Abstract
Purpose
There is increasing clinical utilization of hearts from the donation after circulatory death (DCD) pathway with the aim of expanding the donor pool and mitigating the ever-present discrepancy between the inadequate availability of good quality donor hearts and the rising number of patients with end-stage heart failure.
Methods
This article reviews the rationale, practice, logistical factors, and 5-year experience of DCD heart transplantation at St Vincent’s Hospital, Sydney.
Findings
Between July 2014 and July 2019, 69 DCD donor retrievals were undertaken resulting in 49 hearts being instrumented on an ex situ normothermic cardiac perfusion device. Seventeen (35%) of these hearts were declined and the remaining 32 (65%) were used for orthotopic DCD heart transplantation. At 5 years of follow-up, the 1-, 3-, and 5-year survival was 96%, 94%, and 94% for DCD hearts compared with 89%, 83%, and 82% respectively for donation after brain death (DBD) hearts (n.s). The immediate post-implant requirement for temporary extra-corporeal membrane oxygenation (ECMO) support for delayed graft function was 31% with no difference in rejection rates when compared with the contemporaneous cohort of patients transplanted with standard criteria DBD hearts.
Summary
DCD heart transplantation has become routine and incorporated into standard clinical practice by a handful of pioneering clinical transplant centres. The Australian experience demonstrates that excellent medium-term outcomes are achievable from the use of DCD hearts. These outcomes are consistent across the other centres and consequently favour a more rapid and wider uptake of heart transplantation using DCD donor hearts, which would otherwise be discarded.
Keywords: Donation after circulatory death, Cardiac transplantation, Extra-corporeal heart perfusion
Background
Heart transplantation continues to represent the best evidence-based therapy with symptomatic and prognostic benefit for an increasing number of recipient candidates with end-stage heart failure (HF). This includes an increasing number of HF patients who are being bridged to transplantation with mechanical circulatory support (MCS) systems. The contemporary heart transplant wait-list has consequently become largely populated with more complex and older recipients with a higher degree of immunological sensitization and requiring challenging redo procedures including the removal of in situ implantable MCS devices. In addition to this, the continued shortage of suitable hearts from an increasingly older donor pool with more co-morbidities has placed enormous pressures on transplant centres to accept greater risks in the selection and matching of donors and recipients.
Heart transplantation from distantly procured donation after circulatory death (DCD) hearts was first described by the Sydney group 5 years ago as an important alternative expansion of the donor pool [1]. DCD heart transplantation had occurred prior to this, but in limited numbers and with co-localization of both donor and recipients, frequently in adjacent operating theatres, such as that described by Christiaan Barnard in his seminal procedure that took place at Groote Schuur Hospital in Cape Town, South Africa, in 1967 [2] as well as, almost four decades later, by the group in Denver, CO, USA, in describing three successful paediatric heart transplants [3]. Since then, a total of 21 paediatric DCD heart transplants, between 2005 and 2014, have also been recorded by the ISHLT Registry [4].
The successful initiation of the Sydney DCD heart transplant program in July 2014 was closely followed by Papworth Hospital, Cambridge, UK, in February 2015; Harefield Hospital, London, UK, in August 2015; Wythenshawe Hospital, Manchester, UK, in May 2017; University Hospital, Liege, Belgium, in 2019; and the Freeman Hospital, Newcastle, UK, in August 2019 [5–8]. More recently, DCD heart transplantation has also commenced in the USA with Duke University Hospital, Durham, NC, performing its first case in early December 2019 and followed during the same month by the Massachusetts General Hospital, Boston, and the University Hospital, Madison, WI [9–11]. All these programs have utilized the Sydney direct procurement (DP) method with rapid in situ cardioplegic preservation followed by instrumentation onto an ex situ perfusion device Organ Care System (OCS-Heart, TransMedics Inc., Andover, USA) for re-animation and beating heart transportation to the recipient hospital. The exception has been the Cambridge group, which has used both this and their own normothermic regional perfusion (NRP) method. The latter requires the donor to be placed on cardiopulmonary bypass for in situ cardiac re-animation and assessment of myocardial viability prior to transportation on the OCS-Heart [5]. The same group has also described the first report of initial NRP supplemented by a brief period of cold static preservation, rather than with extra-corporeal perfusion, for an in-house recipient [12]. The Papworth NRP technique, with subsequent ex situ beating-heart perfusion during donor organ transportation, has also been described in a single paediatric case by the group in Newcastle, UK [13]. Surgeons at the University Hospital, Liege, Belgium, have also reported the use of a similar NRP technique for cardiac functional assessment followed by brief cold static storage for two adult DCD heart transplants with co-located donors and recipients [14]. The same group has also described the use of NRP followed by 2 h of cold static storage for a distantly procured DCD heart for a successful paediatric heart transplant [15].
