Abstract
The number of deaths from air pollution worldwide is estimated at 8.8 million per year, more than the number of deaths from smoking. Air pollutants, such as PM2.5, are known to induce respiratory and cardiovascular diseases by inducing oxidative stress. Thioredoxin (Trx) is a 12-kDa endogenous protein that exerts antioxidant activity by promoting dithiol disulfide exchange reactions. We previously synthesized human serum albumin-fused thioredoxin (HSA-Trx), which has a longer half-life in plasma compared with Trx, and demonstrated its efficacy against various diseases including respiratory diseases. Here, we examined the effect of HSA-Trx on urban aerosol-induced lung injury in mice. Urban aerosols induced lung injury and inflammatory responses in ICR mice, but intravenous administration of HSA-Trx markedly inhibited these responses. We next analyzed reactive oxygen species (ROS) production in murine lungs using an in vivo imaging system. The results show that intratracheal administration of urban aerosols induced ROS production that was inhibited by intravenously administered HSA-Trx. Finally, we found that HSA-Trx inhibited the urban aerosol-induced increase in levels of neutrophilic extracellular trap (NET) indicators (i.e., double-stranded DNA, citrullinated histone H3, and neutrophil elastase) in bronchoalveolar lavage fluid (BALF). Together, these findings suggest that HSA-Trx prevents urban aerosol-induced acute lung injury by suppressing ROS production and neutrophilic inflammation. Thus, HSA-Trx may be a potential candidate drug for preventing the onset or exacerbation of lung injury caused by air pollutants.
Keywords: Air pollution, Oxidative stress, Thioredoxin, HSA-Trx, Neutrophilic extracellular traps
Graphical abstract
1. Introduction
Air pollution is a major health hazard that causes an estimated 8.8 million deaths per year worldwide, which is greater than the number of deaths due to smoking (Lelieveld et al., 2019). Suspended particulate matter and fine particulate matter (PM2.5), which contain a variety of toxic substances, can remain in the atmosphere for long periods of time and are known to induce respiratory and cardiovascular diseases (Lelieveld et al., 2019; Losacco and Perillo, 2018). Therefore, it is important to establish methods of prevention and treatment for respiratory and cardiovascular diseases caused by these air pollutants.
Recently, the mechanisms by which air pollutants induce various diseases have been analyzed using both in vivo and in vitro systems, and oxidative stress has been suggested to be involved in this mechanism. Riva et al. found that intranasal administration of PM2.5 (15 μg/mouse) significantly increased lung impedance and alveolar collapse, as well as lung tissue inflammation, oxidative stress, and damage in BALB/c mice at 24 h after PM2.5 administration (Riva et al., 2011). Another group also showed that PM2.5 induces the infiltration of inflammatory cells, upregulation of 8-epi-PGF2α (an oxidative stress marker), and downregulation of superoxide dismutase (an anti-oxidative protein) in the lungs of Sprague Dawley rats (Li et al., 2019). Further, an in vitro study revealed that PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 (ICAM-1) expression in A549 cells (lung epithelial cells) and that N-acetylcysteine (NAC), an anti-oxidative compound, clearly suppresses PM2.5-dependent ICAM-1 expression via suppressing oxidative stress (Liu et al., 2018). Although epidemiological evidence in humans is limited, small cohort studies have shown an association between elevated ultrafine particle concentrations and oxidative DNA damage (8-hydroxy-2ʹ-deoxyguanosine; 8-OHdG) in lymphocytes (Sorensen et al., 2005). These reports suggest that compounds capable of inhibiting the oxidative stress caused by air pollutants may prevent the onset or exacerbation of various respiratory diseases.
Thioredoxin (Trx) is a 12-kDa endogenous protein with antioxidant activity that is induced in various oxidative stress diseases (Holmgren, 1989). Trx has a Cys-X-X-Cys motif at the active site and exerts antioxidant activity by promoting dithiol disulfide exchange reactions. Consequently, the protective effects of Trx in various disease models have been analyzed, focusing on its antioxidant activity. The intraperitoneal administration of human Trx ameliorates dextran sulfate sodium-induced colitis and colonic inflammation in mice (Tamaki et al., 2006). Extracellularly injected recombinant Trx showed protective effects against myocardial ischemia/reperfusion injury caused by oxidative stress (Tao et al., 2004). Moreover, daily supplementation with human Trx prevented ethanol-induced oxidative stress, inflammatory cytokine production, and apoptosis in the livers of mice (Cohen et al., 2009). Based on these findings, we hypothesized that Trx may exert a protective effect against urban aerosol-dependent lung injury.
