Abstract
Background
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Despite treatment being in line with current guidelines, mortality remains high in those with septic shock. Intravenous immunoglobulins represent a promising therapy to modulate both the pro- and anti-inflammatory processes and can contribute to the elimination of pathogens. In this context, there is evidence of the benefits of immunoglobulin M (IgM)- and immunoglobulin A (IgA)-enriched immunoglobulin therapy for sepsis. This manuscript aims to summarize current relevant data to provide expert opinions on best practice for the use of an IgM- and IgA-enriched immunoglobulin (Pentaglobin) in adult patients with sepsis.
Main text
Sepsis patients with hyperinflammation and patients with immunosuppression may benefit most from treatment with IgM- and IgA-enriched immunoglobulin (Pentaglobin). Patients with hyperinflammation present with phenotypes that manifest throughout the body, whilst the clinical characteristics of immunosuppression are less clear. Potential biomarkers for hyperinflammation include elevated procalcitonin, interleukin-6, endotoxin activity and C-reactive protein, although thresholds for these are not well-defined. Convenient biomarkers for identifying patients in a stage of immune-paralysis are still matter of debate, though human leukocyte antigen–antigen D related expression on monocytes, lymphocyte count and viral reactivation have been proposed. The timing of treatment is potentially more critical for treatment efficacy in patients with hyperinflammation compared with patients who are in an immunosuppressed stage. Due to the lack of evidence, definitive dosage recommendations for either population cannot be made, though we suggest that patients with hyperinflammation should receive an initial bolus at a rate of up to 0.6 mL (30 mg)/kg/h for 6 h followed by a continuous maintenance rate of 0.2 mL (10 mg)/kg/hour for ≥ 72 h (total dose ≥ 0.9 g/kg). For immunosuppressed patients, dosage is more conservative (0.2 mL [10 mg]/kg/h) for ≥ 72 h, without an initial bolus (total dose ≥ 0.72 g/kg).
Conclusions
Two distinct populations that may benefit most from Pentaglobin therapy are described in this review. However, further clinical evidence is required to strengthen support for the recommendations given here regarding timing, duration and dosage of treatment.
Keywords: Immunoglobulin, IgM- and IgA-enriched immunoglobulin, Sepsis, Pentaglobin, Hyperinflammation, Immunosuppression
Background
Sepsis is a global issue which affects an estimated 49 million people every year, potentially leading to 11 million deaths [1]. It is a clinical syndrome in which profound physiological and biochemical changes often lead to a fatal outcome of an infection; the Third International Consensus (Sepsis-3) defined sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Even after many years of intensive clinical and laboratory research, there is still no specific therapy for sepsis. A subset of sepsis known as septic shock is characterized by profound circulatory, cellular and metabolic abnormalities that are associated with a greater risk of mortality than sepsis alone; with hospital mortality rates > 50% [2, 3].
Immune pathophysiology of sepsis
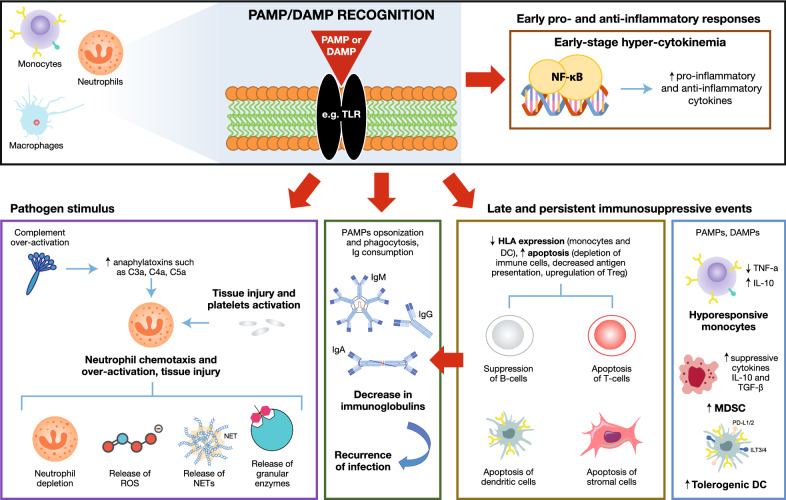
Sepsis is differentiated from uncomplicated infection due to a dysregulated host response to infection. The clinical syndrome of sepsis is initiated by the activation of multiple signaling pathways following the recognition of pathogen-derived molecules [pathogen-associated molecular patterns (PAMPs) e.g. endo- and exotoxins, DNA, lipids] and endogenous host-derived danger signals (damage-associated molecular patterns [DAMPs]) by specific cell-surface receptors on macrophages [toll-like receptors (TLRs)] [4]. Consequently, this leads to the expression of genes involved in inflammation, adaptive immunity, and cellular metabolism [5]. During the course of sepsis, patients often present with multiple features of immunological alterations including systemic inflammatory responses, complement consumption, defects in neutrophil-mediated immunity and decreased serum levels of immunoglobulins finally causing immunosuppression (Fig. 1) [5, 6].
Fig. 1.
Immune pathophysiology of sepsis. DAMP damage-associated molecular pattern, DC dendritic cell, HLA human leukocyte antigen, IgM/G/A immunoglobulin M/G/A, IL interleukin, MDSC myeloid-derived suppressor cell, NET neutrophil extracellular trap, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, PAMP pathogen-associated molecular pattern, PD-1 programmed death protein 1, PD-L1 programmed death ligand 1, ROS reactive oxygen species, TGF-β transforming growth factor β, TLR toll-like receptor, TNF-α tumor necrosis factor α, Treg regulatory T cell
Early stage hypercytokinemia
Activation of the TLRs on macrophages such as monocytes and neutrophils induces signal transduction and translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) to the nucleus. NF-κB induces the expression of early activation genes, including inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1, IL-12, IL-18 and interferons (IFNs), which further initiate a cascade of other inflammatory cytokines (including IL-6, IL-8, IFN-γ), as well as the suppression of adaptive immunity components [5]. Therefore, in the early stages of sepsis, an increase in the presence of both proinflammatory and anti-inflammatory cytokines is observed at diagnosis [7–9].
Effects of complement activation and neutrophil-mediated immunity
In sepsis, there is considerable evidence of complement activation, as reflected by the appearance of complement activation products (anaphylatoxins such as C3a, C4a, C5a) in plasma [10]. Normally, C5a has a beneficial effect and is linked to the recruitment of neutrophils to the site of infection. C5a binding to the C5a receptor (C5aR) transforms the neutrophil into a migratory cell able to invade inflammatory tissue sites and clear pathogens and debris [11]. PAMPs and DAMPs induce oxidative burst leading to the release of reactive oxygen species and granular enzymes, and release neutrophil extracellular traps (NETs). Excessive activation of C5a in the development of sepsis is linked to several processes including apoptosis of lymphocytes, aggravation of systemic inflammation and neutrophil dysfunction [12]. Excessive C5a leads to down-regulation of C5aR during sepsis and can have detrimental effects resulting in homing of neutrophils to the microvasculature, inflammation, tissue damage, thrombosis and multiple organ failure. Blockage of C5a or C5aR inhibits the development of sepsis in mouse models, whereas in patients with sepsis, a downregulated C5aR and high C5a levels correlate with poor prognosis [13].
Decreased levels of immunoglobulins
There have been several observations of decreased immunoglobulins among patients at sepsis diagnosis, in particular decreased levels of the three major immunoglobulin isotypes, immunoglobulins G, M and A (IgG, IgM and IgA, respectively; Table 1. [14–22]). A synergistic role of IgG, IgM and IgA in sepsis and septic shock has been described [21, 24], and the combined presence of low levels of endogenous IgG, IgM and IgA in plasma is associated with reduced survival in patients with severe sepsis or septic shock [21, 25]. The mechanism or the underlying cause for low levels of immunoglobins in sepsis are not entirely clear, but it has been suggested that it may be due to their reduced production/secretion due to immunosuppression, vascular leakage secondary to endothelial dysfunction, redistribution into inflamed tissues, over-utilization by the complement system and excessive catabolism [6, 21, 22, 26, 27].
Table 1.