This slow uptake of DCD heart transplantation, which currently totals just over 130 procedures, has resulted from a number of factors including as follows: logistics; fear of the unavoidable period of warm ischaemia and the ensuing risk of ischaemia-reperfusion injury (IRI); the considerable cost, availability of and familiarity with an ex situ perfusion platform for heart preservation, transportation, and assessment; skilled staff; and any local legal and ethical constraints.
Notwithstanding these limitations, the last decade has witnessed a significant rise in both DCD donation rates and in the use of DCD organs for both thoracic and abdominal transplants [15]. The continued quest to expand the donor pool has further progressed in the riskier utilization of DCD lungs, livers, and kidneys from uncontrolled Maastricht category I donors as well as from the safer but ethically controversial use of Maastricht category V donors from planned euthanasia in jurisdictions that legally permit this pathway [16, 17]. Accepting that here is an ongoing ethical debate over considering the potential use of hearts from euthanasia donors [18], this review will restrict its comments to Maastricht criteria III donors in the context of current DCD heart transplantation.
The clinical DCD heart transplant program at St Vincent’s Hospital in Sydney commenced on the foundation of considerable experimental work [19–22], in modelling the availability of sufficient and suitable DCD donations [23] and clinical experience with marginal donor hearts, often with prolonged cold ischaemic times being common in Australia with the significant geographical separation of donor and recipient hospitals [24]. At the outset, we had accepted that ante mortem interventions or re-establishing in situ donor circulation would not be permitted; that a strategy of using post-conditioning pharmacological agents was necessary; that an ex situ cardiac perfusion device permitting preservation, transportation, and assessment of myocardial viability was essential; and that there was an expectation that almost all the DCD hearts may require post-implant temporary mechanical circulatory assistance for delayed graft function.
The DCD pathway
DCD donation
In Australia, the legal definition of death requires there to be either an “irreversible cessation of all functions of the brain of the person” as in brain death determination or an “irreversible cessation of circulation of blood in the body of the person” as in circulatory death determination [25]. This legal framework therefore denies, in Australia, the option of in situ re-animation of the heart by means of artificial circulatory support in the donor as practiced by the Papworth group in Cambridge, UK.
DCD donation refers to the process of facilitating the wish of organ donation by individuals who typically have suffered irreversible brain damage but exhibit some residual neurological function, thus rendering them ineligible for standard evaluation for brain death criteria. Having established the futility of continued medical intervention, the life-sustaining therapies (WLST) are then withdrawn in a controlled manner following mutual agreement by relatives and treating physicians. Implicit in this process is the understanding that no active resuscitative measures are to take place after WLST and that there must be a mandatory “no-touch” or “stand-off” period to ensure the absence of auto-resuscitation. This no-touch period varies between jurisdictions, ranging from 2 to 30 min in Europe [16], and in Australia is 5 min in all states except New South Wales, where it is 2 min.
Unlike donation after brain death (DBD) donors, where the organ retrieval process avoids any period of warm ischaemia, the DCD process is characterized by an unavoidable but variable period of warm ischaemic time (WIT). This critical period, marked by hypoxia, ischaemia, hypoperfusion, and cardiac distension prior to cessation of circulation, determines significant pathophysiological consequence, referred to as warm ischaemic injury that has been the major limiting factor in DCD heart transplantation. The total WIT, defined as that period from WLST to cardioplegia delivery, contains a “functional WIT”—from a defined systolic blood pressure to cardioplegia delivery. The functional WIT has replaced the earlier “agonal phase” which simply described the time from WLST to asystole. Given the post-WLST variability in haemodynamics and that adequate blood pressure is often maintained for a considerable time, DCD retrieval protocols have moved to consider a functional WIT as being more relevant as it allows more time for cessation of circulation to occur and thereby reduces the donor turn-down rate.
Ante mortem interventions and withdrawal of life-supporting therapies (WLST)
Ante mortem interventions remain controversial and widely variable between countries. For example, of the 12 European nations that have legislation for controlled DCD donation, 7 of them permit ante mortem substances and only 3 of them permit ante mortem peripheral vascular cannulation to institute rapid extra-corporeal circulation following cessation of circulation [17]. The principle issues of doing no harm or that of any intervention conferring no benefit to the patient who has yet to progress to become a donor, and a wider acknowledgement of the potential benefit to any organ recipient and the community at large, requires a balanced consideration that is deemed unsuitable to delegate to the patient’s relatives. So whilst medications to maintain haemodynamics, antibiotics to prevent or treat infection, and investigations to determine organ suitability and identification of suitable recipients are permissible, the use of heparin is variably permitted in Australia. It is an accepted practice in DCD donation in the Australian Capital Territory, Northern Territory, and Victoria. It is officially permitted in South Australia and Western Australia, but the final decision is vested in the intensive care unit (ICU) physician responsible for the end-of-life care. Ante mortem heparin is not permitted in New South Wales and in Queensland; it is only permitted in the circumstance of a brain-dead patient going down a circulatory death pathway. As detailed before, the institution of any extra-corporeal circulatory support, even post-mortem, is prohibited by the Australian definition of death.
