Abstract
Multimodality therapies have improved the survival after tumors like Ewing’s sarcoma and breast cancer. However, cardiotoxicity following chemotherapy remains an important concern. We report a case series of four patients who presented to our heart team with severe dilated cardiomyopathy along with biventricular involvement. Two of the patients were females and had breast cancer for which they were treated with trastuzumab and had developed chemotherapy induced cardiomyopathy (CCMP). The other two patients were males who had Ewing’s sarcoma who developed CCMP following treatment with doxorubicin.
Keywords: Dilated cardiomyopathy, Cardiac transplantation, Doxorubicin, Trastuzumab
Introduction
With the dawn of newer cancer chemotherapeutic agents, survival of cancer patients has improved significantly. The increased survival comes at the cost of increased acute and late side effects of the cancer therapy, the cardiac side effects of which is increasingly being recognized—giving rise to a new branch of “Cardio-Oncology” [1]. A significant number of anti-cancer drugs are known to cause cardiotoxicity particularly anthracyclines, trastuzumab, tyrosine kinase inhibitors, and fluoropyrimidines. Among these anti-cancer agents which are responsible for cardiotoxicity, anthracyclines have been particularly documented to cause end-stage heart failure [1]. The prevalence of end-stage heart failure due to chemotherapy-induced cardiomyopathy (CCMP) is between 3 and 8% causing [2] significant morbidity and mortality [3, 4]. Defining features of CCMP includes development of symptoms of heart failure (HF), overt ventricular dysfunction, and a reduction in ejection fraction (EF) (a reduction in EF of > 15% from baseline or > 10% reduction in EF at any point of time if the EF < 50%) on cardiac imaging. These features are found to be present in 5–65% of CCMP patients. Here we report a case series of four clinical vignettes of CCMP with advanced heart failure who received cardiac transplantation. In our study, the presence of active malignancy was diligently excluded using positron emission tomography/computed tomography scan (PET/CT scan) and biopsy of any suspicion area preoperatively in all patients [5, 6]. The decision to transplant was taken by the heart transplant and oncology team. All patients received standard preoperative evaluation including microbiological culture tests and serological tests for hepatitis B virus, hepatitis C virus, cytomegalovirus, and human immunodeficiency virus (HIV). The immunosuppression protocol intraoperatively, postoperatively, and during follow-up was not different from other transplant patients. For induction immunosuppression, injection basiliximab 20 mg over 45-min infusion was given along with injection methylprednisolone. In maintenance regime, we gave mycophenolate mofetil, tacrolimus, injection methylprednisolone, and oral prednisone in tapering dose. Blood level of tacrolimus was maintained at 10–15 mg/ml for the first 6 months and around 6 mg/ml thereafter. Donor heart harvesting was done using histidine-tryptophan-glutarate (HTK) Custodiol cardioplegia solution. The cold ischemic time varied between 3 and 5 h and transplantation was done using bicaval transplantation technique (BC) in all the cases. The intra-operative and early postoperative period was uneventful in all the patients. The first endomyocardial biopsy was done before discharge and subsequent biopsies were done yearly or whenever there was suspicion of rejection.
Cases with Ewing’s sarcoma (clinical vignette − 1 and 2)
Clinical vignette − 1
A 19-year-old male with no previous cardiac history presented with dyspnea on exertion which had worsened from New York Heart Association classification (NYHA) class II to class IV over 1 month prior to presentation. He also complained of new-onset palpitations for 3 months prior to the presentation. Patient was known to have Ewing’s sarcoma involving the left scapula 3 years back for which he received 16 cycles of chemotherapy (doxorubicin 90 mg/m2 with ifosfamide 10 g/m2 every 3 weeks with a maximum of 375 mg/m2 cumulative dose of doxorubicin) along with surgical removal of the sarcomatous lesion. Enlarged cardiac silhouette was seen on chest X-ray. Electrocardiogram (ECG) showed signs of left ventricular hypertrophy and anterolateral “t” wave inversion. Cardiac marker B-type natriuretic peptide (BNP) was 5390 pg/ml (highly increased) showing left ventricular dysfunction.
