Abstract
Opportunity exists to decrease healthcare-related exposure to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), preserve infection control resources, and increase care capacity by reducing the time to diagnosis of coronavirus disease 2019 (COVID-19). A retrospective cohort analysis was undertaken to measure the effect of targeted rapid molecular testing for SARS-CoV-2 on these outcomes. In comparison with standard platform testing, rapid testing was associated with a 65.6% reduction (12.6 h) in the median time to removal from the isolation cohort for patients with negative diagnostic results. This translated to an increase in COVID-19 treatment capacity of 3028 bed-hours and 7500 fewer patient interactions that required the use of personal protective equipment per week.
Keywords: COVID-19, SARS-CoV-2, Rapid diagnostics
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has infected over 30 million people and caused over 1 million deaths worldwide. Provision of care to patients with or suspected of having COVID-19 requires specialized infection control resources, including dedicated diagnostic and treatment spaces equipped for negative pressure isolation, trained healthcare personnel and large quantities of personal protective equipment (PPE) [1,2]. While most attention has been paid to healthcare resource allocation for patients with confirmed COVID-19 [3,4], most diagnostic tests performed for SARS-CoV-2 are negative [5]. Infection control requirements for persons under investigation (PUIs) for COVID-19 are the same as those for patients who have tested positive. Further, PUIs are commonly cohorted alongside patients with confirmed SARS-CoV-2 infection while awaiting diagnostic test results. This places uninfected patients at excess risk for healthcare-related exposure to SARS-CoV-2 [1,2,6]. There is opportunity to preserve constrained resources, reduce avoidable healthcare costs, and limit the time of risk exposure by decreasing the time spent under investigation for COVID-19 in hospital settings.
More than 120 molecular tests for SARS-CoV-2 have now been granted in-vitro diagnostic emergency use authorization by the US Food and Drug Administration (https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices). Analytical performance of approved nucleic acid amplification tests (NAATs) for SARS-CoV-2 is universally high, but processing times are variable [7]. While rapid (<1 h) NAATs for influenza and other respiratory viruses have had positive effects on patient throughput and appropriateness of antiviral and antibiotic prescribing by physicians [[8], [9], [10], [11]], the benefits of rapid testing for COVID-19 are not well understood.
This study sought to assess the impact of targeted rapid molecular testing on hospital infection control procedures and risk of COVID-19 exposure for uninfected patients. The amount of time that PUIs without microbiological evidence of infection spent in isolation cohorts alongside patients with confirmed COVID-19 was compared for testing with standard and rapid molecular diagnostics. It was hypothesized that rapid testing would drive large decreases in the time to removal from the COVID-19 isolation cohort, thus decreasing the risk of nosocomial infection, preserving infection control resources and increasing COVID-19 treatment capacity.
Methods
Study design, setting and participants
A retrospective cohort analysis of emergency department (ED) visits to two urban academic hospitals (Sites 1 and 2) and a suburban community hospital (Site 3) within a single university-based health system in Maryland, USA between 29th March and 30th June 2020 was undertaken. All adult patients (age ≥18 years) who had a laboratory diagnostic evaluation for SARS-CoV-2 infection initiated during their ED stay and remained in the hospital (ED or inpatient) until their test results were included. Patients who were discharged prior to diagnostic test result were excluded because the clinical decision to remove them from the isolation cohort was independent of the SARS-CoV-2 diagnostic test result. The Johns Hopkins Medicine Institutional Review Board approved this study (IRB No. IRB00246741).