Notably, Trx administered to mice is largely removed through glomerular filtration, resulting in a short plasma half-life of approximately 1 h. Due to its short plasma half-life, Trx must be administered via constant infusions or in frequently repeated doses to achieve a satisfactory therapeutic effect (Liu et al., 2004; Ueda et al., 2006). To improve the pharmacokinetics of Trx, we previously synthesized human serum albumin-fused thioredoxin (HSA-Trx) and found that the plasma half-life of HSA-Trx is over 10-fold higher than that of Trx (Ikuta et al., 2010). Over several subsequent studies, we found that a single intravenous administration of HSA-Trx is effective against a variety of diseases, including ovalbumin-induced lung injury, contrast nephropathy, rhabdomyolysis-associated acute kidney injury, and bleomycin-induced pulmonary fibrosis, via its long-lasting antioxidant action (Furukawa et al., 2011; Kodama et al., 2013; Nishida et al., 2015; Tanaka et al., 2013). Here, we examined the effect of HSA-Trx on urban aerosol-induced lung injury in mice. We also analyzed the mechanism by which HSA-Trx prevents urban aerosol-dependent lung injury, focusing on oxidative stress and neutrophilic inflammation.
2. Materials and methods
2.1. Chemicals and animals
Diff-Quik staining solution was purchased from Sysmex (Kobe, Japan). Antibodies against citrullinated histone H3 (citrulline R2 + R8 + R17) and neutrophil elastase were purchased from Abcam (Cambridge, UK). Luminal-based chemiluminescent probe (L-012) and isoflurane were obtained from Fujifilm Wako Pure Chemical Corporation (Tokyo, Japan). The RNeasy® kit was obtained from Qiagen (Hilden, Germany), PrimeScript® 1st strand cDNA Synthesis kit was obtained from Takara Bio (Ohtsu, Japan), and THUNDERBIRD® SYBR qPCR Mix was obtained from Toyobo (Ohtsu, Japan). Novo-Heparin (5000 units), suitable for injection, was purchased from Mochida Pharmaceutical (Tokyo, Japan). RAW264 cells were purchased from Riken BioResource Center (Tsukuba, Japan). ICR mice (6–7 weeks old, male or female) were purchased from Charles River (Yokohama, Japan). The experiments and procedures described here were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and were approved by the Animal Care Committee of Musashino University. The sex of the mice did not correlate with the extent of urban aerosol-induced lung injury (number of inflammatory cells and levels of protein in bronchoalveolar lavage fluid (BALF)) (Supplementary Fig. S1). Therefore, male mice were used for all analyses in the present study.
2.2. Urban aerosol particle suspensions “urban aerosols”
Urban aerosol particulate matter was obtained from the National Institute for Environmental Studies (NIES, Tsukuba, Japan). These urban aerosol particles were collected in air filters from the central ventilation systems of buildings in central Beijing. Analysis of the urban aerosol particles composition was reported in a previous paper (Mori et al., 2008) and NIES website (https://www.nies.go.jp/labo/crm-e/aerosol.html). For the animal studies, urban aerosol particles were suspended in 0.5% methylcellulose solution and administered to mice. For the cell experiments, urban aerosol particles were suspended in ultrapure water, and the suspension was directly added to the medium.
2.3. Production of HSA-Trx fusion protein
HSA, Trx, and the HSA-Trx fusion protein were produced following previously reported methods (Kodama et al., 2013; Tanaka et al., 2013). Transformed Pichia pastoris cells were incubated in 1.25 L of BMGY liquid media (growth phase) for 2 days (OD600 = 2) at 30 °C and then cultured in 800 ml of BMMY media (protein induction phase) for 3 days at 30 °C. The fusion protein was purified by chromatography on a Blue Sepharose 6 Fast Flow column and HiTrap Phenyl HP column for hydrophobic chromatography. The fusion protein was analyzed by SDS-PAGE using a 10% polyacrylamide gel, with Coomassie blue R250 staining. The purity of the fusion protein was estimated to be in excess of 95%.