Studies reporting on immunoglobulin levels and kinetics in patients with sepsis
| References | Study objective | Study design/enrolled patients | Immunoglobulin findings | Outcomes |
|---|---|---|---|---|
| Taccone et al. [14] | Evaluate the time course of gamma-globulin concentrations in patients with septic shock, to define the frequency of low immunoglobulin concentrations, and to investigate the relationship of immunoglobulin concentrations to disease severity and outcome |
Prospective observational study 21 patients (aged ≥ 18 years old) with community-acquired septic shock |
76% of patients (16/21) had hypo-gammaglobulinemia (single or combined immunoglobulin deficiency) at admission: 7 patients had isolated low IgG concentrations, 4 patients had isolated low IgM concentrations, and 3 patients had low IgG and IgM concentrations Two patients had low concentrations of IgG, IgM, and IgA and died with refractory shock within 2 days Patients with low IgG concentration on Day 1 had persistent low levels throughout the ICU stay. Almost all patients with normal IgG levels maintained normal concentrations throughout their stay (1 patient had a transient decrease in IgG on Day 3) |
Patients with low IgG concentrations were indistinguishable at baseline from patients with normal IgG concentrations but had fewer vasopressor-free days (P = 0.02) and more frequently developed acute lung injury/acute respiratory distress syndrome (P = 0.02) There was no significant difference in outcomes in patients with normal or low IgM levels All deaths occurred in patients with low IgG concentrations (P = 0.01) |
| Myrianthefs et al. [15] | Investigate the time course of IgG and IgM concentrations in patients who developed septic shock during their ICU stay |
Observational cohort study 38 patients who developed septic shock during their ICU stay |
45% of patients (17/38) had hypo-gammaglobulinemia (single or combined immunoglobulin deficiency) on admission: 7 patients had isolated low IgG levels, 5 patients had isolated low IgM concentrations, and 5 patients had low IgG and IgM levels Low levels of IgG were resolved within 10 days in the 5 patients who survived in the group with low IgG IgM concentrations improved over time in patients with and without low IgG levels |
There were no significant differences regarding length of ICU or hospital stay, oxygenation index (PaO2/FIO2), duration of vasopressor use, or duration of mechanical ventilation in those with low or normal IgG levels No comparative analyses were provided for patients with low or normal IgM levels |
| Andaluz-Ojeda et al. [16] | Evaluate the quantitative changes in the status of immunocompetence in severe sepsis over time and its potential influence on clinical outcome |
Prospective observational cohort study 50 patients (aged ≥ 18 years old) with severe sepsis or septic shock |
Survivors exhibited a progressive increase in IgG, IgA, and IgM levels from Day 1 to Day 10 |
Compared to survivors, septic patients who did not survive had significantly lower levels of IgG in the first 24 h following admission to the ICU There was no significant difference in IgA or IgM levels between survivors and non-survivors |
| Venet et al. [17] | Measure the endogenous levels of circulating IgG, IgA, and IgM in a cohort of septic shock patients |
Prospective observational cohort study 62 consecutive patients (aged ≥ 18 years old) with septic shock |
At Days 1–2, 61%, 40%, and 9% of patients had IgG, IgM, and IgA concentrations below the lowest limit of age-matched reference values, respectively Circulating IgG and IgM concentrations increased over time, by Days 5–7, 61% of patients had IgG and IgM levels within the range of normal values |
Changes in immunoglobulin levels did not appear to be associated with increased mortality, morbidity, or severity after septic shock Reduced immunoglobulin level was correlated with reduced protein concentrations at Days 1–4 suggesting an apparent hypogammaglobulinemia is present during this time period in septic shock patients |
| Tamayo et al. [18] | Investigate the relationship between endogenously produced immunoglobulins and the clinical outcome in septic shock |
Retrospective study 42 patients with septic shock and 36 patients with systemic inflammatory response syndrome |
Both patients with systemic inflammatory response syndrome and septic shock showed subnormal levels of total IgG, IgG2, and IgM |
Patients with septic shock who died showed the lowest levels of total IgG and IgG1 Univariate Cox regression analysis showed that levels of IgG1, IgG2, IgG3, IgM, IgA, and total IgG were inversely associated to the probability of death at 28 days Multivariate analysis showed that IgG1, total IgG, IgM, and IgA behaved as independent protective factors against mortality (HR, P): 0.23, 0.026; 0.16, 0.028; 0.11, 0.042; 0.05, 0.010, respectively |
| Giamarellos-Bourboulis et al. [19] | Investigate the kinetics of IgM during the different stages of sepsis |
Prospective observational multicenter cohort study 332 critically ill patients were enrolled |
Serum IgM was decreased in septic shock compared to patients with systemic inflammatory response syndrome and patients with severe sepsis Paired comparisons at distinct time points of the sepsis course showed that IgM was decreased only when patients deteriorated from severe sepsis to septic shock |
Serial measurements in patients who progressed from severe sepsis to septic shock, beginning from the early start of vasopressors, showed that the distribution of IgM over time was significantly greater for survivors than for non-survivors |
| Průcha et al. [20] | Assess the frequency of hypogammaglobulinemia in patients with systemic inflammatory response syndrome, severe sepsis, and septic shock | Retrospective study 708 patients with systemic inflammatory response syndrome, severe sepsis, and septic shock | IgG, IgA, and IgM hypogammaglobulinemia was demonstrated in 25%, 3%, and 12% of patients with severe sepsis, and 24%, 2%, and 13% of septic shock patients, respectively |
Mortality in patients with severe sepsis or septic shock and IgG hypogammaglobulinemia was significantly higher than in those with normal IgG levels Mortality in patients with septic shock and IgM hypogammaglobulinemia was significantly higher than in those with normal IgM levels. In patients with severe sepsis, no significant difference in mortality was observed |
| Bermejo-Martín et al. [21] | Evaluate the association between immunoglobulin levels in plasma and survival in patients with severe sepsis |
Prospective observational multicenter cohort study 172 patients (aged > 18 years old) admitted to the ICU with severe sepsis/septic shock |
At time of diagnosis, 27.9%, 39.2%, and 19.2% of patients had immunoglobulin concentrations below the normal reference values for IgG1, IgM, and IgA, respectively |
Kaplan–Meier analysis showed that levels below normal reference values for IgG1, IgM, and IgA were associated with shorter survival times Multivariate regression analysis showed that low levels of IgG1 were a risk factor for mortality (OR: 2.50, 95% CI 1.04–6.03; P = 0.042) The combined presence of IgG1, IgM, and IgA levels below the normal threshold had a synergistic impact on mortality risk (OR: 5.27, 95% CI 1.41–19.69; P = 0.013). A similar effect was observed for combined low levels of IgG1 and IgA:,and IgG1 and IgM |
| Shankar-Hari et al. [22] | Evaluate the additional mortality risk associated with subnormal IgG concentrations in adults with sepsis managed in an ICU setting |
Systematic review of 8 studies 438 adult patients with sepsis |
IgG concentrations increased over time in most studies | Subnormal IgG levels on the day of sepsis diagnosis did not increase the risk of death in adult patients with severe sepsis and/or septic shock by both fixed effect and random effect meta-analysis (M-H pooled OR: 1.32 [95% CI 0.93–1.87] and D + L pooled OR: 1.48 [95% CI 0.78–2.81], respectively) |
| Tian et al. [23] | Study the relationship between circulating B cells and plasma IgM levels and sepsis survival rate |
Systematic review and meta-analysis of 11 studies 829 patients (aged > 18 years old) with sepsis and/or septic shock |
Plasma IgM level was significantly decreased in septic patients (SMD = − 2.35, 95% CI − 2.94, − 1.76; P < 0.00001, I2 = 0%) compared with healthy controls Plasma IgM level was significantly lower in sepsis survivors versus sepsis non-survivors (SMD = − 0.31, 95% CI − 0.53, − 0.09; P = 0.005, I2 = 50%) |
The reduction of circulating B cells and IgM plasma levels is negatively correlated with sepsis survival |
Previous studies reporting on immunoglobulin levels and kinetics in patients with sepsis were identified by searching PubMed using the following search terms: (“immunoglobulin”[Title/Abstract] AND (“level”[Title/Abstract] OR “kinetic”[Title/Abstract])) AND (“sepsis”[Title/Abstract] OR “septic”[Title/Abstract]). The results were filtered for English language and visually assessed for relevance. Studies which did not report on the levels or kinetics of immunoglobulins in adult patients with sepsis were omitted. Additional studies were identified from reference lists of included studies
CI confidence interval, HR hazard ratio, ICU intensive care unit, IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, M-H Mantel–Haenszel, OR odds ratio, SMD standard mean difference
Late immunosuppressive events
In sepsis, increased circulating levels of myeloid-derived suppressor cells (MDSCs) have been observed; these cells secrete multiple anti-inflammatory cytokines, including IL-10 and transforming growth factor-β (TGF β), which suppress immune function [5, 28]. In addition, an apoptotic decrease in antigen-presenting dendritic cells and monocytes has been observed, along with a loss of their proinflammatory cytokine production [29–33]. Human leukocyte antigen–antigen D related (HLA-DR) expression on monocytes and dendritic cells is also downregulated, which decreases responsiveness, and the failure of monocytes to recover HLA-DR levels predicts a poor outcome from sepsis [34].
Natural killer-cell, B- and T-lymphocyte depletion can also be observed in peripheral blood along with an increase in apoptosis of dendritic cells (antigen-presenting cells [APCs]) and stromal cells [35–40]. In the course of sepsis, inhibitory immune checkpoint molecules, including programmed death protein 1 (PD-1), are upregulated on T cells, APCs or peripheral tissue epithelial cells. These molecules regulate leukocyte functions, leading to immune cell apoptosis (contributing to T cell exhaustion), APC dysfunction and expansion of regulatory T (Treg) cells [5, 39, 41–44]. Although cell death in innate and adaptive immunity is initially beneficial to the host, by downregulating the inflammatory responses in sepsis, the extensive loss of immune cells may compromise the ability of the host to further eliminate invading pathogens. It has been shown that preventing immune cell apoptosis markedly improved survival [45].
Why focus on immunoglobulins?
Polyvalent intravenous immunoglobulins, within the network of inflammation and immunity, represent a promising approach to modulate both the pro- and anti-inflammatory processes [46]. However, studies have observed that polyclonal immunoglobulin formulations containing only IgG do not result in improved mortality rates in patients with sepsis [47–49]. On the other hand, although the underlying mechanisms for IgM- and IgA-enriched immunoglobulins to exert beneficial effects in patients with severe sepsis and septic shock is not completely understood, systematic reviews have generally concluded that IgM- and IgA-enriched immunoglobulin preparations are associated with a reduction in mortality [50, 51]. A recent meta-analysis, with trial sequential analysis that included 19 studies involving a total of 1530 patients, found that mortality was significantly reduced in the IgM- and IgA-enriched immunoglobulin group compared with the control group [52].
Currently, the commercially available IgM- and IgA-enriched immunoglobulin formulation is Pentaglobin (12% IgM, 12% IgA and 76% IgG). A different preparation, trimodulin (approximately 23% IgM, 21% IgA and 56% IgG), is in clinical development [53]. The data on the efficacy and safety of IgM- and IgA-enriched immunoglobulin therapy in patients with sepsis therefore comes from the use of Pentaglobin (Table 2, [54–74]).
Table 2.
