Abstract
Brain angiotensin-II (Ang-II) type-1 receptors (AT1R) exert profound effects on normal cardiovascular, fluid and metabolic homeostasis, effects which are overactive in and contribute to chronic sympathoexcitation and hypertension. There is accumulating evidence that activation of AT2R in the brain exerts effects that are opposite to those of AT1R, i.e., lowering blood pressure and reduced hypertension. Thus, it is of interest to understand the relative cellular localization of AT1R and AT2R in the brain under normal conditions, and whether it changes during hypertension. Here we have developed a novel AT1aR-tdTomato reporter mouse in which the location of brain AT1aR was largely consistent with that determined in previous studies. This AT1aR-tdTomato reporter mouse was crossed with our previously-described AT2R-eGFP reporter mice to yield a novel dual AT1aR/AT2R reporter mouse, which has allowed us to determine that AT1aR and AT2R are primarily localized to different populations of neurons in brain regions controlling cardiovascular, fluid and metabolic homeostasis. We also demonstrated, using the individual AT1aR-tdTomato reporters, that during hypertension induced by deoxycorticosterone acetate-salt administration, there is no shift in expression of AT1aR from neurons to microglia or astrocytes in the paraventricular nucleus, a brain area important in sympathetic regulation. Using AT2R-eGFP reporter mice under similar hypertensive conditions, we demonstrated that the same was true of AT2R expression in the solitary tract nucleus, an area critical for baroreflex control. Collectively, these findings provide novel means to co-localize AT1R and AT2R in the brain, and also a novel view of their cellular localization in hypertension.
Keywords: Renin-angiotensin system, neurogenic hypertension, blood pressure, Transgenic reporter mice
Introduction
The renin-angiotensin system (RAS) plays a fundamental role in body fluid and cardiovascular homeostasis under normal physiological conditions.1–3 An important aspect of these RAS-mediated actions is the central nervous system (CNS)-mediated effects of angiotensin II (Ang-II) to augment thirst, sympathetic nerve activity (SNA) and vasopressin (AVP) secretion by stimulating its type-1 receptor (AT1R).4–6 Importantly, the AT1R-mediated effects of Ang-II within the CNS are exacerbated in and contribute to the chronic sympathoexcitation that underlies resistant or “neurogenic” hypertension.7–9 Consistent with these effects of Ang-II under physiological- and pathological conditions, AT1R are located in brain areas and nuclei that control blood pressure and fluid balance, as demonstrated by the use of receptor autoradiography, radioactive in situ hybridization, and transgenic reporter mice.10–15 Aside from AT1R, it is also clear that the brain contains angiotensin type-2 receptors (AT2R)10–12, 16. While the brain localization of AT2R differs from that of AT1R in general, it is evident that AT2R are found within or adjacent to CNS areas/nuclei that contain AT1R and are involved in cardiovascular regulation, including the solitary tract nucleus (NTS), the rostral ventrolateral medulla (RVLM) and the paraventricular nucleus of the hypothalamus (PVN). This particular localization of AT2R in or around cardiovascular control centers is consistent with studies and an emerging view that their activation results in blood pressure lowering and anti-hypertensive effects, possibly via reduction of sympathetic outflow, i.e., actions that are fundamentally opposite to those of AT1R activation.17–29
Considering this, it is of interest to determine the relative cellular localizations of AT1R and AT2R within cardiovascular control centers of the brain, in order to ultimately help determine whether the opposite cardiovascular effects mediated by these receptors are due to opposing actions on the same cells, or due to modulation of different or interrelated neuronal circuits. Furthermore, it is apparent that the pro-hypertensive actions of AT1R involve stimulation of pro-inflammatory mechanisms in the brain, and certain of the anti-hypertensive actions mediated by brain AT2R involve induction of anti-inflammatory mechanisms.18, 30–32 Thus, it is of interest to determine whether resident inflammatory cells of the CNS, microglia and astrocytes33, 34, express AT1R and AT2R within cardiovascular control centers under baseline conditions, and whether that expression changes during neurogenic hypertension.
To address these issues, we first developed two novel genetically-modified mouse strains: (i) We used the Cre-loxP system to develop a novel AT1aR reporter mouse for visualizing the cellular localization of AT1aR in the brain; (ii) We crossed these AT1aR reporter mice with our previously reported AT2R-eGFP reporter mice16, 35 to develop a novel dual AT1aR/AT2R reporter mouse that allowed us to visualize the location of both receptors at the same time.
Male and female dual AT1aR/AT2R mice were used to determine the relative cellular distributions of these receptors in brain regions involved in cardiovascular control and fluid balance. The AT1aR and AT2R-eGFP single reporter mice were used to determine whether the cellular distribution (neurons vs. microglia vs. astrocytes) of these receptors were altered under conditions of established neurogenic hypertension, induced by administration of deoxycorticosterone acetate (DOCA) and salt. In these latter studies, for AT1aR we focused on the PVN, a brain area that is of major importance in sympathetic regulation, and which contains high levels of this angiotensin receptor subtype. For the AT2R studies, we focused on the NTS, an area that is of major importance in baroreflex control and sympathetic outflow, and which is the major site of expression of this receptor subtype in brain cardiovascular control centers.
Materials and Methods
Animals
Mice
Mice were on a C57BL/6J background. Three genetic mouse models that allow for the identification of cells which contain AT1aR and/or AT2R were used in this study.
(i) AT1aR-tdTomato reporter mice: AT1aR-Cre-Zsgreen knock-in mice were generated by the University of Florida and Biocytogen LLC (Worcester, MA) and were previously-validated.36 AT1aR-Cre-Zsgreen-knockin mice were bred with stop-flox-tdTomato mice (Jackson Laboratory, Stock # 007914) in order to generate AT1aR-tdTomato mice for mapping Cre-recombinase expression throughout the brain regions of interest; (ii) AT2R-eGFP BAC reporter mice, that were generated as detailed previously16; (iii) AT1aR-tdTomato/AT2R-eGFP dual reporter mice, that were produced by crossing the AT1aR-tdTomato reporter mice and the AT2R-eGFP BAC reporter mice. For these mouse strains, the tdTomato (AT1aR) signal reflects AT1aR expressing cells which at any time in their developmental history expressed these receptors. On the other hand, the eGFP (AT2R) signal reflects the current expression of AT2R. Further, while these strains allow for identification of cells that contain AT1aR and AT2R, they do not allow for demonstration of where the receptors are located within the cells.
Rats
Male age-matched 12- and 16-week old male Sprague-Dawley (SD), Wistar Kyoto (WKY) and SHR were purchased from Charles River Farms (Wilmington, MA).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida, complied with the National Institutes of Health guidelines, and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Eight Edition, 2011, published by National Academics Press, 500 Fifth Street NW, Washington, DC, 20001, USA). (AALAC #: 000023; OLAW Assurance #: A3377–01).
Animals were housed in temperature- and humidity-controlled rooms on a 12:12 h light/dark cycle with food and water available ad libitum.
DOCA-Salt Hypertension
In some studies, mice were rendered hypertensive via administration of DOCA and subsequent ad libitum access to isotonic saline drink (0.15M NaCl) in addition to continued access to standard drinking water.37, 38 In these studies, DOCA pellets (50 mg, Innovative Research of America, Sarasota, FL) were implanted subcutaneously (sc) in the interscapular region. Immediately after pellet implantation, mice were provided free access to isotonic saline drink for the three-week duration of the study. Controls underwent sham pellet implantation surgery.
Blood Pressure Recordings
To confirm that DOCA-Salt elevated blood pressure in mice, we measured blood pressure using the volume–pressure recording tail-cuff method (Kent Scientific, Torrington, CT). Briefly, mice were restrained and placed on a warming platform. An occlusion cuff was placed close to the base of the tail and then a VPR cuff was placed next to the occlusion cuff. Both the occlusion cuff and VPR cuff were connected to a CODA controller (Kent Scientific, Torrington, CT) that was connected to the computer. The blood pressure signal from the tail cuff was recorded and analyzed using CODA Non-Invasive Blood Pressure System. Each mouse was recorded for seven cycles to minimize recording error.
Tissue collection and sectioning
Mice or rats were anesthetized with pentobarbital and perfused transcardially with 0.15M NaCl followed by 4% paraformaldehyde. Brains were post-fixed for 4 h, after which they were stored in 30% sucrose until sectioned using a CM3050S cryostat (Leica, Buffalo Grove, Illinois). For in situ hybridization, perfused brains were sectioned at 20 μm into 6 serial sections and immediately mounted onto SuperFrost Plus Gold Microscope Slides. After air-drying at room temperature for 20–30 min, slides were stored at −80 ºC. Tissue collection and sectioning were performed in RNase-free conditions. For immunohistochemistry (IHC) studies, brains were sectioned at 30 μm into 4 serial sections and stored in cryoprotective solution at −20 ºC.
