Abstract
Repaglinide, an oral hypoglycemic agent, is a short-acting insulin secretagogue. We describe a case, in which an extremely low dose of repaglinide caused severe hypoglycemia and novel drug interactions are suggested. A 71-year-old man with type 2 diabetes was taken to the hospital due to consciousness disorder caused by severe hypoglycemia. He was taking repaglinide 0.25 mg once in the morning with nilotinib 400 mg/day and febuxostat 20 mg/day. Endogenous insulin secretion was not suppressed even in hypoglycemia. Detection of plasma repaglinide 10 h after administration in this case indicates delayed elimination of the agent, which might be derived from reduced hepatocyte uptake due to inhibitory effects of nilotinib on OATP1B1 and reduced oxidation of the agents by inhibitory effects of nilotinib, mainly on CYP3A4 activities, and of febuxostat on CYP2C8 activities. Repaglinide is eliminated by the liver, and is a short-acting insulin secretagogue with a good safety profile in patients with type 2 diabetes complicated by renal impairment, including elderly patients; however, its delayed elimination due to drug–drug interactions should be noted.
Keywords: Repaglinide, Nilotinib, Febuxostat, Drug–drug interaction, Hypoglycemia
Introduction
Repaglinide, an oral hypoglycemic agent, is a short-acting insulin secretagogue used to reduce postprandial glucose levels in patients with type 2 diabetes. Since repaglinide and its inactive metabolites are eliminated via the biliary-fecal route, it has a favorable safety profile with a low risk of hypoglycemia in patients with impaired renal function. Repaglinide is extensively metabolized by the hepatic cytochrome P450 (CYP) enzyme system mainly by CYP2C8 and CYP3A4 [1], and agents that inhibit these enzymes raise the plasma concentration of repaglinide [2–4]. Therefore, identification of agents which interact with CYP2C8 and CYP3A4 is important to enable the safe use of repaglinide. A recent nested case–control study suggests that drug interaction between clopidogrel and repaglinide is clinically relevant and could increase the risk for hypoglycemia [5] and that glucuronidation converts clopidogrel to an inhibitor of CYP2C8 [6]. In addition, cases have been reported in which repaglinide caused severe hypoglycemia due to combined use of brotizolam [7], amiodarone [8], and trimethoprim/sulfamethoxazole [9]. In these reports, repaglinide was used at doses from 3 to 10 mg/day, which are higher than the licensed ordinary dose in Japan of 1.5 mg/day. We describe a case, in which an extremely low dose of repaglinide caused severe hypoglycemia and novel drug interactions are suggested.
Case report
A 71-year-old man with type 2 diabetes was taken to the hospital due to consciousness disorder due to severe hypoglycemia.
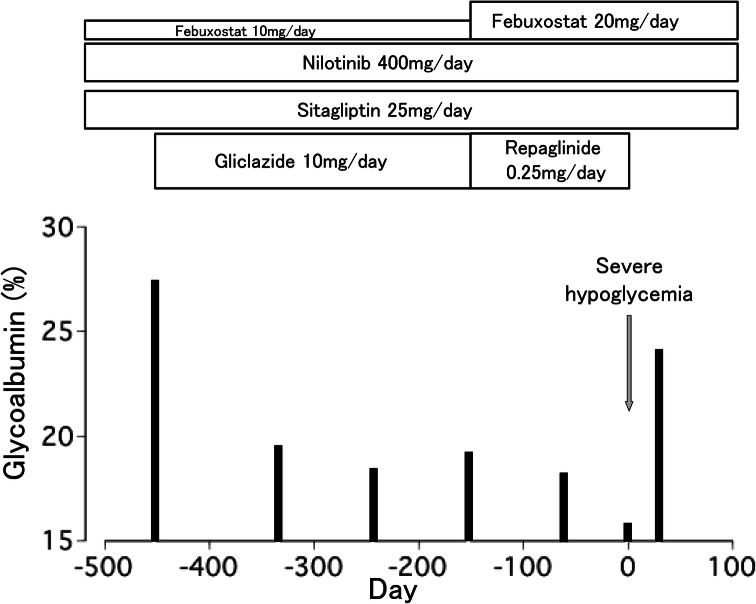
The patient was diagnosed with chronic myelocytic leukemia (CML) 11 years ago. He was taking nilotinib 400 mg/day to treat CML and febuxostat to prevent hyperuricemia. Thirty months prior to admission, his casual plasma glucose level was 267 mg/dL and his HbA1c was 9.3%. His body weight and body mass index were 65.6 kg and 23.2 kg/m2, respectively. He was diagnosed with type 2 diabetes and was taking sitagliptin 25 mg/day. He had intact cognitive function and no impairment of ADL. Glycoalbumin (GA) was used as his glycemic control indicator, since his HbA1c values were apparently low compared to glycemic levels, likely due to alteration in hemoglobin metabolism in CML and related therapy. Fourteen months ago, gliclazide 10 mg/day was added, as his GA had increased to 27.3% (Fig. 1). The agent was effective and his GA was improved to 18.3%. Five months prior to admission, gliclazide was replaced with repaglinide 0.25 mg once in the morning, as his estimated glomerular filtration rate was decreased to 37.2 mL/min/1.73m2, leading to an increased risk of hypoglycemia. In addition, febuxostat was increased from 10 mg/day to 20 mg/day because his hyperuricemia had worsened.