Studies reporting on the outcomes of IgM and IgA-enriched immunoglobin therapy
| References | Study design/enrolled patients | Cumulative dose | Outcome |
|---|---|---|---|
| Just et al. [54] |
Prospective, randomised, controlled clinical trial 104 intensive care patients (50 patients in treatment group, 54 patients in control group) |
Pentaglobin: initially 5 g, then 5 g every 12 h for 36 h (total 20 g) combined with antibiotics | There was a significant decrease in recovery time, ventilation time, and time spent in the ICU in the treatment group compared to the control group |
| Vogel [55] |
Prospective, randomized, controlled study 50 patients with sepsis (25 patients in treatment group, 25 patients in control group) |
Pentaglobin: 10 g/day for 3 days | There was a ~ 20% lower mortality rate in patients receiving Pentaglobin compared with the control group |
| Wesoly et al. [56] |
Prospective, randomized, controlled study 35 patients with septic postoperative complications (18 patients in treatment group, 17 patients in control group) |
Pentaglobin: 250 mg/kg/day | Endotoxin titers decreased, along with a reduction in mortality and shortening of hospitalization and mechanical ventilation time, in patients receiving Pentaglobin compared with control |
| Schedel et al. [57] |
Prospective, randomized, controlled clinical trial 55 patients with gram-negative septic shock (27 patients in treatment group, 28 patients in control group) |
Pentaglobin: for 3 days according to the following schedule: Day 1: 30 g over > 8 h; Days 2 and 3: 15 g over > 8 h | Patients treated with Pentaglobin had a significantly lower rate of sepsis-related mortality compared to the control group |
| Behre et al. [58] |
Prospective pilot study and randomized, controlled trial Pilot study: 21 patients with acute leukemia or non-Hodgkin’s lymphoma and sepsis syndrome Randomized controlled trial: 52 patients with hematological malignancies and sepsis syndrome (30 patients in treatment group, 22 patients in control group) |
Pentaglobin: Initial bolus of 10 g followed by 5 g every 6 h for 3 days | Patients treated with Pentaglobin had a significantly lower rate of all-cause 28-day mortality compared with those who received 5% human albumin |
| Rodríguez et al. [59] |
Multicenter, prospective, randomized, double-blind clinical trial 37 patients with abdominal sepsis (20 patients in treatment group, 17 patients in control group) |
Pentaglobin: 350 mg/kg/day for 5 days | There was no significant difference in organ dysfunction, organ failure, or mortality between the patients receiving Pentaglobin and the control group. The mortality rate was lower in the Pentaglobin versus control group without reaching statistical significance |
| Reith and Mittelkötter [60] |
Prospective, controlled trial 67 patients with severe sepsis or septic shock (35 patients in treatment group, 32 patients in control group) |
Pentaglobin: 15-20 g/day for 3 days | Patients treated with Pentaglobin had a significantly lower mortality rate compared with patients in the control group |
| Tugrul et al. [61] |
Prospective, randomized, controlled study 42 patients with severe sepsis (21 patients in treatment group, 21 patients in control group) |
Pentaglobin: 250 mg/kg/day over 6 h for 3 days | There was no significant difference in organ morbidity, septic shock incidence, or mortality between the treatment and control groups |
| Karatzas et al. [62] |
Prospective, randomized, controlled study 68 patients with severe sepsis (34 patients in treatment group, 34 patients in control group) |
Pentaglobin: 250 mg/kg/day over 6 h for 3 days | Patients treated with Pentaglobin had a significantly lower rate of 28-day mortality compared to the control group |
| Reith et al. [63] |
Prospective, randomized controlled study 64 patients with abdominal infection (31 patients in treatment group, 33 patients in control group) |
Pentaglobin: 10 g within 6 h of surgery followed by 55 g over the next 66 h by continuous perfusion (total: 1300 mL over 3 days) | There was no significant difference in incidence of fever, percentage of days with fever, mean body temperature, or duration of stay in hospital between those receiving Pentaglobin or albumin |
| Rodríguez et al. [64] |
Prospective, randomized, double-blind controlled study 56 patients with severe sepsis and septic shock of intra-abdominal origin (29 patients in treatment group, 27 patients in control group) |
Pentaglobin: 350 mg/kg/day for 5 days | There was a ~ 20% reduction in mortality rate in patients receiving Pentaglobin compared with the control group; however, there was no significant difference in organ dysfunction, organ failure, or mortality between the 2 groups |
| Buda et al. [65] |
Retrospective case-controlled study 66 patients diagnosed with sepsis after cardiac surgery (22 patients in treatment group, 44 patients in control group) |
Pentaglobin: 250 mg/kg daily for 3 days | Pentaglobin did not significantly reduce mortality in the overall study population. However, in the subgroup of patients with severe sepsis, it improved the survival rate significantly |
| Hentrich et al. [66] |
Multicenter, prospective, randomized, controlled study 206 neutropenic patients with sepsis syndrome or septic shock after receiving chemotherapy for severe hematologic disorders (103 patients in treatment group, 103 patients in control group) |
Pentaglobin: 65 g over 3 days according to the following schedule: 10 g initially (0.5 mL/min) followed by 11 infusions of 5 g, repeated every 6 h | There was no significant difference in all-cause 28- or 60-day mortality, or sepsis-related 28-day mortality between patients receiving Pentaglobin or human albumin |
| Yavuz et al. [67] |
Retrospective study 118 patients with sepsis-induced multiple organ dysfunction syndrome (56 patients in treatment group, 62 patients in control group) |
Pentaglobin: 250 mg/kg/day for 3 days | Patients who received IgM-enriched immunoglobulins had significantly lower overall mortality and 28-day case fatality rates and a shorter length of ICU stay compared with the control group |
| Toth et al. [68] |
Prospective, randomized, controlled pilot study 33 patients with early septic shock accompanied by severe respiratory failure (16 patients in treatment group, 17 patients in placebo group) |
Pentaglobin: 250 mg/kg over 8 h for 3 days | There was no significant difference in organ dysfunction between patients who received Pentaglobin and placebo |
| Brunner et al. [69] |
Prospective, randomized, double-blind, placebo-controlled trial 38 critically ill patients with multiple organ failure, systemic inflammatory response syndrome, and early clinical signs of critical illness polyneuropathy and/or myopathy (19 patients in treatment group, 19 patients in placebo group) |
Pentaglobin: 250 mg/kg body weight/day as a continuous intravenous infusion at a rate of 2 g/h for 3 days | Early treatment with Pentaglobin did not significantly improve critical illness polyneuropathy and/or myopathy or influence length of ICU stay or mortality in critically ill patients |
| Cavazzuti et al. [70] |
Retrospective cohort study 168 patients with septic shock (92 patients in treatment group, 76 patients in control group) |
Pentaglobin: 250 mg/kg/day (20 mg/kg/h) for 3 days | Early adjunctive treatment with IgM-enriched immunoglobulins resulted in an approximately 20% reduction in the absolute risk of 30-day mortality in patients with septic shock |
| Giamarellos-Bourboulis et al. [71] |
Retrospective analysis 200 patients with confirmed severe sepsis or septic shock caused by nosocomial multi-drug resistant Gram-negative bacteria infection (100 patients in treatment group, 100 in control group) |
Pentaglobin: Mean daily dose: 30 g/day administered as a 5–6-hour continuous infusion for 5 days | Patients treated with Pentaglobin had a significantly lower rate of all-cause 28-day mortality compared with the control group |
| Berlot et al. [72] |
Retrospective single-center study 355 patients with septic shock |
Pentaglobin: 250 mg/kg/day over 10 h for 3 days (total dose 750 mg/kg) | Earlier administration of Pentaglobin was associated with a decreased risk of in-ICU mortality, both in patients with septic shock caused by any pathogens and in patients with MDR-related septic shock |
| Willuweit et al. [73] |
Retrospective study 21 patients with sepsis-related vasoplegia post-liver transplant |
Pentaglobin: 250 mg/kg over 12 h for 3 days |
Patients who received IgM-enriched immunoglobulins had significantly decreased levels of inflammatory markers and a reduction in vasopressors required to maintain hemodynamic stability 30-day mortality was 14.3%, significantly less than calculated mortality (greater than 90%) based on Sepsis-Related Organ Failure Assessment scores |
| Domizi et al. [74] |
Single-center, randomized, double-blind, placebo-controlled Phase 2 trial 20 patients diagnosed with sepsis or septic shock for less than 24 h (10 patients in the treatment group, 10 patients in the control group) |
Pentaglobin: 250 mg/kg (5 mL/kg)/day for 3 days | A 72-hour infusion of Pentaglobin in patients with sepsis or septic shock was associated with an increase in sublingual microvascular perfusion |
Previous studies reporting on the outcomes of Pentaglobin therapy were identified by supplementing a recent publication which systematically searched PubMed, Cochrane Library, ISI Web of Knowledge, and Embase databases to update the 2013 edition of the Cochrane review from inception to June 2018 [52]. Their search strategy consisted of: [iviggma (All Fields) OR [igm (All Fields) AND enriched (All Fields)] OR [pentaglobulin (Supplementary Concept) OR pentaglobulin (All Fields) OR pentaglobin (All Fields)] AND [sepsis (MeSH Terms) OR sepsis (All Fields)]. We used the same search category in PubMed to complete the search from June 2018 to June 2020
ICU intensive care unit, IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, MDR multidrug-resistant
Relevant mechanisms of action of IgM- and IgA-enriched immunoglobulins include opsonization and phagocytosis of causal pathogens [75], neutralization of virulence factors including bacterial endo- and exotoxins [76, 77], as well as immunomodulation via interaction with complement factors [78, 79] and prevention of hyper-inflammatory responses. Immunoglobulins have also been shown to downregulate IL-2 production, resulting in a significant inhibition of human T-lymphocyte alloproliferative response in vitro as well as in lectin-stimulated peripheral blood mononuclear cells [80]. However, in addition to a modulation of IL-2, IgM and IgA enriched immunoglobulin exhibited differential effects on the release of pro-inflammatory cytokines (IFN-γ, TNF-α and IL-6) during mixed lymphocyte reaction response [80]. Additionally, in vitro and in vivo models have shown an upregulation of IL-10 following IgM and IgA enriched immunoglobulin administration [81, 82]. Furthermore, a recent clinical study in patients treated with either IgM and IgA enriched immunoglobulin or placebo (NaCl) showed a significant decrease of IL-6 and IL-10 levels at 72 h in the IgM and IgA enriched immunoglobulin group only [74]. Ex vivo data also showed that the investigational preparation, trimodulin, lowered monocyte expression of recognition receptors (TLR2 and TLR4) and coagulation receptors (CD11b and CD64) and also reduced lymphocyte proliferation and release of pro- and anti-inflammatory cytokines including TNF-α and IL-10 [83]. Recently, a beneficial effect of IgM administration on microvascular perfusion parameters could be demonstrated in humans [74], which corroborated earlier research in an animal model of endotoxemia [84]. These effects are in line with positive effects of IgM on septic encephalopathy and the integrity of the function of the blood–brain barrier [85, 86].
The benefits of IgM and IgA enriched immunoglobulin have been gathered from different studies with clinically heterogeneous patients, a wide variety of treatment protocols (e.g. dosage) and in settings with variable access to laboratory diagnostics [87]. Understanding which patients may benefit most from Pentaglobin therapy is of high clinical relevance given the need for a balance between a potential reduction in mortality as well as the relatively high cost and availability of treatment. A previous publication sought to provide guidance on optimal IgM- and IgA-enriched immunoglobulin use [88], however, in the intervening years, further clinical data have been generated and more clinical experience has been gathered to warrant an update to this publication. Furthermore, there is increasing interest in the need for ‘personalized medicine’ [89]. Previous immunomodulatory trials in sepsis have often failed in part due to a failure to correctly identify the appropriate target group [45, 90–93]. Therefore, identification of the appropriate target population for IgM- and IgA-enriched immunoglobulin therapy and tailoring an intervention accordingly could be of great benefit.