Diagnostic testing strategy
On 29th March 2020, targeted rapid molecular testing using the Xpert Xpress SARS-CoV-2 test (Cepheid, Sunnyvale, CA, USA) was implemented across the authors' healthcare system. The Xpert testing platform allows diagnostic test results to be generated in approximately 45 min by combining specimen processing, nucleic acid extraction, reverse transcriptase polymerase chain reaction (RT-PCR) and amplicon detection in a single random-access cartridge [13,14]. Limited rapid tests were prioritized for the evaluation of PUIs in the ED who were expected to be hospitalized or could not be discharged to self-isolate at home while awaiting diagnostic test results (e.g. homeless, residing in a group home or assisted living facility). Standard platform NAATs including RealStar SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics, Hamburg, Germany), NeuMoDx SARS-CoV-2 assay (NeuMoDx, Ann Arbor, MI, USA), SARS-CoV-2 reagents for the BD MAX System (Becton Dickinson, Sparks, MD, USA) and GenMark ePlex SARS-CoV-2 test (GenMark, Carlsbad, CA, USA) continued to be used for all PUIs.
All diagnostic orders were placed by treating emergency clinicians. Decision support that included testing algorithms and rapid test prioritization criteria was distributed electronically and made available in real time via electronic health record (EHR)-embedded guidelines and printed guidelines posted at clinical workstations. SARS-CoV-2 testing inclusion criteria were managed by the Department of Hospital Epidemiology and Infection Control, and were standardized across all sites. Testing inclusion criteria broadened over time as guidance from the Centers for Disease Control and Prevention evolved and local capacity increased (i.e. initially high-risk individuals only and then all symptomatic patients), but were standardized across all sites. Rapid test prioritization criteria, described above, remained constant throughout the study period.
Methods of measurement and analysis
Timestamped clinical data were queried from a relational database that underlies the common EHR (Epic, Verona, WI, USA) used at all three study sites. Summary statistics, including demographics (age, sex, race and ethnicity) and rates of test positivity and hospitalization for all patients tested for SARS-CoV-2 infection at one of the three EDs over the study period, were compiled. Daily SARS-CoV-2 testing volume was combined across study sites and stratified by standard and rapid platform (Xpert Xpress) NAATs. Hospitalization was defined as admission to any inpatient unit (e.g. medical or surgical).
Uninfected patient exposure time was defined as the amount of time that patients with negative SARS-CoV-2 NAAT results spent as PUIs subject to isolation precautions and geographically cohorted with patients infected with SARS-CoV-2. It was calculated as the time difference between diagnostic test order entry and first intrahospital treatment space movement (removal from geographic cohort) following a negative SARS-CoV-2 test result. The exposure time of uninfected patients was compared between patients who tested negative by standard and rapid platform NAATs. Exposure times were pooled across sites and compared using boxplot analysis and the logrank test for time-interval data. All data processing and analyses were performed using Python (V 2.7).
Results
In total, 12,263 ED patients were tested for SARS-CoV-2 across the three sites during the study period. Of these, 3245 patients were discharged prior to result availability and were excluded from the study cohort. Of the 9018 patients who remained in hospital (ED or inpatient) until their results became available, 3502 (38.8%) were tested using rapid platform NAATs (Xpert Xpress SARS-Cov-2), with the remainder tested using standard platform NAATs (Table I ). Rates of rapid test use were slightly higher at the urban academic EDs (40.8% for Site 1 and 43.6% for Site 2) than Site 3 (32.1%). Demographics across sites were similar, although patients tested at Site 3 were slightly older, and a higher proportion of patients at Site 1 self-identified as Black or African American (Table I). The overall SARS-CoV-2 positivity rate for the study cohort was 9.9%, and the majority (60%) of patients included were hospitalized after departure from the ED (Table I).
Table I.