2.4. Treatment of mice with urban aerosols and HSA-Trx
Mice were intravenously administered HSA-Trx (3.5 nmol protein/mouse) in sterile saline immediately before and 24 h after the intratracheal administration of urban aerosol particle suspensions. After anesthetization with isoflurane, mice were administered urban aerosol particles (200 μg/mouse) in 0.5% methylcellulose solution intratracheally using a P200 micropipette. During administration, the nostrils of the mice were blocked so that the solutions were inhaled from the mouth into the respiratory tract as the mice breathed. The control group received sterile saline intravenously and 0.5% methylcellulose solution intratracheally, the urban aerosol treatment group received sterile saline intravenously and urban aerosol particle suspension in 0.5% methylcellulose solution intratracheally, and the HSA-Trx group received intravenous HSA-Trx and intratracheal urban aerosol particle suspensions. Additionally, we examined the effect of HSA-Trx alone (intravenously administered HSA-Trx and intratracheally administered 0.5% methylcellulose solution with timing as above), and found that it did not affect protein concentration or number of leukocytes in the BALF, nor did it affect the levels of reactive oxygen species (ROS) in the lung (Supplementary Fig. S2).
2.5. Preparation of BALF and immunoblotting analysis
BALF was collected by cannulating the trachea and lavaging the lung twice with 1 ml of sterile saline containing 50 units/ml heparin. Approximately 1.8 ml of BALF was routinely recovered from each mouse, and the total cell number in the BALF was counted using a hemocytometer. After centrifugation with a Cytospin®4 (Thermo Fisher Scientific, Waltham, MA, USA), the cells were stained with Diff-Quik reagents, and the ratio of neutrophils to the total cell number was determined. The amount of protein or double-stranded DNA (dsDNA) present in the BALF was evaluated by the Bradford method or by using a Quant-iT™ PicoGreen® dsDNA Assay Kit (Thermo Fisher Scientific).
BALF samples (2 μl) were then applied to a NuPAGE® Novex 4%–12% Bis-Tris Gel (Thermo Fisher Scientific) and subjected to electrophoresis. Samples were loaded on NuPAGE® Novex 4%–12% Bis-Tris Protein Gels (Thermo Fisher Scientific) and electrophoresed at a constant voltage of 180 V, after which the proteins were transferred to polyvinylidene difluoride membranes using the iBlot® 7-Minute Blotting System (Thermo Fisher Scientific). Membranes were blocked with 5% non-fat dry milk at room temperature for 1 h, then incubated overnight with rabbit anti-citrullinated histone H3 antibodies (1:1000 dilution) or rabbit anti-neutrophil elastase antibodies (1:1000 dilution) in 5% bovine serum albumin (BSA), 1 × Tris-buffered saline (TBS), and 0.1% Tween 20, followed by incubation for 1 h with HRP-linked goat anti-rabbit IgG antibodies (1:2000 dilution) in 1 × TBS containing 0.1% Tween 20. Finally, the protein bands were visualized using SuperSignal™ West Dura Extended Duration Substrate (Thermo Fisher Scientific). Band intensities were quantitated using ImageJ software (version 1.39u).
2.6. Measurement of ROS by in vivo imaging analysis
In vivo imaging of ROS in mice was performed as previously described (Tanaka et al., 2017). We used the FUSION chemiluminescence imaging system (Vilber Lourmat, Collégien, France). Mice were intraperitoneally administered the ROS-sensing chemiluminescent probe, L-012, in sterile saline (75 mg/kg) 24.5 h after the administration of urban aerosol particle suspensions. At 10 min after the L-012 administration, the mice were euthanized, and their lungs were promptly dissected and imaged (5-min exposure). All data were analyzed using the FUSION chemiluminescence imaging system software.
2.7. Real-time reverse transcription (RT) polymerase chain reaction (PCR) analysis
Total RNA was extracted from lung tissue using an RNeasy kit in accordance with the manufacturer’s protocol. Samples were reverse-transcribed using the PrimeScript® kit described above. The synthesized cDNA was used in real-time PCR experiments with THUNDERBIRD® SYBR qPCR Mix and analyzed with a Bio-Rad (Hercules, CA, USA) CFX96™ real-time system and CFX Manager™ software. Specificity was confirmed by electrophoretic analysis of reaction products and the inclusion of template- or reverse transcriptase-free controls. To normalize the amount of total RNA present in each reaction, glyceraldehyde-3-phosphate dehydrogenase (Gapdh) cDNA was used as an internal standard. Primers were designed using the Primer-BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences will be provided upon request.
