Abstract
Introduction
The type of antithrombotic treatment in cervical artery dissection patients is still a matter of debate. Most physicians prefer anticoagulants over antiplatelet agents for stroke prevention. However, this approach is not evidence-based and antiplatelets might be as safe and as effective. The ‘Biomarkers and Antithrombotic Treatment in Cervical Artery Dissection’ (‘TREAT-CAD’) trial (clinicaltrials.gov: NCT02046460) compares Aspirin to oral anticoagulants (vitamin K antagonists) with regard to efficacy and safety by using both clinical and imaging surrogate outcome measures. TREAT-CAD tests the hypothesis, that aspirin is as safe and effective as vitamin K antagonists.
Patients and methods
TREAD-CAD is a Prospective, Randomised controlled, Open-labelled, multicentre, non-inferiority trial with Blinded assessment of outcome Events (PROBE-design). Key eligibility criteria are (i) clinical symptoms attributable to cervical artery dissection and (ii) verification of the cervical artery dissection diagnosis by established magnetic resonance imaging criteria. Patients are randomised to receive either Aspirin 300 mg daily or vitamin K antagonists for 90 days.
Results
Primary outcomes are assessed at 14 ± 10 days (magnetic resonance imaging and clinical examination) and at 90 ± 30 days (clinical examinations). The primary endpoint is a composite outcome measure – labelled Cerebrovascular Ischemia, major Hemorrhagic events or Death (CIHD) – and includes (i) occurrence of any stroke (including retinal infarction), (ii) new ischaemic lesions on diffusion-weighted magnetic resonance imaging, (iii) any major extracranial haemorrhage, (iv) any symptomatic intracranial haemorrhage, (v) any new haemorrhagic lesion visible on paramagnetic-susceptible sequences and (vi) death.
Discussion
After database closure, (i) central verification of cervical artery dissection diagnosis will be done by two experienced raters, (ii) adjudication of outcome events will be performed by independent adjudication committees, separately for clinical and imaging outcomes. The primary analysis will be done on the per protocol data set. The targeted sample size consists of 169 evaluable patients in the per protocol data set.
Conclusion
TREAT-CAD is testing the non-inferiority of Aspirin versus vitamin K antagonists treatment in patients with symptomatic cervical artery dissection by combined clinical and magnetic resonance imaging outcomes.
Keywords: Cervical artery dissection, stroke, prevention, randomised clinical trial, antiplatelets, anticoagulants
Introduction
Cervical artery dissection (CAD) is the leading cause of stroke in adults aged <50 years.1,2 Most physicians prefer anticoagulants for stroke prevention, while this approach is not evidence-based and antiplatelets might be as safe and effective while less complex in handling.3 Several meta-analyses across observational data comparing antiplatelets to anticoagulants in CAD patients with regard to safety and efficacy outcomes showed inconclusive results.4–8 Three meta-analyses reported no difference between antiplatelets and anticoagulants.4,6,8 In a Cochrane systematic review, Lyrer and Engelter observed a trend in favour of anticoagulants for the primary outcome of ‘death or disability’ (odds ratio (OR): 1.77, 95% confidence interval (CI): 0.98–3.22; P = 0.06), while in turn symptomatic intracranial haemorrhages (5/627; 0.8%) and major extracranial haemorrhages (7/425; 1.6%) occurred solely in the anticoagulation group.7 Using a Bayesian meta-analysis design, Sarikaya et al. showed a benefit of antiplatelets compared to anticoagulants for preventing the composite outcome of ischaemic stroke, intracranial haemorrhage or death (relative risk 0.32, 95% credibility interval 0.12–0.64).5 However, these findings should be interpreted cautiously, as all meta-analyses were based on observational data, which are prone to bias.5,7
In the UK, a randomised-controlled trial (RCT) – CADISS – was designed to show feasibility. While the investigators were able to recruit 250 patients, it took more than 7 years, recruitment at more than 40 centres, and the event rate was much lower than expected (i.e., 2.0% at 3 months, 2.4% at 12 months for ipsilateral stroke and death in the intention-to-treat population).9,10 There was no statistically significant difference between treatment arms. Numerically, there were more ischaemic strokes in the Aspirin group, while the only major haemorrhage occurred in the anticoagulation group. Thus, the clinically important question whether CAD patients should be treated with Aspirin or oral anticoagulation remains to be answered.
Estimates based on the aforementioned observational data and practical experiences from the UK-based CADISS trial show that an RCT based on purely clinical outcomes is a huge venture as a very large sample size (n ≥ 2000–10,000 patients) would be required to render clinically meaningful and statistically reliable results.5,9 The use of surrogate markers enables to significantly reduce the required sample size in such a trial. As a surrogate for ischaemic events, lesions on diffusion-weighted magnetic resonance imaging (MRI DWI) are considered an ideal candidate and have been shown to occur in up to 25% of CAD patients during follow-up.11 Likewise, lesions identified on T2*w gradient echo (GRE) or susceptibility-weighted images (SWI) may serve as surrogates for intracerebral haemorrhage.11
With these considerations in mind, we designed the prospective TREAT-CAD randomised trial which compares Aspirin (ASA) to vitamin K antagonists (VKA) in CAD with regard to efficacy and safety by using both clinical and imaging outcome measures. We here present the trial design and discuss key aspects of the study protocol.