Patient characteristics
| Total | Site 1 | Site 2 | Site 3 | |
|---|---|---|---|---|
| Total tested | 9018 | 3819 | 2383 | 2816 |
| Age (years) | ||||
| 18–44 | 2604 (28.9%) | 1355 (35.5%) | 603 (25.3%) | 646 (22.9%) |
| 45–64 | 3320 (36.8%) | 1548 (40.5%) | 932 (39.1%) | 849 (30.1%) |
| 65–74 | 1506 (16.7%) | 548 (14.3%) | 433 (18.2%) | 525 (18.6%) |
| >74 | 1573 (17.4%) | 367 (9.6%) | 414 (17.4%) | 792 (28.1%) |
| Female | 4453 (49.4%) | 1817 (47.6%) | 1183 (49.6%) | 1453 (51.6%) |
| Race | ||||
| Black or African American | 3727 (41.3%) | 2230 (58.4%) | 715 (30%) | 782 (27.8%) |
| White | 3949 (43.8%) | 1121 (29.4%) | 1332 (55.9%) | 1496 (53.1%) |
| Other | 1342 (14.9%) | 468 (12.3%) | 336 (14.1%) | 538 (19.1%) |
| Ethnicity | ||||
| Latino | 844 (9.4%) | 320 (8.4%) | 277 (11.6%) | 247 (8.8%) |
| Non-Latino | 8174 (90.6%) | 3499 (91.6%) | 2106 (88.4%) | 2569 (91.2%) |
| Admitted | 5409 (60%) | 1951 (51.1%) | 1678 (70.4%) | 1780 (63.2%) |
| Standard platform | 5516 (61.2%) | 2262 (59.2%) | 1343 (56.4%) | 1911 (67.9%) |
| Rapid platform | 3502 (38.8%) | 1557 (40.8%) | 1040 (43.6%) | 905 (32.1%) |
| SARS-CoV-2 positive | 892 (9.9%) | 306 (8%) | 252 (10.6%) | 334 (11.9%) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
All study sites are affiliated with the same university hospital system. Sites 1 and 2 are urban academic emergency departments, and Site 3 is a suburban community emergency department.
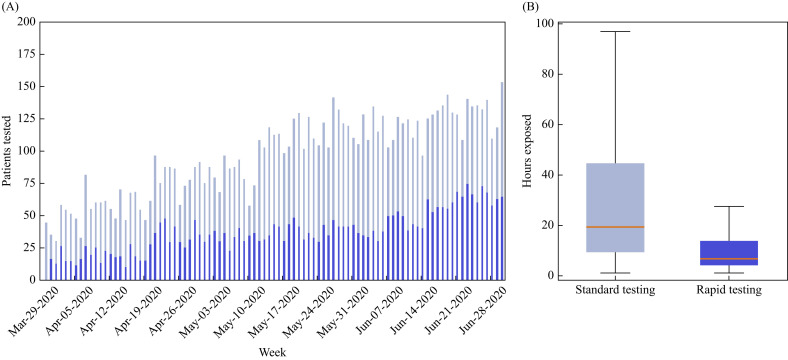
Daily SARS-CoV-2 testing volume increased over time for both standard and rapid testing platforms, as seen in Figure 1 A. The median order to result time, defined as the interval between test order entry by a treating clinician and negative/positive result viewable in the EHR, was 7.8 [interquartile range (IQR) 3.71–11.68] h for standard platform tests and 1.90 (IQR 1.40–2.82) h for rapid platform tests (P<0.001). Results were available prior to ED departure for 50.7% (N=2718) of patients tested using standard NAATs and 92.2% (N=3228) of patients tested using rapid platform NAATs. As shown in Figure 1B, the median exposure time for uninfected patients tested using standard and rapid platform testing was 19.20 (IQR 9.45–44.59) h and 6.62 (IQR 4.13–13.57) h, respectively (P<0.001). Thus, rapid testing for SARS-CoV-2 infection was associated with a 12.6-h decrease (65.6% absolute reduction) in median exposure time of uninfected patients.
Figure 1.
Exposure time for uninfected patients. (A) Daily volume of patients who were tested for severe acute respiratory syndrome (SARS-CoV-2) in the emergency department and remained in the hospital setting until their test results, stratified by standard (light blue) and rapid (dark blue) testing platforms. (B) Boxplot analysis of exposure time of uninfected patients, measured as time from SARS-CoV-2 diagnostic test order to first treatment space re-assignment after a negative result, for both standard (light blue) and rapid (dark blue) testing platforms. Median is represented by an orange horizontal line, interquartile range by boxes, and 95% of range by whiskers.