2.8. Cell culture and ROS measurement in vitro
RAW264 cells (a mouse macrophage-like cell line) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. RAW264 cells were cultured in a humidified atmosphere of 95% air with 5% CO2 at 37 °C. RAW264 cells were pre-cultured in black 96-well microplates (1 × 104 cells/well). After incubation for 24 h, the cells were incubated with the ROS indicator, 2′,7′-dichlorodihydrofluorescein diacetate (DCFHDA, 100 μM). The cells were then treated with HSA-Trx (0.3–2.5 μM) prior to the addition of urban aerosols to the medium. After 1 h, the ROS levels were measured using a microplate reader (Tecan, Kawasaki, Japan; excitation: 480 nm, emission: 530 nm).
2.9. Statistical analysis
All data are expressed as the mean ± SEM. Significant differences among each group were examined using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison. SPSS 24 software was used for all statistical analyses. Probability values of p < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Effect of HSA-Trx on urban aerosol-induced lung injury
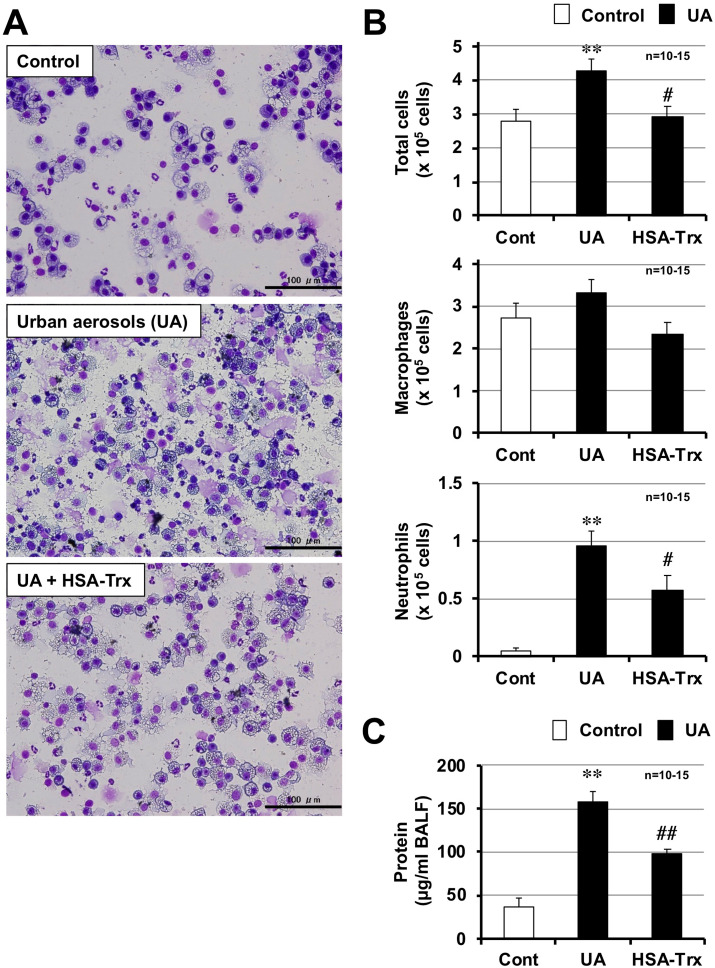
We first examined the effect of HSA-Trx on urban aerosol-induced lung injury by measuring the number of leukocytes in BALF 48 h after an intratracheal administration of urban aerosols. The dose of HSA-Trx used was 3.5 nmol protein per mouse, and this optimal concentration was established in previous studies on lung disease (Tanaka et al., 2013, 2014). As shown in Fig. 1 A and B, the total number of leukocytes, and especially the number of neutrophils, in the BALF was increased by urban aerosol treatment. The number of macrophages was not increased by this treatment. In contrast, mice pre-treated with HSA-Trx had significantly lower total numbers of leukocytes, particularly neutrophils, following urban aerosol exposure. The number of neutrophils after treatment with a control, urban aerosols, or urban aerosols plus HSA-Trx was 0.05 ± 0.02, 0.96 ± 0.12, or 0.58 ± 0.12 (x 105 cells), respectively. The protein concentration in BALF is an indicator of lung injury and edema. As shown in Fig. 1C, treatments with urban aerosols increased the protein concentration in BALF, whereas, pre-treatment with HSA-Trx significantly suppressed the urban aerosol-dependent increase in protein concentration. These results suggest that HSA-Trx suppresses urban aerosol-induced lung injury.
Fig. 1.
