Abstract
Background
Small vessel disease causes a quarter of ischaemic strokes (lacunar subtype), up to 45% of dementia either as vascular or mixed types, cognitive impairment and physical frailty. However, there is no specific treatment to prevent progression of small vessel disease.
Aim
We designed the LACunar Intervention Trial-2 (LACI-2) to test feasibility of a large trial testing cilostazol and/or isosorbide mononitrate (ISMN) by demonstrating adequate participant recruitment and retention in follow-up, drug tolerability, safety and confirm outcome event rates required to power a phase 3 trial.
Methods and design
LACI-2 is an investigator-initiated, prospective randomised open label blinded endpoint (PROBE) trial aiming to recruit 400 patients with prior lacunar syndrome due to a small subcortical infarct. We randomise participants to cilostazol v no cilostazol and ISMN or no ISMN, minimising on key prognostic factors. All patients receive guideline-based best medical therapy. Patients commence trial drug at low dose, increment to full dose over 2–4 weeks, continuing on full dose for a year. We follow-up participants to one year for symptoms, tablet compliance, safety, recurrent vascular events, cognition and functional outcomes, Trails B and brain MRI. LACI-2 is registered ISRCTN 14911850, EudraCT 2016–002277-35.
Trial outcome: Primary outcome is feasibility of recruitment and compliance; secondary outcomes include safety (cerebral or systemic bleeding, falls, death), efficacy (recurrent cerebral and cardiac vascular events, cognition on TICS, Trails B) and tolerability.
Summary
LACI-2 will determine feasibility, tolerability and provide outcome rates to power a large phase 3 trial to prevent progression of cerebral small vessel disease.
Keywords: Lacunar stroke, small vessel disease, cilostazol, isosorbide mononitrate, randomised clinical trial
Introduction
Stroke and vascular dementia are increasing in prevalence,1 have enormous economic and societal costs, share many risk factors, and are major Government targets for health improvement.2,3 Cerebral small vessel disease (SVD) is a central link between stroke and dementia.4 It accounts for 20–25% of clinical strokes as either recent small subcortical or ‘lacunar’ infarcts5 (hereafter referred to as ‘lacunar ischaemic stroke’) and most intracerebral haemorrhages in older people,6 and up to 45% of all dementias, alone or combined with Alzheimer’s disease.7 Patients presenting with a lacunar ischaemic stroke are often younger than with other stroke subtypes,6 and about a third experience post-stroke cognitive impairment or dementia,8,9 which may restrict their independence, including ability to return to work.9
The causes of lacunar ischaemic stroke are still poorly understood.4 Most appear due to an intrinsic cerebral perforating arteriolar disease. This includes impaired vasoreactivity, increased blood-brain barrier permeability and perivascular inflammation, all causing brain damage4 visible on magnetic resonance imaging (MRI) including white matter hyperintensities (WMH), lacunes, prominent perivascular spaces and microbleeds. Additionally, the damaged endothelium may precipitate local thrombosis and ischaemia, which worsens the damage.4
Despite the profound health impacts, few RCTs specifically targeted either SVD lesion progression or lacunar stroke.10 The largest trial in lacunar ischaemic stroke, the Secondary Prevention of Small Subcortical Stroke (SPS3) trial, tested 3.4 years of aspirin + clopidogrel versus aspirin11 and 3.7 years of target versus guideline blood pressure (BP) reduction12 in 3020 lacunar ischaemic stroke patients, to prevent recurrent stroke and cognitive decline. The aspirin + clopidogrel versus aspirin arm stopped early due to increased death;11 target (versus guideline) BP lowering was consistent with a small reduction in recurrent stroke, but neither it nor aspirin + clopidogrel versus aspirin reduced cognitive decline.13
Given the long timeline to develop new drugs, available licensed drugs with relevant actions should be evaluated.10 Prostacyclin (PGI2)-cyclic AMP-phosphodiesterase 3 pathway or nitric oxide (NO)-cyclic GMP-phosphodiesterase 5 modulators may improve vasodilatation, reduce inflammation and smooth muscle hypertrophy and improve cerebral endothelial integrity.10 Amongst several drugs licensed in Europe, cilostazol and isosorbide mononitrate (ISMN) show promise; two drugs with complementary actions could have synergistic effects.
Cilostazol, a phosphodiesterase 3-inhibitor (PDE3-inhibitor), enhances the PGI2-cAMP pathway, with weak antiplatelet effects (so low bleeding risk).14 Routine cilostazol use reduced dementia incidence in the Taiwanese registry,15 reduced infarct size16 and improved myelin repair in animal models,17 and has been trialled for secondary stroke prevention in more than 6400 patients.18–22 Trials where >50% of participants had lacunar stroke (n = 4780) found long-term cilostazol (versus placebo or aspirin) reduced recurrent stroke (OR 0.62, 95%CI 0.49–0.77) with no evidence of an increase in cerebral haemorrhage (OR 0.52, 95%CI 0.36–0.75), or death (OR 0.90 95%CI 0.53–1.52) over median one year treatment. However, there are limited randomised data on cilostazol’s effects on cognition or imaging features of SVD, and data are from Asia-Pacific countries where the epidemiology and risk factors may differ from western patients.
ISMN is an NO-donating organic nitrate that enhances vasodilation, is widely used in ischaemic heart disease with a known safety profile and has no antiplatelet activity.23 NO maintained cerebral perfusion short term after subacute stroke,24 but drugs that increase NO availability are rarely used in stroke.25
The LACI-1 trial26 tested short-term dose escalation protocols for cilostazol and ISMN individually and together, tolerability and early markers of safety and efficacy in patients with lacunar stroke.27 LACI-1 recruited 56 patients in two centres, showed that headache, nausea, palpitations were common pre-randomisation, increased on starting either trial drug, but by incrementing the dose slowly, symptoms returned to background levels within two weeks.27 Most patients achieved the target dose27 without safety concerns.28 The LACI-2 trial aims to determine if cilostazol and ISMN, alone or together, are tolerated at the target dose for a year, provide feasibility, safety and efficacy data, prior to proceeding to a large phase III trial.