Cardiovascular imaging showed dilated left ventricle with wall thinning as depicted in Fig. 1. Diffuse global hypokinesia involving left ventricle was significant. Left ventricular ejection fraction was 22%. He was diagnosed with dilated cardiomyopathy. Patient could not be managed with medical treatment. Patient was advised heart transplantation. The standard protocol for preoperative evaluation was followed and PET-CT carried out to rule out malignancy.
Fig. 1.
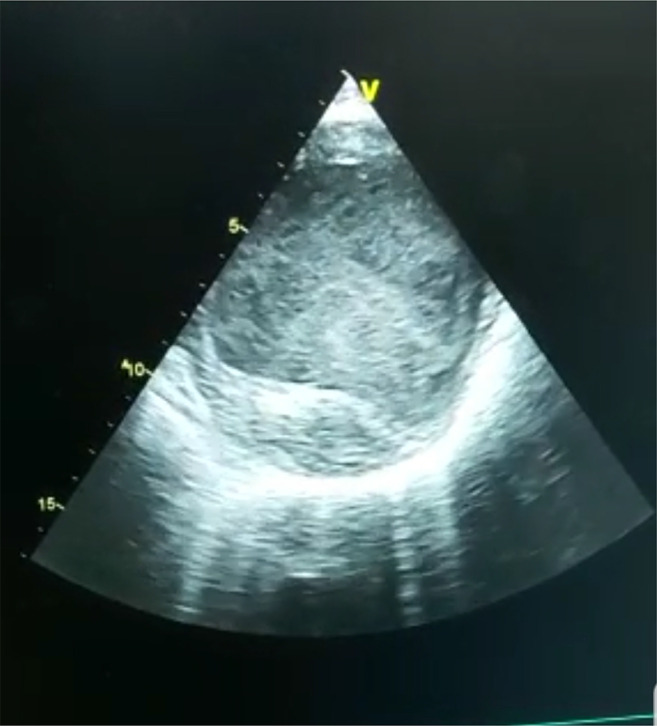
Cardiac imaging showing thinned and dilated left ventricle with spontaneous echo contrast suggesting very poor ventricular function
Clinical vignette − 2
A 35-year-old male, known case of localized Ewing’s sarcoma of lower extremity, had undergone doxorubicin-based chemotherapy. He received doxorubicin 90 mg/m2 doxorubicin as a continuous infusion with a cumulative dose of approximately 400 mg/m2 and 10 g/m2 ifosfamide for up to 6 cycles. Four years later, the patient developed symptoms of dilated cardiomyopathy with biventricular dysfunction. Cardiotoxicity was assessed by echocardiogram and elevated serum brain natriuretic peptide and troponin levels. Patient could be managed medically for 6 months but gradually the symptoms worsened. After obtaining negative PET scan, he was stabilized and heart transplantation was performed. He underwent successful heart transplantation and is doing well at 2 years post cardiac transplantation.
Cases with breast carcinoma (clinical vignette − 3 and 4)
Clinical vignette − 3
A 58-year-old woman, a known case of right-sided stage II-A breast carcinoma, underwent mastectomy after receiving neoadjuvant chemotherapy. She had no past history of cardiovascular morbidity, smoking, or hypertension. She was found to have overexpressed human epidermal growth factor-2 receptor (HER-2 or HER-2/neu receptors), and hence started on paclitaxel l80 mg/m2 with trastuzumab (4 mg/kg IV over 90 min then 2 mg/kg over 30 min) weekly. After 1 year, she developed shortness of breath which progressed to severe cardiomyopathy over a 2-year period. She was managed with supportive pharmacological treatment for a period of nearly 6 years but when her left ventricular ejection fraction became less than 25% with intolerant exertion, she was referred to our heart team for cardiac transplantation. She underwent successful heart transplantation and she is doing well now 3 years post heart transplant surgery.