WLST continues to occur predominantly in the ICU setting, which is viewed as a more natural and conducive environment for the family and friends during this terminal phase. The time required to transport the donor following determination of circulatory death from the ICU to the retrieval operating theatre is now recognized as a significant variable that can be influenced to reduce the WIT. Better outcomes for liver transplantation have been reported with WLST occurring in an operating theatre [26]. There is an increasing trend towards WLST being permitted nearer or ideally adjacent to the retrieval theatre with donor family and friends present. Following WLST, the determination of circulatory death can only be made from having observed mechanical asystole by the absence of pulsatility ideally from intra-arterial pressure monitoring; having observed the mandatory stand-off period; and in satisfying that other features of death such as immobility, apnoea, and absent skin perfusion are also present.
In the event that circulatory arrest does not happen within a defined length of time following WLST, then the donation process is stopped with institution of continued end-of-life care. In Australia, this process is typically stopped at 90 min with rare exceptions.
Donor selection
A highly recipient-protective donor selection criterion was used during the first three and a half years of the Sydney program. This required donors to be under 40 years of age with no relevant cardiac history, to have normal haemodynamics on minimal vasopressor/inotropic support, and to accept a 30-min limit from WLST to cardioplegia delivery. This time limitation was based on an extensive pre-clinical evaluation in porcine models of DCD heart transplantation that demonstrated irreversible myocardial damage beyond a WIT of 30 min [19, 20]. Encouraged by the excellent outcomes with the initial DCD heart transplants, which included more complex recipients requiring redo sternotomies, some with in situ MCS devices, the program then relaxed its donor selection criteria from January 2018. This new protocol increased the donor age to < 55 years and limited 30-min functional WIT with a clock start at a systolic blood pressure (BP) of < 90 mmHg, this value being on modelling of WLST in a porcine model of DCD donation. In keeping with the aim to have a recipient protective protocol, we felt that the standard but arbitrarily accepted commencement of functional WIT at a systolic BP of 50 mmHg, which originated from the renal literature, did not apply to the heart. Our studies in the pig model had demonstrated that the heart was already profoundly ischaemic by the time the arterial systolic blood pressure had fallen to 50 mmHg [22]. Table 1 shows this evolution in the donor selection criteria.
Table 1.
Donor selection criteria
| Donor factors | Initial protocol July 2014–December 2017 |
Updated protocol Since January 2018 |
|---|---|---|
| Age | < 40 years | < 55 years |
| Cardiac function LVEF/ECHO* | Stable haemodynamics with minimal inotrope/vasopressor support | Unchanged |
| Medical history | No history of heart disease ± previous cardiac surgery | Unchanged |
| Acceptable WIT** | ± 30 min from WLST | ± 30 min from systolic BP < 90 mmHg |
| Ante mortem heparin | Variable | Variable |
Surgical retrieval
The following provides a brief account of the surgical retrieval process for DCD hearts which has been previously reported in detail [1, 27, 28]. Immediately on arrival into the retrieval theatre, the donor is re-intubated, prepped, and draped before sternotomy and cannulation of the right atrium for the necessary collection of 1.2–1.5 L of blood into a heparinized bag for priming the OCS-Heart circuit. If permitted, pre-optimization of haemoglobin level above 100 g/dL prior to WLST and the use of Trendelenburg positioning during the retrieval process facilitate and speed up the blood collection. This is followed by application of an aortic cross-clamp and delivery, via the aortic root, of cold crystalloid St Thomas’ cardioplegic solution containing 100 mg/L of glyceryl trinitrate and 5000 Units/L of erythropoietin as post-conditioning agents. The heart is vented as per standard donor heart explant procedure and kept cold. The heart is then rapidly explanted and transferred for preparation and instrumentation onto the OCS device, whilst the assistant surgeon is able to retrieve the lungs if required. The technique has always permitted the retrieval of lungs and has never delayed the retrieval of abdominal organs.