Fig. 1.
Clinical course of the patient
On the day of the attack, he had breakfast and took 0.25 mg repaglinide at 7:00 am and had lunch at noon as usual according to the information provided by his family living together. At 4:30 pm, he became disturbed and could not speak clearly, and he was taken to hospital by ambulance. His vital signs and neurological signs were normal except consciousness disorder. His arterial blood gas including PaO2, PaCO2, HCO3−, and pH was normal. His renal function had recovered to the level it had been 8 months prior and apparent liver damage and electrolyte disorder was not observed (Table 1). He was diagnosed with severe hypoglycemia as his plasma glucose was 29 mg/dL and intravenous administration of glucose improved consciousness. Glucose was continuously infused intravenously for 12 h and he recovered completely.
Table 1.
Results of the blood laboratory test carried out immediately after emergency transportation
| Laboratory test | Value |
|---|---|
| Alanine aminotransferase (U/L) | 13 |
| Aspartate aminotransferase (U/L) | 33 |
| Albumin (g/dL) | 3.5 |
| Creatinine (mg/dL) | 1.11 |
| eGFR (mL/min/1.73m2) | 50.9 |
| Uric acid (mg/dL) | 3.8 |
| Na (mmol/L) | 140 |
| K (mmol/L) | 3.9 |
| Hb (g/dL) | 9.7 |
| Platelet (104/μL) | 16.8 |
| White blood cell (103/μL) | 7.3 |
| Glucose (mg/dL)* | 29 |
| IRI (μU/mL)* | 17.5 |
| CPR (pg/mL)* | 4.80 |
| Cortisol (μg/dL)* | 12.8 |
| Repaglinide (ng/mL)* | 1.2 |
eGFR estimated glomerular filtration rate, IRI immunoreactive insulin, CPR C-peptide immunoreactivity
*Items measured from the identical plasma sample
We concluded that severe hypoglycemia was caused by repaglinide because endogenous insulin secretion was not suppressed, even in hypoglycemia, apparent adrenal insufficiency was not diagnosed because of no hyponatremia and a normal cortisol level and repaglinide was detected by LC/MS/MS in plasma sample about 10 h after administration of the agent (Table 1).
He has not experienced hypoglycemia since repaglinide was stopped. Thirty days after the attack, his GA was elevated to 24.0% (Fig. 1).
Discussion
Increased attention has been placed on avoiding treatment-related hypoglycemia in the medical management of diabetes. Especially in elderly patients, frequent hypoglycemia increases the risk of falls and consequently decreases quality of life in these patients [10], and severe hypoglycemia increases the risk of dementia, cardiovascular disease, and death from any cause [11–13]. Moreover, because of polypharmacy in the elderly and age-related changes in pharmacokinetics and pharmacodynamics, mainly due to decline in renal function, adverse effects of drugs and drug–drug interactions are exacerbated in elderly patients.
Since repaglinide, a short-acting insulin secretagogue, is mainly metabolized in the liver and its metabolites, which are eliminated via the biliary–fecal route [14], are inactive, repaglinide has a good safety and efficacy profile in patients with type 2 diabetes complicated by renal impairment [15]. The half-life of the agent at doses of 0.5–4 mg is 1.0–1.4 h in patients with type 2 diabetes [16] and that at doses of 0.25–1.0 mg is 0.8–1.1 h in healthy Japanese individuals [17]. The half-life of the agent at a dose 1 mg in Japanese patients with type 2 diabetes was not significantly different from that in healthy individuals [17]. In this case, detection of plasma repaglinide 10 h after administration indicates delayed elimination of the agent, as plasma concentration of repaglinide at a dose 0.25 mg is not detectable 5 h after administration in healthy individuals [17].
Repaglinide, a liver-eliminated drug, is taken up to hepatocytes from blood via organic anion transporting polypeptide 1B1 (OATP1B1), the expression of which is exclusive to the basolateral membrane of hepatocytes [18]. Several stains are OATP1B1 substrates and cyclosporine is an inhibitor of the transporter [19]. Cyclosporine raises the plasma concentration of repaglinide by inhibiting its OATP1B1-mediated hepatic uptake [19]. Irbesartan, which also has inhibitory effects on the transporter, raises the plasma concentration of repaglinide [20].