Trial design
Overview
The ‘Biomarkers and Antithrombotic Treatment in Cervical Artery Dissection’ – TREAT-CAD trial (NCT02046460) is a Prospective, Randomised controlled, Open-labelled, multicentre, non-inferiority trial with Blinded assessment of outcome Events (PROBE-design). Recruitment is performed in 10 stroke centres (n = 7 Switzerland, n = 2 Germany, n = 1 Denmark) with special expertise and interest in CAD diagnosis and treatment. Participants with clinical and MRI-based definite diagnosis of CAD are enrolled within two weeks of first symptoms (ischaemic and/or non-ischaemic) attributable to CAD. Participants are randomised to receive open-label ASA or VKA for a period of 90 days after randomisation (see ‘Intervention’ section). The primary hypothesis is that ASA treatment is non-inferior to VKA treatment with regard to the primary composite efficacy and safety outcome measure (see ‘Study outcomes’ section).
Study population
Patients aged > 18 years presenting at the participating centres with acute ischaemic (ischaemic stroke or transient ischaemic attack) or non-ischaemic (i.e., ‘local signs’) symptoms of CAD within the preceding two weeks (prior to enrolment) are eligible for participation. Particularly in patients presenting at the study centres with stroke or transient ischaemic attack, the presence of inaugural CAD symptoms (i.e., local symptoms including pain), which is used to define the symptom onset and thus to determine the time window for enrolment, is assessed in detail. Centres are encouraged to enrol all patients as soon as possible once admitted to the hospital and after verification of the clinical diagnosis of CAD (of the internal carotid artery dissection (ICAD) and/or vertebral artery dissection (VAD)) by MR techniques (see ‘Imaging’ section) according to pre-defined, widely accepted CAD diagnostic criteria (see Table 1 and ‘Imaging’ section). The inclusion and exclusion criteria of the trial are presented in Table 1. A written informed consent by the patient, a next-of kin or an independent physician is required before enrolment. The targeted sample size consists of 169 evaluable patients for the primary per protocol (PP) analysis.
Table 1.
Participant inclusion and exclusion criteria.
| Inclusion criteria | |
| 1 | Acute ischaemic or non-ischaemic symptoms within two weeks |
| 2 | Verification of CAD diagnosis (carotid and/or vertebral) by MR techniques (at least one): |
| mural hematoma or | |
| pseudo-aneurysm or | |
| long filiform stenosis or | |
| intimal flap or | |
| double lumen or | |
| occlusion situated more than 2 cm above the bifurcation of the carotid artery, revealing a pseudo aneurysm or a long filiform stenosis after recanalisation | |
| 3 | Written informed consent by patient |
| 4 | 24-h latency period in case of thrombolysis |
| 5 | Age > 18 years by time of inclusion |
| Exclusion criteria | |
| 1 | MR contraindications (claustrophobia precluding MRI: patients agreeing to undergo MRI scanning with mild sedation may be entered into the study) |
| 2 | Contraindications to the use of anticoagulation (vitamin k antagonists, heparin) or ASA (according to the Swiss ‘Arzneimittelkompendium’ http://www.compendium.ch/search/de) or the ‘Rote Liste’ (German centres) or Lægemiddelstyrelsen – produktresume for the Danish centre (https://laegemiddelstyrelsen.dk/da/bivirkninger/find-medicin/produktresumeer/) and the judgment of the treating physician |
| 3 | Pregnancy (note: for women in child bearing age a pregnancy test has to be done prior to study entry) |
| 4 | Insufficient transtemporal bone window (for TCD-substudy patients only) |
TCD: transcranial Doppler; ASA: Aspirin; MRI: magnetic resonance imaging; CAD: cervical artery dissection.
Imaging
For verification of CAD diagnosis, all study participants undergo a routine MRI scan of brain and neck before entering the study. In these scans, the diagnosis of CAD has to be established according to the following criteria (presence of at least one)12: (i) mural hematoma, (ii) pseudo-aneurysm, (iii) long filiform stenosis, (iv) intimal flap, (v) double lumen and (vi) occlusion situated more than 2 cm above the bifurcation of the carotid artery revealing a dissecting aneurysm or a long filiform stenosis after recanalisation. Inclusion of specific sequences (e.g., fat-saturated T1) into baseline scans to depict the mural hematoma is encouraged. A follow-up MRI (3 Tesla) scan of the brain includes the following sequences 14 ± 10 days after study treatment onset: (i) diffusion-weighted imaging (DWI+) including apparent diffusion coefficient (ADC) maps to detect new acute ischaemic brain lesions, (ii) T2*w gradient echo (GRE) or susceptibility-weighted images (SWI) to detect new haemorrhagic brain lesions and (iii) contrast-enhanced magnetic resonance angiography (CE-MRA) to improve delineation of the wall hematoma against the perfused vessel lumen and to assess the degree of obstruction of the affected artery, as done in prior research.11
Central imaging analysis
All baseline and follow-up MRI imaging of all trial participants are transferred to the leading centre at the University Hospital Basel, where central imaging analyses is performed. This includes (i) verification of the CAD-diagnosis by consensus reading of two experienced investigators (STE and CT) and (ii) central analysis of the baseline and follow-up brain MRI by an independent Imaging Core Lab (ICL). The latter consists of two experienced neuroradiologists who will assess the imaging component of the composite outcome measure (i.e., new – compared to baseline imaging – acute lesions on diffusion-weighted MRI (DWI+) or any new micro- or macrobleed on T2* or SWI). The assessors of the imaging core lab are blinded to the type of allocated treatment within the trial and clinical outcome.