Clinical vignette − 4
A 51-year-old lady, presented with right-sided stage III breast cancer, underwent radical mastectomy after receiving neoadjuvant chemotherapy with cyclophosphamide, epirubicin, and 5-fluorouracil. Immunohistochemistry revealed tumor 3+ve for HER-2/neu receptor. She was given 14 cycles of paclitaxel and radiotherapy for metastatic breast carcinoma of grade pT2N1M0. After confirming normal cardiac evaluation on 2D echocardiogram, she was started on trastuzumab in standard doses (8 mg/kg over 90 min at first cycle followed by 4 mg/kg over 30 min in next cycles). Her quality of life was good for the first 3 months but deteriorated rapidly after that. She could tolerate only 12 cycles of trastuzumab and presented with severe dyspnea and palpitation. Her left ventricle was dilated along with global hypokinesia and left ventricular ejection fraction of 20% on two-dimensional echocardiogram (2D echo). On this basis, a diagnosis of dilated cardiomyopathy was made which was confirmed by CT thorax. She could not be maintained on pharmacological treatment of tablets carvedilol 6.25 mg, enalapril 2.5 mg, and combination of frusemide–spironolactone (20/50 mg). Her PET scan was negative, so she was referred to us for receiving cardiac transplantation. After 1 year of transplantation, the patient hemodynamically deteriorated and liver metastasis developed. We followed the same immunosuppression regime but she could not respond. She unfortunately died after 1 year. In this case, particularly implantation of ventricular assist device was not practically possible due to its high cost.
Discussion
Multimodality therapies have improved the survival after tumors like Ewing’s sarcoma and breast cancer. However, cardiotoxicity following chemotherapy remains an important concern [7, 8].
Pathogenesis of CCMP and strategies to mitigate the cardiotoxic effects of chemotherapeutic drugs
Dilated cardiomyopathy (DCM) is characterized by dilation of the ventricle and reduced performance of myocardium [9]. The extent of remodeling of heart correlates with the severity of heart failure clinically in dilated cardiomyopathy patients [10].
We have presented a case series of four patients in this article. Two male patients of Ewing’s sarcoma developed dilated cardiomyopathy after treatment with anthracycline.
Anthracyclines causes irreversible cardiomyopathy by generation of free radicals which is related to total dose of the drug. Oliveira et al. reported that this irreversible damage is characterized by decrease in cardiomyocyte density and myofibrillar dropout with vacuolization of sarcoplasm [1, 2]. Cardiomyopathy is the most important long-term toxicity of anthracyclines. In the case of anthracycline therapy, cardiac dysfunction is classically related in an exponentially dose-dependent manner. Moreover, the incidence of heart failure (HF) and left ventricular systolic dysfunction (LVSD) increases as time progresses after treatment and ranges from 1 to 16% [5, 11]. Moreover, it has been illustrated recently in a study that in mammalian cardiomyocytes, doxorubicin-induced cardiotoxicity may be mediated by topoisomerase-IIβ [12]. Other theories suggested that cardiotoxicity is facilitated by release of vasoactive amines, impairment of mitochondrial membrane binding, and assembly and creatine kinase activity, whereas decreased production of adenosine triphosphate, increased formation of toxic metabolites, inhibition of nucleic acid, and protein synthesis are also defined to cause cardiotoxicity [13, 14]. The total cumulative dose of anthracyclines is the most predictive measure of the development of cardiotoxicity [15].
The other two female patients of breast cancer mentioned in our study developed cardiotoxicity after treatment with trastuzumab-based chemotherapy. HER2/neu epidermal growth factor receptor is overexpressed in about 15–20% of breast tumors. Trastuzumab is a humanized monoclonal antibody which acts by blocking these receptors and hence it is used in the treatment of HER2/neu receptor–positive breast cancer [16].
However, it has been documented that combination of trastuzumab and anthracyclines resulted in cardiac dysfunctions and heart failures in up to 27% of HER2-positive metastatic breast cancer patients compared with less than 7% in anthracyclines-only group [17].
Pretransplant malignancy and heart transplantation
Ewing’s sarcoma affects children between 10 and 20 years of age commonly, and with current multimodality therapy, the 5-year survival is 70% and long-term survival is 50%. Chemokine receptors can be used as prognostic markers, with low level of expression of C-X-C chemokine receptor type 4 (CXCR-4) and SRY-related HMG box–containing transcription factor-2 (SOX2) receptors associated with 5-year survival of > 90% and high level of expression with < 30% survival.