Immunohistochemistry
All primary antibodies were characterized by the manufacturers and in previously-published studies [Supplementary Table 1].39–48 Secondary antibodies were purchased from Jackson Immunoresearch, raised in donkey and used at a 1:500 dilution. All IHC protocols used have been described by us previously16, 35, 49 and were performed in at least 4 separate mice/rats. Importantly, for studies conducted in the AT1aR-tdTomato/AT2R-eGFP dual reporter mice, GFP IHC was performed using a Cy5 secondary antibody. This protocol does not produce significant labeling in mice that express only AT1aR-tdTomato, but not AT2R-eGFP.
RNAscope in situ hybridization
RNAscope in situ hybridization for AT1aR mRNA was performed as described.16 The probes were designed using the proprietary Advanced Cell Diagnostics (ACD; Newark, CA) RNAscope Probe Design pipeline, and contain 20 short double-Z oligonucleotide probe pairs that are specific to the genes of interest (i.e., AT1aR, UBC [positive control] and DapB [negative control]). Details concerning the probes for the RNAscope in situ hybridization can be found in Supplementary Table 2. The color label was assigned to FAR RED (Excitation 647 nm; Emission 690 ± 10 nm). Importantly, using this technique, each punctate dot represents a single mRNA molecule.
Image capture and processing
Images were captured and processed using Axiovision 4.8.2 software and a Zeiss AxioImager fluorescent Apotome microscope. In order to generate images of the entire coronal sections depicted in Supplementary Figure 1 and Figure 1, 2.5 × images were captured across the various points of the sections. Subsequently, ImageJ was used to generate stitched mosaics images comprising coronal sections taken through specific levels of the forebrain or hindbrain. These mosaics were used for the analysis reported in Supplementary Table 3.
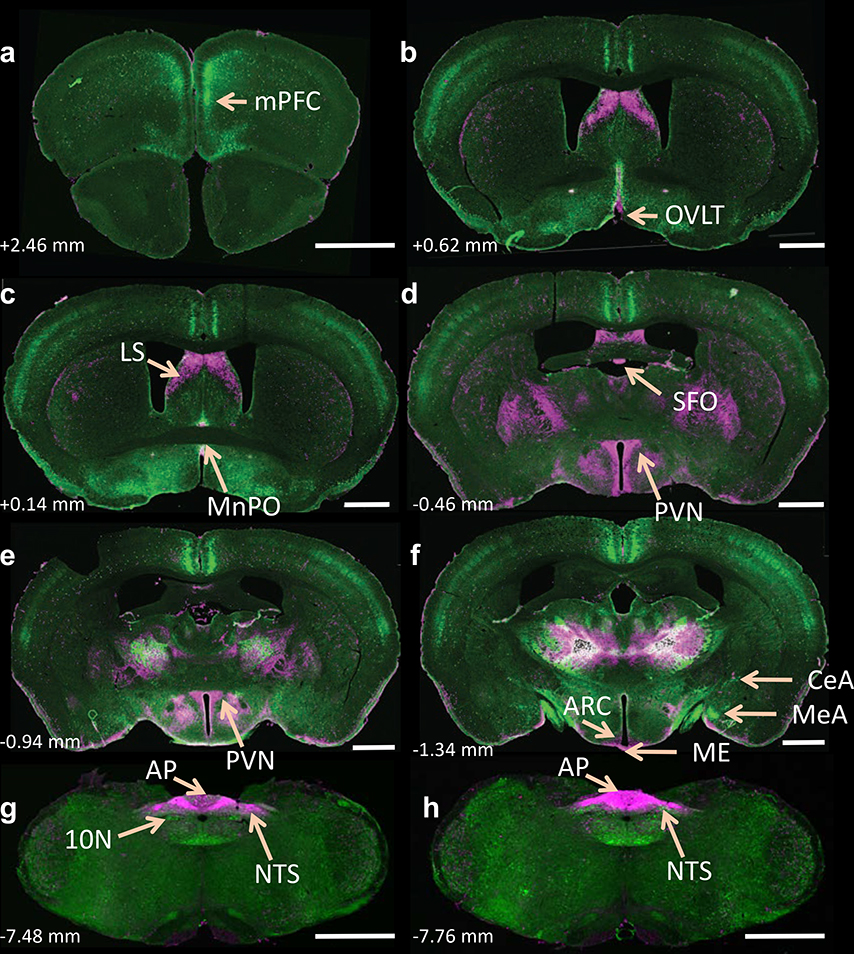
Figure 1. Localization of tdTomato- and eGFP fluorescence throughout the brain of dual AT1aR-tdTomato/AT2R-eGFP reporter mice.
(a-h) Low magnification coronal sections through selected brain regions of a male dual AT1aR-tdTomato/AT2R-eGFP reporter mouse. AT1aR-tdTomato fluorescence is magenta; AT2R-eGFP fluorescence is green. The number in the lower left of each image indicates the approximate distance rostral/caudal from bregma, in accordance with the mouse brain atlas 50. mPFC = medial prefrontal cortex, MnPO = median preoptic nucleus, LS = lateral septum, AH = anterior hypothalamus, PVN = paraventricular nucleus of the hypothalamus, MeA = medial amygdala, CeA = central amygdala, NA = nucleus ambiguus, NTS = nucleus of the solitary tract, 10N = dorsal motor nucleus of the vagus, AP = area postrema. Scale bars = 1 mm. These images are representative of 8 mice.
For IHC studies included in Figures 2, 3 and 5, images were captured at 5 × - 10 × and z-stacks were captured at 20 × throughout the ROIs using neuroanatomical landmarks found in a mouse brain atlas 50. In these cases, the exposure time was adjusted using the best fit feature in Axiovision in order to provide optimal visualization. For dual IHC/RNAscope ISH included in Figure 4, z-stacks of the proteins and transcripts of interest were captured at 20 × magnification throughout the ROIs. In all cases, z-steps were set at 0.5 μm, with an average of 20 optical sections per image and these z-stacks were used to generate the projection images displayed in the photomicrographs. For each dual IHC/RNAscope ISH experiment, sections hybridized with the positive control probes were used to determine the exposure time and image processing required to provide optimal visualization of RNA signal. As described, these same parameters were then used for visualization of mRNA transcripts of interest and to assess background fluorescence in sections hybridized with negative control probe (DapB).16 Importantly, using these exposure times and image processing parameters there was negligible fluorescence in sections hybridized with the negative control probe.
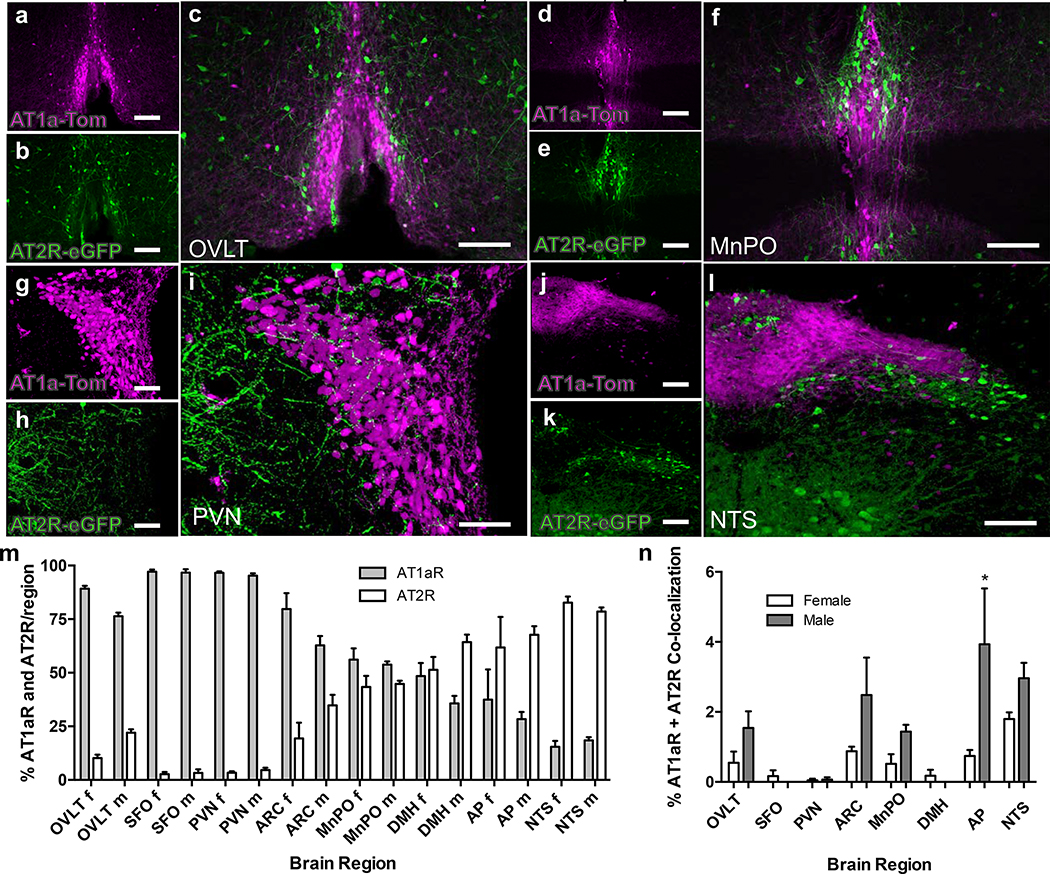
Figure 2. Relative localization of tdTomato- and eGFP fluorescence within the OVLT, MnPO, PVN and NTS of dual AT1aR-tdTomato/AT2R-eGFP reporter mice.