Discussion
Strategic delivery of SARS-CoV-2 rapid tests to a subset of ED patients with anticipated prolonged stays (e.g. hospitalization or limited capacity to self-isolate) resulted in large decreases in time spent as a PUI by patients without microbiological evidence of SARS-CoV-2 infection. While important, the finding that rapid platform NAAT results were available an average of 5.9 h earlier than standard platform NAAT results is not surprising. However, the finding that rapid testing led to larger decreases (12.6 h) in the interval from test order to removal from the isolation cohort was somewhat unexpected. The magnitude of this effect is likely explained by patient location at time of diagnostic result and operational constraints of bed re-assignment in each setting. Over 90% of rapid NAAT results became available during the ED encounter, enabling immediate intradepartmental transfers and optimal initial inpatient cohort assignment. However, the results for nearly half of the patients tested in the ED using standard platform NAAT were delayed until after their arrival in the inpatient setting, where removal from isolation is complicated by the need for transfer between distinct wards and clinical teams.
Targeted ED rapid testing was associated with a considerable decrease in healthcare-associated exposure to SARS-CoV-2 for uninfected patients. Over the study period, an aggregate saving of 40,106 h (3028 h/week) in the time that uninfected patients were treated in COVID-19 isolation units alongside confirmed positive patients was observed. The intervention was also associated with drastically decreased infection control resource consumption and healthcare expenditures. Using a conservative estimate of 2.5 healthcare worker–patient contacts per hour [12,13], this simple intervention prevented over 100,000 patient interactions under isolation precautions that would have required consumption of non-reusable infection control supplies (e.g. N95 respirators, gloves, gowns, sanitizing wipes) and decontamination of reusable equipment (e.g. face shields, goggles, powered air purifying respirators). Under assumptions of conventional surge capacity [15] and current supply costs [14], with each interaction consuming one N95 respirator (mean estimated cost US$4.02 each), four gloves (US$0.20), one gown (US$2.33) and two sanitizing wipes (US$0.08), this intervention translated to cost-savings of over US$650,000 in non-reusable PPE alone. Under assumptions of contingency or crisis capacity, with repeated use of disposable N95 respirators across multiple encounters [15], there were cost-savings of more than US$250,000 in PPE. Further, this intervention increased negative pressure isolation bed capacity in the ED and inpatient units, increasing capacity to care for additional patients who were under investigation for, or had been diagnosed with, COVID-19.
Previous studies on the effectiveness of rapid testing for respiratory viruses have primarily focused on influenza, and have evaluated impacts on therapeutic decision-making and reductions in ED and hospital lengths of stay [9,10,16]. To the authors' knowledge, this study is unique in that it is the first to evaluate the clinical effectiveness of rapid testing for SARS-CoV-2, and because it focuses on both risk exposure time and resource savings. The finding that a relatively small number of rapid tests, if properly targeted, can be used to drive large decreases in the time that patients spend under investigation for infectious diseases in the hospital setting is novel. This finding is particularly relevant to the management of COVID-19, but is also broadly applicable to seasonal respiratory viruses and emerging viruses.
This study has several limitations. First, these data are from a single hospital system with high SARS-CoV-2 testing capacity. While other sites may not have the same testing capacity, the value of strategically targeting rapid tests to a specific population translates to other settings, and may inform the use of rapid testing protocols generally. Secondly, SARS-CoV-2 testing criteria changed over the course of this study, creating a heterogeneous study population. However, testing criteria were standardized across sites, and the study outcome is reflective of real-world clinical management. Finally, the true clinical sensitivity of all diagnostic tests for SARS-CoV-2 is still unknown, and it is possible that this study may include patients with false-negative results. This limitation was mitigated by the use of first physical transfer after a negative test result as the outer bound of the time interval outcome. This transfer served as a proxy for the removal of isolation precautions based on clinical determination that the patient was at low risk for SARS-CoV-2 infection.