Breast cancer is the most common malignancy affecting women of all age groups, with 5-year survival depending on the stage, and tumor biology can vary between 80 and 90%. The use of anthracyclines, radiotherapy, and trastuzumab for HER2 receptor–positive patients has incremental risk of cardiotoxicity [18].
Incidence of cardiotoxicity caused by doxorubicin and trastuzumab has been depicted in Table 1 [19–24]. Some other drugs also cause cardiotoxicity but the incidence is low.
Table 1.
Incidence of cardiotoxicity caused by doxorubicin and trastuzumab
| Class of drug | Chemotherapeutic agents causing cardiac toxicity | Incidence of heart failure (%) |
|---|---|---|
| Anthracyclines | Doxorubicin in cumulative dose of | |
| 400 mg/m2 | 3 | |
| 550 mg/m2 | 7 | |
| 700 mg/m2 | 18 | |
| Humanized monoclonal antibody against human epidermal growth factor receptor(HER-2) | Trastuzumab | 27 |
It has been recommended for all cancer survivors who are exposed to cardiotoxic therapies to monitor their cardiac function for early diagnosis of cardiac toxicity. More sophisticated techniques like tissue velocity and strain imaging by echocardiography, and delayed contrast enhancement by cardiac magnetic resonance imaging (CMRI), might be useful in detection of subclinical cardiotoxicity [25, 26].
Cardiac troponins and brain natriuretic peptides are important biomarkers to assess the development of left ventricular dysfunction due to chemotherapy [27–29].
Due to lack of ionizing radiation, echocardiography is preferred as screening and monitoring modality among pediatric patients [30, 31]. A recent study reported sensitivity of multigated acquisition (MUGA) as 90% and its specificity was found to be 72%.
Role of PET/CT scan in CCMP patients
Positron emission tomography uses 18 fluorodeoxyglucose (FDG) to identify areas of uptake. Malignant cells have increased glucose transporter −1(GLUT1) and glucose transporter −3(GLUT 3) which takes up the radioactive tracer. This accumulates in the tumor cells as 18 FDG phosphate which cannot be metabolized further. The positron released by the tracer is detected by lutetium-based sensors. This in combination with computerized tomography can give anatomical detail with spatial resolution of 4–5mm. The sensitivity and specificity of PET/CT for detecting residual or active malignancy is up to the tune of 90% while the negative predictive value is 75%. These facts have to be borne in mind when we transplant on the basis of negative PET/CT scans and why recurrences and relapses can occur in spite of negative scans. However, these are the best modalities to ensure if the tumor is in remission and to detect any early relapse and response to treatment [30–32].
Interestingly, PET/CT can also be used to monitor heart transplant rejection, and its ability to detect relapse of malignancy might increase its use as a surveillance tool post transplant in CCMP patients [32].We could find increased BNP among our patients. Moreover, B-type natriuretic peptides, and brain natriuretic peptide/N-terminal pro-brain natriuretic peptide (BNP and NTproBNP), have also been acknowledged to play an important role in predicting LVSD among cancer survivors [2, 33].
Medical therapies for prevention and treatment of CCMP
Interaction of doxorubicin with iron significantly increases the production of free radicals.
Superoxide dismutase and catalase are enzymatic defenses which protects against the toxicity of anthracyclines. Exogenous antioxidants like alpha-tocopherol or an iron chelator dexrazoxane thus help in augmenting these enzymatic defenses.
Bosch et al. documented that use of cardioselective beta blocker with angiotensin-converting enzyme inhibitor (OVERCOME Trial) may result in improving the left ventricular functions. Another study added the role of beta blocker with angiotensin-converting enzyme inhibitor with angiotensin receptor blocker (PRADA Trial) in improvement of cardiac functions. The role of 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitors (statins) was also studied in improving CCMP [34].
An early involvement by a cardiologist, carefully monitoring the cardiac status by strain imaging, and preventive institution of beta blockers, angiotensin-converting enzyme inhibitors, and statins can help mitigate if not eliminate the cardiotoxic side effects of cancer chemotherapeutic agents.