Gemfibrozil, a CYP2C8 inhibitor, raised the area under the plasma concentration–time curve (AUC) of repaglinide 8.1-fold and prolonged its half-life from 1.3 to 3.7 h in healthy individuals. Itraconazole, a CYP3A4 inhibitor, raised repaglinide AUC 1.4-fold, and the gemfibrozil-itraconazole combination raised the AUC 19.4-fold and prolonged the half-life of repaglinide to 6.1 h [2]. These results indicate that both CYP2C8 and CYP3A4 participate in the elimination of repaglinide in vivo and display additive inhibitory effects. Clopidogrel [5] and trimethoprim/sulfamethoxazole [9] which possess CYP2C8-inhibitory effects, and brotizolam [7] and amiodarone [8], which possess CYP3A4-inhibitory effects, have been reported to cause severe hypoglycemia during administration of repaglinide.
In this case, a very small dose 0.25 mg repaglinide unexpectedly caused severe hypoglycemia due to delayed elimination, which may be due to drug–drug interactions between repaglinide and nilotinib and between repaglinide and febuxostat. Nilotinib, a tyrosine-kinase inhibitor indicated for the treatment of CML, is primarily metabolized via CYP3A4-mediated oxidation and to a minor extent by CYP2C8 [21]. Nilotinib increases plasma concentration of orally administrated midazolam, a substrate of CYP3A4 [21], and selectively inhibits CYP2C8 activities in human liver microsomes [22]. Nilotinib also inhibits uptake of OATP1B1 substrates [23, 24]. Febuxostat, an inhibitor of xanthine oxidase, is extensively metabolized by conjugation via uridine diphosphate glucuronosyltransferase (UGT) enzymes and by oxidation via CYP enzymes, including CYP2C8 [25]. Febuxostat inhibits CYP2C8 activities [26], but does not inhibit CYP3A4 activities [27]. Taken together, delayed elimination of repaglinide in this case may be derived from reduced uptake to hepatocytes due to the inhibitory effects of nilotinib on OATP1B1 and reduced oxidation of the agents due to the inhibitory effects of nilotinib, mainly on CYP3A4 activities, and of febuxostat on CYP2C8 activities.
In cases of severe repaglinide-induced hypoglycemia due to drug-drug interactions including Japanese cases, administered doses of the agent have been reported to range from 0.75 mg to 10 mg/day [7–9, 28, 29], far larger than the dose taken in this case. Severe hypoglycemia induced by a very small dose of repaglinide could be due to multiple mechanisms leading to reduced elimination of the agent.
In this case, it took approximately 150 days for the hypoglycemic attack to appear after the dose of febuxostat was increased. In speculation, repaglinide, a lipophilic agent, may slowly accumulate in lipophilic tissues, including adipose, which could form a drug reservoir, contributes to the long residence time of the drug in the plasma, and affects trough concentrations. Since the administrated dose of repaglinide was small, it may take a prolonged period of time for the trough concentration of the agent to reach the concentration which causes insufficient suppression of endogenous insulin secretion during hypoglycemia.
In conclusion, this is the first case of repaglinide-induced severe hypoglycemia with concurrent use of nilotinib and febuxostat. In addition, this is the smallest dose of the agent reported in the literatures to cause severe hypoglycemia. Repaglinide, a liver-eliminated and short-acting insulin secretagogue, has a good safety profile in patients with type 2 diabetes complicated by renal impairment, including elderly patients. However, delayed elimination due to drug–drug interactions should be noted. Consequently, further studies regarding such interactions are necessary to allow safer use of the agents.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This article does not contain any studies with human or animal subjects performed by the any of the authors. The identity of the patient has been protected. The patient provided informed consent for this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/23/2020
In the original publication, the unit of C-peptide immunoreactivity.