Intervention
Patients are randomised to receive either ASA or anticoagulants (ratio 1:1). ASA means Aspirin™ 300 mg once a day (q.d.) given orally. In patients who cannot swallow safely, ASA can be given intravenously (e.g., Aspegic™ 250 mg q.d.) until swallowing function has recovered. Anticoagulants means the use of vitamin K antagonists (e.g., phenprocoumon [Marcumar™] or acenocoumarol [Sintrom™] or warfarin [MarevanTM]) with a target INR of 2.0–3.0. Until the target INR has been reached, patients receive anticoagulation with intravenous heparine or low molecular weight heparine. The procedure of INR monitoring is left to the discretion of the treating physicians according to usual practice in each centre. The choice of the distinct agent (e.g., phenprocomon or acenocoumarol or warfarin) is left to the discretion of the treating physician and the patient, taking into account the experience with these agents (phenprocoumon is commonly used in the German-speaking part of Switzerland and in Germany, acenocoumarol in the French-speaking part of Switzerland and warfarin in Denmark). The use of direct oral anticoagulants (DOACs) is not allowed. Although in the design phase of the trial, the use of DOACs was suggested, this was rejected by regulatory authorities in Switzerland due to the lack of safety data on the use of DOACs in CAD. Treatment duration within the trial is 90 days (± 30 days, i.e., until follow-visit 2) or until occurrence of a primary outcome event.
Follow-up
After enrolment into the study, participants are scheduled for a first clinical and imaging (MRI) follow-up after 14 (±10) days. A second, purely clinical follow-up visit is scheduled for 90 (±30) days after enrolment. Selected centres perform additional, yet optional, 180-day (±30 days) long-term follow-up. Centres are encouraged to perform follow-up visits and collect data as foreseen in the trial protocol even when outcome events or change of allocated treatment occur prior to regular end of study visits.
Outcome events
The primary endpoint is a composite outcome measure – labelled Cerebrovascular Ischemia, major Hemorrhagic events or Death (CIHD) – and includes the following efficacy and safety outcomes during the treatment period of 90 days since randomisation: (1) Cerebral ischaemic events (clinical) or surrogate findings for cerebral ischemia: (a) any ischaemic stroke, defined as new symptomatic neurologic deterioration lasting at least 24 h that was not attributable to a non-ischaemic cause, or a new symptomatic neurologic deterioration that was not attributable to a non-ischaemic cause and was accompanied by neuroimaging evidence of a new brain infarction.13 Retinal infarction is included in this component and is defined according to prior research14; (b) new acute ischaemic lesions on DWI MRI since baseline are defined as (i) those with an unequivocal lack of continuity between new and existing (on baseline imaging) lesions on the same slice as well as on adjacent slices – irrespective of vascular territories and lesion pattern11 and (ii) ‘acute’ being defined as a hyperintense signal alteration on DWI with a corresponding hypointense or isointense signal on ADC maps.11 (2) Haemorrhages (clinical) or surrogate findings: (a) any major extracranial haemorrhage: any clinically apparent bleeding requiring any kind of intervention (including hospitalisation or prolongation of hospitalisation) or leading to death; (b) any symptomatic intracranial haemorrhage defined as any documented intracranial hemorrhage – i.e., an acute extravasation of blood into the brain parenchyma13 – that is temporally associated to any deterioration in the patient’s clinical condition; (c) any new, asymptomatic micro- or macrobleeds (visible on follow-up brain T2*-MRI or SWI, which were absent on the baseline MR scan) defined as (1) those with an unequivocal lack of continuity between new and existing (on baseline imaging) lesions on the same slice as well as on adjacent slices – irrespective of vascular territories and lesion pattern,11 and lesions being defined as hypointense brain lesions with clear margins on T2*-MRI or SWI. (3) Death of any cause: In an adjudication procedure, causes of death will be distinguished as cardio/cerebrovascular (i.e., fatal acute coronary syndrome, fatal stroke, fatal intracranial haemorrhage, fatal pulmonary embolism, sudden death and unobserved or unexpected death)13 versus all other causes.