However, current international guidelines for the management of sepsis and septic shock from the Surviving Sepsis Campaign advise against the use of intravenous immunoglobulins (IVIGs) in these conditions [94]. This recommendation was graded as weak, with low quality of evidence, and was based largely on a Cochrane meta-analyses which predominantly included relatively small trials performed with IgG. The only large study included used IgG and showed no effect [47].
With this in mind, and given the relatively new concept of sepsis being a ‘dysregulated’ host/immune response, as well as how excessive consumption and insufficient production of immunoglobulins could result in (acquired) deficiency, an expert meeting was organized in March 2019 in Brussels, Belgium during the 39th International Symposium on Intensive Care and Emergency Medicine (ISICEM) congress (Additional file 1: Appendix S1). This working group consisted of six experienced academic critical care physicians from Italy, Germany and Hungary, who had more than a decade of both scientific and clinical experience using immunoglobulins in the context of adjunctive sepsis therapy. The participants discussed which septic patients most benefit from IgM- and IgA-enriched immunoglobulins, current best practice management in different patient populations and how the sepsis treatment landscape has changed over recent years. A consensus report was produced from this expert meeting, which formed the basis of this manuscript, and literature searches using the relevant databases were carried out to identify further evidence of the topics discussed. Additional references were then included during the preparation of the manuscript.
Which patients may benefit most from IgM- and IgA-enriched immunoglobulin therapy?
Defining patient phenotypes
Sepsis is a complex syndrome shaped by pathogen and host factors with specific characteristics that progress over time [2] and a ‘one size fits all’ approach to treatment with IgM- and IgA-enriched immunoglobulins seems inappropriate. We have identified two distinct patient groups who may benefit most from treatment with IgM and IgA enriched immunoglobulin, which can be defined as: (1) those with an acute disease onset, who are heavily inflamed, showing signs of imminent or overt septic shock (patients in a hyperinflammatory stage); and (2) those with an immunocompromised phenotype, often with a long-term intensive care unit (ICU) stay and a higher incidence of viral reactivation and/or nosocomial infections (patients in an immunosuppressive stage). Two-thirds of patients who have combatted initial sepsis may suffer from persistent hyperinflammation, elevated immunosuppression biomarkers and catabolism syndrome developing ‘persistent critical illness’ while still on the ICU; these patients often experience poor long-term outcomes such as high 1-year mortality rates and are frequently disabled by cognitive dysfunction, neuromyopathies, immunological dysfunction and other complications [95].
It must be noted that evidence for the clinical phenotypes and management for these two patient populations can be variable and recommendations made in this review are, therefore, based on both published evidence and the authors’ clinical experience.
Patients with hyperinflammation
Clinical phenotype
Scientific evidence
There are several clinical consequences of hyperinflammation, which affect almost all organs in the body and result in a marked elevation of many biomarkers such as procalcitonin [PCT], IL-6 and C-reactive protein [CRP] [2, 96]. A post hoc analysis of a randomized, controlled study in patients with severe community-acquired pneumonia and elevated baseline CRP, reduced IgM or both, showed a reduction in mortality rate, ventilation requirements and length of hospital stay with the investigational IgM-preparation trimodulin compared with placebo [53].
Another potential method of identifying patients who may best benefit from IgM- and IgA-enriched immunoglobulin treatment could be the use of an adapted version of the predisposition, insult/infection, response and organ dysfunction (PIRO) score—the Torino (TO)-PIRO score [97]. However, the score has its limitations and requires validation through use in clinical practice and results gathered from large databases.
The shock index is an effective, low-cost, easily available bedside measurement tool for the initial assessment of patients at risk for sepsis; patients who present with a normal shock index (< 0.7) have been found to be at very low risk for severe sepsis [98]. The shock index may also help in the evaluation of fluid resuscitation as well as predict the presence of lactic acidosis, development of organ failure and mortality [99]. According to the international consensus definition, septic shock is defined by a vasopressor requirement to maintain a mean arterial pressure of ≥ 65 mmHg and serum lactate level > 2 mmol/L (> 18 mg/dL) in the absence of hypovolemia [2, 100].
Clinical experience
The biomarker thresholds for starting IgM and IgA enriched immunoglobulin therapy have not been well-defined for most of the listed parameters (PCT, IL-6 and CRP). As an example of biomarker-driven interventions, Branche et al. [101] suggest a PCT cut-off of > 0.5 μg/L for antibiotic use. Conversely, rather than threshold values serving as an indicator for starting therapy, observing the kinetics of these biomarkers may better serve to indicate the effectiveness of overall treatment and assist in the determination of the required duration of therapy. Although there is little published evidence with immunoglobulin treatment to support this recommendation [74], there have been studies with ICU patients treated with antibiotics [102, 103].
Timing of therapy
Scientific evidence
Among ICU patients with septic shock caused by any pathogens [including those that are multidrug-resistant (MDR)], those who received IgM- or IgA-enriched immunoglobulins earlier (median delay 12 h versus 14 h) were more likely to survive than those who received them later [71, 72]. This suggests that the timing of treatment may play a critical role in treatment efficacy and patients with hyperinflammation should be treated with IgM- and IgA-enriched immunoglobulins as soon as possible.
Clinical experience
Patients with particularly low IgM levels should be treated as soon as possible; the threshold for low IgM is uncertain, but we suggest ≤ 40–80 mg/dL. Although starting treatment as soon as possible (within 24 h) may lead to overtreatment in some patients, this is felt to outweigh the increased risk of mortality in some patients if treatment is delayed. Given the benefit of early treatment, IgM- and IgA-enriched immunoglobulin administration should be initiated prior to the cause of sepsis/severe infection being identified.
Appropriate dosage
Current recommendation
The summary of product characteristics (SmPC) currently recommends Pentaglobin therapy at a dose of 5 mL (0.25 g)/kg body weight/day for 3 consecutive days with an infusion rate of 0.4 mL/kg/h, further infusions may be required depending on the clinical course. Dosing depends on the immunological status of the patient and the severity of the disease. A higher dosage (7 mL/kg/day for 5 days) of IgM- and IgA-enriched immunoglobulin was used in a prospective study assessing the impact of adjuvant therapy in combination with antibiotics in patients with abdominal sepsis [64]. Dosing of IgM- and IgA-enriched immunoglobulin is also an important consideration in two ongoing Pentaglobin trials; in one septic shock study (IgM-FAT trial), the dosage based on IgM serum levels is compared with the dosage recommended in the SmPC (NCT04182737). In the randomized controlled PEPPER trial, which is currently recruiting patients, a single-mode continuous infusion of 0.4 mL/kg/hour without initial bolus is administered until a total dose of 7 mL/kg/day has been reached; this administration is repeated for 5 consecutive days (NCT02810704) [104].
Clinical experience
Dosing varies between hospitals; however, it may be reasonable to consider an initial bolus since reaching higher IgM levels earlier could be beneficial, i.e. an initial bolus of Pentaglobin at a rate of up to 0.6 mL (30 mg)/kg/h for the first 6 h, followed by a continuous maintenance rate of 0.2 mL (10 mg)/kg/h for 72 h for at least 3 days (total dose ≥ 0.9 g/kg). If possible, IgM levels should be determined upon admittance and monitored regularly. It is not currently known which target values are appropriate to achieve in patients with sepsis (i.e. ‘normal’ or ‘supranormal’), however, the doubling of a patient’s IgM level from the start of treatment has been observed to greatly increase their likelihood of survival. If IgM levels do not increase after 3 days, treatment should be prolonged for at least 2 additional days. In settings where it is not feasible to measure IgM levels regularly, treatment should be started independently from the initial level of IgM. Blood should, however, be drawn at admission prior to treatment and the initial IgM levels may be determined later.
Which patients are not eligible for treatment?
Patients ineligible for therapy are those with a standing do not resuscitate (DNR) order or limitation of therapy, incurable metastatic malignant disease or unstable hematological malignancies.
Recommendations for patients with hyperinflammation
Clinical phenotype:
Laboratory evidence of hyperinflammation e.g. high values of PCT, IL-6, CRP [105]
Septic shock markers: serum lactate [2, 100] and arterial pressure of <65 mmHg
Clinical examples include meningococcal sepsis, toxic shock syndrome, necrotizing fasciitis and severe community-acquired pneumonia (sCAP) [53, 106, 107]
Timing:
As early as possible, particularly in those with low IgM levels and high inflammatory load, and within 24 hours [72]
Dosage:
Total dose of ≥0.9 g/kg
Rate of 0.6 mL (30 mg)/kg/hour for the first 6 hours followed by a continuous maintenance rate of 0.2 mL (10 mg)/kg/hour for 72 hours (Expert Opinion)
Determine IgM levels if possible; if no increase is observed prolong treatment for at least 2 additional days (Expert Opinion)
Exclusion criteria:
Standing DNR order or limitation of therapy, incurable metastatic malignant disease, unstable hematological malignancies
Patients with immunosuppression
Clinical phenotype
Scientific evidence
Our understanding of dysregulated immunity in sepsis has shifted in the last decade. Excessive immune activation has previously been the focus of attention in sepsis; however, more recent evidence has highlighted the important role of immunosuppression (or ‘sepsis-induced immunoparalysis’) as the prevailing immune dysfunction associated with morbidity and mortality [96]. The clinical symptoms/phenotypes of immunosuppression are not as well defined as those of hyperinflammation, though it is recognized that these patients have increased susceptibility to secondary infections [96]. Many patients with septic shock remain in the ICU for weeks with chronic critical illness, and mortality rates increase after 28–30 days following repeated nosocomial infections [108, 109]. These chronic critically ill patients with persistent immunosuppression eventually succumb following viral infections (reactivation and de novo infection) as well as bacterial and fungal infections, and successfully managing and treating these patients is a significant challenge [71, 110–112]. Low HLA-DR expression can also be a marker of immune dysfunction and a predictor of mortality in severe sepsis and septic shock patients [113–115].