High magnification images through the OVLT (a, b), MnPO (d, e), PVN (g, h,) and NTS (j, k) of a male dual AT1aR-tdTomato/AT2R-eGFP reporter mouse showing AT1aR-tdTomato (magenta) and AT2R-eGFP (green). The respective merged images are shown in panels c (OVLT), f (MnPO), i (PVN) and l (NTS). Scale bars = 100 μm (a, b, d, e, g, h, j and k) and 50 μm (c, f, i, l). These images are representative of 8 mice. Bar graphs in panels m and n are analyses of the relative levels of AT1aR- and AT2R-positive cells within selected brain regions of female and male AT1aR-tdTomato/AT2R-eGFP reporter mice. (m) The percentage of AT1aR and AT2R within the regions indicated are plotted as means ± SEM for both female [f] and male [m] mice. OVLT = organum vasculosum of the lamina terminalis; SFO = subfornical organ; PVN = paraventricular Nucleus; ARC = arcuate nucleus; MnPO = median preoptic nucleus; DMH = dorsomedial hypothalamus; AP = area postrema; NTS = nucleus of the solitary tract. Data are from 4 female and 4 male AT1aR-tdTomato/AT2R-eGFP reporter mice. (n) The percentage of AT1aR and AT2R co-localization on the same cell within the selected regions are plotted as means ± SEM for both female and male mice. * P<0.05 vs. female AP.
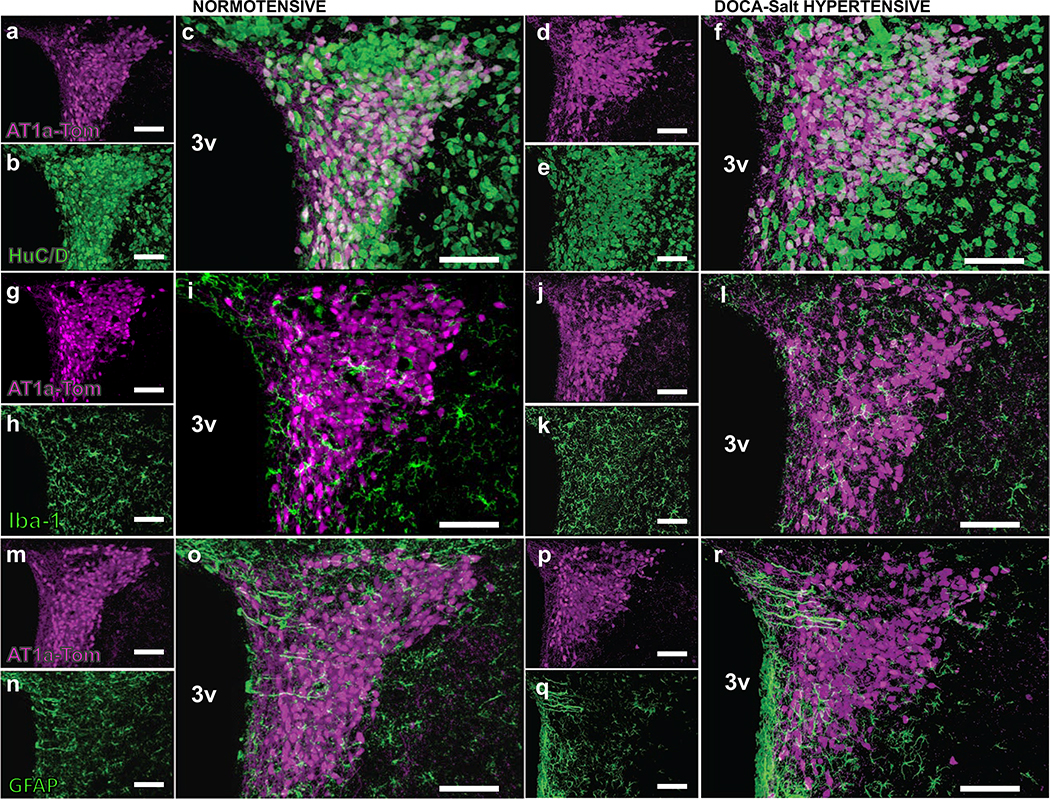
Figure 3. Cellular localization of AT1aR-positive cells within the PVN of normotensive - and DOCA-salt hypertensive mice.
Projection images from the PVN of male normotensive and DOCA-salt hypertensive mice showing the location of AT1aR-tdTomato fluorescence in relation to neurons, microglia and astrocytes. The location of tdTomato fluorescence (magenta) is illustrated in panels a, g and m (control normotensive mice) and d, j and p (DOCA-salt hypertensive mice). Panels b and e demonstrate HuC/D (neuron-specific marker; green) immunostaining of the same sections as shown in a and d, respectively. The merged images (c and f) illustrate that the tdTomato fluorescence is entirely associated with HuC/D. Panels h and k demonstrate Iba-1 (microglial marker; green) immunostaining of the same sections as shown in g and j, respectively, while panels n and q are GFAP (astrocyte marker; green) immunostaining of the same sections as shown in m and p. The merged images (i and l for Iba-1; o and r for GFAP) indicate that none of the tdTomato fluorescence is associated with microglia or astrocytes. 3V = 3rd cerebroventricle. These images are representative of 4 mice/group. Scale = 100 μm.
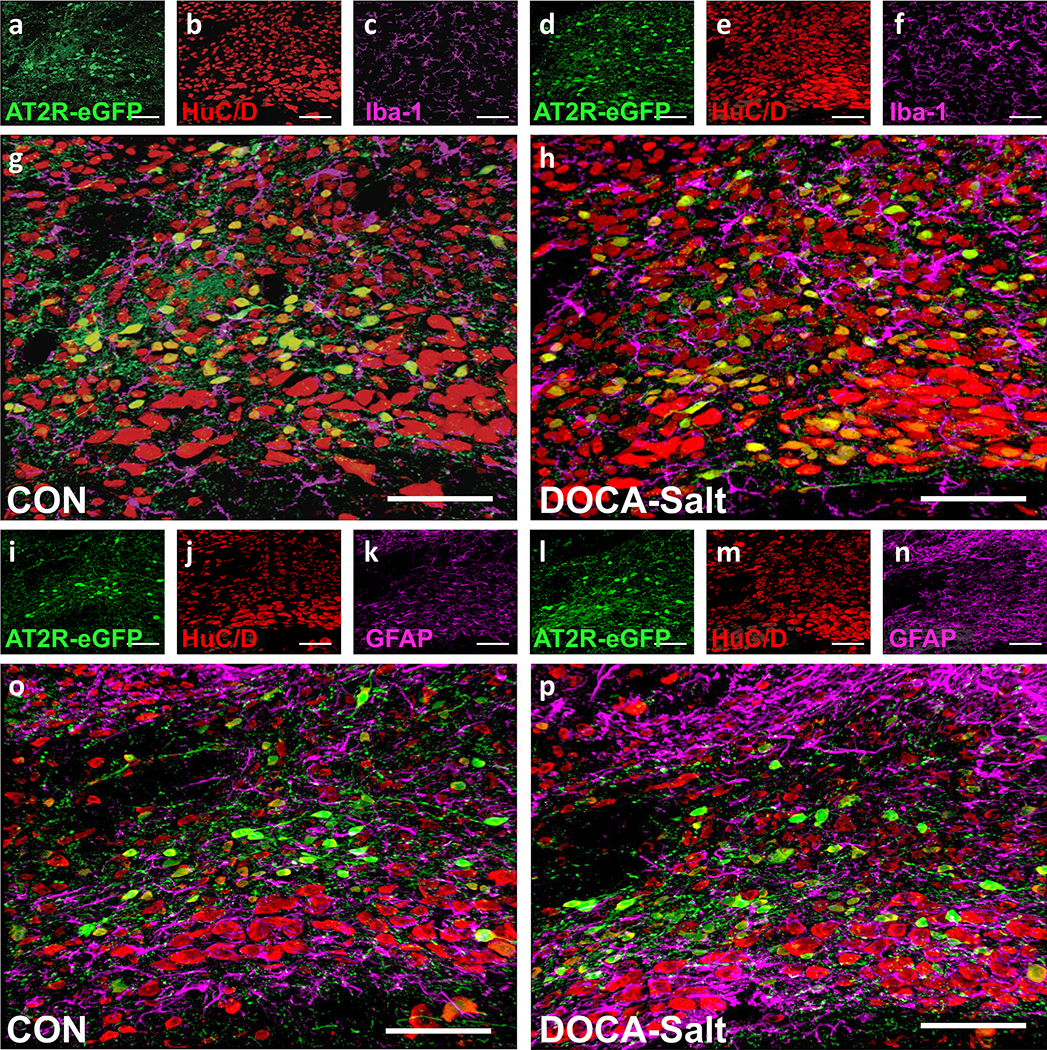
Figure 5. Cellular localization of AT2R-positive cells within the intNTS of normotensive - and DOCA-salt hypertensive mice.