Management of ischaemia-reperfusion injury
The inability to give ante mortem medication, as discussed before, precludes a pharmacological pre-conditioning strategy. We have therefore focused on leveraging post-conditioning mechanisms of myocardial protection originally described as ischaemic post-conditioning [29, 30] and which occurs via recruitment of several signal transduction pathways that are activated on reperfusion or reflow under mitochondrial control: reperfusion injury salvage kinase (RISK) pathway, survival activating factor enhancement (SAFE) pathway, and nitric oxide synthase pathway [28]. The use of agents that activate these pathways such as erythropoietin (a glycoprotein cytokine secreted by the kidneys in response to tissue hypoxia), glyceryl trinitrate (nitric oxide donor), and zoniporide (sodium-hydrogen exchanger inhibitor) have been previously described by us in their synergistic activation of post-conditioning protection, in their ability to reduce IRI in rodent Langendorff working heart model and in the porcine brain-dead model [19, 31–34]. Furthermore, the finding that these agents, when supplemented in Celsior solution, were able to significantly extend the myocardial tolerability to warm ischaemic insult in a porcine DCD model confirmed the ability to recruit the protective post-conditioning pathways even after determination of circulatory death [20].
Ex situ cardiac perfusion
Assessment of myocardial viability is an essential component of DCD heart transplantation given the expected but variable degree of warm ischaemic injury. Considering that even an echocardiogram is not always available in the Australian setting, it would be negligent to base the final acceptance of a DCD heart solely on cardiac information prior to WLST. There are two tested options for the evaluation of myocardial viability of DCD hearts: the Sydney DP technique of rapid explant post-cardioplegia and then re-animation in an ex situ normothermic perfusion device for transportation and assessment; and in situ cardiac re-animation in the donor using the Papworth NRP technique followed by either ex situ normothermic perfusion or cold static storage for transportation. The options of primary cardioplegic arrest followed by either cold static storage or cold continuous perfusion and then instrumentation of the donor heart onto a full working-heart perfusion device are still in development.
Currently, the only available ex situ device that permits blood-based normothermic perfusion is the cardiac developed by TransMedics Inc., Boston, USA. This has been extensively trialled and tested since 2006 with excellent safety and benefit profile with standard hearts, with extended-criteria DBD hearts, and is accepted as being indispensable for the current DCD heart transplant practice involving distant procurement of the donor organ [1, 6–11, 35–41]. An added advantage of this system is that in the event of suspected or palpable coronary artery disease, the heart can undergo routine angiography [42]. This is particularly helpful in Australia where coronary angiography for those below 50 years is often denied and never carried out in the DCD donor from the procedure being viewed as of no benefit to the patient who has yet to become a donor.
Normothermic ex situ perfusion allows for the restitution of myocardial metabolic haemostasis, reduction of oxidative stress, and provision of nutrient and oxygen stores. Once the heart is re-animated on the OCS device, the team is able to commence their journey back to the recipient hospital with the heart now beating in resting mode. Regular assessment is made of the following parameters: coronary flow, mean aortic pressure, perfusion temperature, haematocrit, and arteriovenous lactate levels (Table 2). Initially, we respected the recommendation of accepting hearts based on achieving a perfusate lactate value of < 5 mM but eventually, from experience, realized that hearts with higher lactate values were acceptable as long as there was a down-trending lactate level with an arteriovenous differential in favour of lactate metabolism as an energy source rather than its accumulation indicating signs of myocardial injury.
Table 2.
Donor and recipient characteristics (mean ± std. dev.)
| Donor | Recipient | |
|---|---|---|
| Age (years) | 30 ± 8 | 53 ± 13 |
| Gender (M:F) | 28 ± 4 | 26 ± 6 |
| Height (cm) | 176 ± 9 | 176 ± 7 |
| Weight (kg) | 82 ± 15 | 77 ± 13 |
| LVEF (%) | 64 ± 5* | 24 ± 10 |
*n = 24, echocardiography was not performed in 8 donors
Clinical experience and outcomes
The transplant program at St Vincent’s Hospital (SVH) in Sydney represents the entire experience of clinical DCD heart transplantation in Australia. It commenced in July 2014 under strict institutional Human Research Ethics Committee approval (HREC/13/SVH/68) and for the following 5-year period performed DCD heart transplants in 32 recipients. The donor-recipient characteristics are detailed in Table 2. Ten patients (31%) required post-implant mechanical support in the form of extra-corporeal membrane oxygenation (ECMO) for delayed graft function (Table 3). The previously reported rate of ECMO use at 23 transplants was 35% [28]. Increasing familiarity with managing the donor heart on the ex situ OCS-Heart device and increased standardization of the implant process have reflected in a further reduction to 22% over the last 9 recipients described in this series.
Table 3.
Key intra- and peri-operative details for all 32 DCD heart recipients
| (mean ± std. dev.) | |
|---|---|
| Cross-clamp time (min) | 89 ± 32 |
| Bypass time (min) | 188 ± 66 |
| Mechanical support (MCS) | 12*/32 (34%) |
| ECMO | 10/32 (31%) |
| IABP | 3/32 (9%) |
*One patient was supported with both intra-aortic balloon pump (IABP) and ECMO
Table 4 describes the comparative times from WLST to instrumentation onto the OCS for the cohorts, which did and not require post-implant MCS support. Despite a shorter time to asystole and a comparable total WIT, the critical time between asystole and delivery of cardioplegia appears to have the strongest correlation with post-transplant need for ECMO support. This time period is made up of the mandatory stand-off time of 2 or 5 min and then the necessary time for transporting the donor from the place of WLST to the operating theatre. As previously discussed, continued understanding and facilitation of WLST to occur in the theatre complex, ideally in the adjacent room, would reduce the warm ischaemic damage that is most likely responsible for this correlation.