Methods
LACI-2 is a phase IIb randomised, partial factorial, open label, blinded end-point trial, aiming to recruit 400 patients from UK Stroke Network Centres, with follow-up to one year. In addition to experience from LACI-1, LACI-2 trial design benefitted from:
a National Institutes of Health Research (NIHR) Stroke Research Network (SRN) Portfolio Development Expert Writing Group (members indicated*), which discussed trial design options in detail;
the USA NIH-funded SPS3 trial11,12 (3020 patients with lacunar stroke): Chief Investigator (CI) O Benavente*, which informed on recruitment and outcome event rates;
the CSPS, CSPS-2 (3740 patients)18,20 and CSPS-3 trials22 testing cilostazol to prevent stroke in Japan: CI K Toyoda, informed on cilostazol dose and escalation to increase tolerability;
monogenic SVDs:29 CI H Chabriat*, informed on timing and practicality of potential outcome assessments, and the relative merits and rates of functional, cognitive and other outcomes to optimise sensitive outcomes against the burden of assessments on the participants;
the PRESERVE trial:30 CI H Markus*, informed on approaches to patient recruitment, cognitive assessments, and MRI rates;
the PODCAST trial:31 CI P Bath*, Co-CI LACI-2, informed on minimisation algorithms, data collection, likely availability of informants, approaches to loss of mental capacity during the trial, cognitive assessments and frequency of outcome assessments;
CROMIS-2 and the Microbleeds International Collaborative Network,32,33 informed on clinical and imaging assessments, frequency and practicalities of potential outcome assessments.
The UK SRN Prevention Clinical Studies Group strongly supported testing cilostazol and ISMN in SVD prevention and many UK Clinical Research Network Centres expressed interest in joining LACI-2.
Patients undergo guideline-based investigations as per usual stroke prevention practices in the UK including carotid and cardiac investigations and for other rarer causes in young strokes as appropriate.
Patients are eligible if they are independent in activities of daily living, have capacity to give consent, are aged over 30 years, have suffered a minor stroke with clinical features compatible with a lacunar syndrome (most frequently, but not limited to, a pure motor hemiparesis, pure sensory stroke, ataxic hemiparesis, sensorimotor stroke, or dysarthria-clumsy hand syndrome; designated as the ‘index stroke’) and have, on contemporaneous CT or MRI brain imaging, either a visible relevant recent small subcortical (lacunar) infarct or no alternative pathology to explain symptoms (Table 1). There is no maximum time limit for recruitment because (a) lacunar stroke indicates the presence of SVD, which is a longstanding condition, (b) recurrent stroke after lacunar stroke and signs of SVD progression are thought to occur slowly and inexorably (in contrast to atherothromboembolic stroke where the risk of recurrence is immediate then declines), (c) to avoid guideline dual antiplatelets and minimise the burden of participation on the patient soon after the stroke, (d) since both drugs are likely to affect long-term chronic vascular dysfunctions, (e) to increase recruitment by avoiding clashes with high periods of recruitment to acute treatment and secondary prevention trials and (f) since any time limit would be very arbitrary after considering all the above. However, time after stroke is accounted for in minimisation and will be accounted for in the statistical analysis.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Independent in activities of daily living (modified Rankin ≤2), • Capacity to give consent,• Aged over 30 years,• Minor stroke with clinical features compatible with a lacunar syndrome. Contemporaneous brain imaging shows either: a) a recent, relevant (in time and location) acute small subcortical infarct on MRI diffusion imaging, b) or MR FLAIR, T2 or T1 MR imaging or CT brain imaging shows a recent-appearing small subcortical infarct in a relevant location (i.e. no cavitation or ex-vacuo effect, may be slightly swollen, ill-defined edges), c) or MR FLAIR, T2 or T1 MR imaging or CT brain imaging shows a new small subcortical infarct, in a relevant location, appearing between a scan obtained prior to, or just after, the stroke and one done hours to days after the stroke, d) or if there is no visible acute small subcortical infarct on MR or CT brain imaging, then there is no alternative pathology as a cause for stroke (i.e. no acute cortical infarct, no acute intra-cerebral haemorrhage, no stroke mimic such as tumour, subdural haematoma). | General: – Other significant active neurological illness e.g. recurrent seizures, multiple sclerosis, brain tumour (well-controlled epilepsy present prior to the stroke, a single seizure at onset of the stroke or provoked seizure is not an exclusion); – Modified Rankin ≥3; – Formal clinical diagnosis of dementia; – Hypotension, defined as sitting systolic BP <100 mmHg; – Unable to swallow tablets; – Planned surgery during the trial period; – Other concurrent life threatening illness; – Unlikely to be available for follow-up; – History of drug overdose, attempted suicide, significant active mental illness; – Pregnant or breastfeeding women, women of childbearing age not taking contraception; – Renal impairment (creatinine clearance <25 ml/min); – Hepatic impairment; – Current enrolment in another Clinical Trial of Investigational Medicinal Product (CTIMP); – Unable to tolerate, or contraindication to, MRI. |
| Specific to one or other but not both trial drugs (note patients with a contraindication to one trial drug can still be randomised to the other trial drug): – Definite indication for, or already prescribed, or contraindication to, a trial medication;Cilostazol: – Bleeding tendency, e.g. known platelets < 100, active peptic ulcer, history of intracranial haemorrhage (not asymptomatic haemorrhagic transformation of infarction, or a few microbleeds); – Prescribed anticoagulant medication or dual antiplatelet drugs; – Prescribed prohibited medications including omeprazole (can be switched to other proton pump inhibitor), erythromycin, clarithromycin, diltiazem, some antifungal or antiviral agents; – Active cardiac disease (atrial fibrillation, myocardial infarct in last six months, active angina, symptomatic cardiac failure). Isosorbide mononitrate:– Lactose intolerance, where site uses ISMN preparations which contain lactose monohydrate; – prescribed prohibited medications including avanafil, sildenafil, tadalafil, vardenafil. |
CTIMP: Clinical Trial of Investigational Medicinal Product; FLAIR: fluid attenuated inversion recovery; MR: magnetic resonance.