Projection images through the intNTS of male control normotensive- and DOCA-salt hypertensive mice showing the location of AT2R-eGFP fluorescence (green) in relation to neurons, microglia and astrocytes.
(a-c, g) CON or (d-f, h) DOCA-salt AT2R-eGFP mice depicting eGFP (a, d), HuC/D (b, e), and Iba-1 (c, f) immunoreactivity, and the respective merged images (g, h).
(i-k, o) CON or (l-n, p) DOCA-salt AT2R-eGFP mice depicting eGFP (i, l), HuC/D (j. m), and GFAP (k, n) immunoreactivity and the respective merged images (o, p). These images are representative of 8 mice/group. Scale bars are 100 μm.
Figure 4. Cellular localization of AT1aR mRNA in the PVN of normotensive rats and SHR.
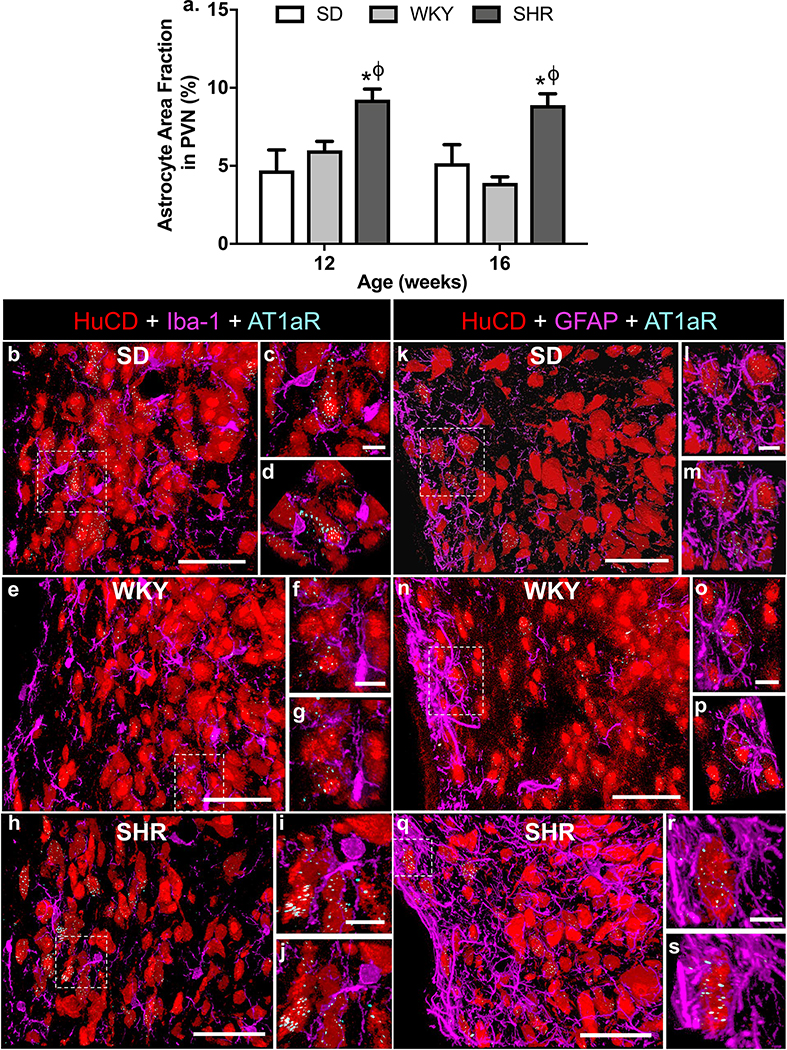
(a) Naïve male SD and WKY rats, or SHR, were prepared for GFAP immunostaining in the PVN as detailed in the methods. Bar graph is quantification of GFAP immunoreactivity within the PVN of 12- and 16-week old SD and WKY rats, and SHRs, using Image J. Data are presented as a summation of the astrocyte area fraction in the medial and posterior PVN (means ± SEM), for each set of rats, and are from 4 SHR, and 3 WKY- and 4 SD rats at 12- and 16-weeks of age. * p < 0.05 SHR vs. SD; Φ p < 0.05 SHR vs. WKY. (b - s) Representative fluorescence images showing the localization of Iba-1 (left, panels b - j) and GFAP (right, panels k - s) immunoreactivities (both magenta) in the PVN of SD and WKY rats, and SHR. Immunoreactivity for the neuronal marker HuCD is red, and AT1aR mRNA is indicated by the cyan dots. The higher power images (c, d; f, g; I, j; l, m; o, p; r, s) are from the areas indicated by hashed-lines in the respective lower power images. Each higher power image was taken at two different angles, to enable discrete cellular localization of the AT1aR mRNA. Sections are from the medial PVN, ~ −1.8 mm relative to bregma 51, and are representative of 3 (WKY) and 4 (SD; SHR) rats. Sections taken from more rostral or caudal parts of the PVN exhibited a similar cellular distribution of AT1aR. Scale for lower power images = 50 μm; Scale for higher power insets = 10 μm.
All final figures were then prepared using Adobe Photoshop 7.0 where the brightness and contrast was adjusted to provide optimal visualization.
Analysis of AT1aR- and AT2R-expressing neurons
Mosaics of coronal sections through 4 separate mouse brains were analyzed to determine the number of tdTomato positive cell bodies and/or the presence of tdTomato fibers or terminals within brain regions important for cardiovascular function, fluid balance and metabolism. The number of sections analyzed are dependent on the rostrocaudal length of the particular ROI analyzed. For example, only two sections are analyzed through the OVLT of each mouse, whereas approximately 15 sections throughout the NTS are analyzed for each mouse. Criteria for scoring the number of AT1aR-positive cell bodies were as follows: 0 = no tdTomato cells within the ROI, 1 = 1–25% of the area occupied by tdTomato-positive cell bodies, 2 = 26–50% of the area occupied by tdTomato-positive cell bodies, 3 = 51–75% of the area occupied by tdTomato-positive cell bodies, and 4 = 76–100% of the area occupied by tdTomato-positive cell bodies. All sections were analyzed by two separate investigators and mean score for each brain region was calculated and rounded to the nearest whole number. Brain regions that received a score between 0 and 0.5 are listed as <1. The presence of fibers or terminals was assessed in the same sections and are scored as + (indicating that there are scattered fibers/terminals present within the ROI), ++ (indicating that there are dense fibers/terminals present within the ROI) or – (indicating that no fibers/terminals were observed within the ROI).
Analysis of AT1aR-tdTomato and AT2R-eGFP co-localization was performed by two independent investigators on 10 × z-stacks throughout the ROIs of four male and four female AT1aR-tdTomato/AT2R-eGFP dual reporter mice. In order to generate percent expression of AT1aR vs. AT2R and their co-localizations within these areas, the total number of AT2R-eGFP, AT1aR-tdTomato, and double-labeled neurons were assessed. In order to determine co-localization of AT1aR-tdTomato and the microglial (Iba-1), astroglial (GFAP) and neuronal (HuC/D) markers within the PVN of normotensive (n = 4) vs. DOCA-salt hypertensive (n = 4) mice, sections throughout the area were evaluated by two independent investigators. A similar procedure was used to evaluate co-localization of AT2R-eGFP and the microglial (Iba-1), astroglial (GFAP) and neuronal (HuC/D) markers within the NTS (n = 4/group).
Analysis of astroglial area fraction
Astroglial area fraction within rat PVN, as indicated by the level of GFAP immunostaining, was quantified by Image J (NIH) analysis of fluorescence micrographs obtained from the anterior, medial and posterior PVN, as identified using neuroanatomical landmarks described by Paxinos and Watson 51. PVN regions of interest (ROI) were then converted into gray scale and binary formats, and thresholds for black and white balance were adjusted to the same level for each ROI. At each PVN level, counts from both PVN sides were collected and averaged. Quantified results were plotted as percentages of cells that were GFAP-positive in each ROI. Astroglial area fraction in the anterior portion of the PVN exhibited no changes under any of the tested conditions, and so the data presented in the Results section of this manuscript are from the medial (−1.44 mm) and posterior (−2.16 mm) levels of the PVN [coordinates from bregma].