Secondary endpoints include – among all components of the composite primary outcome measure as separately analysed endpoints – (i) any increase in volume of the vessel wall hematoma at the follow-up cervical MRI (as compared to baseline), (ii) functional outcome at three months as assessed with the modified Rankin Scale score and (iii) any transient ischaemic attack (defined as new neurologic symptoms or deficit lasting less than 24 h with no new infarction on neuroimaging13), as well as (iv) recurrent CAD during the treatment period of 90 days since randomisation.
Within two substudies (see ‘Substudies’ section) further, tertiary endpoints will be analysed. All primary, secondary and tertiary endpoints are listed in Table 2.
Table 2.
Primary, secondary and tertiary outcome measures.
| Primary outcome measure | |
| The primary composite outcome measure – labelled cerebrovascular ischaemia, major Hemorrhagic events or Death (CIHD) – includes the following efficacy and safety outcome measures during the treatment period | |
| 1 | Cerebral ischaemic events (clinical) or surrogate findings for cerebral ischemia |
| Occurrence of any ischaemic strokea | |
| New acute lesions on DWI since baseline | |
| 2 | Haemorrhages (clinical) or surrogate findings: |
| Any major extracranial haemorrhage (defined as any clinical apparent bleeding requiring any kind of intervention (including hospitalisation or prolongation of hospitalisation) or leading to death) | |
| Any symptomatic intracranial haemorrhage (any documented intracranial haemorrhage that was temporally related to any deterioration in the patient’s clinical condition) | |
| Any asymptomatic micro- or macrobleeds (visible on follow-up brain T2*-MRI (or SWI), which were absent on the baseline MR scan) | |
| 3 | Death (of any course) |
| Secondary outcome measures | |
| 1 | New ischaemic strokes since baseline |
| 2 | New acute lesions on DWI since baseline |
| 3 | Any major extracranial haemorrhage (defined as any clinical apparent bleeding requiring any kind of intervention [including hospitalisation or prolongation of hospitalisation] or leading to death) since baseline |
| 4 | Any symptomatic intracranial haemorrhage (any documented intracranial haemorrhage that was temporally related to any deterioration in the patient’s clinical condition since baseline |
| 5 | Any asymptomatic micro- or macrobleeds (visible on follow-up brain T2*-/SWI-MRI, any asymptomatic micro- or macrobleeds (visible on follow-up brain T2*-/SWI-MRI, |
| 6 | Any death |
| 7 | Any increase in volume of the vessel wall hematoma at the follow-up cervical MRI as compared to the baseline MR scan |
| 8 | Independence in activity of daily living (modified Rankin scale 0-2) at three months and at six months |
| 9 | Excellent functional outcome (modified Rankin scale 0-1) at three months and at six months |
| 10 | Any TIA (classical definition)13 |
| 11 | Recurrent cervical artery dissection |
| Tertiary outcome measures (substudies) | |
| 1 | TCD recording |
| (i) Frequency of MES in CAD patients, stratified to the type of treatment (aspirin vs.) anticoagulation and compared with the presence of stroke/TIA (at three months) | |
| (ii) The presence of new ischaemic lesion (DWI) | |
| (iii) Microbleeds (SWI) at the first follow-up MRI (14 ± 10 days after treatment onset), not present a the ‘baseline MRI’ (by which diagnosis was established) | |
| 2 | Biomarker |
| (i) Interaction between MMP9 and the primary endpoint measure stratified to type of antithrombotic treatment (i.e., ASA vs. anticoagulation) | |
| (ii) Interaction between MMP9/TIMP2 ratio and the primary endpoint measure stratified to type of antithrombotic treatment (i.e., ASA vs. anticoagulation) | |
| (iii) Interaction between other biomarkers (e.g., inflammatory or neurodegenerative) and the primary endpoint measure stratified to the type of antithrombotic treatment (i.e., ASA vs. anticoagulation) | |
| 3 | (i) Occurrence of haemodynamic or morphological arterial alterations (as assessed by MRI and ultrasound) and (ii) their interaction with study endpoints stratified to type of antithrombotic treatment (i.e., ASA/anticoagulation) |
SWI: susceptibility-weighted image; ASA: Aspirin; MRI: magnetic resonance imaging; DWI: diffusion-weighted magnetic resonance imaging; TIA: transient ischaemic attack; TCD: transcranial Doppler; MES: microembolic signal; CAD: cervical artery dissection; MMP9: matrix-metalloproteinase 9; TIMP2: tissue inhibitor of metalloproteinases 2; CIHD: Cerebrovascular Ischemia, major Hemorrhagic events or Death.
aIncluding retinal infarction.