Treatment of end-stage CCMP
Among anthracycline-induced cardiomyopathy patients, 2–4% of patients might progress to end-stage heart failure. These patients require advanced therapies such as inotropic support, orthotopic heart transplantation (OHT), or left ventricular assist device (LVAD) [2]. Increased level of immunosuppression is required in heart transplantation which results in post-OHT malignancy. Thus, potential risk of relapse of the primary malignancy is greater in OHT in survivors of cancer than any other solid organ transplant [3, 35].
Despite higher rates of post-transplant malignancies, the overall survival of patients was satisfactory [3, 35].
Nonetheless, when all other existing traditional therapies for the treatment of end-stage heart failure get exhausted, mechanical circulatory support devices (MCS) come in role. LVAD may be the only option in some of these cases. LVAD can be used as a bridge to transplantation or as destination therapy in CCMP patients [2, 36, 37].
As reported in a study, right ventricular assist device (RVAD) was required either concomitantly or following LVAD implantation among a statistically significant proportion of CCMP patients.
The cause for RVAD requirement is the demonstration of biventricular nature of anthracycline-mediated cardiomyopathy as documented in cardiac imaging studies [38].
Higher incidence of pulmonary emboli in patients with cancer can also contribute to right ventricular dysfunction. In contradiction to this, Oliveira et al. reported comparable pulmonary vascular resistance preceding MCS implantation among the CCMP group and the non-ischemic/ischemic cardiomyopathy (NICMP/ICMP) groups [1, 2].
Moreover, this is a matter of great concern that requirement of RVAD after LVAD implantation might be a plausible cause of consequential escalation in morbidity and mortality.
Though patients with CCMP have higher incidence of recurrence of malignancy and higher need for RVAD after LVAD implantation, the overall survival of these patients post OHT/LVAD is similar to patients with isolated dilated cardiomyopathy [38].
It has been reported that in any large heart failure clinic, 3% of advanced heart failure patients have CCMP. In those with non-ischemic cardiomyopathy, up to 8% have CCMP. LVAD is an option for those with CCMP with advanced age and comorbidities where it can be used as destination therapy. It can also be used for those in remission and with rapidly worsening cardiac status as bridge to decision and bridge to transplant, the advantage in this setting being the absence of immunosuppression and the absence of waiting time for a donor heart, and the disadvantage being the cost of LVAD and the high incidence of post LVAD, right ventricular failure in this group (up to 40%) as CCMP affects both the ventricles equally [2, 39].
The young age of patients, evidence of remission by negative PET scan, lack of serious comorbidities, and cost factor made us prefer OHT as management option. Other than cost, the significant incidence of right ventricular dysfunction post-LVAD insertion with its attendant morbidity and mortality made us prefer OHT over LVAD.
Why is transplantation justified in the presence of previous malignancy?
The decision to offer transplantation is not easy and needs to be done in close consultation with the oncologist, the family, and the cardiologist. While transplantation in patients with treated malignancy carries a risk of recurrence, it is difficult not to offer them a therapeutic option in the presence of severe symptoms. This is further justified when the patients are found to be in remission on a PET scan. The waiting period has to be decided in consultation with the oncologist, based on tumor biology and the rapidity of clinical worsening of the patient and not on an arbitrary period of remission.
Studies uniformly show transplantation outcomes similar to those with dilated cardiomyopathy in these subset of patients with CCMP. The patients in remission cannot be considered cured of cancer; at best, they can be certified as no evidence of disease (NED) and there is always a risk of relapse. This has to be explained and understood before offering transplantation as what happened in one of our cases.
Follow-up after transplant in CCMP patients
Ascribable to its soaring spatial and temporal resolution and immense diagnostic sensitivity and accuracy, PET scan is the gold standard technique to assess myocardial glucose metabolism and perfusion [39–41].
In our study, one of the four patients developed recurrent metastatic breast cancer and died 1 year after transplantation, despite having a negative PET scan. Furthermore, it is advocated in recent studies that newer hybrid PET/CT scanners have increased the interest in using diverse PET tracers to evaluate skeletal disease including [18F]fluoride (NaF) as a bone-specific tracer and [18F]fluorodeoxyglucose and [18F]choline as tumor-specific tracers [41, 42]. Judicious use of these newer imaging techniques is the need of the hour in restaging and treatment response of assessment of the malignancy. However, there is insufficient data regarding outcomes of heart transplantation post CCMP.
