuracy for the complementary nature of given information. PCT was optimal for etiological diagnosis and antimicrobial therapy management12, whereas MR-proADM was significantly correlated with organ failure and worse prognosis. In the present study, ROC analysis showed that besides clinical scores, PCT measurement represent the best diagnostic accuracy in sepsis, as previously described12,21–24,41,42 allowing early tailored antimicrobial therapy administration and daily follow-up. It should be reliable to combine bedside SIRS criteria or qSOFA with PCT laboratory determination for early identification of sepsis, followed by SOFA score calculation for severity and prognosis evaluation. In this study, about 35% of patients were negative for SIRS criteria or qSOFA, and SOFA score or for all, despite evidence of positive blood culture and documented microbiological isolate or clinical diagnosis of infection. In these patients, the use of MR-proADM was essential to provide early diagnosis and confirm the suspicion of sepsis.
These results suggest that in case of suspected sepsis, SIRS criteria or qSOFA should be bedside evaluated together with PCT measurement. These combinations reach a post-test probability of 99.9%. Besides PCT and MR-proADM, a marker of organ failure, even if comparable to SOFA score in sepsis severity prediction, showed the ability to anticipate SOFA and qSOFA score and the advantage to be more objective and fasten measured, as previously described outside ICU24. Exactly, in case of clinical suspicious of infection the presence of SIRS criteria ≥ 2, qSOFA ≥ 2, PCT ≥ 0.5 and MR-proADM ≥ 1.5 nmol/L identifies sepsis in 99.9% of cases. This approach, reliable in about 45 min, could allow an early diagnosis of sepsis within the first hour, even outside the intensive care contest to reduce the need for ICU transfer and mortality, as previously reported24,43.
Data from the prospective study highlighted comparable diagnostic accuracy between SIRS criteria from the First Consensus Conference of 1991, Criteria from the Second Consensus Conference of 2001 and from the Third Consensus Conference of 2016. Moreover, the use of the modified Second Consensus Conference Criteria of 2001, based on SIRS criteria plus SOFA score for sepsis diagnosis, did not improve diagnostic accuracy more than PCT and MR-proADM. In this study, SIRS criteria allowed a diagnosis in 97% of patient and, when combined with PCT measurement, identified 99.9% of septic patients. Moreover, MR-proADM values > 1 nmol/L showed the ability to identify septic patients when SIRS, SOFA and PCT were still negative. These results confirm those reported by other authors where MR-proADM anticipates by 24 h the organ failure development44.
Through SIRS criteria, qSOFA, PCT and MR-proADM determination, sepsis diagnosis can be achieved within the first hour from suspicion as recommended35 to improve outcome and decrease mortality. Furthermore, MR-proADM ≥ 1 nmol/L, even in case of negative PCT, qSOFA, SOFA, absence of Second Consensus Conference Criteria, identified septic patients with positive blood culture.
In conclusion, data from this study could suggest a diagnostic protocol for sepsis management outside ICU setting including, within 30 min from sepsis suspicion, bedside SIRS criteria or qSOFA score evaluation; within 1 h, PCT and MR-proADM measurement, microbiological culture collection, empiric sepsis therapy set up and SOFA score calculation. From these prompt actions, rapid diagnostic and prognostic evaluation of sepsis could be achieved also in case of negative SIRS, qSOFA or SOFA score with high post-test probability to reduce mortality and improve outcome.
Informed consent
Written consent for publication was obtained from the patient before submission of this article.