Effect of HSA-Trx on urban aerosol-induced lung injury. Male ICR mice were intravenously administered with HSA-Trx (3.5 nmol protein/mouse in sterile saline) or sterile saline alone, immediately before and 24 h after intratracheal administration of urban aerosol particle suspensions administration (200 μg/mouse) or 0.5% methylcellulose solution alone (Cont). BALFs were prepared 48 h after the intratracheal administration. (A) BALF cells were deposited onto slides using a Cytospin® 4 cytocentrifuge then stained with Diff-Quik reagents and visualized under light microscopy (scale bar, 100 μm). (B) The numbers of total cells, macrophages, and neutrophils were determined. (C)The amount of protein present in the BALF was determined by the Bradford method. Values are the mean ± S.E.M.; ∗ or #p < 0.05; ∗∗ or ##p < 0.01. (∗, vs Control; #, vs UA).
3.2. Effect of HSA-Trx on urban aerosol-induced inflammatory responses
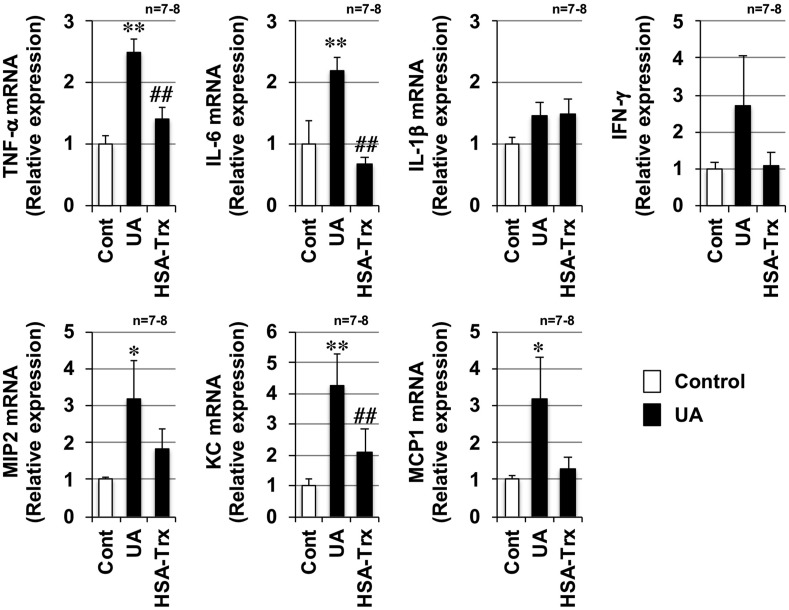
Air pollutants including PM2.5 are reported to induce pro-inflammatory cytokine production, which is involved in the promotion of inflammatory responses in the lungs (He et al., 2017). Thus, we monitored the expression of pro-inflammatory cytokines and chemokines by using real-time RT-PCR. As shown in Fig. 2 , the mRNA expressions of tumor necrosis factor-α (Tnf-α), interleukin-6 (Il-6), keratinocyte-derived chemokines (Kc), macrophage inflammatory protein 2 (Mip2), and monocyte chemoattractant protein 1 (Mcp1) were induced by urban aerosol treatment. The expressions of Il-1β and interferon-γ (Ifn-γ) after urban aerosol treatment trended higher compared with mock-treated controls, but these differences were not statistically significant. In contrast, pre-treatment with HSA-Trx significantly inhibited the urban aerosol-dependent increases in Tnf-α, Il-6, and Kc expression. While, a trend toward suppression of the urban aerosol-dependent increase in Mip2 and Mcp1 expression was observed, these differences were not statistically significant. Together, the data suggest that HSA-Trx inhibits urban aerosol-induced lung injury through the inhibition of inflammatory responses.
Fig. 2.
Effect of HSA-Trx on urban aerosol-dependent inflammatory responses. Male ICR mice were intravenously administered with HSA-Trx (3.5 nmol protein/mouse in sterile saline) or sterile saline alone, immediately before and 24 h after intratracheal administration of urban aerosol particle suspensions administration (200 μg/mouse) or 0.5% methylcellulose solution alone (Cont). Total RNA was extracted from the lungs 48 h after urban aerosol particle suspensions administration and subjected to real-time RT-PCR using a specific primer set for each gene. Values were normalized to Gapdh and are expressed relative to the Control. Values are the mean ± S.E.M.; #p < 0.05; ∗∗ or ##p < 0.01 (∗, vs Control; #, vs UA).