Statistical considerations and analyses
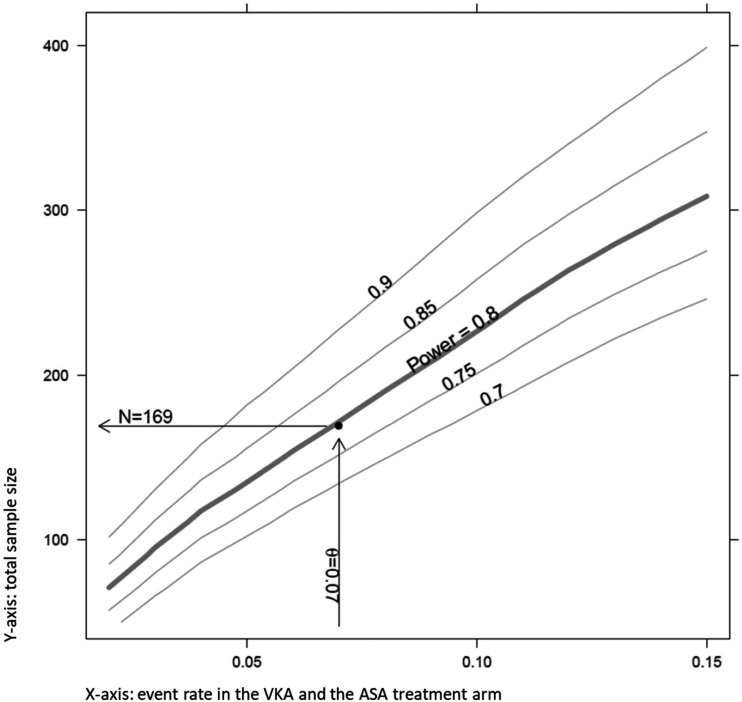
The sample size of the trial was calculated to be able to show the non-inferiority of ASA to VKA treatment in CAD patients with regard to the primary study endpoint. The calculation was based on an expected CIHD rate of 7% for patients in both the ASA and the VKA treatment arms, which yields a target sample size of 169 evaluable participants in the primary – PP – analysis. The estimation of the event rate was based on the frequency of clinical outcome events in prior observational studies and a conservative estimate of the frequency of imaging surrogates which we expect to occur more frequently than clinical events. A pre-planned interim analysis after recruitment of 80% (i.e., n = 136) of the target sample size indicated that a total of 194 participants have to be recruited in order to have at least 169 evaluable data sets in the PP population.
Non-inferiority can be declared if the upper limit of the two-sided 95% confidence interval of the absolute risk difference is smaller than 12% (noninferiority margin). Sample size was set to ensure at least 80% power (1 − β = 0.8) at a significance level of alpha = 5%.
Primary analysis
The primary analysis will be performed on the PP data set. The difference in the CIHD rate between the ASA and the VKA treatment will be compared with the non-inferiority margin using a two-sided 95% confidence interval. We will use Wilson’s method to calculate CI (continuity-corrected modification of the Wilson’s score method Newcombe, 1998). The primary analysis will be repeated on the intention-to-treat (ITT) population.
Exploratory analyses of the secondary endpoints will be performed on the PP as well as the ITT population. As outlined in the statistical analysis plan (SAP), pre-specified sensitivity analyses including assessment of the impact of MR surrogates (DWI and T2*/SWI lesions) versus clinical events will be performed on the PP as well as the ITT population. The full SAP will be made public prior to database closure and final analysis.
All analyses will be conducted using the statistical software package R, version 3.6.
Safety and event adjudication
As specified within the trial data safety monitoring board (DSMB) charter, an independent DSMB consisting of two independent experienced stroke neurologists and an independent statistician assessed patient safety after 80% of the original target sample size (i.e., n = 136 participants). The DSMB concluded that there were no safety issues at that time point and the enrolment can be completed as planned.
Both clinical and imaging components of the composite primary study endpoint will be independently adjudicated by a Clinical Event Adjudication Committee and the ICL.
Substudies
Two substudies within TREAT-CAD are performed in selected centres also participating in the main study. Enrolment in these studies is optional for TREAT-CAD participants.
TCD monitoring substudy
The objective of the TREAT-CAD transcranial Doppler (TCD) substudy is to (i) detect the frequency of microembolic signals (MES) in CAD patients, stratified to the type of treatment (aspirin vs. anticoagulation) – in the setting of an RCT – and (ii) to evaluate the meaning of MES by addressing the following questions: (a) Is there an association of MES (presence or number) with the occurrence of clinical and/or surrogate MR outcome measures; (b) Is there an interaction between MES, type of treatment and outcome events. Participants are asked to allow a 6-h TCD monitoring in between day 1 and day 4 since start of the allocated study treatment. Recordings were allowed to be split in up to three episodes (2 h each). In patients with ICAD, the ipsilateral middle cerebral artery is investigated. In patients with VAD, the ipsilateral posterior cerebral artery is investigated.
Biomarker substudy
The objective of the biomarker study is to investigate whether the plasma level of MMP9 (matrix-metalloproteinase 9) and the ratio of the plasma MMP9 to TIMP2 (tissue inhibitor of metalloproteinases 2) is associated with efficacy and safety measures in CAD patients, when stratified to the allocated treatment regime. Plasma samples are collected from participants at baseline (i.e., prior to start of the allocated treatment within the TREAT-CAD main study) and at Follow-up visit 1. The specific focus on MMP9 and TIMP2 is based on preliminary observational data pointing to higher MMP9 and MMP9/TIMP2 ratios in CAD versus control patients.
Figure 1.