Table 4.
Comparison of withdrawal and retrieval timings between DCD transplant recipients who required initial ECMO support and those that did not (mean ± std. dev.)
| Time interval (min) | No-ECMO (n = 22) | ECMO (n = 10) | p value |
|---|---|---|---|
| Time to asystole | 12 ± 5 | 9 ± 3 | 0.036 |
| Warm ischaemic time | 24 ± 6 | 23 ± 3 | 0.458 |
| Asystole to cardioplegia | 12 ± 2 | 15 ± 3 | 0.002 |
| Cold ischaemic time | 29 ± 5 | 27 ± 6 | 0.197 |
| OCS run time | 281 ± 68 | 306 ± 60 | 0.155 |
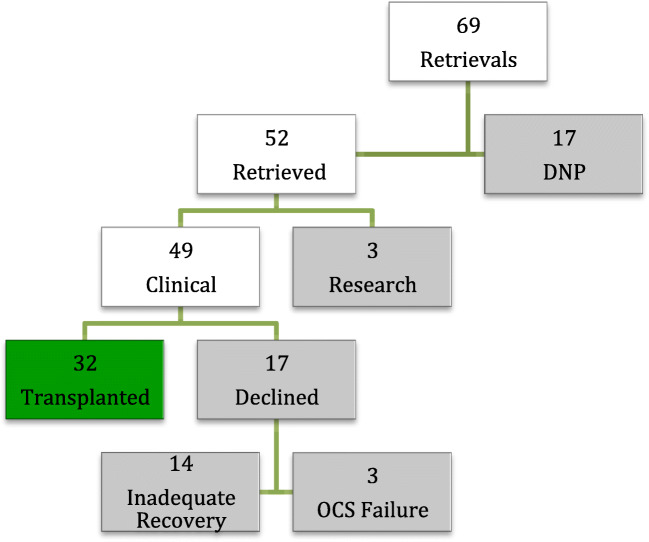
Over the 5-year period, the team attended 69 DCD heart retrievals with a 25% failure rate for progression to circulatory arrest. Of the 52 hearts that were recovered, 49 (94%) were considered suitable for instrumentation on the OCS. Of these, 17 (35%) were declined predominantly for inadequate recovery and further 3 cases after failure of the OCS device. Thirty-two of the retrieved hearts (62%) were finally accepted for transplantation (Fig. 1).
Fig. 1.
Breakdown of all DCD heart retrievals between July 2014 and July 2019. Did Not Progress (DNP)
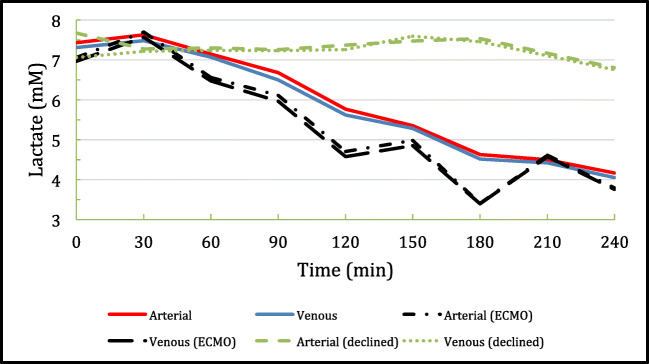
Three of the recovered hearts defaulted into the research protocol by virtue of the WIT exceeding the 30-min cutoff that was strictly imposed in the early phase of the DCD program. Upon ex situ perfusion, acceptability for transplantation depends on a combination of arteriovenous perfusate lactate values suggesting extraction, physiological parameters of aortic pressure, coronary flow, cardiac rhythm, and visual assessment. Figure 2 demonstrates the expected smoothly down-trending arteriovenous lactate profile unlike the less predictable trends seen in patients requiring ECMO post-transplant. The lactate profiles of those hearts rejected for transplantation show the classic uptrend suggesting lactate release from myocardial injury.
Fig. 2.
Conglomerate arterial and venous lactate profiles of the 46 DCD hearts during ex situ reperfusion on the TransMedics OCS-Heart, of which 14 were declined for inadequate functional recovery
Figure 3 shows the recipient survival curves for the DCD against the DBD cohorts over the same time period. This data continues to show excellent medium-term outcomes following DCD heart transplantation.