References
- 1.Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305–314. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–351. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 3.Niemi M, Kajosaari LI, Neuvonen M, Backman JT, Neuvonen PJ. The CYP2C8 inhibitor trimethoprim increases the plasma concentrations of repaglinide in healthy subjects. Br J Clin Pharmacol. 2004;57:441–447. doi: 10.1046/j.1365-2125.2003.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58–65. doi: 10.1067/mcp.2001.116511. [DOI] [PubMed] [Google Scholar]
- 5.Wei Y, Lin FJ, Lin SY, Wang CC. Risk of hypoglycemia and concomitant use of repaglinide and clopidogrel: a population-based nested case-control study. Clin Pharmacol Ther. 2019;106:1346–1352. doi: 10.1002/cpt.1556. [DOI] [PubMed] [Google Scholar]
- 6.Tornio A, Filppula AM, Kailari O, et al. Glucuronidation converts clopidogrel to a strong time-dependent inhibitor of CYP2C8: A phase II metabolite as a perpetrator of drug-drug interactions. Clin Pharmacol Ther. 2014;96:498–507. doi: 10.1038/clpt.2014.141. [DOI] [PubMed] [Google Scholar]
- 7.Khamaisi M. Severe hypoglycaemia from repaglinide–brotizolam drug interaction: a case report and literature review. Diabet Med. 2012;29:1214–1215. doi: 10.1111/j.1464-5491.2012.03631.x. [DOI] [PubMed] [Google Scholar]
- 8.Mennecart M, Mondon K, Malherbe C, Constans T. Delayed hypoglycemia induced by repaglinide in a frail elderly adult with diabetes mellitus. J Am Geriatr Soc. 2014;62:2460–2462. doi: 10.1111/jgs.13144. [DOI] [PubMed] [Google Scholar]
- 9.Roustit M, Blondel E, Villier C, Fonrose X, Mallaret MP. Symptomatic hypoglycemia associated with trimethoprim/sulfamethoxazole and repaglinide in a diabetic patient. Ann Pharmacother. 2010;44:764–767. doi: 10.1345/aph.1M597. [DOI] [PubMed] [Google Scholar]
- 10.Chiba Y, Kimbara Y, Kodera R, et al. Risk factors associated with falls in elderly patients with type 2 diabetes. J Diabetes Complicat. 2015;29:898–902. doi: 10.1016/j.jdiacomp.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 13.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed]
- 14.van Heiningen PN, Hatorp V, Kramer Nielsen K, et al. Absorption, metabolism and excretion of a single oral dose of 14C-repaglinide during repaglinide multiple dosing. Eur J Clin Pharmacol. 1999;55:521–525. doi: 10.1007/s002280050667. [DOI] [PubMed] [Google Scholar]
- 15.Hasslacher C, Multinational Repaglinide Renal Study Group Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care. 2003;26:886–891. doi: 10.2337/diacare.26.3.886. [DOI] [PubMed] [Google Scholar]
- 16.Novo Nordisk Inc.; PRANDIN® (repaglinide) prescribing information. Princeton, NJ; 2008.
- 17.Sumitomo Dainippon Pharma; Package inserts of prescription drugs of SUREPOST® (repaglinide) (in Japanese). Osaka, Japan; 2016.
- 18.Niemi M, Backman JT, Kajosaari LI, et al. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468–478. doi: 10.1016/j.clpt.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005;78:388–399. doi: 10.1016/j.clpt.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Pei Q, Liu JY, Yin JY, et al. Repaglinide-irbesartan drug interaction: effects of SLCO1B1 polymorphism on repaglinide pharmacokinetics and pharmacodynamics in Chinese population. Eur J Clin Pharmacol. 2018;74:1021–1028. doi: 10.1007/s00228-018-2477-6. [DOI] [PubMed] [Google Scholar]
- 21.Novartis Pharmaceuticals Corporation; TASIGNA® (Nilotinib) prescribing information. East Hanover, NJ; 2019.
- 22.Kim MJ, Lee JW, Oh KS, et al. The tyrosine kinase inhibitor nilotinib selectively inhibits CYP2C8 activities in human liver microsomes. Drug Metab Pharmacokinet. 2013;28:462–467. doi: 10.2133/dmpk.DMPK-13-RG-019. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Mathijssen RHJ, de Bruijn P, Baker SD, Sparreboom A. Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro–in vivo correlations. Br J Cancer. 2014;110:894–898. doi: 10.1038/bjc.2013.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koide H, Tsujimoto M, Takeuchi A, et al. Substrate-dependent effects of molecular-targeted anticancer agents on activity of organic anion transporting polypeptide 1B1. Xenobiotica. 2018;48:1059–1071. doi: 10.1080/00498254.2017.1393582. [DOI] [PubMed] [Google Scholar]
- 25.Takeda Canada Inc. ULORIC® (Febuxostat) prescribing information. Deerfield, IL; 2019.
- 26.Teijin Pharma. Package inserts of prescription drugs of Febric® (febuxostat). Tokyo, Japan; 2019 (in Japanese).
- 27.Mukoyoshi M, Nishimura S, Hoshide S, et al. In vitro drug-drug interaction studies with febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibition. Xenobiotica. 2008;38:496–510. doi: 10.1080/00498250801956350. [DOI] [PubMed] [Google Scholar]
- 28.Imai K, Ichiman Y, Matsumiya T, Tsujii T, Tada S. Case report of hypoglycemia caused by the combination of repaglinide and clopidogrel. Jpn J Pharm Diabetes. 2017;6:112–6. [Google Scholar]
- 29.Ishii T, Wakasugi M, Nagamura T, et al. A case of prolonged severe hypoglycemia induced by the combined use of repaglinide and clopidogrel. J Japan Diab Soc. 2017;60:461–465. [Google Scholar]