Study flow chart – overview of study visits. aApplicable for TCD and biomarker substudy; *optional. AE: adverse event; IC: informed consent; MRI/A: magnetic resonance imaging/angiography; MES: microembolic signal; mRS: modified Rankin scale; NIHSS: National Institute of Health Stroke Scale; TCD: transcranial Doppler.
Figure 2.
Power and sample size calculation indicating 169 evaluable participants needed in the per protocol data set to achieve 80% power at an event rate of 7%. ASA: Aspirin; VKA: vitamin K antagonists.
Discussion
In light of the conflicting results of observational studies, the need for data from RCTs comparing antiplatelets to anticoagulants in CAD patients has long been stipulated. Realisation of a RCT in patients with CAD is a huge challenge. The UK-based CADISS trial has shown that recruitment of CAD patients in an RCT is feasible, but completion took several years despite recruitment at >40 centres. Furthermore, in one out of five CADISS patients, the diagnosis of CAD could not be confirmed in central imaging reading.9 This finding demonstrates the challenge of correctly and uniformly diagnosing CAD in the setting of a multicentre trial. In CADISS, at three months, 3 out of 124 ASA patients versus 1 out 126 VKA patients had recurrent strokes, while the only major haemorrhage occurred in the anticoagulation group.9 Whether these data are sufficient to convincingly advocate the use of ASA is a matter of debate, taking into account the numerically higher rate of events in the ASA group. Furthermore, the lower-than-expected rate of events requires confirmation. ‘The CADISS investigators estimated that a future trial with a similar design would need to enrol 10,000 participants to detect a 1% difference in occurrence of ipsilateral stroke or death or major bleeding between anticoagulation and antiplatelet treatment’.15 In his editorial on CADISS, Kasner estimated that ‘recruitment into CADISS occurred at an average of one participant per centre per year’.15 Thus, ‘a study of 5000 participants might require 500 sites and 10 years to complete recruitment’ and he concluded that a pivotal trial – based on clinical outcomes – ‘is not feasible’.15
Taking into account both (i) the infeasibility of a conventional RCT based on pure clinical outcomes and (ii) the uncertainty, whether ASA can unequivocally be considered the treatment of choice in CAD patients, we designed TREAT-CAD with elements addressing the aforementioned challenges and shortcomings. First, we designed TREAT-CAD as a non-inferiority trial, taking into account that – in case that non-inferiority is shown – ASA as the more convenient, and less costly option might become the default treatment.15 Second, we used the combination of clinical and MRI outcome events in order to lower the sample size required to a feasible magnitude. For this approach, the ICSS-MR substudy16 served as a role model, as the comparative analysis of new lesions on MR images in both treatment arms (i.e., stenting vs. carotid endarterectomy) resulted in virtually the same results as those in the main study based on pure clinical outcomes – with less than 10% of patients.16 More recently, the usefulness of implementing MR surrogates in RCTs has also been shown in a PFO-closure trial (the REDUCE trial)17 and in the MRI substudies of the NAVIGATE ESUS18 and the COMPASS19 trials (to be published).
Third, considering the low rate of clinical events seen in the aforementioned meta-analyses and particularly the CADISS RCT and a much higher rate of imaging endpoints (i.e., 25%) to be expected according to prior research, we assumed that most outcome events in TREAT-CAD will be imaging rather than clinical endpoints. These assumptions have also driven our decision to set the non-inferiority margin (i.e.12%) which might be criticised as being too broad. However, the clinical meaning of pure imaging endpoints is debated20 and indeed unclear. It is possible that such imaging endpoints are associated with the occurrence of psychosocial sequela, which have been reported in a high rate in otherwise well-recovered CAD patients.21,22 As several CAD-patients are both well educated23,24 and in their 30s–50s lacking relevant comorbidities, we assume that the occurrence of new ischaemic or haemorrhagic brain lesions is of disadvantage, no matter if they do reflect in abnormalities, detectable in neurological exams, or not.
Fourth, we focused the selection of our study sites on centres experienced in both diagnosis and treatment of CAD. Furthermore, eligibility to the trial requires the verification of the CAD-diagnosis solely by means of MRI (most frequently mural hematoma). This includes fat-saturated T1 sequences with their known value in the MR-based verification of CAD diagnosis. With this, we assume to minimise the number of patients in which the CAD diagnosis cannot be confirmed in central reading.
Observational data have shown that most ischaemic events occur very early in the time course of CAD.25 This includes clinical26and imaging outcomes.11 Thus, we encourage sites to include patients as soon as possible. Furthermore, the study MR is scheduled at 14 ± 10 days in order to detect new lesions preferably in the early treatment phase which is supported by the fact that no lesion was detected later than on day 11 in an observational study.11 Likewise, the treatment period within the study was set to 90 days as events might be expected in the early phase after treatment initiation and centres tend to switch to longer term treatments beyond a three-month treatment period.27 Furthermore, optional 180-day follow-up will allow to detect variability in treatment regimens across centres. The rather broad time windows for the follow-up visits were chosen to ensure feasibility, particularly of the imaging follow-up in the early phase of study treatment and acute hospitalisation. This might bear the risk of missing outcome events. However, we assume that any variation in follow-up times would be comparable in both treatment groups. Furthermore, centres are encouraged to follow-up patients at the exact date as foreseen in the study schedule.