Exclusion criteria include other significant active neurological illness, alternative pathologies visible on imaging (cortical infarct, subcortical infarct exceeding 2 cm diameter likely to be large artery, haemorrhage, tumour, etc), athero- or cardio-embolic source requiring specific treatment, renal or hepatic impairment, unlikely to complete follow-up due to illness or location, current enrolment in another Clinical Trial of Investigational Medicinal Product (CTIMP), and other general and specific reasons listed in Table 1. Co-enrolment is allowed in non-CTIMPs.
Consent
Participants must have capacity and consent is taken from them for the trial and for secondary data uses; consent is taken from a relative or other informant to provide outcome data.
Intervention
ISMN 40–60 mg (or equivalent formulation) daily only; cilostazol 200 mg daily only; or both ISMN 40–60 mg and cilostazol 200 mg daily. The range of ISMN doses is allowed to account for different formulations provided in hospital pharmacies. The trial is open-label since placebo tablets were not available in the UK and masking by encapsulation was impractical and expensive due to dose escalation and factorial trial design. However, outcomes including structured symptom questionnaires, ascertainment of clinical outcomes and MRI analysis are collected by individuals blinded to treatment allocation. Participants with indications for or contraindications to one drug can be randomised to the other drug only. The partial factorial design allows testing of both drugs when given alone and together.
Comparator
Best medical guideline stroke prevention therapy as per UK guidelines, which include neither cilostazol nor ISMN. Throughout the trial, participants continue to take their normal prescribed medication, information on which is collected at randomisation.
Randomisation, minimisation
Randomisation is by central computer-generated allocation at the University of Nottingham after recording key patient variables and minimisation data. Minimisation uses key prognostic factors including: age, sex, stroke severity (National Institute of Health Stroke Scale), dependency resulting from the stroke, systolic BP ≤/>140, smoker status, time after stroke, and years of education. Years of education give an estimate of pre-morbid cognitive ability and predicts post-stroke cognitive impairment;9 BP and smoking predict recurrent stroke; delay since stroke reflects disease activity; age, sex and stroke severity are standard minimisation variables.27 This approach ensures concealment of allocation, minimises differences in key baseline prognostic variables, and improves statistical power. Randomisation is not minimised by Centre because this may result in high rates of allocation prediction, but a pre-specified post-hoc analysis by centre will be performed to investigate and adjust for heterogeneity of treatment effect by centre.
Dispensing
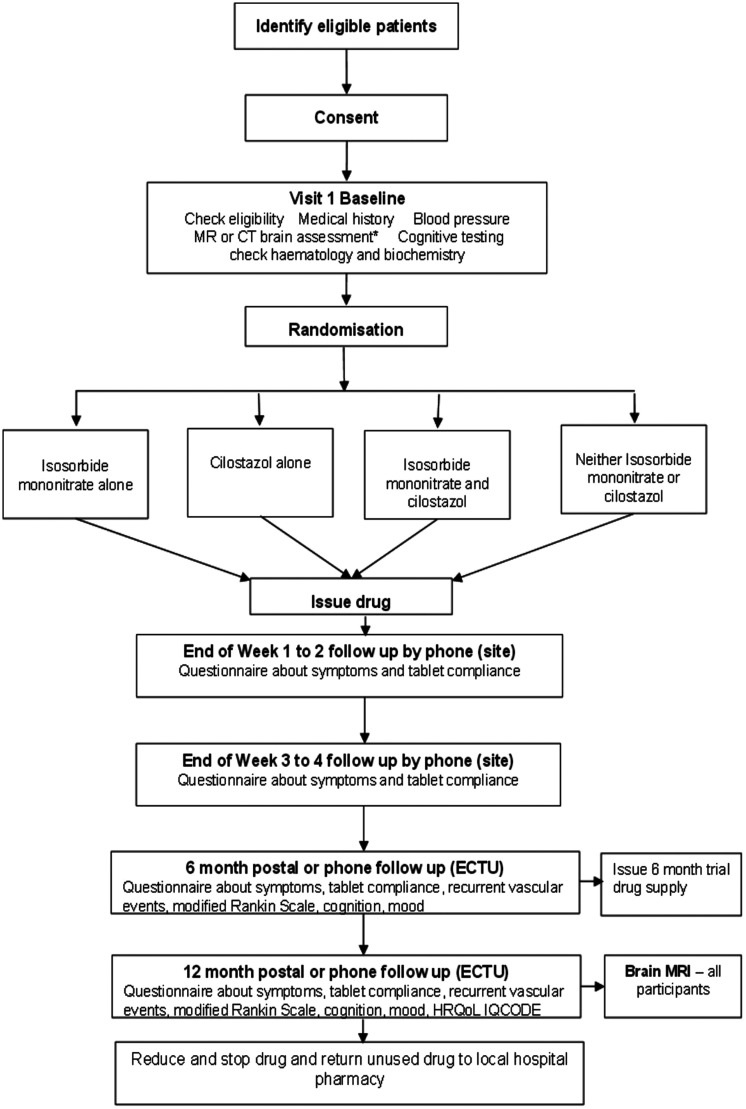
Participants are allocated in a 1:1 ratio to cilostazol versus no cilostazol and to ISMN versus no ISMN, resulting in a partial factorial comparison of cilostazol versus ISMN versus both drugs versus neither drug. The algorithm generates a unique trial ID and number corresponding to a prescription of allocated trial treatment, which is prepared and dispensed by the local Pharmacy (Figure 1).