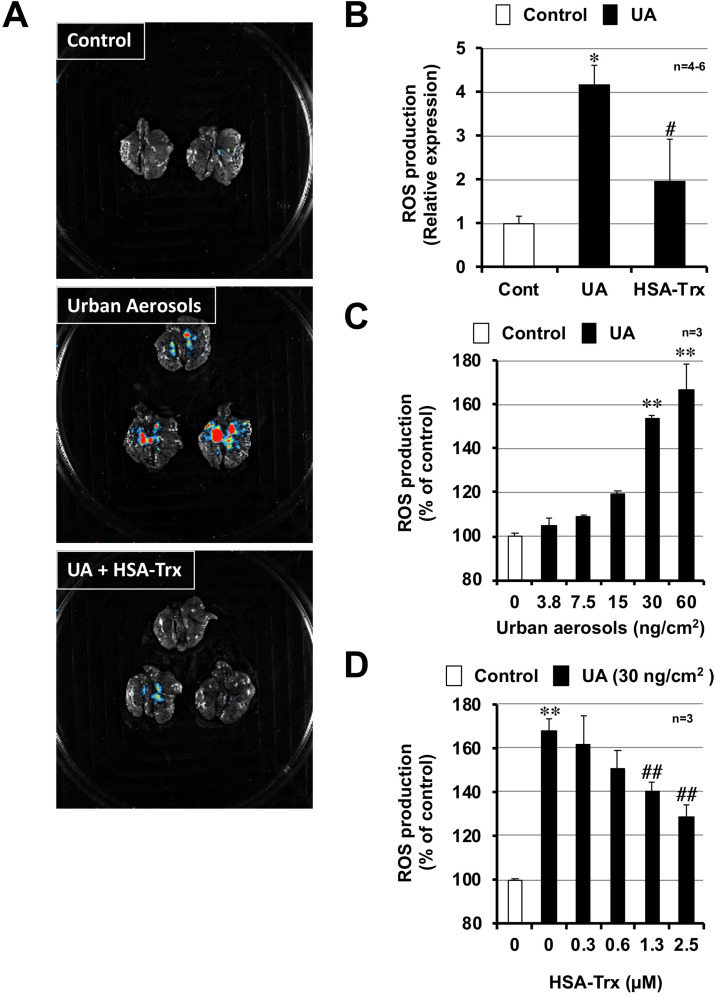
3.3. Effect of HSA-Trx on urban aerosol-induced ROS production
ROS are involved in the onset and progression of various lung injuries, such as acute respiratory distress syndrome (ARDS) and chronic obstructive lung disease (COPD) (Sarma and Ward, 2011; Tasaka et al., 2008). In addition, air pollutants are reported to induce lung injury and inflammatory responses via promoting oxidative stress (Li et al., 2015; Zhang et al., 2018). Thus, we monitored ROS levels using the FUSION in vivo imaging system. As shown in Fig. 3 A, intratracheal the administration of urban aerosols increased ROS levels in the lung. The area that is stained red is the place where high levels of reactive oxygen were generated. In contrast, mice pre-treated with HSA-Trx did not display such red staining after urban aerosol treatment, indicating that HSA-Trx pre-treatment markedly suppressed ROS production. A quantitative analysis using the standard software provided with FUSION showed that HSA-Trx significantly inhibits ROS production (Fig. 3B). We next used an in vitro system to investigate whether HSA-Trx directly inhibits urban aerosol-dependent ROS production. As shown in Fig. 3C, the treatment of RAW264 cells with urban aerosols induced ROS production in a dose-dependent manner. In contrast, urban aerosol-dependent ROS production was significantly inhibited in RAW264 cells that were pre-treated in vitro with HSA-Trx (Fig. 3D). These results suggest that HSA-Trx inhibits urban aerosol-induced lung injury and inflammatory responses through reducing ROS production.
Fig. 3.
Effect of HSA-Trx on urban aerosol-induced ROS production. Male ICR mice were intravenously administered with HSA-Trx (3.5 nmol protein/mouse in sterile saline) or sterile saline alone, immediately before and 24 h after intratracheal administration of urban aerosol particle suspensions administration (200 μg/mouse) or 0.5% methylcellulose solution alone (Cont). Luminescent probe (L-012, 75 mg/kg) was administered 24.5 h after the urban aerosol particle suspensions administration. (A) Isolated lungs were imaged using a FUSION chemiluminescence imaging system. (B) The summed pixel intensity of the ROS signal was determined using standard software for FUSION. (C, D) RAW264 cells were pre-cultured with DCFHDA (100 μM). The cells were then treated with urban aerosols (3.8–60 ng/cm2) (C) or HSA-Trx (0.3–2.5 μM) prior to the addition of urban aerosols (30 ng/cm2) (D) to the medium. After 1 h, the ROS levels were measured using a microplate reader. Values represent the mean ± S.E.M. #p < 0.05; ∗∗ or ##p < 0.01 (B: ∗ vs Control; #, vs UA, C, D: ∗ vs Control, # vs UA (30 ng/cm2)).