Results
AT1aR-tdTomato reporter mice
We developed an AT1aR-tdTomato reporter mouse that was to be used in two ways in this manuscript: (i) To generate a novel AT1aR-tdTomato/AT2R-eGFP dual reporter mouse line by crossing it with our existing AT2R-eGFP BAC reporter mice; (ii) To study the cellular localization of AT1aR in neurons vs. glia during hypertension. Before being used in these ways, it was important to perform certain characterization experiments with the AT1aR-tdTomato reporter mice. First, as noted in the Methods, the tdTomato (AT1aR) fluorescence signal not only indicates cells that currently express AT1aR, but also reflects cells that may have expressed AT1aR at an earlier developmental stage. The extent to which this tdTomato fluorescence is indicative of current AT1aR expression was assessed in a previous study via in situ hybridization for AT1aR mRNA in tissue sections collected from the AT1aR tdTomato reporter mouse 36, where we demonstrated that ~97% of tdTomato neurons within the PVN contain AT1aR mRNA 36. Second, it was important to demonstrate that the pattern of AT1aR expression within the AT1aR-tdTomato reporter mouse brain, as indicated by tdTomato fluorescence, was generally consistent with that observed in previous studies which had utilized receptor autoradiography, radioactive in situ hybridization, and transgenic reporter mice to assess AT1aR localization in rodent brains.10–15 This is illustrated by Supplementary Figure 1, which contains representative fluorescence micrographs of coronal brain sections through the subfornical organ (SFO), the organum vasculosum of the lamina terminalis (OVLT), the PVN, the MnPO and the area postrema (AP) of a male mouse. These illustrations demonstrate that, within the AT1aR-tdTomato reporter mouse brain, the tdTomato fluorescence is present in areas that are important for cardiovascular function, fluid balance and metabolism. These include the subfornical organ (SFO), the organum vasculosum of the lamina terminalis (OVLT), the PVN, the median preoptic nucleus (MnPO), the area postrema (AP), and the NTS. In addition, Supplementary Table 3 presents a survey of AT1aR-tdTomato-positive cells and fibers throughout these specific sections, with emphasis on the brain nuclei important for cardiovascular function, fluid balance and metabolism. This distribution is largely consistent with the known distribution of AT1aR in the brain, as demonstrated in previous studies10–15. An important point to note is that although the AT1aR-Cre knock-in mouse used to generate AT1aR-tdTomato reporter mice were intended to also express zsGreen in AT1aR-containing cells, zsGreen fluorescence is not observed in the brains of these mice, nor does immunohistochemistry using primary antibodies that detect GFP amplify any type of zsGreen signal. Based on these collective results studies, we believed that the AT1aR tdTomato reporter mouse was an appropriate model to cross breed with our AT2R-eGFP BAC reporter mice and produce a novel AT1aR-tdTomato/AT2R-eGFP dual reporter mouse, and also for use in investigating the neuronal/glial localization of AT1aR in hypertension.
AT1aR and AT2R are localized primarily to separate populations of neurons
Several lines of evidence suggest that AT1R and AT2R activation counteract one another via opposing intracellular signaling cascades52; however, direct evidence localizing AT1R and AT2R to the same cells within the brain is limited. For the present studies, we generated a dual AT1aR-tdTomato/AT2R-eGFP mouse line by cross-breeding the AT1aR-tdTomato and AT2R-eGFP BAC reporter mice, and performed an analysis of the co-localization of the two receptor subtypes within brain areas important for regulating the cardiovascular system, fluid balance and metabolism. Figure 1 depicts representative low power fluorescence micrographs from coronal brain sections of a male dual reporter mouse and provides a stark visualization of the mostly distinct localization of AT1R (magenta)- and AT2R (green)-positive cells in the mouse brain. This is particularly apparent in the brain areas of specific interest to the current study, as shown in Figure 2, which includes representative higher magnification fluorescence images of AT1aR-tdTomato/AT2R-eGFP fluorescence in the OVLT (a-c), MnPO (d-f), PVN (g-i), and nucleus of the solitary tract (NTS; j-l). Analyses of the relative levels of AT1aR- and AT2R-positive cells within brain sections obtained from male and female mice are shown in Figure 2m, and revealed that: (i) The OVLT, SFO, PVN and arcuate nucleus (ARC) contain greater levels of AT1aR vs. AT2R-containing cells, while the opposite can be said for the NTS and AP; (ii) Levels of AT1aR- and AT2R positive cells in the MnPO are roughly equal; (iii) In most cases, there are no gender differences in the absolute levels of AT1aR and AT2R-positive cells from region to region. An exception is the dorsomedial nucleus of the hypothalamus (DMH), where females express almost equal levels but males contain more AT2R-positive cells. Analyses of the co-localization of the two receptor subtypes on the same cell revealed that only a minority of the cells within these regions of interest contain both AT1aR and AT2R (Figure 2n). In fact, within the SFO, PVN and DMH, AT1aR and AT2R co-localization represents <0.2% of the total population of these subtypes present. In the other regions examined, the co-localization of these receptor subtypes is scarcely more, ranging from 0.5 to 3.9 %, but interestingly is greater in the males in all cases, reaching significance in the AP (Figure 2n). However, the overall implication from these studies is that, in these brain regions, AT1aR and AT2R are localized primarily to separate neurons.
Cellular localization of AT1aR in the PVN under baseline conditions and during hypertension
Both the normotensive dual AT1aR-tdTomato/AT2R-eGFP reporter mouse and the AT1aR-tdTomato reporter also revealed that the AT1aR-positive cells within the brain are mostly neuronal-like. Considering that the pro-hypertensive actions of AT1R involve stimulation of pro-inflammatory mechanisms in the brain53, we investigated the cellular distribution of AT1aR during DOCA-salt-induced hypertension, an experimental model of neurogenic hypertension. Specifically, we utilized the AT1aR-TdTomato reporter mice to determine whether AT1aR expression increases in the resident inflammatory cells of the CNS, microglia and astrocytes36, during hypertension. We focused on the PVN because it is a major center for sympathetic- and neuroendocrine control 54, because lesioning the PVN prevents the development of hypertension in DOCA-salt treated rats and in SHR55, 56, and also because previous studies demonstrated increased expression of AT1R and AT1R mRNA in this area in hypertensive animals.18, 57 AT1aR-tdTomato reporter mice were implanted with sham or DOCA pellets and given ad libitum access to isotonic saline drink, in addition to their normal drinking water. After three weeks, systolic blood pressure in the DOCA-treated mice (112.0 ± 4.57 mmHg; n = 4) was significantly higher than that of the controls (87.99 ± 2.88 mmHg; n=4; t (6) = 4.45; p = 0.002). Following blood pressure measurements, mice were euthanized for immunohistochemical studies. Figure 3 depicts representative coronal sections through the PVN of these DOCA-salt-treated or control AT1aR reporter mice that were immunohistochemically labeled for microglial, astrocyte or neuronal markers. As expected, under either baseline or hypertensive conditions there was significant overlap between AT1aR-tdTomato fluorescence (magenta) and the neuronal marker HuC/D (green; Figure 3a–f). The control normotensive mice exhibited no overlap between AT1aR-tdTomato fluorescence and either the microglial (Iba-1) or astrocyte (GFAP) markers throughout the PVN (Figures 3g–i; 3m–o). This was largely expected given previous publications which indicate that within the brain in vivo, AT1R are almost exclusively localized to neurons.15, 58 Interestingly, in DOCA-salt-treated hypertensive mice there was also no overlap between AT1aR-tdTomato fluorescence and the microglial (Iba-1) or astrocyte (GFAP) markers in the PVN (Figures 3j–l; 3p–r). Similar results were obtained when the MnPO, SFO, AP and NTS were probed for co-localization of tdTomato with HuC/D, GFAP or Iba-1 (data not shown).
SHR are another experimental model of neurogenic hypertension, and previous studies indicated that SHR with established hypertension (12–21 weeks-old) exhibit increased numbers and activation of microglia within the PVN.59 Our current immunostaining experiments and quantification of the GFAP-positive astrocyte area fraction within the PVN (medial to posterior regions) of 12- and 16-week old SHR indicate that numbers of astrocytes are significantly increased compared with normotensive SD and WKY rats (Figure 4a). Despite the increased numbers and activation of astrocytes and microglia in the PVN of SHR with established hypertension, RNAscope analyses of AT1aR mRNA provided no evidence for the presence of this angiotensin receptor subtype on these glial cells within SHR PVN. This is illustrated in Figures 5b–s, which contain representative coronal sections through the medial PVN of a 12-week old SHR and age-matched WKY- and SD rats. Panels b, e and h are respective lower power images from the PVN of SD- and WKY rats and SHR, indicating that the AT1aR mRNA (cyan dots) is associated with neurons (red, HuCD-positive) but not with microglia (magenta, Iba-1-positive). This cellular distribution was confirmed by the higher power micrographs which were taken in two different planes (panels c, d [SD]; panels f, g [WKY]; panels i, j [SHR]). Similarly, panels k - m, n - p and q - s show that AT1aR mRNA is associated with neurons and not astrocytes (magenta, GFAP-positive) in the PVN of SD- and WKY rats, and SHR, respectively.
Cellular localization of AT2R in the NTS under baseline conditions and during hypertension
Preliminary observations from the normotensive AT1aR-tdTomato/AT2R-eGFP dual reporter mouse suggested that the AT2R-positive cells within the brain are mostly neuronal-like, consistent with our previous observation from the AT2R-eGFP BAC reporter mice.16 However, some anti-hypertensive actions mediated by brain AT2R involve induction of anti-inflammatory mechanisms.18 Thus, using AT2R-eGFP reporter mice, we examined the expression of AT2R on microglia and astrocytes during DOCA-salt-induced hypertension. For these experiments we focused on the intermediate NTS (intNTS) because it contains one of the major populations of AT2R in the brain (Figure 2)60, and studies have indicated that activation of AT2R in the NTS modulates blood pressure and baroreflex function.23, 27–29 The fluorescence micrographs in Figures 5a–c, g and i–k, o is that under control normotensive conditions, the AT2R-eGFP in the intNTS is co-localized entirely with neurons (HuC/D-positive), and there is no co-localization of AT2R-eGFP with either Iba-1-positive microglia or GFAP-positive astrocytes, as expected. The cellular localization of AT2R was unchanged during DOCA-salt hypertension, i.e., the AT2R-eGFP was associated with neurons, and not with microglia nor astrocytes (Figures 5d–f, h; l–n, p).