Figure 1.
CONSORT diagram. Diagnostic MR or CT brain scan assessment (Baseline) refers to a visual assessment of the scan that has already been performed as part of the patient’s routine stroke clinical assessment and diagnosis. Features present on the scan are scored for their presence and severity to create a total sum of SVD score. This is used in the minimisation algorithm. Dispensing may be at 3-monthly intervals if preferred in particular centres.
Drug initiation and maintenance
After randomisation, patients are given instructions to start on a low dose and escalate to target dose over two-to-four weeks. For ISMN, 25 mg once daily in the first week, 40–60 mg daily in the second week and thereafter. For Cilostazol, 50 mg once daily for the first week, twice daily in the second week rising to 100 mg twice daily over the next two weeks until participants are on their full dose by one month, remaining on full dose for the rest of the year. Escalation can proceed more slowly if necessary. Participants can remain on less than full dose if they are unable to tolerate the full dose, since some drug may be better than no drug and dose is recorded.
Assessments
At baseline, the study researcher records details of the presenting stroke, past medical history, medications, socioeconomic status, education, lifestyle factors, neurological findings, brain imaging findings (index stroke, background WMH, lacunes, microbleeds), BP, cognitive testing (Montreal Cognitive Assessment, Trails B), haematology and biochemistry (Table 2).
Table 2.
Study assessments.
| Assessment | Prior to baseline | Visit 1 baseline | Week 1–2 FU | Week 3–4 FU | 6 month FU | 12 month FU |
|---|---|---|---|---|---|---|
| Screening for eligibility and Consenta | X | |||||
| Confirm and document ongoing consent | X | |||||
| Medical including drug history | X | |||||
| Assess MR or CT diagnostic scan and send a copy to Edinburgh | X | |||||
| Randomisation | X | |||||
| Haematology (full blood count) and Biochemistry (urea, electrolytes, creatinine) | X | |||||
| BP | X | X * | ||||
| Cognitive test: years of education;Montreal Cognitive Assessment (MOCA), timed Trail Making Test B | X | |||||
| Dispense trial medicationb | X | X | ||||
| Record symptoms; medication history and IMP adherence | X | X | X | X | ||
| Record recurrent vascular events, mRS, TICS, TMOCA, SIS, ZUNG,* timed Trail Making Test B | X | X * | ||||
| IQCODE (post/phone) | X | |||||
| Follow-up brain MRI | X | |||||
| Health Economics data: EQ-5D-5L, EQ-VAS | X | |||||
| AE reporting as necessary | X | X | X | X |
Consent will be obtained before the data collection procedures commence or randomisation is performed. Randomisation occurs at the end of the baseline visit.
Dispensing in 3-monthly intervals is allowed.
At 12 months in some centres only.
AE: adverse event; FU: follow-up; SIS: stroke impact scale; TICS: telephone interview for cognitive status; TMOCA: telephone MOCA.
After commencing trial treatment, local researchers telephone the participants at 1–2 and 3–4 weeks post randomisation, with intermediary phone calls as necessary, to advise on dose escalation, symptoms, events, check they have achieved full dose or are established on a lower tolerated dose by four weeks.
At 6 and 12 months, central trial staff (Edinburgh, Nottingham), masked to treatment allocation, perform follow up by postal questionnaire and phone. Additionally, at 12 months, the patient attends their local trial centre for Trails B and brain MRI (to assess SVD lesion burden, new stroke lesions). Information recorded at 6 and 12 months follow-up includes symptoms, recurrent ischaemic or haemorrhagic stroke or transient ischaemic attack (TIA), cardiac events, smoking status, current medications, modified Rankin scale (mRS), stroke impact scale34 and separately the amount of trial medication (Table 2). Date and cause of death are recorded. Participants are encouraged to contact their local Trial Centre to record vascular events occurring between follow-up points. Unused drug is returned to pharmacy at the participating hospital for accountability and destruction at the end of the trial.
Brain imaging
All MR or CT scans performed for stroke diagnosis, to assess new neurological events during the trial, and at one-year follow-up, are collected centrally, anonymised, for blinded assessment. MRI at one year must include diffusion-weighted imaging (DWI), T2-weighted, fluid attenuated inversion recovery (FLAIR), susceptibility-weighted (blood sensitive), and T1-weighted sequences (exemplar sequences are provided). Scans are rated, blind to patient characteristics and treatment allocation, to assess the index infarct location, size, WMH, lacunes, old cortical infarcts, old haemorrhages, microbleeds, siderosis, perivascular spaces and brain atrophy, with validated scores using a web-based system.5
Adverse events are recorded as serious (SAEs), reported to the Sponsor, if they result in hospitalisation, persistent disability, death or are life threatening. Recurrent vascular events (stroke, myocardial infarction (MI), TIA) and bruising, bleeding or other types of haemorrhage are collected as outcomes, not SAEs. Serious adverse reactions (SARs) and suspected unexpected SARs (SUSARS) are also reported. Adverse events (AEs) that are common in patients with stroke and do not meet criteria for seriousness or drug related are recorded.