Although limited to exploratory analyses, the implementation of two substudies will likely provide further insight into CAD pathogenesis (biomarker substudy) and will clarify the significance of monitoring of CAD patients with TCD (TCD substudy).
In conclusion, we anticipate that TREAT-CAD will generate data to support the primary hypothesis of non-inferiority of ASA as compared to VKA treatment and thus will represent a breakthrough trial in clinical CAD research.
Acknowledgements
None.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CT has received funding for travel from Bayer, has received a personal scholarship from the University of Basel and was supported by the Swiss Heart Foundation as well as the Swiss National Science Foundation. HG reports no COI. SS reports no COI. AL reports no COI. MA reports personal fees from Speaker honoraria from Bayer, Medtronic and Covidien and scientific advisory board honoraria from Amgen, Bayer, Boehringer Ingelheim, BMS, Pfizer, Covidien, Daichy Sankyo, Medtronic and Nestlé Health Science. PM received within the last two years research grants from the Swiss National Science Foundation, the Swiss Heart Foundation and the ERISTA program (BMS/Pfizer); and consulting and speaker fees from Medtronic. All these support go to his institution and are used for stroke education and research. GK received research funds from the Swiss heart foundation and the Swiss Parkinson Association as well as honoraria for scientific advisory boards for Bayer, Zambon and Medtronic. TK received research funds from the Cantonal Hospital Forschungsrat as well as travel grants, speaker honorary or served on scientific advisory boards for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo and Medtronic not related to this study. All this support is utilised for stroke education and research at the Cantonal Hospital Aarau Stroke Center. CHN has received funding for travel or speaker honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer Pharma, W.L. Gore and Associates and Abbott. He has received funding for research from Deutsche Zentrum für Herz-Kreislaufforschung (DZHK) and Deutsches Zentrum für neurodegenerative Erkrankungen (DZNE) and from German Ministry of Research and Education. LK has received fees for travel and speaker honaria from Bayer vital, Boehringer Ingelheim, Brystol Myers Squpp, Daiichi-Synkyo and Pfizer outside of this study. SR reports no COI. RS reports no COI. AB reports no COI. CS, The Department of Radiology, University Hospitals Basel, Switzerland, receives financial support from Bayer Healthcare, Bracco and Guerbet and has a research agreement with SIEMENS Medical Solutions. The Department of Neuroradiology receives support from Guerbet. The submitted work is not related to these agreements. CS receives funding from the Swiss National Science Foundation as principal investigator and as co-investigator and from the University Hospital Zurich as co-investigator. MP reports no COI. PL has served on scientific advisory boards for Bayer Schering Pharma and Boehringer Ingelheim; has received funding for travel or speaker honoraria from Bayer Schering Pharma, Boehringer Ingelheim and Shire plc; he serves as coeditor for Neurologie und Psychiatrie and on the editorial board of Swiss Archives of Neurology and Psychiatry; and has received research support from AstraZeneca, Boehringer Ingelheim, Sanofi-Aventis, PhotoThera, the Swiss National Science Foundation and the Swiss Heart Foundation. STE has received funding for travel or speaker honoraria from Bayer, Boehringer Ingelheim and Daiichi-Sankyo. He has served on scientific advisory boards for Bayer, Boehringer Ingelheim, BMS/Pfizer and MindMaze and on the editorial board of Stroke. He has received an educational grant from Pfizer, research support from Daiichi-Sankyo, compensation from Stago for educational efforts and research support from the Science Funds [Wissenschaftsfonds] of the University Hospital Basel, the University Basel, the ‘Freiwillige Akademische Gesellschaft Basel (FAG)’, the Swiss Heart Foundation and the Swiss National Science Foundation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The TREAT-CAD trial was supported by grants received from (1) The Swiss National Science Foundation, (2) the University Hospital Basel, Department of Neurology, (3) the University of Basel, (4) the ‘Freiwillige Akademische Gesellschaft Basel (FAG)’ and (5) the Swiss Heart Foundation.
Ethical approval
The study protocol was approved by relevant local authorities in all centres and complied with national regulations concerning ethics committee approval and informed consent.
Informed consent
All patients gave written informed consent prior to study participation.
Guarantors
CT and STE.
Contributorship
CT drafted the manuscript, served as medical network adviser and scientific trial manager during the study period and collected data. HG designed/conceptualised the study, collected data and performed critical review of the manuscript and edited the manuscript for content. STE initiated, designed, conceptualised and supervised the study; serves as the sponsor investigator of the trial; acquired funding for the trial; drafted the manuscript and revised the manuscript and collected data. PL contributed to the trial design, concept and to the acquisition of funding for the trial; serves as a Co-PI of the trial; performed critical review of the manuscript, edited the manuscript for content; and collected data. MA contributed to the acquisition of funding for the trial, serves as a local PI of the study, performed critical review of the manuscript and edited the manuscript for content. MP and AB serve as independent adjudicators of the core imaging lab, performed critical review of the manuscript and edited the manuscript for content. CS contributed to the trial design and concept with regard to imaging aspects. Performed critical review of the manuscript and edited the manuscript for content. SS serves as independent trial statistician and contributed to design and concept of the trial, performed critical review of the manuscript and edited the manuscript for content. AL, PM, GK, TK, CHN, LK, SR and RS serve as local PIs of participating centres, performed critical review of the manuscript and editing the manuscript for content.