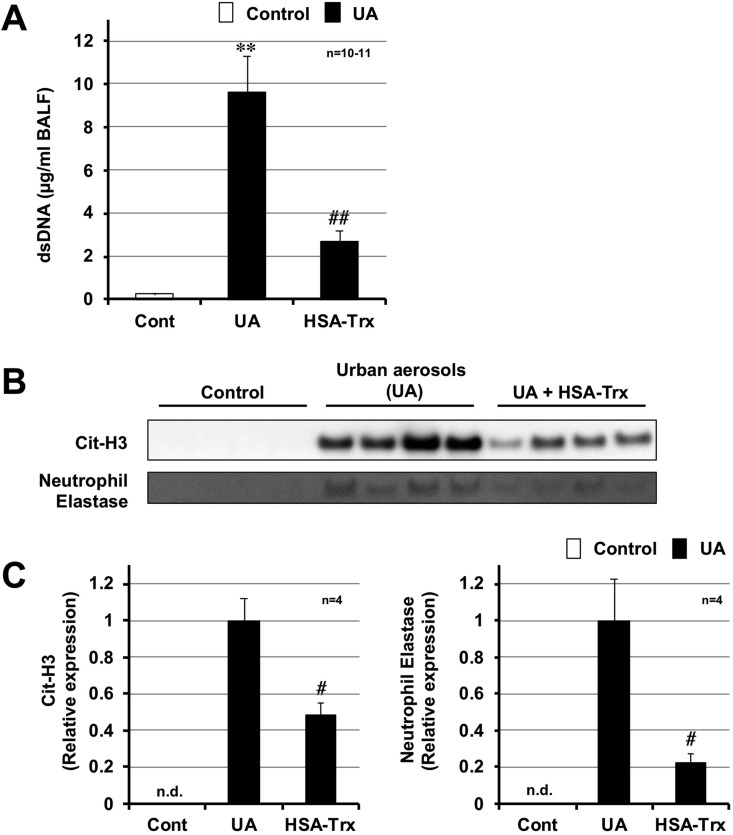
3.4. Effect of HSA-Trx on urban aerosol-induced neutrophilic inflammation
ROS has been reported to play a major role in the formation of neutrophilic extracellular traps (NETs) (Stoiber et al., 2015). NETs are composed of decondensed chromatin fibers and cytoplasmic protein, such as neutrophil elastase and peptidylarginine deiminase 4 (PAD4). Citrullination of histones H3 and H4 by PAD4 is essential for the disaggregation of chromatin, which is required for the formation of NETs (Wang et al., 2009). Thus, we monitored the dsDNA levels in BALF as an indicator of NET formation. As shown in Fig. 4 A, the levels of dsDNA in BALF markedly increased following the intratracheal administration of urban aerosols. In contrast, HSA-Trx pre-treatment significantly reduced this increase. We then analyzed the expression levels of two other NET indicators, citrullinated histone H3 (Cit-H3) and neutrophil elastase, in BALF. As shown in Fig. 4B and C, higher levels of Cit-H3 and neutrophil elastase were detected in the urban aerosol treatment group, compared with the control group. The expression of these proteins was not detectable in the control group. Pre-treatment with HSA-Trx significantly reduced the urban aerosol-dependent increases in these proteins. Based on these results, the intravenous administration of HSA-Trx protects against urban aerosol-induced lung injury by suppressing neutrophilic inflammation (NET formation).
Fig. 4.
Effect of HSA-Trx on urban aerosol-induced neutrophil extracellular trap formation. Male ICR mice were intravenously administered with HSA-Trx (3.5 nmol protein/mouse in sterile saline) or sterile saline alone, immediately before and 24 h after intratracheal administration of urban aerosol particle suspensions administration (200 μg/mouse) or 0.5% methylcellulose solution alone (Cont). BALFs were prepared 48 h after the intratracheal administration. (A) The amount of double-stranded DNA (dsDNA) present in the BALF was determined using the Quant-iT™ PicoGreen® dsDNA Assay Kit in accordance with the manufacturer’s protocol. (B) BALF samples (2 μl) were analyzed by immunoblotting with an antibody against citrullinated histone H3 (Cit-H3) or neutrophil elastase. (C) The intensities of these bands were determined using Image J software. n.d. indicates not detected. Values are the mean ± S.E.M.; #p < 0.05; ∗∗ or ##p < 0.01 (∗, vs Control; #, vs UA).