Discussion
The major novel findings of this study are that: (i) We have developed a AT1aR-tdTomato reporter mouse that was crossed with our existing AT2R-eGFP BAC reporter mouse to yield a novel AT1aR/AT2R dual reporter mouse; (ii) This AT1aR-tdTomato/AT2R-eGFP dual reporter mouse has revealed the relative cellular localization of AT1aR/AT2R in the adult brain, and while they are primarily localized to different populations of neurons, in a few cases, there are neurons that express both; (iii) In AT1aR-tdTomato reporter mice made hypertensive via chronic DOCA-salt treatment, there is no shift in expression of AT1aR from neurons to microglia or astrocytes in the PVN. This was confirmed in SHR, another model of neurogenic hypertension, where there was similarly no difference in the neuronal vs. glial distribution of AT1aR in the PVN compared with WKY and SD normotensive rats; (iv) In AT2R-eGFP BAC reporter mice made hypertensive via chronic DOCA-salt treatment, there is no shift in expression of AT2R from neurons to microglia or astrocytes in the intNTS.
In the transgenic AT1aR-tdTomato reporter mouse used in the present study, the distribution of tdTomato fluorescence was predominantly consistent with the previous in situ hybridization, receptor autoradiography and AT1aR reporter mouse studies with respect to regional localization.10–15, 61 For example, and as expected, key cardiovascular-, fluid homeostasis- and neuroendocrine control centers in the brain such as the SFO, OVLT, PVN, MnPO, NTS and AP are rich in AT1aR-positive cells and fibers, consistent with previous studies. The location of AT1aR at these areas, as evidenced by the findings from the AT1aR reporter mice, is consistent with the well-described AT1R-mediated actions of Ang-II at these brain sites: the SFO and OVLT in water- and salt intake 62–65; the PVN in sympathetic outflow, blood pressure regulation, energy balance and corticotropin releasing hormone (CRH) secretion60, 66–68; the MnPO in renal and cardiovascular regulation, and water intake 69, 70; the NTS in baroreflex and blood pressure control71, 72; and the AP in sympathetic outflow and blood pressure control.73, 74 Therefore, the present studies corroborate the validity of the AT1aR-Cre mouse line, as well as the utility of the AT1aR-tdTomato mouse to report for AT1aR localization. Based upon this, our AT1aR-tdTomato mouse could be used to provide a detailed characterization of the phenotype of cells that AT1aR are localized to throughout the brain. Knowledge of the identity of AT1aR-containing cells could then be used to assess function of these receptors. For example, in a recent study using this mouse, we demonstrated that with regard to neurons that originate in the PVN, the AT1aR are located on corticotropin releasing hormone (CRH) and thyrotropin releasing hormone neurons that project to the median eminence 36, consistent with the ideas put forth by Lenkei et al.58 and Oldfield et al.75 using traditional IHC and in situ hybridization approaches. Furthermore, optogenetic activation of these AT1aR-containing neurons resulted in significant elevations of plasma adrenocorticotrophic hormone and corticosterone, as well as thyroid-stimulating hormone and thyroxine.36 In the same study, we demonstrated that pre-autonomic neurons that project to the RVLM do not contain AT1aR36, suggesting that the increased blood pressure resulting from stimulation of AT1aR in the PVN is not a direct effect of Ang-II on sympathetic pathways. With the anatomical knowledge gained from the AT1aR reporter mouse, our future studies will target the functionality of AT1aR-containing neuron populations in areas beyond the PVN, such as the MnPO.
However, using the AT1aR-tdTomato mouse to perform a detailed analysis in the phenotype of AT1aR-containing neurons was not a goal of the present study. Rather, we utilized this mouse to help produce an AT1aR/AT2R dual reporter, and to help determine the cellular distribution of brain AT1aR during hypertension. With regard to the former, the AT1aR-tdTomato/AT2R-eGFP dual reporter mouse has allowed for visualization and comparison of the discrete cellular localization of AT1aR and AT2R in the brain. Previous receptor autoradiography techniques in rats and humans 11, 12, 61, 76–78 had illustrated the regional/area distribution of AT2R in the brain, and early conclusions were that AT1R and AT2R exhibit mostly exclusive localization, with the former present in areas that are important in cardiovascular and fluid balance regulation, and the latter in brain areas that control sensory and cortical functions. However, in situ hybridization studies had revealed that AT2R do exist in areas that are important in cardiovascular regulation, such as the NTS.10 Further, our recent study using adult AT2R-eGFP reporter mice has provided a more discrete view of AT2R localization in the brain, including the presence of these sites within or adjacent to areas that are important for cardiovascular regulation and fluid balance, such as the NTS, AP, MnPO, OVLT and SFO.35 The AT1aR-tdTomato/AT2R-eGFP dual reporter mouse developed here and used in the present study has re-emphasized that localization. Any apparent differences in brain location of AT1aR and AT2R in rats and humans gained from receptor autoradiography compared with their location in the reporter mice used in the present study could be for a number of reasons: (i) Species differences; (ii) As mentioned earlier, the tdTomato fluorescence in the AT1aR reporter, and indeed the eGFP fluorescence in AT2R-eGFP BAC reporters, do not reveal where the receptors reside within the cells. So, for example, the presence of eGFP fluorescence in cell bodies of neurons close to the OVLT may not necessarily mean that AT2R are located on the cell bodies; they may be located where the terminals of those neurons reside, potentially at a distant site. This could explain possible discrepancies with autoradiographic studies, which should reveal the general area where the receptors are located; (iii) As the reporter mouse model enables discrete detection of these receptors to individual cells, it means that cells with low expression of AT1aR or AT2R in mice can now be visualized, whereas this may not have been possible with the receptor autoradiography technique that was used in rats and humans.
Our current studies using the AT1aR-tdTomato/AT2R-eGFP dual reporter mouse have revealed that in certain brain areas the number of AT2R-positive neurons exceeds that, or is at least equal to, the number of AT1R-positive neurons (Figure 2m). Our studies have also demonstrated that the expression of AT1R- and AT2R is largely (>95%) on separate populations of neurons, and that there are no gender differences in the expression of these receptors (Figure 2m). Interestingly, however, in situations where there is co-localization of these receptors on the same neuron (OVLT, ARC, MnPO, AP and NTS; ~1–4% overlap), there is a strong trend for more co-localization in males vs. females (Figure 2n). Based on the largely exclusive expression of AT1R- and AT2R to separate populations of neurons in brain regions that control cardiovascular function, fluid homeostasis and metabolism, one might speculate that functional influences of these receptors are via separate mechanisms; for example, opposite effects on blood pressure and baroreflex function, mediated by different neuronal circuitry, as we proposed previously 19. Despite this, and even though the co-localization of AT1R and AT2R on the same cell never exceeds 4%, we cannot exclude at this point the possibility that intracellular interactions between these receptors have a role in controlling cardiovascular function, fluid homeostasis and metabolism.
One thing that is apparent from our present and previous studies using the AT1aR-tdTomato reporter-, the AT2R-eGFP reporter-, and the dual reporter mice is that in normal, adult animals the AT1R and AT2R are localized to neurons, and not to astrocytes nor to microglia, in the areas studied (PVN, MnPO, NTS, AP, OVLT). This is in concert with previous publications which indicate that within the normal brain in vivo, AT1R and AT2R are almost exclusively localized to neurons, rather than astrocytes or microglia.15, 58, 79–81 Previous studies have demonstrated increased expression of AT1R in the PVN of hypertensive animals.18, 57 Thus, we wanted to determine whether AT1R are also expressed in glia at this site, during hypertension. Our results indicate that under conditions of neurogenic hypertension, AT1aR-tdTomato reporter mice made hypertensive by administration of DOCA-salt [Figure 3], and SHR [Figure 4], the AT1aR-tdTomato fluorescence remains localized only to PVN neurons, and are not found on glia. Interestingly, in other studies we have observed increased levels of AT2R mRNA within the same population of neurons in the intNTS of DOCA-salt hypertensive mice (de Kloet et al., unpublished data). Consistent with this, in the current study it is clear from the data in Figure 5 that in DOCA-salt hypertension that the AT2R-eGFP fluorescence remains localized to neurons, and is not associated with astrocytes or microglia. It will be interesting to determine whether a similar pattern of cellular location of AT1aR and AT2R is evident during other forms of hypertension, such as obesity-induced hypertension or renovascular hypertension.