Outcomes
The primary outcome is feasibility, i.e. that eligible participants can be identified correctly, in sufficient numbers, enrolled and >95% retained in follow-up at one year, to achieve target sample of 400 participants in 24 months in the UK. The secondary outcomes are tolerability (can patients tolerate trial medication at least in half dose for up to a year?), safety (systemic or intracranial bleeding, vascular and non-vascular causes of death) and efficacy (stroke, TIA, myocardial ischaemia, cognitive impairment and dementia).
Sample size
Data to estimate sample size were limited. Based on LACI-1, we estimated that 75% of participants would take trial medications, in at least half dose, up to one year. We calculated mean annual event rates (Table 3) from trials (SPS3,11 lacunar participants in ENOS,25 IST-3,35 of cilostazol18,20,36) and observational data (LADIS;37 our9,38,39 and other40 studies). We expect deaths including vascular deaths of median 2.0% pa, with 4% being the upper 95% CI of 2% in 400 participants.11 We propose that the absolute risk of death, including fatal haemorrhage, will not exceed 4% per year on trial drugs versus no trial drugs, given in addition to guideline drugs, and will not increase bleeding or ischaemic SVD lesions significantly (at the p < 0.01 level) on MRI. LACI-2 therefore has a sample size of 400. Considering a future Phase III trial, recurrent stroke 2.5% pa, MI 0.6% pa and vascular death 1.8% pa,6,11 (major adverse cardiovascular events, MACE are infrequent); in contrast, new cognitive impairment and dependency approximate 16% and 15%, respectively, at one year,8,40,41 and are important to healthcare professionals, patients and carers,9 thereby justifying their inclusion in a composite primary outcome. Thus, the combined rate of recurrent stroke, MI, death, cognitive impairment and dependency was estimated at 40–50% at one year after enrolment.42 We estimate main Phase III trial sample size at 1100 (Table 4), assuming 80% power, alpha 5%, 10% loss to follow-up, composite event rate (45%) and conservative estimate of cilostazol effect (20% RRR is the lower 95% CI of effect; there were no data for ISMN). A primary outcome including MRI SVD progression was considered, but at least 15% of participants miss repeat MRI, sample sizes were similar or larger due to variance in WMH change,39,43 the cost of an MRI-based trial is much higher and the WMH intermediary measure is less relevant to patients.
Table 3.
Annual absolute risks (%) of outcome events after lacunar stroke – see text for data sources.
| Vascular death | Non-vascular death | Non-fatal ischaemic stroke or TIA | Non-fatal ICH | MI | MACE | Dependent (mRS 3–5) | Any cognitive impairment | Dementia |
|---|---|---|---|---|---|---|---|---|
| 1.8 | 0.5 | 2.5 | 0.5 | 0.6 | 3 | 15 | 30 | 15 |
ICH: intracerebral haemorrhage; MACE: major adverse cardiovascular events; MI: myocardial infarction; mRS: modified ranking scale.
Table 4.
Sample size for composite outcome in main trial, estimated event rates – see text for data sources.
| Composite model | A | B | Ci | Cii | D |
|---|---|---|---|---|---|
| Composite outcome for Phase III includes: | MACE, dementia, non-vasc death, new MR signs | MACE, dementia, death | MACE, cog↓, dependency↓, death | MACE, cog imp, depend, all death | |
| 1-Beta (power) | 80% | 80% | 80% | 80% | 80% |
| Event rate, control, pa | 50% | 10% | 30% | 30% | 45% |
| Relative risk reduction | 20% | 20% | 20% | 30% | 20% |
| Event rate, active, pa | 40% | 8% | 24% | 21% | 36% |
| Total sample size | 950 | 6626 | 1784 | 778 | 976 |
| Total trial size, inc losses | 1250 | 7400 | 2000 | 900 | 1100 |
Assume: 1:1 randomisation and Fleiss adjustment; alpha 5%; primary outcome incomplete in 10%.
MACE: major adverse cardiovascular events; MR: magnetic resonance.
Statistical analysis
A statistical analysis plan will be published prior to recruitment ending, blinded to data. A brief summary of planned analyses are as follows. We will compare cilostazol versus no cilostazol, ISMN versus no ISMN, cilostazol and ISMN v neither, by intention to treat (ITT) analysis. The proportion of patients achieving and sustaining target dose and adverse symptoms will be assessed using odds ratios. ITT analysis will use logistic regression adjusted for minimisation variables and other baseline prognostic variables (to maximise power44) including SVD score, age and cilostazol+/–ISMN versus control on efficacy and safety outcomes, with secondary tests of subgroup interactions. Ordinal logistic regression will be used for ordinal categorical outcomes (e.g. mRS) to increase power.45 To analyse safety, we will use Kaplan-Meier and Cox proportional regression for time-to-event outcomes of death, recurrent stroke, MI; binary logistic regression for SAEs, recurrent stroke, MI, and in those completing 12 month MRI, microbleeds, siderosis, new infarcts, and WMH burden.
Trial and data management
LACI-2 has an operational management committee meeting alternate weeks, a Trial Steering Committee (TSC) with an independent chair, two scientific advisors, participant, funder and sponsor representatives meeting six monthly, a Data Monitoring Committee (DMC) with four independent members meeting yearly, and an International Advisory Board that provides informal advice. The Sponsor, the University of Edinburgh/NHS Lothian joint Academic and Clinical Central Office for Research and Development (ACCORD) approves all trial procedures and provides monitoring. The trial is managed from the Edinburgh Clinical Trials Unit, registered with UKCRC. The secure, password-protected, electronic database and case record form is held at the University of Nottingham; statistical support is provided by the Nottingham Stroke Trials Unit and UKCRC-registered Nottingham Clinical Trials Unit; image data management and analysis is at the University of Edinburgh. Only the DMC members see unblinded data during the trial. Members of each group are listed in Appendix 1.