ORCID iDs
Christopher Traenka https://orcid.org/0000-0002-7600-1005
Timo Kahles https://orcid.org/0000-0002-1569-6376
Christian H Nolte https://orcid.org/0000-0001-5577-1775
References
- 1.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009; 8: 668–678. [DOI] [PubMed] [Google Scholar]
- 2.Engelter ST, Traenka C, Lyrer P. Dissection of Cervical and Cerebral Arteries. Curr Neurol Neurosci Rep 2017; 17: 59. [DOI] [PubMed] [Google Scholar]
- 3.Menon RK, Markus HS, Norris JW. Results of a UK questionnaire of diagnosis and treatment in cervical artery dissection. J Neurol Neurosurg Psychiatry 2008; 79: 612. [DOI] [PubMed] [Google Scholar]
- 4.Menon R, Kerry S, Norris JW, et al. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2008; 79: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 5.Sarikaya H, da Costa BR, Baumgartner RW, et al. Antiplatelets versus anticoagulants for the treatment of cervical artery dissection: Bayesian meta-analysis. PLoS One 2013; 8: e72697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy F, Lanfranconi S, Hicks C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012; 79: 686–689. [DOI] [PubMed] [Google Scholar]
- 7.Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev 2010; 3: CD000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury MM, Sabbagh CN, Jackson D, et al. Antithrombotic treatment for acute extracranial carotid artery dissections: a meta-analysis. Eur J Vasc Endovasc Surg 2015; 50: 148–156. [DOI] [PubMed] [Google Scholar]
- 9.Markus HS, Hayter E, Levi C, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol 2015; 14: 361–367. [DOI] [PubMed] [Google Scholar]
- 10.Markus HS, Levi C, King A, et al. Cervical artery dissection in stroke study i. antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the Cervical Artery Dissection in Stroke Study (CADISS) randomized clinical trial final results. JAMA Neurol 2019; 76: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gensicke H, Ahlhelm F, Jung S, et al. New ischaemic brain lesions in cervical artery dissection stratified to antiplatelets or anticoagulants. Eur J Neurol 2015; 22: 859– 865.e861. [DOI] [PubMed] [Google Scholar]
- 12.Debette S, Grond-Ginsbach C, Bodenant M, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology 2011; 77: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 13.Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med 2018; 378: 2182–2190. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol 2005; 140: 376–391. [DOI] [PubMed] [Google Scholar]
- 15.Kasner SE. CADISS: a feasibility trial that answered its question. Lancet Neurol 2015; 14: 342–343. [DOI] [PubMed] [Google Scholar]
- 16.Bonati LH, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol 2010; 9: 353–362. [DOI] [PubMed] [Google Scholar]
- 17.Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017; 377: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. Eur Stroke J 2016; 1: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma M, Hart RG, Smith EE, et al. Rationale, design, and baseline participant characteristics in the MRI and cognitive substudy of the cardiovascular outcomes for people using anticoagulation strategies trial. Int J Stroke 2019; 14: 270–281. [DOI] [PubMed] [Google Scholar]
- 20.Bae HJ, Debette S. Commentary on the cervical artery dissection in stroke study trial. Stroke 2016; 47: 1413–1415. [DOI] [PubMed] [Google Scholar]
- 21.Speck V, Noble A, Kollmar R, et al. Diagnosis of spontaneous cervical artery dissection may be associated with increased prevalence of posttraumatic stress disorder. J Stroke Cerebrovasc Dis 2014; 23: 335–342. [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Ledermann I, Nedeltchev K, et al. Quality of life in survivors after cervical artery dissection. J Neurol 2009; 256: 443–449. [DOI] [PubMed] [Google Scholar]
- 23.Kellert L, Grau A, Pezzini A, et al. University education and cervical artery dissection. J Neurol 2018; 265: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 24.Grond-Ginsbach C, Lichy C, Debette S, et al. Cervical Artery Dissection (CeAD) in physicians. Cerebrovasc Dis 2015; 39: 72–74. [DOI] [PubMed] [Google Scholar]
- 25.Morris NA, Merkler AE, Gialdini G, et al. Timing of incident stroke risk after cervical artery dissection presenting without ischemia. Stroke 2017; 48: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biousse V, D’Anglejan-Chatillon J, Touboul PJ, et al. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke 1995; 26: 235–239. [DOI] [PubMed] [Google Scholar]
- 27.Debette S. Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol 2014; 27: 20–28. [DOI] [PubMed] [Google Scholar]