In the absence of effective characterization of immune status, nosocomial MDR infection can be considered a surrogate marker for immunosuppression, although this must be considered within the context of local resistance patterns [116]. Cytomegalovirus (CMV) and herpes simplex virus (HSV) reactivation also reflect acquired immunosuppression manifesting as T-cell exhaustion [117]. Measurement of the immune status, such as PD-1/programmed death ligand 1 (PD-L1) expression on T cells and dendritic cells, lymphocyte count, HLA-DR expression on monocytes, immunoglobulin levels and inflammatory markers (e.g. CRP, IL-6 or PCT) are potential diagnostic biomarkers to be considered [105, 118, 119]. Low HLA-DR expression, in particular, may correlate with low lymphocyte counts in the differential blood count and lymphocyte count is also readily available in most hospitals.
Clinical experience
There are currently insufficient means to characterize the immune status of a patient on a day-to-day basis, particularly between different centers. Therefore, choosing the most meaningful biomarkers for identifying patients with immune paralysis is still a matter of debate; until now, repeated measurement of HLA-DR expression on monocytes, lymphocyte count and viral reactivation have been proposed as potential biomarkers [119–122]. Measuring IgM level may be of additional benefit in immunocompromised patients, and persistently low IgM levels (≤ 40–80 mg/dL) may prompt substitution. As previously mentioned, however, actionable thresholds for IgM in this patient group are largely elusive and further data are required to confirm this hypothesis. It is also acknowledged that in some settings monitoring IgM levels is not feasible and as yet cannot be considered a mandatory criterion for treatment [123]. Further research and technological development regarding the identification and monitoring of patients with immunosuppression is certainly warranted.
Timing of therapy
Clinical experience
Providing an exact recommendation on timing of IgM therapy in this population is difficult as the most appropriate data are from patients with severe sepsis or septic shock. However, in our experience, the timing of IgM therapy may be less critical in this phenotype, though it’s largely agreed that patients should be treated early, taking into account that the clinical manifestations of septic shock are more subtle in immunosuppressed patients compared with non-immunosuppressed patients. Either way, the 6-h sepsis bundle should be completed, and the patient should fulfil the clinical criteria for septic shock.
Appropriate dosage
Current recommendation
The SmPC currently recommends Pentaglobin therapy at a dose of 5 mL (0.25 g)/kg body weight/day for 3 consecutive days with an infusion rate of 0.4 mL/kg/h. Further infusions may be required depending on the clinical course. Dosing depends on the immunological status of the patient and the severity of the disease.
Clinical experience
Pentaglobin should be administered with a continuous maintenance rate of about 0.2 mL (10 mg)/kg/h for 72 h (total dose of ≥ 0.72 g/kg), and an initial bolus is not considered beneficial. IgM levels should be determined if possible and if no increase is observed, treatment should be prolonged for at least 2 additional days. Given the lack of supporting evidence and clinical experience in treating this population, we acknowledge that dose and a timeline for immunosuppressed patients with late-onset septic shock have yet to be elucidated.
Which patients are not eligible for treatment?
Exclusion criteria are in accordance with those for patients with hyperinflammation.
Recommendations for patients with immunosuppression
Clinical phenotype:
Increased susceptibility to secondary infections in the blood and lungs [96]
Persistence of septic shock with ≥2 organ dysfunctions after initial resuscitation treatment (Expert Opinion)
Persistent immunosuppression determined by e.g. high PD-1, lymphopenia, low IgM levels, low HLA-DR expression on monocytes, expansion of MDSCs [111, 124–126] (Expert Opinion)
Clinical examples: nosocomial infections, secondary fungal infections (e.g. Aspergillosis), viral reactivation, insufficient clearance of primary infective focus, multi-morbid elderly patient (diabetes mellitus, liver disease, renal insufficiency, malnutrition), patients with viral (co-)reactivation [110]
Timing:
Exact recommendation is difficult, but suggest that patients with severe sepsis or septic shock require rapid infusions to counteract the potential downstream effects (Expert Opinion)
Dosage:
Total dose at least: 0.72 g/kg
Continuous maintenance rate of 0.2 mL (10 mg)/kg/hour for 72 hours; IgM levels should be monitored if possible, and if no increase is observed, treatment should be prolonged for at least 2 additional days (Expert Opinion)
Exclusion criteria:
Standing DNR order or limitation of therapy, incurable metastatic malignant disease, unstable hematological malignancies
Monitoring immunoglobulin levels during therapy
Understanding when to stop therapy is important to prevent overtreatment and for economic reasons. We believe that there is a synergistic impact of simultaneously low levels of IgGAM during sepsis, and we suggest that immunoglobulin level kinetics may be a suitable marker for monitoring and modifying treatment, although we emphasize that the required minimum levels of circulating IgM, IgG and IgA are unclear at this point and further data are required to determine the scale of changes in immunoglobulins after treatment (Table 1). Based on current experience, we propose that patients with pathologically low levels of IgM should reach a sustained elevation to values > 80 mg/dL. Serial measurements of IgA, IgG, and IgM could help to correlate supplementation with outcome and important secondary endpoints in the future and define the optimal immunoglobulin levels required [21, 24, 94].
It is also important to consider that immunoglobin levels may be influenced by other treatment interventions such as fresh frozen plasma (which increases IgM) and rituximab (which significantly lowers IgM). Another consideration is the accumulation of IgM and IgA among chronic kidney disease patients; due to their high molecular weights, IgM and IgA are not removed by conventional renal replacement treatments such as continuous veno-venous hemodialysis and diafiltration (CVVH and CVVHD, respectively). Additionally, the possible effect of other blood purification techniques on immunoglobulin levels is not yet well established [127]. Even though not commonly used in septic shock patients, plasma exchange methods are able to remove both IgM and IgA due to the high sieving coefficient of the membranes used in this technique [128].
A novel situation in COVID-19
In 2019, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) caused a pandemic with an unprecedented global crisis. Current data suggest a link between the severity of coronavirus disease 2019 (COVID-19), viral production, and the severe dysregulation of the inflammatory immune reaction (‘cytokine storm’). It is still unclear, however, which molecular mechanisms trigger the onset of the immune disbalance and why it can rapidly progress to multiorgan dysfunction or acute respiratory distress syndrome (ARDS) with a fatal outcome in a considerable subset of patients [129, 130].
Clinical observation of fatal courses of COVID-19 often includes severe ARDS, which is caused by alveolar injury and multiple organ failure—both of which are associated with hyperinflammation and cytokinemia [131]. Both mild and severe/fatal cases display changes in cytokine production, particularly IL-1β, IL-1ra, IL-6, IL-10, TNF-α, GM-CSF, IL-17, and pathological shifts of circulating leukocyte subsets [132, 133]. This leads to the disturbed development of protective immunity against the infection. The most severe complications of COVID-19 include sepsis-like inflammation, pulmonary or cardiovascular complications, and coagulopathy [134–136].
As discussed above, the innate host immune system is activated in response to the virus to limit infection. Subsequently, the adaptive immune system develops specific immunoglobulins and activates T-cells in direct response to the virus. If this inflammation is unmodulated or excessive, there is a risk of chronic hyperinflammation resulting in functional inhibition of the adaptive immune system. In addition to virus-induced lymphopenia, this can result in progressive tissue and organ damage, and failure of the adaptive immune system to develop functional immunoglobulins and clear the pathogen [137]. In theory, the use of IgGAM in patients showing signs of both hyper- and hypoinflammation could therefore be an effective therapeutic strategy. Investigations with Ig M- and IgA-enriched immunoglobulin are on the way. The beneficial use was reported in a first case report in a patient with hyperinflammation [138].
Unwanted side effects and adverse reactions
The use of IVIG as supportive therapy in sepsis is not entirely without controversy or risk. In some patients, serious adverse reactions consist of the development of a hyperviscosity syndrome with thromboembolic events. Further, acute renal failure has been observed, which was presumably associated with stabilizers contained in the IVIG preparations. IVIG-associated renal failure is most common in patients with pre-existing conditions such as renal impairment, diabetes mellitus, advanced age, volume depletion, or concomitant use of other substances known to cause renal toxicity [139]. However, most of these potential complications can be prevented by taking appropriate countermeasures. For example, slow infusion rates and adequate hydration may help to avoid renal failure as well as thromboembolic events [140].
Conclusions and potential future research
It is evident that there are still many uncertainties associated with the diagnosis and particularly the management of different types of patients with sepsis. Research to effectively phenotype and characterize patient populations which correlate with a propensity to respond to treatment will be essential in tailoring management to the individual patient [141]. In this article we have described two distinct populations we believe would most benefit from therapy with IgM- and IgA-enriched immunoglobulins. For patients with hyperinflammation, clinical phenotypes are better recognized compared with patients with immunosuppression. Whilst there are more tools and biomarkers available for diagnosing patients with hyperinflammation compared to patients with immunosuppression, universally valid thresholds for these biomarkers (PCT, IL-6 and CRP) need to be elucidated. We also suggest that the timing of therapy with IgM- and IGA enriched immunoglobulin may be critical for patients with hyperinflammation, with early treatment showing the greatest benefit. These patients may further benefit from an initial bolus of Pentaglobin followed by a maintenance dose. However, further clinical or real-world evidence is required to make decisive recommendations regarding timing and dosage of treatment.
Among patients with immunosuppression, relevant biomarkers are largely debated, and research into developing technologies or identifying easily measured markers would be very valuable. Timing and dosage of therapy with IgM- and IgA-enriched immunoglobulins among immunosuppressed patients with chronic critical illness is also uncertain since the only available evidence is taken from patients with sepsis or septic shock. Therefore, clinical trials to identify optimal target parameters are critical to define the appropriate therapy parameters for this patient population.
For both patient populations, deciding when to discontinue therapy is also important. Pharmacokinetic and dose-response studies that monitor IgM, IgA and IgG levels in patients on IgM-immunoglobulin therapy should be carried out. It may also be of interest to study the impact of treatment with IgM- and IgA-enriched immunoglobulins on sepsis-related complications including critical illness polyneuropathy.
Within this manuscript, we characterized two different phenotypes of patients with sepsis and/or septic shock. This segregation is supported at a genomic level by a recent cohort study looking at the transcriptome variation of a large group of patients with severe community-acquired pneumonia (sCAP). Two distinct sepsis response signatures (SRS 1 and SRS 2) were identified, of which one group (SRS 1) showed clear signs of relative immunosuppression, endotoxin tolerance, and T-cell exhaustion, and was accompanied by a significantly worse outcome [142]. We hypothesize that patients exhibiting this phenotype might be likely to benefit from the administration of IgGAM. We acknowledge, however, that clinical reality currently excludes genetic/transcriptomic analyses, and that there is considerable overlap between these types of host response.