Discussion
LACI-2 is testing drugs that aim to improve the endothelial dysfunction seen in lacunar ischaemic stroke, given for one year. LACI-2 builds on experience in LACI-1 including inclusion and exclusion criteria, data collection, randomisation and statistical analysis, has a similar TSC, the same DMC and International Advisory Boards, to enhance and retain knowledge and experience. LACI-2 will provide methods for balancing randomisation, streamlining of follow-up and reliable data on key event rates for lacunar stroke/SVD trials, preparatory to a Phase III trial of these drugs for definitive efficacy and safety data. LACI-2 aims to promote more personalised approaches to brain vascular disease by recognising and addressing key mechanistic and susceptibility differences between ischaemic stroke subtypes.
LACI-2 is pragmatic; we faced several practical issues in designing the trial and recognise that some of our decisions were, of necessity, empirical since there have been few trials in lacunar stroke to guide design. In LACI-1, target sample 60 patients in two centres, recruitment time was reduced by 8 months and the total cost by £85,000 (equivalent to a third of total available funding) using enrolment based on CT or MRI compared with only allowing enrolment based on MRI.27 Although an MRI-based trial could be more specific for lacunar stroke, we showed that it would delay trial completion by at least 33% thus inflating staff costs significantly, it would exclude patients unable to tolerate MRI, and delay scientific progress since the time to finding a treatment for lacunar ischaemic stroke would take much longer. Since no imaging modality is perfect, and not all acute ischaemic lesions are visible on MRI even with diffusion imaging (e.g. 30% of patients with lacunar or mild cortical stroke do not have an acute lesion on DWI yet have similar rates of recurrent stroke, functional and cognitive impairments46) appropriate use of CT and MRI can exclude alternative causes of symptoms, and CT can detect moderate to severe WMH, lacunes, atrophy, and old stroke lesions including differentiating some old haemorrhages from old infarcts.47,48 LACI-2 also does not set a maximum time from stroke to recruitment after consideration of pathological (lacunar stroke due to intrinsic cerebral arteriolar pathology indicates the presence of a chronic condition, SVD, and disease progression is thought to occur steadily and slowly), pharmacological (putative effects of cilostazol and ISMN are on chronic vascular dysfunctions), and practical reasons (avoid subacute post-stroke period when guideline dual antiplatelet drugs are used, and minimise clashes with other acute treatment and short term secondary prevention trials).
There is no placebo in LACI-2 since, after considerable searching, none was available, but central follow-up and MRI are blinded to allocation. LACI-2 encourages best medical management in all patients including good BP control using guideline medications. Good BP control may help reduce WMH progression49 and prevents stroke. LACI-2 is therefore testing cilostazol or ISMN or both plus best medical management versus best medical management alone.
LACI-2 started recruiting in March 2018 in one site, had opened 26 sites and recruited 258 participants by February 2020. Apart from delays in opening sites due to the large administrative burden of clinical trials, it is on course to complete recruitment by Dec 2020, to complete follow-up by Dec 2021, and report in spring 2022. The DMC have met twice (August 2018 and 2019) and recruitment continues. To the best of our knowledge, LACI-2 appears to be the only ongoing trial specifically testing agents for long term prevention of recurrent lacunar stroke, MACE and worsening of SVD. It is one of only five trials listed in the Alzheimer’s Drug Discovery Foundation Report 2018 (Drug testing in Dementia, https://www.alzdiscovery.org/research-and-grants/clinical-trials-report) that is addressing prevention of vascular dementia, and the only one at Phase IIb level. We hope that LACI-2 will help inform the future design of trials in lacunar stroke and other clinical presentations of SVD.
Trial registration
The trial is registered: EudraCT 2016–002277-35, ISRCTN 14911850.
Acknowledgements
We thank the patients and their relatives for their time and effort to participate in LACI-2, the Trial Steering Committee, Sponsor, Data Monitoring Committee, Edinburgh Clinical Trials Unit staff, UK Clinical Research Network, Scottish Stroke Research Network, International Advisory Panel, and all staff at participating sites, for their support.
Appendix 1. The LACI-2 Trial Investigator Group
Writing Committee: Joanna M Wardlaw, Philip MW Bath, Fergus Doubal, Anna Heye, Niki Sprigg, Lisa Woodhouse, Gordon Blair, Jason P Appleton, Vera Cvoro, Timothy J England, Ahamed Hassan, David Werring, Alan Montgomery.
Trial Steering Committee: John Bamford, Independent Chair; Christine Roffe, Stoke, John T. O’Brien, Cambridge, Independent Advisors; Euan Haig, Patient Representative; Joanna M Wardlaw, CI, Fergus Doubal, PI, Edinburgh; Philip MW Bath, Co CI, Nikola Sprigg, Safety Adjudication, Alan Montgomery, Statistical Oversight, Lisa Woodhouse, Statistician, Richard Dooley, Programmer, Nottingham; Timothy England, PI Derby, David Werring, PI UCL London, Ahamad Hassan, PI Leeds, Vera Cvoro, PI Fife; Anna Heye, Trial Manager, Edinburgh; F O’Mahoney, Sponsor Representative, Edinburgh; Shannon Amoils, British Heart Foundation, Funder Representative.
Independent Advisors: Oscar Benavente, Vancouver, Canada; Kasuo Toyoda, Kyoto, Japan; Hugues Chabriat, Paris, France.
Data Monitoring Committee: Colin Baigent, Oxford; Gary Ford, Oxford; Alison D Murray, Aberdeen; Jonathan Emberson, Oxford.