Fig. 3.
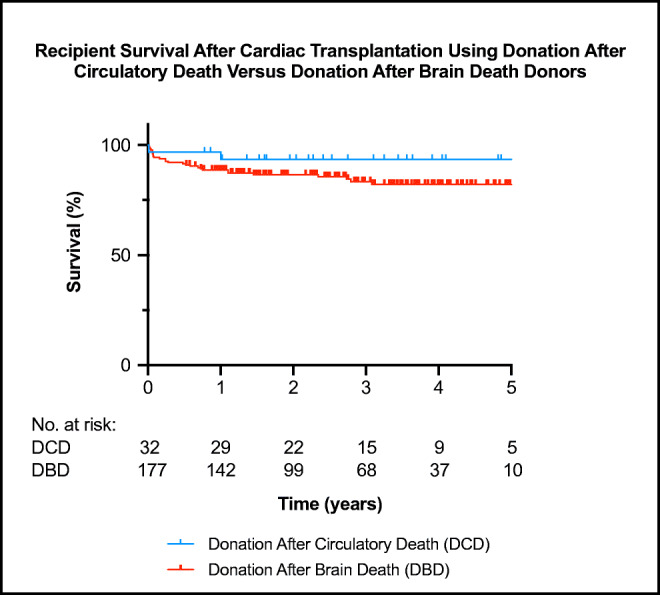
The Kaplan-Meijer survival curve for all DCD and DBD heart transplants between July 2014 and July 2019. There is no statistically significant difference between the two survival curves
Conclusion
DCD heart transplantation is now an accepted reality supported by these excellent medium-term outcomes that are aligned with the experience from other centres which currently is limited to data from the heart transplant centres in the UK in respect of volume. The outcomes, which are comparable with those from the use of standard criteria DBD donors out to 5 years, are encouraging and support the utilization of DCD hearts as a standard-of-care practice in the early adopter centres. Notwithstanding these positive results, there are several factors pertinent to DCD transplantation that still require attention.
The lack of a unifying definition of death across jurisdictions continues to raise ethical concerns when applied to the necessarily progressive aim to expand the donor pool. The ability to restart the donor circulation with NRP or the ability to even re-animate the heart extra-corporeally requires a collective review of what we have for so long accepted as the inviolable “dead donor” rule (DDR). Whilst the timings of the DCD process as practiced must obey the requirement for irreversible cessation of all functions of the entire brain on basic physiological principles, the same compliance becomes questionable for the requirement of irreversible cessation of circulatory and respiratory functions. This difficulty is further reflected by the varying legislative times set for the stand-off period after cessation of circulation. Although the majority of jurisdictions respect a period of 2–5 min, some have longer stipulations such as 10 min in Austria, Ireland, and Poland, 20 min in Italy, and a full 30 min in Russia [16]. This difference in the stand-off periods means that the acceptance and compliance of the DDR can be viewed in another jurisdiction as a practice of euthanasia.
The rapid advances in artificial ventilation and the growing demand for organs for transplantation led to the formation of the ad hoc Committee of the Harvard Medical School for the task of examining the definition of brain death. It chose to extrapolate the neurological determination of brain death to defining a novel concept of Irreversible Coma and published its findings on 5 August 1968 [43]. On the same day, the 22nd World Medical Assembly gathered in Sydney, Australia, to deliberate on the same topic. The brief Sydney Declaration stated “…death is a gradual process at the cellular level with tissues varying in their ability to withstand deprivation of oxygen. But clinical interest lies not in the state of preservation of isolated cells but in the fate of a person” [44]. The widespread and growing practice of DCD transplantation, but particularly heart transplantation that appears to stir-up greater ethical questions, requires us to find a more realistic approach in bridging the DDR concept with the loss of an individual defined by integrated higher cerebral functions and therefore exhibiting some level of consciousness. Without an open societal discussion, we are unlikely to justly resolve this debate which is having to deal with further complexity from the recent suggestion of possibly using living donated hearts from Maastricht category V euthanasia donors [18].
The search remains for novel agents that may further extend the resilience of DCD hearts against warm ischaemic injury. Our understanding of extra-corporeal organ perfusion is still in its infancy, and a necessary first step is to reduce the degree of myocardial oedema so that even longer perfusion times and therefore even more distant organ procurements can take place. We are unclear of a reliably safe upper time limit of extra-corporeal perfusion at normothermia, and it is possible that continuous cold perfusion for transportation followed by working heart assessment may be the more practical solution although this technology, despite excellent laboratory results, has yet to commence clinical trials [45].