Tacrolimus is the preferred immunosuppressive agent used post-heart transplant, but does not have tumor-suppressive properties. Everolimus has the theoretical advantage of acting both as immunosuppressant and anti-tumor agent. Its role as an alternative immunosuppressant (after 4–6 weeks) and in reducing chronic allograft vasculopathy is proven [43]. Further studies are required to establish its role as the preferred immunosuppressive/anticancer drug for patients undergoing heart transplant for CCMP.
Conclusion
Early detection of subclinical cardiotoxicity and timely intervention might be helpful in better long-term prognosis. Inter-specialty collaboration may result in better care of cancer survivors. We suggest that OHT can be offered to chemotherapy-induced cardiomyopathy even within five years of its occurrence provided that the PET scan is negative for better survival of the patient and depending upon the tumor biology, grade of the tumor, and heart condition of the patient. The decision for transplantation is not easy and has to be made in close consultation with oncologist and family, understanding the risk of recurrence. Finally, the treating surgeon has to be convinced that the progression of heart failure is more likely to be fatal than the risk of recurrence before embarking on this major step. The availability of more affordable and durable MCS would help simplify some of the difficult decision-making challenges posed by these increasing subset of heart failure patients.
Funding
No funding was required for this article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Consent of the patient
Written informed consent was obtained from all the patients for publication of this case series and accompanying images.
Ethical approval
Ethical approval was not required as this is case series and review.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oliveira GH, Qattan MY, Al-Kindi S, Park SJ. Advanced heart failure therapies for patients with chemotherapy-induced cardiomyopathy. Circ Heart Fail. 2014;7:1050–8. [DOI] [PubMed]
- 2.Oliveira GH, Hardaway BW, Kucheryavaya AY, Stehlik J, Edwards LB, Taylor DO. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant. 2012;31:805–810. doi: 10.1016/j.healun.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh N, Hilton J. Orthotopic heart transplantation and mechanical circulatory support in cancer survivors: challenges and outcomes. J Oncol. 2015; 10.1155/2015/232607. [DOI] [PMC free article] [PubMed]
- 4.Shakir DK, Rasul KI. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piper SE, McDonagh TA. Chemotherapy-related cardiomyopathy. Eur Cardiol. 2015;10:19–24. doi: 10.15420/ecr.2015.10.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardinale D, Colombo A, Cipolla CM. Prevention and treatment of cardiomyopathy and heart failure in patients receiving cancer chemotherapy. Curr Treat Options Cardiovasc Med. 2008;10:486–495. doi: 10.1007/s11936-008-0041-x. [DOI] [PubMed] [Google Scholar]
- 7.Longhi A, Ferrari S, Tamburini A, et al. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: the Italian Sarcoma Group Experience (1983-2006). Cancer. 2012;118:5050–9. [DOI] [PubMed]
- 8.Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41:2836–2845. [DOI] [PubMed]
- 9.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.CIR.93.5.841. [DOI] [PubMed] [Google Scholar]
- 10.Singh TP, Sleeper LA, Lipshultz S, et al. Association of left ventricular dilation at listing for heart transplant with postlisting and early posttransplant mortality in children with dilated cardiomyopathy. Circ Heart Fail. 2009;2:591–8. [DOI] [PubMed]
- 11.Jones RL, Ewer MS. The hallmark of doxorubicin induced cardiomyopathy is a HF syndrome arising from dilated cardiomyopathy (DCM) Expert Rev Anticancer Ther. 2006;6:1249–1269. doi: 10.1586/14737140.6.9.1249. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 13.Ryberg M, Nielsen D, Cortese G, Nielsen G, Skovsgaard T, Andersen PK. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100:1058–1067. doi: 10.1093/jnci/djn206. [DOI] [PubMed] [Google Scholar]
- 14.Wouters KA, Kremer LCM, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol. 2001;12:S35–S41. doi: 10.1093/annonc/12.suppl_1.S35. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 18.Bennani-Baiti IM, Cooper A, Lawlor ER, et al. Intercohort gene expression co- analysis reveals chemokine receptors as prognostic indicators in Ewing’s sarcoma. Clin Cancer Res.2010;16: 3769-78. [DOI] [PMC free article] [PubMed]