Concerning the PVN and neurogenic hypertension, if the AT1aR are localized exclusively to neurons at this site, then this has a number of implications for the mechanisms underlying the chronic sympathoexcitation and neuroinflammatory processes which occur during sustained high blood pressure.30, 82, 83 For example, this suggests that the microgliosis which occurs in the PVN in hypertension that involves Ang II/AT1aR is not due to direct actions of Ang II at AT1R on microglia. Rather, it may be due to an effect of Ang II via neurons, which then exert a paracrine action to recruit microglia to the area, where they subsequently proceed to increase neuroinflammation via secretion of cytokines. The fact that our previous studies indicate that the AT1aR in the PVN are not localized to pre-autonomic neurons, but to neuroendocrine cells36, adds another layer of complexity to the cellular mechanisms by which Ang II can exert neuroinflammation. There are at least two possibilities for these mechanisms: (i) That Ang II acts directly in the PVN to influence the neuroendocrine cells which, via a local paracrine effect can lead to neuroinflammation that influences the pre-autonomic neurons; (ii) Another possibility is that the AT1R-containing neuroendocrine cells influence the pre-autonomic neurons via local neuronal circuitry, and there is subsequent secretion of chemokines that influence microglial migration and activation.
Despite our novel findings, multiple questions and avenues for further research remain. For example, within the realm of cellular distribution of AT1aR and AT2R, we have not used the respective reporter mice to evaluate their presence on cerebral vessels. Along the same lines of cellular location of AT1aR, our current in vivo data indicate that these receptors are located exclusively to neurons in the PVN of the AT1aR-tdTomato reporter mouse conflict with two previous studies that suggested the presence of AT1R on astrocytes and microglia at this hypothalamic nucleus.84, 85 In those studies, Percoll density gradients were used to isolate either astrocytes from PVN punches or microglia from macro-dissected hypothalamus, and the presence of AT1R in the isolated cells was confirmed by RT-PCR.84, 85 However, it is extremely difficult to dissect only the hypothalamus (or indeed the PVN) for such isolation procedures, and so it is possible that the isolates used in the above studies contain glia from brain tissue surrounding the hypothalamus. The presence of AT1R on glia in these surrounding tissues might explain the discrepancy between our current findings and these previous studies. Thus, our future studies will include a more detailed investigation of whether AT1R exist on astrocytes and microglia in other areas throughout the AT1aR-tdTomato reporter mouse brain. This is warranted as most, but not all, studies using brain cell cultures prepared from whole brain or large brain areas, have suggested that functional AT1R exist on glia86–92
In summary, the AT1aR reporter, AT2R reporter- and AT1aR/AT2R dual reporter mice used in this study have provided essential neuroanatomical information on the cellular location of angiotensin receptor subtypes in the brain, under normal and diseased (resistant hypertension) conditions. As such, they lay the foundation for further anatomical studies to identify the phenotype of neurons that harbor AT1aR and AT2R, and also for functional (optogenetic) approaches to identify the roles of specific AT1aR- and AT2R- containing neuron populations in cardiovascular and fluid homeostasis and in neuroendocrine control, under normal and disease states.
Supplementary Material
Acknowledgements
This work was supported by AHA grant 17GRNT33660969 and NIH grants HL-125805 (ADdK), HL-145028 (ADdK), HL-093186 (CS), HL-136595 (EGK/CS), HL-096830 (EGK) and HL-122494 (EGK).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Hollenberg NK. The renin-angiotensin system and sodium homeostasis. J Cardiovasc Pharmacol 1984; 6 Suppl 1: S176–183. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation 1988; 77 (6 Pt 2): I4–13. [PubMed] [Google Scholar]
- 3.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 1998; 78 (3): 583–686. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: Critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 2009; 89 (4): 370–376. [DOI] [PubMed] [Google Scholar]
- 5.McKinley MJ, Allen AM, Mathai ML, May C, McAllen RM, Oldfield BJ, et al. Brain angiotensin and body fluid homeostasis. Jpn J Physiol 2001; 51 (3): 281–289. [DOI] [PubMed] [Google Scholar]
- 6.Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leenen FH. Actions of circulating angiotensin ii and aldosterone in the brain contributing to hypertension. Am J Hypertens 2014; 27 (8): 1024–1032. [DOI] [PubMed] [Google Scholar]
- 8.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: Implications for new treatment. Progress in neurobiology 2011; 95 (2): 89–103. [DOI] [PubMed] [Google Scholar]
- 9.Young CN, Davisson RL. Angiotensin-ii, the brain, and hypertension: An update. Hypertension 2015; 66 (5): 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (at1) and type-2 (at2) receptor mrnas in the adult rat brain: A functional neuroanatomical review. Front Neuroendocrinol 1997; 18 (4): 383. [DOI] [PubMed] [Google Scholar]
- 11.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of at1 and at2 angiotensin ii receptor subtypes in the rat brain during development. Proceedings of the National Academy of Sciences of the United States of America 1991; 88 (24): 11440–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin ii receptor subtypes (at1 and at2) in rat brain. The American journal of physiology 1991; 261 (1 Pt 2): R209–216. [DOI] [PubMed] [Google Scholar]
- 13.Carter DA, Choong YT, Connelly AA, Bassi JK, Hunter NO, Thongsepee N, et al. Functional and neurochemical characterization of angiotensin type 1a receptor-expressing neurons in the nucleus of the solitary tract of the mouse. American journal of physiology. Regulatory, integrative and comparative physiology 2017; 313 (4): R438–R449. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, et al. Angiotensin type 1a receptors in c1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32 (6): 2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 2012; 226: 489–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, et al. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain structure & function 2016; 221 (2): 891–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwers S, Smolders I, Wainford RD, Dupont AG. Hypotensive and sympathoinhibitory responses to selective central at2 receptor stimulation in spontaneously hypertensive rats. Clin Sci (Lond) 2015; 129 (1): 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai SY, Peng W, Zhang YP, Li JD, Shen Y, Sun XF. Brain endogenous angiotensin ii receptor type 2 (at2-r) protects against doca/salt-induced hypertension in female rats. Journal of neuroinflammation 2015; 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kloet AD, Steckelings UM, Sumners C. Protective angiotensin type 2 receptors in the brain and hypertension. Curr Hypertens Rep 2017; 19 (6): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens 2011; 24 (6): 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension 2008; 51 (2): 521–527. [DOI] [PubMed] [Google Scholar]
- 22.Dai SY, Zhang YP, Peng W, Shen Y, He JJ. Central infusion of angiotensin ii type 2 receptor agonist compound 21 attenuates doca/nacl-induced hypertension in female rats. Oxid Med Cell Longev 2016; 2016: 3981790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanch GT, Freiria-Oliveira AH, Speretta GF, Carrera EJ, Li H, Speth RC, et al. Increased expression of angiotensin ii type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension 2014; 64 (4): 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens 2014; 27 (10): 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L, Zucker IH. At2 receptor signaling and sympathetic regulation. Current opinion in pharmacology 2011; 11 (2): 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legat L, Smolders I, Dupont AG. Gabaergic signaling mediates central cardiovascular angiotensin ii type 2 receptor effects. Trends in endocrinology and metabolism: TEM 2018; 29 (9): 605–606. [DOI] [PubMed] [Google Scholar]
- 27.Ruchaya PJ, Speretta GF, Blanch GT, Li H, Sumners C, Menani JV, et al. Overexpression of at2r in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides 2016; 60: 29–36. [DOI] [PubMed] [Google Scholar]
- 28.Speretta GF, Ruchaya PJ, Delbin MA, Melo MR, Li H, Menani JV, et al. Importance of at1 and at2 receptors in the nucleus of the solitary tract in cardiovascular responses induced by a high-fat diet. Hypertens Res 2019; 42 (4): 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steckelings UM, Kloet A, Sumners C. Centrally mediated cardiovascular actions of the angiotensin ii type 2 receptor. Trends in endocrinology and metabolism: TEM 2017; 28 (9): 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han C, Rice MW, Cai D. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. American journal of physiology. Endocrinology and metabolism 2016; 311 (1): E32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montaniel KR, Harrison DG. Is hypertension a bone marrow disease? Circulation 2016; 134 (18): 1369–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santisteban MM, Zubcevic J, Baekey DM, Raizada MK. Dysfunctional brain-bone marrow communication: A paradigm shift in the pathophysiology of hypertension. Curr Hypertens Rep 2013; 15 (4): 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology 2007; 28 (3): 138–145. [DOI] [PubMed] [Google Scholar]