A DCD heart transplant program has added logistical challenges and requires more resources including cost and personnel. The DCD donor pool should be seen as a reliable source of good-quality hearts that are supplementary to having exhausted the availability of standard DBD hearts which continues to offer the advantage of controlled organ retrieval and maximizes the number of retrievable organs per donor. However, in the absence of suitable DBD hearts, with sufficient local resources and legislative permission, it appears unjustifiable to discard young DCD hearts given the very positive global experience of DCD heart transplantation. The recent initiation of DCD heart transplantation at several programs in the USA is encouraging and further reinforces the need for a broader uptake.
Acknowledgements
The authors thank Dr. Helen Opdam, the National Medical Director of the Australian Organ & Tissue Authority, for collating information of the use of ante mortem heparin in the various Australian states.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 2.Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–1274. [PubMed] [Google Scholar]
- 3.Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359:709–714. doi: 10.1056/NEJMoa0800660. [DOI] [PubMed] [Google Scholar]
- 4.Kleinmahon JA, Patel SS, Auerbach SR, et al. Hearts transplanted after circulatory death in children: Analysis of the International Society for Heart and Lung Transplantation registry. Pediatr Transplant. 2017;21:e13064. doi: 10.1111/petr.13064. [DOI] [PubMed] [Google Scholar]
- 5.Messer S, Large S. Resuscitating heart transplantation: the donation after circulatory determined death donor. Eur J Cardiothorac Surg. 2016;49:1–4. doi: 10.1093/ejcts/ezv357. [DOI] [PubMed] [Google Scholar]
- 6.Garcia Saez D, Bowles CT, Mohite PN, et al. Heart transplantation after donor circulatory death in patients bridged to transplant with implantable left ventricular assist devices. J Heart Lung Transplant. 2016;35:1255–1260. doi: 10.1016/j.healun.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Mehta V, Taylor M, Hasan J, et al. Establishing a heart transplant programme using donation after circulatory-determined death donors: a United Kingdom based single-centre experience. Interact Cardiovasc Thorac Surg. 2019;29:422–429. doi: 10.1093/icvts/ivz121. [DOI] [PubMed] [Google Scholar]
- 8.http://www.newcastle-hospitals.org.uk/news/news-item-23933.aspx.
- 9.https://surgery.duke.edu/news/doctors-duke-university-hospital-perform-first-dcd-heart-transplant-us.
- 10.https://www.massgeneral.org/news/press-release/Massachusetts-general-hospital-performs-first-of-its-kind-heart-transplant-in-new-england.
- 11.https://www.surgery.wisc.edu/2020/01/10/division-of-cardiothoracic-surgery-makes-uw-hospital-the-third-center-in-the-u-s-to-perform-a-donation-after-circulatory-death-dcd-heart-transplant/.
- 12.Messer S, Page A, Colah S, et al. Human heart transplantation from donation after circulatory- determined death donors using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2018;37:865–869. doi: 10.1016/j.healun.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Kushnood A, Butt TA, Jungschleger J, et al. Paediatric donation after circulatory determined death heart transplantation using donor normothermic regional perfusion and ex situ heart perfusion: A case report. Pediatr Transplant. 2019;23:e13536. doi: 10.1111/petr.13536. [DOI] [PubMed] [Google Scholar]
- 14.Tchana-Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38:593–598. doi: 10.1016/j.healun.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Tchana-Sato V, Ledoux D, Vandendriessche K, et al. First report of successful pediatric heart transplantation from donation after circulatory death with distant procurement using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2019;38:1112–1115. doi: 10.1016/j.healun.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Lomero M, Gardiner D, Coli E, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33:76–88. doi: 10.1111/tri.13506. [DOI] [PubMed] [Google Scholar]
- 17.Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29:749–759. doi: 10.1111/tri.12776. [DOI] [PubMed] [Google Scholar]
- 18.Bollen JAM, Shaw D, de Wert G, et al. Euthanasia through living organ donation: ethical, legal, and medical challenges. J Heart Lung Transplant. 2019;38:111–113. doi: 10.1016/j.healun.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Watson A, Gao L, Sun L, et al. Enhanced preservation of pig cardiac allografts by combining erythropoietin with glyceryl trinitrate and zoniporide. Am J Transplant. 2013;13:1676–1687. doi: 10.1111/ajt.12249. [DOI] [PubMed] [Google Scholar]
- 20.Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant. 2014;14:1744–1752. doi: 10.1111/ajt.12782. [DOI] [PubMed] [Google Scholar]
- 21.Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15:371–380. doi: 10.1111/ajt.12994. [DOI] [PubMed] [Google Scholar]
- 22.Iyer A, Chew HC, Gao L, et al. Pathophysiological trends during withdrawal of life support: implications for organ donation after circulatory death. Transplantation. 2016;100:2621–2629. doi: 10.1097/TP.0000000000001396. [DOI] [PubMed] [Google Scholar]
- 23.Iyer A, Wan B, Kumarasinghe G, et al. What is the potential source of heart allografts from donation after circulatory death (DCD) donors? Transplantation. 2013;96:S217. doi: 10.1097/TP.0b013e31829807aa. [DOI] [Google Scholar]
- 24.Listijono DR, Watson A, Pye R, et al. Usefulness of extracorporeal membrane oxygenation for early cardiac allograft dysfunction. J Heart Lung Transplant. 2011;30:783–789. doi: 10.1016/j.healun.2011.01.728. [DOI] [PubMed] [Google Scholar]
- 25.https://donatelife.gov.au/sites/default/files/Brain_Death_Determination_Statement.pdf.