Trial Staff: Anna Heye, Carol Williams, Anna Foster, Kaye Ferguson, Guen Innes, Edinburgh; Di Havard, Sharon Ellender, Patricia Robinson, Lisa Woodhouse, Richard Dooley, Lee Hayward, Nottingham.
Image Management: Eleni Sakka, Image Data Manager, Jeb Palmer, Programmer, David Buchanan, Programmer, Edinburgh.
Independent blinded event adjudication: Nikki Sprigg, Nottingham.
Participating Sites:
Edinburgh, Royal Infirmary, C001: Fergus Doubal (PI)
Nottingham, University Hospitals, C002; Kailash Krishnan (PI)
Kircaldy, Victoria Hospital, C004: Vera Cvoro (PI)
Glasgow, Queen Elizabeth Hospital, C005: Jesse Dawson (PI)
Bradford, Royal Infirmary, C006: Christopher Patterson (PI)
Aberdeen Royal Infirmary, C007: German Gutierrez (PI)
Leeds General Infirmary, C008: Ahamed Hassan (PI)
Derby, Royal Derby Hospital, C009: Timothy England (PI)
Inverness Raigmore Hospital, C010: Stephen Makin (PI)
London, St George’s Hospital, C011: Usman Khan (PI)
London, Kings College Hospital, C012: Laszlo Sztriha (PI)
Essex, Broomfield Hospital, C013: Ramanathan Kirthivasan (PI)
Stockton-on-Tees, University Hospital of North Tees, C014: Anwar Ijaz (PI)
Sheffield, Royal Hallamshire Hospital, C015: Kirsty Harkness (PI)
Sandwell, General Hospital, West Bromich, C016: Sissi Ispoglou (PI)
Winchester, Royal Hampshire County Hospital, C017: Nigel Smith (PI)
London, University College, C018: David Werring (PI)
Harrow, Northwick Park Hospital, C019: Aravinth Sivagnanaratnam (PI), David Cohen,
Luton, Luton and Dunstable NHSFT University Hospital, C020: Lakshmanan Sekaran (PI)
Doncaster Royal Infirmary, C021: Dinesh Chadha (PI)
Wolverhampton, New Cross Hospital, C022: Kenneth Fotherby (PI)
Halifax, Calderdale Hospital, C023: Pratap Rana (PI)
Taunton, Musgrove Park Hospital, C025: Malik Hussain (PI)
Southampton General Hospital, C026: Nic Weir (PI)
London, Homerton University Hospital, C027: Thomas Harrison (PI)
Exeter, Royal Devon and Exeter Hospital, C028: Salim Elyas (PI)
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by British Heart Foundation (CS/15/5/31475), the Alzheimer’s Society (AS-PG-14–033), EU Horizon 2020 SVDs@Target (666881), MRC UK DRI, Fondation Leducq (16/05 CVD), NHS Research Scotland, The Stroke Association and Garfield-Weston Foundation, Chief Scientist Office (UC), and National Institute of Health Research. PB is Stroke Association Professor of Stroke Medicine and a NIHR Senior Investigator.
Ethical approval
The trial is conducted in accordance with the principles of the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice (ICH GCP). Ethics approval was granted by the East Midlands Nottingham 2 Research Ethics Committee of the Health Research Authority number 17/EM/0077 on 10/05/2017. NHS Research and Development Approval is given by each participating Centre. The Medicines and Healthcare Regulatory Authority approved the trial on 01/06/2017. LACI-2 was adopted by the UK Clinical Research Network and Scottish Stroke Research Network.
Informed consent
Participants must have capacity and consent is taken from them for the trial and for secondary data uses; consent is taken from a relative or other informant to provide outcome data.
Guarantor
JMW.
Contributorship
JMW, PMWB, FD, NS, VC, THE, AH, DW designed the trial and secured funding; JMW is the Chief Investigator, obtained ethics and regulatory approvals; GB, JPA helped design the assessments, and with JMW and PMWB, designed the case record form; AH is the trial manager, responsible for daily running of the trial including regulatory compliance; FD, THE, AH, DW are Principle Investigators; NS provides independent blinded event review; AM provides statistical expertise; LW is the trial statistician; JMW drafted the manuscript; all other authors commented and edited it; all authors approved the final version for submission.
ORCID iD
Joanna Wardlaw https://orcid.org/0000-0002-9812-6642
References
- 1.Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J 2018; 3: 309–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. The top 10 causes of death, www.who.int/mediacentre/factsheets/fs310/en/. (2014, accessed 29 January 2015).