Abbreviations
- APACHE
Acute Physiology and Chronic Health
- AUCs
Areas under the curve
- CBC
Complete blood counts
- CRP
C-reactive protein
- ICU
Intensive care unit
- IL-6
Interleukin 6
- MR-proADM
Mid-Regional pro-Adrenomedullin
- PCR
Polymerase chain reaction
- PCT
Procalcitonin
- PPV
Positive predictive value
- qSOFA
Quick
- ROC
Receiver operating characteristic
- SIRS
Systemic inflammatory response syndrome
- SOFA
Sequential sepsis-related organ failure assessment
- TRACE
Time-resolved amplified cryptate emission
- WBC
White blood cell
Author contributions
S.S. led the study design, data collection, data analysis, data interpretation, and manuscript writing; E.N. and E.P.R.C. assisted with data collection and analysis of the validation dataset; L.D.F. performed microbiology specimens analysis; M.F. and E.V. assisted with computer queries, data analysis and manuscript preparation; D.C. and D.B. assisted with data collection and analysis of the validation dataset; M.C. assisted with data collection and analysis of the development dataset as well as study design, data interpretation and manuscript writing; S.C. and S.A. assisted with chart review and data analysis and supervised all aspects of the investigation, as well as assisting with study design, data interpretation and manuscript writing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fleischmann C, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Churpek MM, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am. J. Respir. Crit. Care Med. 2017;195:906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 5.Kopczynska M, et al. Red-flag sepsis and SOFA identifies different patient population at risk of sepsis-related deaths on the general ward. Medicine. 2018;97:e13238. doi: 10.1097/MD.0000000000013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprung CL, Schein RMH, Balk RA. The new sepsis consensus definitions: the good, the bad and the ugly. Intensive Care Med. 2016;42:2024–2026. doi: 10.1007/s00134-016-4604-0. [DOI] [PubMed] [Google Scholar]
- 8.Horeczko T, Green JP, Panacek EA. Epidemiology of the systemic inflammatory response syndrome (SIRS) in the emergency department. West J. Emerg. Med. 2014;15:329–336. doi: 10.5811/westjem.2013.9.18064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 10.Bates DW, et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J. Infect. Dis. 1997;176:1538–1551. doi: 10.1086/514153. [DOI] [PubMed] [Google Scholar]
- 11.Sridharan P, Chamberlain RS. The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg. Infect. 2013;14:489–511. doi: 10.1089/sur.2012.028. [DOI] [PubMed] [Google Scholar]
- 12.Spoto S, et al. Procalcitonin and MR-proAdrenomedullin combination in the etiological diagnosis and prognosis of sepsis and septic shock. Microb. Pathog. 2019;137:103763. doi: 10.1016/j.micpath.2019.103763. [DOI] [PubMed] [Google Scholar]
- 13.Briel M, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 2008;168:2000–2007. doi: 10.1001/archinte.168.18.2000. [DOI] [PubMed] [Google Scholar]
- 14.Stolz D, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 15.Larsen FF, Petersen JA. Novel biomarkers for sepsis: a narrative review. Eur. J. Intern. Med. 2017;45:46–50. doi: 10.1016/j.ejim.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Thomas-Rüddel DO, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit. Care. 2018;22:128. doi: 10.1186/s13054-018-2050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leli C, et al. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis. Markers. 2015;2015:701480. doi: 10.1155/2015/701480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angeletti S, et al. Procalcitonin and mid-regional pro-Adrenomedullin test combination in sepsis diagnosis. Clin. Chem. Lab. Med. 2013;51:1059–1067. doi: 10.1515/cclm-2012-0595. [DOI] [PubMed] [Google Scholar]
- 19.Arai T, et al. Procalcitonin levels predict to identify bacterial strains in blood cultures of septic patients. Am. J. Emerg. Med. 2016;34:2150–2153. doi: 10.1016/j.ajem.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Li S, et al. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J. Res. Med. Sci. 2016;21:39. doi: 10.4103/1735-1995.183996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angeletti S, et al. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS. 2015;123:740–748. doi: 10.1111/apm.12406. [DOI] [PubMed] [Google Scholar]