- 26.Cao Y, Shahrestani S, Chew H, et al. Donation after circulatory death for liver transplantation: A meta-analysis on the location of life support withdrawal affecting outcomes. Transplantation. 2016;100:1513–1524. doi: 10.1097/TP.0000000000001175. [DOI] [PubMed] [Google Scholar]
- 27.Connellan M, Dhital K. Donor heart procurement from the donation after circulatory death pathway. Oper Tech Thorac Cardiovasc Surg. 2017;22:58–67. doi: 10.1053/j.optechstcvs.2017.09.005. [DOI] [Google Scholar]
- 28.Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73:1447–1459. doi: 10.1016/j.jacc.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 29.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischaemic preconditioning. AM J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy DJ, Barrabes JA, Botker HE, et al. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao L, Hicks M, Macdonald PS. Improved preservation of the rat heart with Celsior solution supplemented with cariporide plus glyceryl trinitrite. Am J Transplant. 2005;5:1820–1826. doi: 10.1111/j.1600-6143.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 32.Hing AJ, Watson A, Hicks M, et al. Combining cariporide with glyceryl trinitrite optimizes cardiac preservation during porcine heart transplantation. Am J Transplant. 2009;9:2048–2056. doi: 10.1111/j.1600-6143.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Tsun J, Sun L, et al. Critical role of the STAT 3 pathway in the cardioprotective efficacy of zoniporide in a model of myocardial preservation- The rat isolated working heart. Br J Pharmacol. 2011;162:633–647. doi: 10.1111/j.1476-5381.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson AJ, Gao L, Sun L, et al. Enhanced preservation of the rat heart after prolonged hypothermic ischaemia with erythropoietin supplemented Celsior solution. J Heart Lung Transplant. 2013;32:633–640. doi: 10.1016/j.healun.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomized, non-inferiority trial. Lancet. 2015;385:2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 36.Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl Int. 2015;28:634–642. doi: 10.1111/tri.12361. [DOI] [PubMed] [Google Scholar]
- 37.Garcia Saez D, Zych B, Sabashnikov A, et al. Evaluation of the organ care system in heart transplantation with adverse donor/recipient profile. Ann Thorac Surg. 2014;98:2099–2105. doi: 10.1016/j.athoracsur.2014.06.098. [DOI] [PubMed] [Google Scholar]
- 38.Connellan M, Chew H, Iyer A, et al. Ex-vivo perfusion of marginal donor hearts: is normal allograft function assured post-transplant? J Heart Lung Transplant. 2019;25:e92. doi: 10.1016/j.hlc.2015.12.018. [DOI] [Google Scholar]
- 39.Sponga S, Ferrara V, Beltrami AP, et al. Ex-vivo perfusion on marginal donors in heart transplantation: Clinical results and pathological findings. J Heart Lung Transplant. 2019;38:s42–s43. doi: 10.1016/j.healun.2019.01.089. [DOI] [Google Scholar]
- 40.Schroder JN, D’Alessandro D, Esmailian F, et al. Successful utilization of extended criteria donor (ECD) hearts for transplantation – results of the OCS heart EXPAND trial to evaluate the effectiveness and safety of the OCS heart system to preserve and assess ECD hearts for transplantation. J Heart Lung Transplant. 2019;38:s42. doi: 10.1016/j.healun.2019.01.088. [DOI] [Google Scholar]
- 41.Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36:1311–1318. doi: 10.1016/j.healun.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Anthony C, Michel J, Christofi M, et al. Ex vivo coronary angiographic evaluation of a beating donor heart. Circulation. 2014;130:e341–e343. doi: 10.1161/CIRCULATIONAHA.114.010289. [DOI] [PubMed] [Google Scholar]
- 43.No authors listed A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205:337–340. doi: 10.1001/jama.1968.03140320031009. [DOI] [PubMed] [Google Scholar]
- 44.Machando C, Korein J, Ferrer Y, et al. The declaration of Sydney on human death. J Med Ethics. 2007;33:699–703. doi: 10.1136/jme.2007.020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steen S, Paskevicius A, Liao Q, Sjoberg T. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J. 2016;50:193–200. doi: 10.3109/14017431.2016.1154598. [DOI] [PMC free article] [PubMed] [Google Scholar]