- 19.Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 20.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 21.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A, Kennedy J, O’Byrne K, Conte PF, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC, Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 23.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. doi: 10.1016/S0025-6196(11)60896-3. [DOI] [PubMed] [Google Scholar]
- 24.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 25.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 26.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, ESMO Guidelines Working Group Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 27.Herman EH, Zhang J, Lipshultz SE, Rifai N, Chadwick D, Takeda K, Yu ZX, Ferrans VJ. Correlation between serum levels of cardiac troponin-T and the severity of the chronic cardiomyopathy induced by doxorubicin. J Clin Oncol. 1999;17:2237–2243. doi: 10.1200/JCO.1999.17.7.2237. [DOI] [PubMed] [Google Scholar]
- 28.Morandi P, Ruffini PA, Benvenuto GM, la Vecchia L, Mezzena G, Raimondi R. Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high-dose (7 g/m(2)) cyclophosphamide. Bone Marrow Transplant. 2001;28:277–282. doi: 10.1038/sj.bmt.1703132. [DOI] [PubMed] [Google Scholar]
- 29.Specchia G, Buquicchio C, Pansini N, di Serio F, Liso V, Pastore D, Greco G, Ciuffreda L, Mestice A, Liso A. Monitoring of cardiac function on the basis of serum troponin I levels in patients with acute leukemia treated with anthracyclines. J Lab Clin Med. 2005;145:212–220. doi: 10.1016/j.lab.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Purkayastha K, Seth R, Seth S, Lyon AR. Cancer therapy-induced cardiotoxicity: review and algorithmic approach toward evaluation. J Pract Cardiovasc Sci. 2017;3:82–93. doi: 10.4103/jpcs.jpcs_33_17. [DOI] [Google Scholar]
- 31.Wieczorek SJ, Wu AHB, Christenson R, et al. A rapid B-type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: A multicenter evaluation. Am Heart J. 2002;144:834–9. [DOI] [PubMed]
- 32.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, Colan SD, Neuberg DS, Dahlberg SE, Henkel JM, Asselin BL, Athale UH, Clavell LA, Laverdière C, Michon B, Schorin MA, Sallan SE. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzó M, Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 35.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122:173–183. doi: 10.1161/CIRCULATIONAHA.109.858076. [DOI] [PubMed] [Google Scholar]
- 36.Cavigelli-Brunner A, Schweiger M, Knirsch W, Stiasny B, Klingel K, Kretschmar O, Hubler M. VAD as bridge to recovery in anthracycline-induced cardiomyopathy and HHV6 myocarditis. Pediatrics. 2014;134:e894–e899. doi: 10.1542/peds.2013-2272. [DOI] [PubMed] [Google Scholar]
- 37.Appel JM, Sander K, Hansen PB, Møller JE, Krarup-Hansen A, Gustafsson F. Left ventricular assist device as bridge to recovery for anthracycline-induced terminal heart failure. Congest Heart Fail. 2012;18:291–294. doi: 10.1111/j.1751-7133.2012.00291.x. [DOI] [PubMed] [Google Scholar]
- 38.Jurczak W, Szmit S, Sobociński M, et al. Premature cardiovascular mortality in lymphoma patients treated with (R)-CHOP regimen—a national multicenter study. Int J Cardiol. 2013;168:5212–5217. doi: 10.1016/j.ijcard.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced Cardiotoxicity. Maedica (Buchar) 2013;8:59–67. [PMC free article] [PubMed] [Google Scholar]
- 40.Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, Butler J. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9:e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awadalla M, Hassan MZO, Alvi RM, Neilan TG. Advanced imaging modalities to detect cardiotoxicity. Curr Probl Cancer. 2018;42:386–396. doi: 10.1016/j.currproblcancer.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook GJR. PET and PET/CT imaging of skeletal metastases. Cancer Imaging. 2010;10:1–8. doi: 10.1102/1470-7330.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potena L,Pellegrini C, Grigioni F, et al. Optimizing the safety profile of everolimus by delayed initiation in De Novo heart transplant recipients:Results of the prospective randomized study EVERHEART. Transplantation. 2018;102:493–501. [DOI] [PMC free article] [PubMed]