- 4.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration: A united approach. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson CA, Hutchison A, Dennis MS, et al. Differences between ischemic stroke subtypes in vascular outcomes support a distinct lacunar ischemic stroke arteriopathy. A prospective, hospital-based study. Stroke 2009; 40: 3679–3684. [DOI] [PubMed] [Google Scholar]
- 7.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017; 134: 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makin S, Turpin S, Dennis M, et al. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke sub-types. J Neurol Neurosurg Psychiatry 2013; 84: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHutchison CA, Cvoro V, Makin S, et al. Functional, cognitive and physical outcomes 3 years after minor lacunar or cortical ischaemic stroke. J Neurol Neurosurg Psychiatry 2019; 90: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015; 10: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012; 367: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the sps3 randomised trial. Lancet 2013; 382: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce LA, McClure LA, Anderson DC, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the sps3 randomised trial. Lancet Neurol 2014; 13: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comerota AJ. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl 2006; 6: 13–19. [DOI] [PubMed] [Google Scholar]
- 15.Tai SY, Chien CY, Chang YH, et al. Cilostazol use is associated with reduced risk of dementia: a nationwide cohort study. Neurotherapeutics 2017; 14: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedder H, Vesterinen H, Macleod M, et al. A systematic review and meta-analysis of interventions tested in animal models of lacunar stroke. Stroke 2014; 45: 563–570. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto N, Pham LD, Hayakawa K, et al. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 2013; 44: 2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh F, Tohgi H, Hirai S, et al. Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000; 9: 147–157. [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama S, Demaerschalk BM, Goto S, et al. Stroke prevention by cilostazol in patients with atherothrombosis: meta-analysis of placebo-controlled randomized trials. J Stroke Cerebrovasc Dis 2009; 18: 482–490. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (csps 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9: 959–968. [DOI] [PubMed] [Google Scholar]
- 21.Dinicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of cilostazol versus aspirin for the secondary prevention of stroke. Am J Cardiol 2013; 112: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda K, Uchiyama S, Yamaguchi T, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol 2019; 18: 539–548. [DOI] [PubMed] [Google Scholar]
- 23.Bath PM, Pathansali R, Iddenden R, et al. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and platelet function in acute stroke. Cerebrovasc Dis 2001; 11: 265–272. [DOI] [PubMed] [Google Scholar]
- 24.Willmot M, Ghadami A, Whysall B, et al. Transdermal glyceryl trinitrate lowers blood pressure and maintains cerebral blood flow in recent stroke. Hypertension 2006; 47: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 25.The ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (enos): a partial-factorial randomised controlled trial. Lancet 2015; 385: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair GW, Appleton JP, Law ZK, et al. Preventing cognitive decline and dementia from cerebral small vessel disease: the laci-1 trial. Protocol and statistical analysis plan of a phase iia dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int J Stroke 2018; 13: 530–538. [DOI] [PubMed] [Google Scholar]
- 27.Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the lacunar intervention-1 (laci-1) trial, a randomised clinical trial. EClinicalMedicine 2019; 11: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleton JP, Blair GW, Flaherty K, et al. Effects of isosorbide mononitrate and/or cilostazol on haematological markers, platelet function and haemodynamics in patients with lacunar ischaemic stroke: safety data from the lacunar intervention-1 (laci-1) trial. Front Neurol 2019; 10: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabriat H, Hervé D, Duering M, et al. Predictors of clinical worsening in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: prospective cohort study. Stroke 2016; 47: 4–11. [DOI] [PubMed] [Google Scholar]
- 30.Croall ID, Tozer DJ, Moynihan B, et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: the preserve randomized clinical trial. JAMA Neurol 2018; 75: 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bath PM, Scutt P, Blackburn DJ, et al. Intensive versus guideline blood pressure and lipid lowering in patients with previous stroke: main results from the pilot ‘prevention of decline in cognition after stroke trial’ (podcast) randomised controlled trial. PLoS One 2017; 12: e0164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (cromis-2): a multicentre observational cohort study. Lancet Neurol 2018; 17: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson D, Ambler G, Lee K, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol 2019; 18: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson C, Fitzpatrick R, Crocker H, et al. The stroke impact scale: validation in a UK setting and development of a sis short form and sis index. Stroke 2013; 44: 2532–2535. [DOI] [PubMed] [Google Scholar]
- 35.IST-3 collaborative group. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third international stroke trial. 18-month follow-up of a randomised controlled trial . Lancet Neurol 2013; 12: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyoda K, Uchiyama S, Hoshino H, et al. Protocol for cilostazol stroke prevention study for antiplatelet combination (csps.Com): a randomized, open-label, parallel-group trial. Int J Stroke 2015; 10: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt R, Berghold A, Jokinen H, et al. White matter lesion progression in LADIS: frequency, clinical effects, and sample size calculations. Stroke 2012; 43: 2643–2647. [DOI] [PubMed] [Google Scholar]
- 38.Wardlaw JM, Doubal FN, Valdes-Hernandez MC, et al. Blood-brain barrier permeability and long term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013; 44: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell FM, Del Carmen Valdes Hernandez M, Makin SD, et al. Sample size considerations for trials using cerebral white matter hyperintensity progression as an intermediate outcome at 1 year after mild stroke: results of a prospective cohort study. Trials 2017; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlovic AM, Pekmezovic T, Tomic G, et al. Baseline predictors of cognitive decline in patients with cerebral small vessel disease. J Alzheimers Dis 2014; 42: S37–S43. [DOI] [PubMed] [Google Scholar]
- 41.Makin SD, Doubal FN, Shuler K, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J 2018; 3: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makin SDJ, Doubal FN, Quinn TJ, et al. The effect of different combinations of vascular, dependency and cognitive endpoints on the sample size required to detect a treatment effect in trials of treatments to improve outcome after lacunar and non-lacunar ischaemic stroke. Eur Stroke J 2017; 3: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wardlaw JM, Chappell FM, Valdes Hernandez MDC, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017; 89: 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Optimising the Analysis of Stroke Trials (OAST) Collaboration, Gray LJ, Bath PM, Collier T. Should stroke trials adjust functional outcome for baseline prognostic factors? Stroke 2009; 40: 888–894. [DOI] [PubMed] [Google Scholar]
- 45.The Optimising Analysis of Stroke Trials (OAST) Collaboration. Calculation of sample size for stroke trials assessing functional outcome: comparison of binary and ordinal approaches. Int J Stroke 2008; 3: 78–84. [DOI] [PubMed] [Google Scholar]
- 46.Makin SDJ, Doubal FN, Dennis MS, et al. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging. Longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 2015; 46: 3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson KJ, Cvoro V, MacLullich AMJ, et al. Visual rating scales of white matter hyperintensities and atrophy: comparison of computed tomography and magnetic resonance imaging. J Stroke Cerebrovasc Dis 2018; 27: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Simoni M, Kuker W, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013; 44: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 49.SPRINT MIND Investigators for the SPRINT Research Group, Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]