- 34.Norris GT, Kipnis J. Immune cells and cns physiology: Microglia and beyond. The Journal of experimental medicine 2019; 216 (1): 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Kloet AD, Pitra S, Wang L, Hiller H, Pioquinto DJ, Smith JA, et al. Angiotensin type-2 receptors influence the activity of vasopressin neurons in the paraventricular nucleus of the hypothalamus in male mice. Endocrinology 2016; 157 (8): 3167–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, et al. A unique “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017; 37 (13): 3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, et al. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (doca)-salt in c57 mice. Hypertension 2011; 57 (3): 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, et al. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 2013; 61 (3): 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessberger S, Toni N, Clemenson GD, Jr., Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nature neuroscience 2008; 11 (8): 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, et al. Blood-borne angiotensin ii acts in the brain to influence behavioral and endocrine responses to psychogenic stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011; 31 (42): 15009–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, et al. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav (in press) 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood–cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. Journal of Comparative Neurology 2013; 521 (15): 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kádár A, Sánchez E, Wittmann G, Singru PS, Füzesi T, Marsili A, et al. Distribution of hypophysiotropic thyrotropin-releasing hormone (trh)-synthesizing neurons in the hypothalamic paraventricular nucleus of the mouse. The Journal of comparative neurology 2010; 518 (19): 3948–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautron L, Rutkowski JM, Burton MD, Wei W, Wan Y, Elmquist JK. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. Journal of Comparative Neurology 2013; 521 (16): 3741–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mousa SA, Shaqura M, Schäper J, Treskatsch S, Habazettl H, Schäfer M, et al. Developmental expression of δ-opioid receptors during maturation of the parasympathetic, sympathetic, and sensory innervations of the neonatal heart: Early targets for opioid regulation of autonomic control. The Journal of comparative neurology 2011; 519 (5): 957–971. [DOI] [PubMed] [Google Scholar]
- 46.Liu M, Shi P, Sumners C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. Journal of neurochemistry 2016; 136 (1): 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ, et al. Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Exp Physiol 2011; 96 (10): 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, et al. Centrally administered angiotensin-(1–7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp Physiol 2014; 99 (2): 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, et al. A unique ‘angiotensin sensitive’ neuronal population coordinates neuroendocrine, cardiovascular and behavioral responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin KBJ, Paxinos G. The mouse brain: In stereotaxic coordinates. New York, NY: Elsevier, 2008. [Google Scholar]
- 51.Paxinos G, Watson C. The rat brain in stereotaxic coordinates: Elsevier Life Sciences, 2013. [Google Scholar]
- 52.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, et al. International union of basic and clinical pharmacology. Xcix. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacological reviews 2015; 67 (4): 754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension 2010; 56 (2): 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clinical and experimental pharmacology & physiology 1998; 25 (6): 461–463. [DOI] [PubMed] [Google Scholar]
- 55.Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain research 1984; 310 (2): 355–359. [DOI] [PubMed] [Google Scholar]
- 56.Nakata T, Takeda K, Itho H, Hirata M, Kawasaki S, Hayashi J, et al. Paraventricular nucleus lesions attenuate the development of hypertension in doca/salt-treated rats. Am J Hypertens 1989; 2 (8): 625–630. [DOI] [PubMed] [Google Scholar]
- 57.Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin ii binding sites in selected brain areas of spontaneously hypertensive rats. Journal of hypertension 1988; 6 (1): 79–84. [DOI] [PubMed] [Google Scholar]
- 58.Lenkei Z, Corvol P, Llorens-Cortes C. Comparative expression of vasopressin and angiotensin type-1 receptor mrna in rat hypothalamic nuclei: A double in situ hybridization study. Brain research. Molecular brain research 1995; 34 (1): 135–142. [DOI] [PubMed] [Google Scholar]
- 59.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res 2015; 117 (2): 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013; 33 (11): 4825–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obermuller N, Unger T, Culman J, Gohlke P, de Gasparo M, Bottari SP. Distribution of angiotensin ii receptor subtypes in rat brain nuclei. Neuroscience letters 1991; 132 (1): 11–15. [DOI] [PubMed] [Google Scholar]
- 62.Daniels D Diverse roles of angiotensin receptor intracellular signaling pathways in the control of water and salt intake In: De Luca LA Jr., Menani JV, Johnson AK, eds. Neurobiology of body fluid homeostasis: Transduction and integration. Boca Raton (FL)2014. [PubMed] [Google Scholar]
- 63.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: Implications for long term control of autonomic output. Clinical and experimental pharmacology & physiology 1997; 24 (1): 96–101. [DOI] [PubMed] [Google Scholar]
- 64.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin ii mediated by at1 and at2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl 1996; 3: S99–104. [PubMed] [Google Scholar]
- 65.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ang ii in conscious rats. American journal of physiology. Regulatory, integrative and comparative physiology 2010; 299 (6): R1564–1571. [DOI] [PubMed] [Google Scholar]
- 66.Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin-ii. Neuroendocrinology 1995; 61 (4): 437–444. [DOI] [PubMed] [Google Scholar]
- 67.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. The American journal of physiology 1995; 268 (3 Pt 2): R625–633. [DOI] [PubMed] [Google Scholar]
- 68.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ang ii into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 2002; 282 (6): H2039–2045. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham JT, Beltz T, Johnson RF, Johnson AK. The effects of ibotenate lesions of the median preoptic nucleus on experimentally-induced and circadian drinking behavior in rats. Brain research 1992; 580 (1–2): 325–330. [DOI] [PubMed] [Google Scholar]
- 70.McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: Front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015; 214 (1): 8–32. [DOI] [PubMed] [Google Scholar]
- 71.Abegaz B, Davern PJ, Jackson KL, Nguyen-Huu TP, Bassi JK, Connelly A, et al. Cardiovascular role of angiotensin type1a receptors in the nucleus of the solitary tract of mice. Cardiovascular research 2013; 100 (2): 181–191. [DOI] [PubMed] [Google Scholar]
- 72.Colombari E, Colombari DS. Nts at1a receptor on long-term arterial pressure regulation: Putative mechanism. Cardiovascular research 2013; 100 (2): 173–174. [DOI] [PubMed] [Google Scholar]
- 73.Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin ii. Clinical and experimental pharmacology & physiology 2000; 27 (5–6): 432–436. [DOI] [PubMed] [Google Scholar]
- 74.Nahey DB, Collister JP. Ang ii-induced hypertension and the role of the area postrema during normal and increased dietary salt. Am J Physiol Heart Circ Physiol 2007; 292 (1): H694–700. [DOI] [PubMed] [Google Scholar]
- 75.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (at1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. Journal of neuroendocrinology 2001; 13 (2): 139–146. [DOI] [PubMed] [Google Scholar]
- 76.Rowe BP, Saylor DL, Speth RC. Analysis of angiotensin ii receptor subtypes in individual rat brain nuclei. Neuroendocrinology 1992; 55 (5): 563–573. [DOI] [PubMed] [Google Scholar]
- 77.MacGregor DP, Murone C, Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin ii receptor subtypes in the human central nervous system. Brain research 1995; 675 (1–2): 231–240. [DOI] [PubMed] [Google Scholar]
- 78.Song K, Allen AM, Paxinos G, Mendelsohn FA. Mapping of angiotensin ii receptor subtype heterogeneity in rat brain. The Journal of comparative neurology 1992; 316 (4): 467–484. [DOI] [PubMed] [Google Scholar]
- 79.Guimond MO, Gallo-Payet N. The angiotensin ii type 2 receptor in brain functions: An update. International journal of hypertension 2012; 2012: 351758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (at1) and type-2 (at2) receptor mrnas in the adult rat brain: A functional neuroanatomical review. Frontiers in neuroendocrinology 1997; 18 (4): 383. [DOI] [PubMed] [Google Scholar]
- 81.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin ii type-2 receptor (at2) mrna expression in the adult rat brain. The Journal of comparative neurology 1996; 373 (3): 322–339. [DOI] [PubMed] [Google Scholar]
- 82.Haspula D, Clark MA. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton Neurosci 2018; 210: 10–17. [DOI] [PubMed] [Google Scholar]
- 83.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: Implications for hypertension and related therapeutics. Circ Res 2016; 118 (8): 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between at1 receptors and toll-like receptor 4 in microglia contributes to angiotensin ii-derived ros production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 2016; 310 (3): H404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP. Astrocytes contribute to angiotensin ii stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the mptp model of parkinson’s disease is mediated by brain angiotensin: Relevance to progression of the disease. Journal of neurochemistry 2009; 109 (2): 656–669. [DOI] [PubMed] [Google Scholar]
- 87.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, et al. Angiotensin ii sustains brain inflammation in mice via tgf-beta. The Journal of clinical investigation 2010; 120 (8): 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Negussie S, Lymperopoulos A, Clark MA. Role of betaarrestin1 in at1 r-mediated mitogen-activated protein kinase activation in wistar and shr brainstem astrocytes. Journal of neurochemistry 2019; 148 (1): 46–62. [DOI] [PubMed] [Google Scholar]
- 89.Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin ii receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proceedings of the National Academy of Sciences of the United States of America 1991; 88 (17): 7567–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tallant EA, Higson JT. Angiotensin ii activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia 1997; 19 (4): 333–342. [PubMed] [Google Scholar]
- 91.Wu CY, Zha H, Xia QQ, Yuan Y, Liang XY, Li JH, et al. Expression of angiotensin ii and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Molecular and cellular biochemistry 2013; 382 (1–2): 47–58. [DOI] [PubMed] [Google Scholar]
- 92.O’Callaghan EL, Bassi JK, Porrello ER, Delbridge LM, Thomas WG, Allen AM. Regulation of angiotensinogen by angiotensin ii in mouse primary astrocyte cultures. Journal of neurochemistry 2011; 119 (1): 18–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.