Clearly, more evidence is required to determine several specific aspects of treatment with IgM- and IgA-enriched immunoglobulins in patients with hyperinflammation and immunosuppression [143]. We conclude that, compared with IgG-only formulations which did not improve survival rates in patients with sepsis [47–49], treatment with IgM- and IgA-enriched immunoglobulins is very likely associated with a reduction in mortality and morbidity in terms of length of ventilatory support, length of ICU stay, and risk of secondary infectious complications [50–52, 71].
Supplementary information
Additional file 1: Appendix S1. Participants at the Expert Meeting, which took place at the 39th International Symposium on Intensive Care and Emergency Medicine (ISICEM) congress in Brussels, Belgium in March 2019.
Abbreviations
- APC
Antigen-presenting cells
- ARDS
Acute respiratory distress syndrome, CI confidence interval
- CMV
Cytomegalovirus
- CRP
C-reactive protein
- DAMP
Damage-associated molecular pattern
- CVVH
Continuous veno-venous hemodialysis
- CVVHD
Continuous veno-venous hemodialysis and diafiltration
- DNA
Deoxyribose nucleic acid
- DNR
Do not resuscitate
- HR
Hazard ratio
- HLA-DR
Human leukocyte antigen–antigen D related
- HSV
Herpes simplex virus
- ICU
Intensive care unit
- IFN
Interferon
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IL
Interleukin
- IVIG
Intravenous immunoglobulin
- MDR
Multidrug-resistant
- MDSC
Myeloid-derived suppressor cell
- NET
Neutrophil extracellular traps
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- M-H
Mantel–Haenszel
- OR
Odds ratio
- PAMP
Pathogen-associated molecular pattern
- PCT
Procalcitonin
- PD-1
Programmed death protein 1
- PD-L1
Programmed death ligand 1
- PIRO
Predisposition: insult/infection: response and organ dysfunction
- sCAP
Severe community-acquired pneumonia
- SMD
Standard mean difference
- SmPC
Summary of product characteristics
- TGF β
Transforming growth factor-β
- TLR
Toll-like receptor
- TNF-ɑ
Tumor necrosis factor α
- TO
Torino
- Treg
Regulatory T
Authors’ contributions
All authors contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Medical writing support was provided by Selene Jarrett and Tom Mitchell, Elements Communications Ltd and funded by Biotest AG. The opinions expressed in this manuscript reflect those of the authors.
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AN: research funds, speaker honoraria and travel reimbursement from Biotest AG, CytoSorbents Europe, ThermoFisher Scientific. GB: declares that he has no competing interests. DKM: speaker honoraria from Biotest AG. EM: speaker, consultant and/or advisory board member honoraria from Astellas, AstraZeneca, Basilea, Bayer Vital, Biosyn Arzneimittel, Biotest AG, Fresenius Medical Care, GE Healthcare, Gilead Sciences, Janssen–Cilag, Merck Sharp & Dohme, Merck, Novartis, Pfizer, Sanofi–Aventis, Wyeth. MG: speaker and/or advisory board member honoraria from Amomed, BioMerieux, Biotest AG, Estor, Merck Sharp & Dohme, Nordic Pharma, NovoNordisk, Orion Pharma, Pfizer, Shinogi Europe, Thermofisher.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Axel Nierhaus, Email: nierhaus@uke.de.
Giorgio Berlot, Email: berlotg@virgilio.it.
Detlef Kindgen-Milles, Email: Kindgen-Milles@med.uni-duesseldorf.de.
Eckhard Müller, Email: e.mueller@evk-herne.de.
Massimo Girardis, Email: girardis@unimore.it.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13613-020-00740-1.
References
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23(1):196. doi: 10.1186/s13054-019-2478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;9(12):e422–e436. doi: 10.1016/S1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermejo-Martin JF, Andaluz-Ojeda D, Almansa R, Gandía F, Gómez-Herreras JI, Gomez-Sanchez E, et al. Defining immunological dysfunction in sepsis: a requisite tool for precision medicine. J Infect. 2016;72(5):525–536. doi: 10.1016/j.jinf.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Tamayo E, Fernández A, Almansa R, Carrasco E, Heredia M, Lajo C, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22(2):82–87. doi: 10.1684/ecn.2011.0281. [DOI] [PubMed] [Google Scholar]
- 8.Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, et al. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–336. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. Vivo. 2013;27(6):669–684. [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PA, Gao H. Sepsis, complement and the dysregulated inflammatory response. J Cell Mol Med. 2009;13(10):4154–4160. doi: 10.1111/j.1582-4934.2009.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denk S, Taylor RP, Wiegner R, Cook EM, Lindorfer MA, Pfeiffer K, et al. Complement C5a-induced changes in neutrophil morphology during inflammation. Scand J Immunol. 2017;86(3):143–155. doi: 10.1111/sji.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, et al. Alterations in antigen-specific naive CD4 T cell precursors after sepsis impairs their responsiveness to pathogen challenge. J Immunol. 2015;194(4):1609–1620. doi: 10.4049/jimmunol.1401711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R, Lin F, Bao C, Huang H, Ji C, Wang S, et al. Complement 5a receptor-mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell Mol Immunol. 2016;3(1):103–109. doi: 10.1038/cmi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taccone FS, Stordeur P, De Backer D, Creteur J, Vincent JL. Gamma-globulin levels in patients with community-acquired septic shock. Shock. 2009;32(4):379–385. doi: 10.1097/SHK.0b013e3181a2c0b2. [DOI] [PubMed] [Google Scholar]
- 15.Myrianthefs PM, Boutzouka E, Baltopoulos GJ. Gamma-globulin levels in patients with community-acquired septic shock. Shock. 2010;33(5):556–557. doi: 10.1097/01.shk.0000370606.30525.21. [DOI] [PubMed] [Google Scholar]
- 16.Andaluz-Ojeda D, Iglesias V, Bobillo F, Almansa R, Rico L, Gandía F, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. 2011;15(5):R243. doi: 10.1186/cc10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venet F, Gebeile R, Bancel J, Guignant C, Poitevin-Later F, Malcus C, et al. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int Immunopharmacol. 2011;11(12):2086–2090. doi: 10.1016/j.intimp.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Tamayo E, Fernández A, Almansa R, Carrasco E, Goncalves L, Heredia M, et al. Beneficial role of endogenous immunoglobulin subclasses and isotypes in septic shock. J Crit Care. 2012;27(6):616–622. doi: 10.1016/j.jcrc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Giamarellos-Bourboulis EJ, Apostolidou E, Lada M, Perdios I, Gatselis NK, Tsangaris I, et al. Kinetics of circulating immunoglobulin M in sepsis: relationship with final outcome. Crit Care. 2013;17(5):R247. doi: 10.1186/cc13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Průcha M, Zazula R, Herold I, Dostál M, Hyánek T, Bellingan G. Presence of hypogammaglobulinemia—a risk factor of mortality in patients with severe sepsis, septic shock, and SIRS. Prague Med Rep. 2013;114(4):246–257. doi: 10.14712/23362936.2014.14. [DOI] [PubMed] [Google Scholar]
- 21.Bermejo-Martín JF, Rodriguez-Fernandez A, Herrán-Monge R, Andaluz-Ojeda D, Muriel-Bombín A, Merino P, et al. Immunoglobulins IgG1, IgM and IgA: a synergistic team influencing survival in sepsis. J Intern Med. 2014;276(4):404–412. doi: 10.1111/joim.12265. [DOI] [PubMed] [Google Scholar]
- 22.Shankar-Hari M, Culshaw N, Post B, Tamayo E, Andaluz-Ojeda D, Bermejo-Martín JF, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Intensive Care Med. 2015;41(8):1393–1401. doi: 10.1007/s00134-015-3845-7. [DOI] [PubMed] [Google Scholar]
- 23.Tian L, Zhu J, Jin J, et al. Prognostic value of circulating lymphocyte B and plasma immunoglobulin M on septic shock and sepsis: a systematic review and meta-analysis. Am J Transl Res. 2019;11(12):7223–7232. [PMC free article] [PubMed] [Google Scholar]
- 24.Bermejo-Martin JF, Giamarellos-Bourboulis EJ. Endogenous immunoglobulins and sepsis: new perspectives for guiding replacement therapies. Int J Antimicrob Agents. 2015;46(Suppl 1):S25–S28. doi: 10.1016/j.ijantimicag.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Krautz C, Maier SL, Brunner M, Langheinrich M, Giamarellos-Bourboulis EJ, Gogos C, et al. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis—a meta-analysis. J Crit Care. 2018;45:71–75. doi: 10.1016/j.jcrc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Shankar-Hari M, Spencer J, Sewell WA, Rowan KM, Singer M. Bench-to-bedside review: immunoglobulin therapy for sepsis—biological plausibility from a critical care perspective. Crit Care. 2012;16(2):206. doi: 10.1186/cc10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almansa R, Tamayo E, Heredia M, Gutierrez S, Ruiz P, Alvarez E, et al. Transcriptomic evidence of impaired immunoglobulin G production in fatal septic shock. J Crit Care. 2014;29(2):307–309. doi: 10.1016/j.jcrc.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, LaFace DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17(3–4):281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cazalis MA, Friggeri A, Cavé L, Demaret J, Barbalat V, Cerrato E, et al. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care. 2013;17(6):R287. doi: 10.1186/cc13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhardwaj N, Mathur P, Kumar S, Gupta A, Gupta D, John NV, et al. Depressed monocytic activity may be a predictor for sepsis. J Lab Physicians. 2015;7(1):26–31. doi: 10.4103/0974-2727.154785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan X, Liu Z, Jin H, Yan J, Liang HP. Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int. 2015;2015:903720. doi: 10.1155/2015/903720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kjaergaard AG, Nielsen JS, Tønnesen E, Krog J. Expression of NK cell and monocyte receptors in critically ill patients–potential biomarkers of sepsis. Scand J Immunol. 2015;81(4):249–258. doi: 10.1111/sji.12272. [DOI] [PubMed] [Google Scholar]
- 33.Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernández-Jiménez E, et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1a. Immunity. 2015;42(3):484–498. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Hynninen M, Pettilä V, Takkunen O, Orko R, Jansson SE, Kuusela P, et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock. 2003;20:1–4. doi: 10.1097/01.shk.0000068322.08268.b4. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 36.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. MyD88-dependent expansion of an immature GR-1 + CD11b + population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taneja R, Sharma AP, Hallett MB, Findlay GP, Morris MR. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock. 2008;30(6):618–622. doi: 10.1097/SHK.0b013e318173ef9c. [DOI] [PubMed] [Google Scholar]
- 38.Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34(4):358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Pablo R, Monserrat J, Prieto A, Alvarez-Mon M. Role of circulating lymphocytes in patients with sepsis. Biomed Res Int. 2014;2014:671087. doi: 10.1155/2014/671087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, et al. Marked elevation of human circulating CD4+ CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31(7):2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 43.Hein F, Massin F, Cravoisy-Popovic A, Barraud D, Levy B, Bollaert PE, et al. The relationship between CD4+ CD25+ CD127-regulatory T cells and inflammatory response and outcome during shock states. Crit Care. 2010;14(1):R19. doi: 10.1186/cc8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao C, Ma T, Chai YF, Shou ST. The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med. 2015;6(1):5–9. doi: 10.5847/wjem.j.1920-8642.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 46.Busani S, Damiani E, Cavazzuti I, Donati A, Girardis M. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016;82(5):559–572. [PubMed] [Google Scholar]