- 22.Angeletti S, et al. Procalcitonin, MR-Proadrenomedullin, and cytokines measurement in sepsis diagnosis: advantages from test combination. Dis. Markers. 2015;2015:951532. doi: 10.1155/2015/951532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeletti S, et al. Procalcitonin and MR-proAdrenomedullin combined score in the diagnosis and prognosis of systemic and localized bacterial infections. J. Infect. 2016;72:395–398. doi: 10.1016/j.jinf.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Spoto S, et al. Procalcitonin and MR-Proadrenomedullin combination with SOFA and qSOFA scores for sepsis diagnosis and prognosis: a diagnostic algorithm. Shock. 2018;50:44–52. doi: 10.1097/SHK.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 25.Önal U, Valenzuela-Sánchez F, Eshwara Vandana K, Rello J. Mid-regional pro-adrenomedullin (MR-proADM) as a biomarker for sepsis and septic shock: narrative review. Healthcare (Basel). 2018;6:110. doi: 10.3390/healthcare6030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christ-Crain M, et al. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit. Care. 2005;9:816–824. doi: 10.1186/cc3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, et al. Circulating biologically active adrenomedullin predicts organ failure and mortality in sepsis. Ann. Lab. Med. 2019;39:454–463. doi: 10.3343/alm.2019.39.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenzuela-Sánchez F, Valenzuela-Méndez B, Rodríguez-Gutiérrez JF, Estella-García Á, González-García MÁ. New role of biomarkers: mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016;4:329. doi: 10.21037/atm.2016.08.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour CW, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling SA, et al. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit. Care Med. 2015;43:1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zasowski EJ, Bassetti M, Blasi F, Goossens H, Rello J, Sotgiu G, Tavoschi L, Arber MR, McCool R, Patterson JV, Longshaw CM, Lopes S, Manissero M, Nguyen ST, Tone K, Aliberti S. A systematic review of the effect of delayed appropriate antibiotic treatment on the outcomes of patients with severe bacterial infections. Chest. 2020;S0012–3692:31497–31505. doi: 10.1016/j.chest.2020.03.087. [DOI] [PubMed] [Google Scholar]
- 32.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin. Biochem. Rev. 2008;29:S83–87. [PMC free article] [PubMed] [Google Scholar]
- 33.Albert A. On the use and computation of likelihood ratios in clinical chemistry. Clin. Chem. 1982;28:1113–1119. doi: 10.1093/clinchem/28.5.1113. [DOI] [PubMed] [Google Scholar]
- 34.Howell MD, Davis AM. Management of sepsis and septic shock. JAMA. 2017;317:847–848. doi: 10.1001/jama.2017.0131. [DOI] [PubMed] [Google Scholar]
- 35.Angeletti S, et al. Role of neutrophil gelatinase-associated lipocalin in the diagnosis and early treatment of acute kidney injury in a case series of patients with acute decompensated heart failure: a case series. Cardiol. Res. Pract. 2016;2016:3708210. doi: 10.1155/2016/3708210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortés-Puch I, Hartog CS. Opening the debate on the new sepsis definition change is not necessarily progress: revision of the sepsis definition should be based on new scientific insights. Am. J. Respir. Crit. Care Med. 2016;194:16–18. doi: 10.1164/rccm.201604-0734ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aublanc M, Richard JC. Assessment of clinical criteria for sepsis-was the cart put before the horse? J. Thorac. Dis. 2016;8:E816–818. doi: 10.21037/jtd.2016.07.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent JL, Martin GS, Levy MM. qSOFA does not replace SIRS in the definition of sepsis. Crit. Care. 2016;20:210. doi: 10.1186/s13054-016-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moskowitz A, Andersen LW, Cocchi M, Donnino MW. The misapplication of severity-of-illness scores toward clinical decision making. Am. J. Respir. Crit. Care Med. 2016;194:256–258. doi: 10.1164/rccm.201605-1005ED. [DOI] [PubMed] [Google Scholar]
- 40.Filbin MR, et al. Challenges and opportunities for emergency department sepsis screening at triage. Sci. Rep. 2018;8:11059. doi: 10.1038/s41598-018-29427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spoto S, et al. The role of procalcitonin in the diagnosis of bacterial infection after major abdominal surgery: advantage from daily measurement. Medicine (Baltimore). 2018;97:e9496. doi: 10.1097/MD.0000000000009496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincenzi B, et al. Procalcitonin as diagnostic marker of infection in solid tumors patients with fever. Sci. Rep. 2016;6:28090. doi: 10.1038/srep28090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spoto S, et al. An algorithm of good clinical practice to reduce intra-hospital and 90-days mortality and need for ICU transfer: a new approach for septic patient management. Ital. J. Med. 2020;14:14–21. doi: 10.4081/itjm.2020.1215. [DOI] [Google Scholar]
- 44.Viaggi B, et al. Mid regional pro-adrenomedullin for the prediction of organ failure in infection. results from a single centre study. PLoS ONE. 2018;13:e0201491. doi: 10.1371/journal.pone.0201491. [DOI] [PMC free article] [PubMed] [Google Scholar]