In summary, this study found that targeted rapid testing was effective in reducing the time that uninfected patients spent in hospital under investigation for COVID-19. Implementation of this strategy was associated with decreased risk for healthcare-associated SARS-CoV-2 infection, conservation of limited infection control resources and increased COVID-19 treatment capacity.
Conflict of interest statement
KC and HM participated in a multi-centre validation of the Cepheid SARS-CoV-2 test. SL and RR are principal investigators of a research contract sponsored by Cepheid. No financial or material support was provided from Cepheid to directly support this work.
Funding sources
This work was supported by the Agency for Healthcare Research and Quality Connected Emergency Care Patient Safety Learning Lab (R18 HS26640 to JH and SL), the Biomedical Advanced Research and Development Authority BARDA (IDSEP150023-01-00 to RR), the Centers for Disease Control, Modeling in Infectious Disease Network (U01CK000536 to EK) and the National Institute of Allergy and Infectious Diseases (HHSN272201400007C to RR).
References
- 1.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html Available at: [last accessed July 2020] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2020. Coronavirus disease (COVID-19) technical guidance: infection prevention and control.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control Available at: [last accessed August 2020] [Google Scholar]
- 3.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Blickman A. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 4.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 5.COVIDView: a weekly summary of U.S. COVID-19 activity. Centers for Disease Control and Prevention; Atlanta, GA: 2020. https://coronavirus.jhu.edu/us-map Available at: [last accessed April 2020] [Google Scholar]
- 6.Chopra V., Toner E., Waldhorn R., Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172:621–622. doi: 10.7326/M20-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostafa H.H., Hardick J., Morehead E., Miller J.-A., Gaydos C.A., Manabe Y.C. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J Clin Virol. 2020;130:104578. doi: 10.1016/j.jcv.2020.104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss ZF, Cunha CB, Chambers AB, Carr AV, Rochat C, Raglow-Defranco M. Opportunities revealed for antimicrobial stewardship and clinical practice with implementation of a rapid respiratory multiplex assay. J Clin Microbiol. 2019;57:e00861–19. doi: 10.1128/JCM.00861-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinot M, Greigert V, Gravier S, Klein S, Eyriey M, Pachart A. Positive impact of a point-of-care molecular influenza test in the emergency department during the 2017–2018 seasonal influenza epidemic. Open Forum Infect Dis. 2019;6:ofz312. doi: 10.1093/ofid/ofz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brendish NJ, Malachira AK, Armstrong L, Houghton R, Aitken S, Nyimbili E. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabattoni E, Le V, Pilmis B, Pean de Ponfilly G, Caisso C, Couzigou C. Implementation of Alere influenza A & B point of care test for the diagnosis of influenza in an ED. Am J Emerg Med. 2018;36:916–921. doi: 10.1016/j.ajem.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Klein EY, Tseng KK, Hinson J, Goodman KE, Smith A, Toerper M. The role of healthcare worker-mediated contact networks in the transmission of vancomycin-resistant enterococci. Open Forum Infect Dis. 2020;7:ofaa056. doi: 10.1093/ofid/ofaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan DJ, Pineles L, Shardell M, Graham MM, Mohammadi S, Forrest GN. The effect of contact precautions on healthcare worker activity in acute care hospitals. Infect Control Hosp Epidemiol. 2013;34:69–73. doi: 10.1086/668775. [DOI] [PubMed] [Google Scholar]
- 14.ProjectN95. Brooklyn, NY. Available at: https://www.projectn95.org/supply [last accessed August 2020].
- 15.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed August 2020] [Google Scholar]
- 16.Iyer S.B., Gerber M.A., Pomerantz W.J., Mortensen J.E., Ruddy R.M. Effect of point-of-care influenza testing on management of febrile children. Acad Emerg Med. 2006;13:1259–1268. doi: 10.1197/j.aem.2006.07.026. [DOI] [PubMed] [Google Scholar]