- 47.Werdan K, Pilz G, Bujdoso O, Fraunberger P, Neeser G, Schmieder RE, et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med. 2007;35(12):2693–2701. doi: 10.1097/01.CCM.0000297164.40980.F0. [DOI] [PubMed] [Google Scholar]
- 48.Werdan K, Pilz G, Müller-Werdan U, Maas Enriquez M, Schmitt DV, Mohr FW, et al. Immunoglobulin G treatment of postcardiac surgery patients with score-identified severe systemic inflammatory response syndrome–the ESSICS study. Crit Care Med. 2008;36(3):716–723. doi: 10.1097/01.CCM.0B013E3181611F62F. [DOI] [PubMed] [Google Scholar]
- 49.Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365(13):1201–1211. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 50.Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013;9:1090. doi: 10.1002/14651858.CD001090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35(12):2677–2685. [PubMed] [Google Scholar]
- 52.Cui J, Wei X, Lv H, Li Y, Li P, Chen Z, Liu G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (Pentaglobin) in sepsis or septic shock: a meta-analysis with trial sequential analysis. Ann Intensive Care. 2019;9(1):27. doi: 10.1186/s13613-019-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welte T, Dellinger RP, Ebelt H, Ferrer M, Opal SM, Singer M, et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicentre, phase II trial (CIGMA study) Intensive Care Med. 2018;44(4):438–448. doi: 10.1007/s00134-018-5143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Just HM, Metzger M, Vogel W, Pelka RB. Effect of adjuvant immunoglobulin therapy on infections in patients in an surgical intensive care unit. Results of a randomized controlled study. Klinische Wochenschrift. 1986;64(6):245–256. doi: 10.1007/BF01711930. [DOI] [PubMed] [Google Scholar]
- 55.Vogel F. Bewertung der intravenösen IgM-Therapie bei schweren nosokomialen infektionen (Ergebnis einer kontrollierten randomisierten Studie), Klinisch angewandte Immunologie. Berlin: Springer; 1988. pp. 30–41. [Google Scholar]
- 56.Wesoly C, Kipping N, Grundmann R. Immunoglobulin therapy of postoperative sepsis. Z Exp Chir Transplant Kunstliche Organe. 1990;23(4):213. [PubMed] [Google Scholar]
- 57.Schedel IN, Dreikhausen UR, Nentwig BI, Höckenschnieder MA, Rauthmann DI, Balikcioglu SA, et al. Treatment of gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med. 1991;19(9):1104–1113. doi: 10.1097/00003246-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Behre G, Ostermann H, Schedel I, Helmerking M, Schiel X, Rothenberger M, et al. Endotoxin concentrations and therapy with polyclonal IgM-enriched immunoglobulins in neutropenic cancer patients with sepsis syndrome: pilot study and interim analysis of a randomized trial. AntiinfectDrugs Chemother. 1995;13:129–134. [Google Scholar]
- 59.Rodríguez A, Palizas F, Neira J, Maskin B, Raimondi N, Alvarez J, et al. Inmunoglobulina (Ig) polivalente en el tratamiento de la sepsis abdominal. Medicina Intensiva. 2001;18(1):11–15. [Google Scholar]
- 60.Reith HB, Mittelkötter U. Markers of inflammation for prognosis and therapy control in patients with abdominal sepsis—options for using adjuvant intravenous immunoglobulins. In: Faist E, editor. Immunological screening and immunotherapy in critically ill patients with abdominal infections. New York: Springer; 2001. pp. 15–38. [Google Scholar]
- 61.Tugrul S, Ozcan PE, Akinci O, Seyhun Y, Cagatay A, Cakar N, et al. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis. Crit Care. 2002;6(4):357–362. doi: 10.1186/cc1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karatzas S, Boutzouka E, Venetsanou K, Myrianthefs P, Fildisis G, Baltopoulos G. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis: another point of view. Crit Care. 2002;6(6):543–545. doi: 10.1186/cc1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reith HB, Rauchschwalbe SK, Mittelkötter U, Engemann R, Thiede A, Arnold A, et al. IgM-enriched immunoglobulin (pentaglobin) positively influences the course of post-surgical intra-abdominal infections. Eur J Med Res. 2004;9(10):479–484. [PubMed] [Google Scholar]
- 64.Rodríguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, et al. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock. 2005;23(4):298–304. doi: 10.1097/01.shk.0000157302.69125.f8. [DOI] [PubMed] [Google Scholar]
- 65.Buda S, Riefolo A, Biscione R, Goretti E, Cattabriga I, Grillone G, et al. Clinical experience with polyclonal IgM-enriched immunoglobulins in a group of patients affected by sepsis after cardiac surgery. J Cardiothorac Vasc Anesth. 2005;19(4):440–445. doi: 10.1053/j.jvca.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Hentrich M, Fehnle K, Ostermann H, Kienast J, Cornely O, Salat C, et al. IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med. 2006;34(5):1319–1325. doi: 10.1097/01.CCM.0000215452.84291.C6. [DOI] [PubMed] [Google Scholar]
- 67.Yavuz L, Aynali G, Aynali A, Alaca A, Kutuk S, Ceylan BG. The effects of adjuvant immunoglobulin M-enriched immunoglobulin therapy on mortality rate and renal function in sepsis-induced multiple organ dysfunction syndrome: retrospective analysis of intensive care unit patients. J Int Med Res. 2012;40(3):1166–1174. doi: 10.1177/147323001204000337. [DOI] [PubMed] [Google Scholar]
- 68.Toth I, Mikor A, Leiner T, Molnar Z, Bogar L, Szakmany T. Effects of IgM-enriched immunoglobulin therapy in septic-shock–induced multiple organ failure: pilot study. J Anesth. 2013;27(4):618–622. doi: 10.1007/s00540-012-1553-9. [DOI] [PubMed] [Google Scholar]
- 69.Brunner R, Rinner W, Haberler C, Kitzberger R, Sycha T, Herkner H, et al. Early treatment with IgM-enriched intravenous immunoglobulin does not mitigate critical illness polyneuropathy and/or myopathy in patients with multiple organ failure and SIRS/sepsis: a prospective, randomized, placebo-controlled, double-blinded trial. Crit Care. 2013;17(5):R213. doi: 10.1186/cc13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavazzuti I, Serafini G, Busani S, Rinaldi L, Biagioni E, Buoncristiano M, et al. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med. 2014;40(12):1888–1896. doi: 10.1007/s00134-014-3474-6. [DOI] [PubMed] [Google Scholar]
- 71.Giamarellos-Bourboulis EJ, Tziolos N, Routsi C, Katsenos C, Tsangaris I, Pneumatikos I, et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect. 2016;22(6):499–506. doi: 10.1016/j.cmi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 72.Berlot G, Vassallo MC, Busetto N, Nieto Yabar M, Istrati T, Baronio S, et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann Intensive Care. 2018;8(1):122. doi: 10.1186/s13613-018-0466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willuweit K, Bezinover D, Herzer K, Nowak KM, Paul A, Saner FH. Efficacy of IgM-enriched immunoglobulin for vasopressor-resistant vasoplegic shock after liver transplantation. Transplantation. 2019;103(2):381–386. doi: 10.1097/TP.0000000000002344. [DOI] [PubMed] [Google Scholar]
- 74.Domizi R, Adrario E, Damiani E, Scorcella C, Carsetti A, Giaccaglia P, et al. IgM-enriched immunoglobulins (Pentaglobin) may improve the microcirculation in sepsis: a pilot randomized trial. Ann Intensive Care. 2019;9:135. doi: 10.1186/s13613-019-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stehr SN, Knels L, Weissflog C, Schober J, Haufe D, Lupp A, et al. Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock. 2008;29(2):167–172. doi: 10.1097/SHK.0b013e318067df15. [DOI] [PubMed] [Google Scholar]
- 76.Berger D, Schleich S, Seidelmann M, Beger HG. Antiendotoxic Therapy with polyclonal and polyvalent immunoglobulins: in vitro and in vivo studies. Host Defense Dysf Trau Shock Sepsis. 1993;1993:1163–1174. doi: 10.1007/978-3-642-77405-8_151. [DOI] [Google Scholar]
- 77.Wand S, Klages M, Kirbach C, Warszawska J, Meybohm P, Zacharowski K, et al. IgM-enriched immunoglobulin attenuates systemic endotoxin activity in early severe sepsis: a before-after cohort study. PLoS ONE. 2016;11(8):e0160907. doi: 10.1371/journal.pone.0160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rieben R, Roos A, Muizert Y, Tinguely C, Gerritsen AF, Daha MR. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood. 1999;93(3):942–951. doi: 10.1182/blood.V93.3.942. [DOI] [PubMed] [Google Scholar]
- 79.Walpen AJ, Laumonier T, Aebi C, Mohacsi PJ, Rieben R. Immunoglobulin M-enriched intravenous immunoglobulin inhibits classical pathway complement activation, but not bactericidal activity of human serum. Xenotransplantation. 2004;11(2):141–148. doi: 10.1046/j.1399-3089.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 80.Nachbaur D, Herold M, Gächter A, Niederwieser D. Modulation of alloimmune response in vitro by an IgM-enriched immunoglobulin preparation (Pentaglobin) Immunology. 1998;94(2):279–283. doi: 10.1046/j.1365-2567.1998.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaschetto R, Clemente N, Pagni A, Esposito T, Longhini F, Mercalli F, et al. A double blind randomized experimental study on the use of IgM-enriched polyclonal immunoglobulins in an animal model of pneumonia developing shock. Immunobiology. 2017;222(12):1074–1080. doi: 10.1016/j.imbio.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Barratt-Due A, Sokolov A, Gustavsen A, Hellerud BC, Egge K, Pischke SE, et al. Polyvalent immunoglobulin significantly attenuated the formation of IL-1β in Escherichia coli-induced sepsis in pigs. Immunobiology. 2013;218(5):683–689. doi: 10.1016/j.imbio.2012.08.268. [DOI] [PubMed] [Google Scholar]
- 83.Duerr C, Bacher A, de Martin A, Sachet M, Sadeghi K, Baumann S, et al. The novel polyclonal Ab preparation trimodulin attenuates ex vivo endotoxin-induced immune reactions in early hyperinflammation. Innate Immunity. 2019;25(6):374–388. doi: 10.1177/1753425919853333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman JN, Fertmann JM, Vollmar B, Laschke MW, Jauch KW, Menger MD. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock. 2008;29:133–139. doi: 10.1097/shk.0b013e318123e5a6. [DOI] [PubMed] [Google Scholar]
- 85.Esen F, Senturk E, Ozcan PE, Ahishali B, Arican N, Orhan N, et al. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med. 2012;40(4):1214–1220. doi: 10.1097/CCM.0b013e31823779ca. [DOI] [PubMed] [Google Scholar]
- 86.Esen F, Orhun G, Ozcan PE, Senturk E, Kucukerden M, Giris M, et al. Neuroprotective effects of intravenous immunoglobulin are mediated through inhibition of complement activation and apoptosis in a rat model of sepsis. Intens Care Med Exp. 2017;5:1. doi: 10.1186/s40635-016-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakoullis L, Pantzaris ND, Platanaki C, Lagadinou M, Papachristodoulou E, Velissaris D. The use of IgM-enriched immunoglobulin in adult patients with sepsis. J Crit Care. 2018;47:30–35. doi: 10.1016/j.jcrc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Molnár Z, Nierhaus A, Esen F. Immunoglobulins in Sepsis: Which Patients will Benefit the Most? In: Vincent JL, eds. Annual Update in Intensive Care and Emergency Medicine. Berlin: Springer; 2013.
- 89.Antcliffe DB, Gordon AC. Why understanding sepsis endotypes is important for steroid trials in septic shock. Crit Care Med. 2019;47(12):1782–1784. doi: 10.1097/CCM.0000000000003833. [DOI] [PubMed] [Google Scholar]
- 90.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 92.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126(1):23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santacruz CA, Pereira AJ, Celis E, Vincent JL. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit Care Med. 2019;47(12):1680–1691. doi: 10.1097/CCM.0000000000004000. [DOI] [PubMed] [Google Scholar]
- 94.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 95.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters van Ton AM, Abdo WF, Abdo WF, Pickkers P. Precision immunotherapy for sepsis. Front Immunol. 2018;9:1926. doi: 10.3389/fimmu.2018.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Rosa FG, Corcione S, Tascini C, Pasero D, Rocchetti A, Massaia M, et al. A position paper on IgM-enriched intravenous immunoglobulin adjunctive therapy in severe acute bacterial infections: the TO-PIRO SCORE proposal. New Microbiol. 2019;42(3):176–180. [PubMed] [Google Scholar]
- 98.Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14(2):168–174. doi: 10.5811/westjem.2012.8.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng J, Nugent K. Utility of the shock index in patients with sepsis. Am J Med Sci. 2015;349(6):531–535. doi: 10.1097/MAJ.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 100.Lee SM, An WS. New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J Thorac Dis. 2016;8(7):1388–1390. doi: 10.21037/jtd.2016.05.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Branche A, Neeser O, Mueller B, Schuetz P. Procalcitonin to guide antibiotic decision making. Curr Opin Infect Dis. 2019;32(2):130–135. doi: 10.1097/QCO.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 102.Beye F, Vigneron C, Dargent A, Prin S, Andreu P, Large A, et al. Adhering to the procalcitonin algorithm allows antibiotic therapy to be shortened in patients with ventilator-associated pneumonia. J Crit Care. 2019;53:125–131. doi: 10.1016/j.jcrc.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 103.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 104.Kalvelage C, Zacharowski K, Bauhofer A, Gockel U, Adamzik M, Nierhaus A, et al. Personalized medicine with IgGAM compared with standard of care for treatment of peritonitis after infectious source control (the PEPPER trial): study protocol for a randomized trial. Trials. 2019;20(1):156. doi: 10.1186/s13063-019-3244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weidhase L, Wellhöfer D, Schulze G, Kaiser T, Drogies T, Wurst U, et al. Is interleukin-6 a better predictor of successful antibiotic therapy than procalcitonin and C-reactive protein? A single center study in critically ill adults. BMC Infect Dis. 2019;19:150. doi: 10.1186/s12879-019-3800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tascini C, Fraganza F, Salani F, Sozio E, Rossi M, Sbrana F, et al. Potential role of IgM-enriched immunoglobulin as adjuvant treatment for invasive meningococcal disease. Intensive Care Med. 2018;44(2):261–262. doi: 10.1007/s00134-017-4957-z. [DOI] [PubMed] [Google Scholar]
- 107.Pota V, Passavanti MB, Sansone P, Pace MC, Peluso F, Fiorelli A, et al. Septic shock from descending necrotizing mediastinitis—combined treatment with IgM-enriches immunoglobulin preparation and direct polymyxin B hemoperfusion: a case report. J Med Case Rep. 2018;12(1):55. doi: 10.1186/s13256-018-1611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winters BD, Eberlein M, Leung EM, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 109.Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29(2):87–95. doi: 10.1177/0885066612467162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14(2):121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 111.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Efron PA, Mohr AM, Bihorac A, Horiguchi H, Hollen MK, Segal MS, et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery. 2018;164(2):178–184. doi: 10.1016/j.surg.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schefold JC. Measurement of monocytic HLA-DR (mHLA-DR) expression in patients with severe sepsis and septic shock: assessment of immune organ failure. Intensive Care Med. 2010;36(11):1810–1812. doi: 10.1007/s00134-010-1965-7. [DOI] [PubMed] [Google Scholar]
- 114.Drewry AM, Ablordeppey EA, Murray ET, Beiter ER, Walton AH, Hall MW, et al. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit Care. 2016;20(1):334. doi: 10.1186/s13054-016-1505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: utility of innate immune response markers. Intensive Care Med Exp. 2017;5(1):49. doi: 10.1186/s40635-017-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tosi M, Roat E, De Basi S, Munari E, Venturelli S, Coloretti I, et al. Multidrug resistant bacteria in critically ill patients: a step further antibiotic therapy. J Emerg Crit Care Med. 2018;2:103. doi: 10.21037/jeccm.2018.11.08. [DOI] [Google Scholar]
- 117.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adrie C, Lugosi M, Sonneville R, Souweine B, Ruckly S, Cartier JC, et al. Persistent lymphopenia is a risk factor for ICU-acquired infections and for death in ICU patients with sustained hypotension at admission. Ann Intensive Care. 2017;7(1):30. doi: 10.1186/s13613-017-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winkler MS, Rissiek A, Priefler M, Schwedhelm E, Robbe L, Bauer A, et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: a diagnostic tool for immunosuppression? PLoS ONE. 2017;12(8):e0182427. doi: 10.1371/journal.pone.0182427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Traylen CM, Patel HR, Fondaw W, Mahatme S, Williams JF, Walker LR, et al. Virus reactivation: a panoramic view in human infections. Future Virol. 2011;6(4):451–463. doi: 10.2217/fvl.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lim TY, Heneghan M. Biomarkers of immunosuppression. Clin Liver Dis (Hoboken). 2016;8(2):34–38. doi: 10.1002/cld.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cajander S, Rasmussen G, Tina E, Magnuson A, Söderquist B, Källman J, et al. Dynamic of monocytic HLA-DR expression differs between bacterial etiologies during the course of bloodstream infection. PLoS ONE. 2018;13(2):e0192883. doi: 10.1371/journal.pone.0192883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Landry ML. Immunoglobulin M for acute infection: true or false? Clin Vaccine Immunol. 2016;23(7):540–545. doi: 10.1128/CVI.00211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riché F, Chousterman BG, Valleur P, Mebazaa A, Launay JM, Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care. 2018;22(1):42. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cross D, Drury R, Hill J, Pollard AJ. Epigenetics in sepsis: understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front Immunol. 2019;10:1363. doi: 10.3389/fimmu.2019.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(Suppl 3):1–14. doi: 10.1159/000499520. [DOI] [PubMed] [Google Scholar]
- 128.Hanaoka A, Naganuma T, Takemoto Y, Uchida J, Nakatani T, Kabata D, et al. Efficacy of selective plasma exchange as pre-transplant apheresis in ABO-incompatible kidney transplantation. Ren Replace Ther. 2019;5:6. doi: 10.1186/s41100-019-0204-0. [DOI] [Google Scholar]
- 129.Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang Y, Xu J, Zhou C, Wu Z, Zhong S, Liu J, et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 134.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020:M20–2003. [DOI] [PMC free article] [PubMed]
- 136.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20(5):277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carannante N, Fiorentino G, Corcione A, Di Sarno R, Spatarella M, Maturo N, et al. Administration of immunoglobulins in SARS-CoV-2-positive patient is associated with fast clinical and radiological healing: case report. Front Med. 2020;7:388. doi: 10.3389/fmed.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dantal J. Intravenous immunoglobulins: in-depth review of excipients and acute kidney injury risk. Am J Nephrol. 2013;38(4):275–284. doi: 10.1159/000354893. [DOI] [PubMed] [Google Scholar]
- 140.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6(4):257–259. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 141.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Resp Med. 2016;4(4):259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jarczak D, Kluge S, Nierhaus A. Use of intravenous immunoglobulins in sepsis therapy - a Clinical View. Int J Mol Sci. 2020;21:5543. doi: 10.3390/ijms21155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix S1. Participants at the Expert Meeting, which took place at the 39th International Symposium on Intensive Care and Emergency Medicine (ISICEM) congress in Brussels, Belgium in March 2019.
Data Availability Statement
Not applicable