4. Discussion
Here, we examined the effect of HSA-Trx on urban aerosol-induced acute lung injury. As shown in Fig. 1, Fig. 2, we found that HSA-Trx clearly suppresses urban aerosol-induced lung injury and inflammatory responses. Moreover, focusing on oxidative stress and neutrophilic inflammation, we analyzed the mechanism by which HSA-Trx prevents urban aerosol-dependent lung injury. As shown in Fig. 3, Fig. 4, we found that HSA-Trx suppresses urban aerosol-induced ROS production in vivo and in vitro, and urban aerosol-induced NET formation in vivo. Based on these results, we suggest that HSA-Trx prevents urban aerosol-induced acute lung injury by suppressing ROS production and neutrophilic inflammation. Although it has been known from previous studies that air pollutants such as PM2.5 produce ROS in vivo and in vitro (Li et al., 2015, 2018b; Zhang et al., 2018), this is the first study to detect the production of ROS by urban aerosol exposure using an in vivo imaging system. In the future, we would like to detect the production of ROS following long-term exposure to urban aerosols and analyze the effects of candidate drugs in animal models.
Oxidative stress has been shown to play a major role in the development of various lung injuries via activation of neutrophil inflammation, vascular permeability, and the coagulation system (Sarma and Ward, 2011; Tasaka et al., 2008). For example, in ARDS patients, ROS levels have been reported to increase in plasma or BALF (Lamb et al., 1999; Quinlan et al., 1997; Tasaka et al., 2008). Recently, Delgado-Roche et al. speculated that oxidative stress may also play an important role in severe acute respiratory syndrome coronavirus (SARS-CoV) infection (Delgado-Roche and Mesta, 2020). Moreover, ROS has also been suggested to be involved in chronic obstructive pulmonary disease (COPD), a respiratory disease with a high number of patients caused by smoking and air pollution. Specifically, the levels of oxidative radicals, 8-OHdG, or lipid peroxide have been reported to be higher in lung tissues and BALF from COPD patients or smokers compared with healthy nonsmokers (Kluchova et al., 2007; Nadeem et al., 2005; Pinamonti et al., 1998). In fact, we have previously found that HSA-Trx exerts its effects against influenza virus-dependent acute lung injury and bleomycin-dependent pulmonary fibrosis via antioxidant action (Tanaka et al., 2013, 2014). Considering these results, we believe that HSA-Trx may be a potential candidate for drugs to prevent the onset or exacerbation of lung injury caused by air pollutants.
NETs play an important role as a defense system against bacterial and viral infections. However, the overactivation of NETs is thought to lead to systemic exacerbation of inflammation, including inflammatory lung disease (Porto and Stein, 2016; Yang et al., 2016). In fact, a clinical trial of asthma, which is a lung disease characterized by airflow limitation, detected neutrophil elastase, an indicator of NETs, in 67% of sputum samples from asthmatic patients (compared with 0% in the control group) (Simpson et al., 2007). An analysis using sputum from COPD patients revealed that the presence of large amounts of NETs is associated with disease severity. Specifically, such high NET amounts were observed in more than 90% of subjects with exacerbated COPD, in more than 45% of subjects with stable COPD, and in more than 25% of smoking control subjects, but in less than 5% of nonsmoking subjects (Grabcanovic-Musija et al., 2015). Furthermore, it has also been shown that the NET-DNA concentration correlates with disease severity in patients with gastric aspiration-induced ARDS (Li et al., 2018a). In contrast, to the best of our knowledge, no studies have previously shown that urban aerosols induce NETs in the lungs of humans or mice. Therefore, it is notable that we have demonstrated here for the first time that urban aerosols induce NETs in vivo and that HSA-Trx inhibited the NET formation in this study.
Considering the future potential clinical application of HSA-Trx, it is important to analyze the effect of HSA-Trx on urban aerosol-induced exacerbations of various respiratory diseases, not only on lung injury caused directly by administration of urban aerosols. For example, a meta-analysis published in 2016 shows that short-term exposure to air pollutants (e.g., O3, CO, NO2, SO2, PM10, and PM2.5) significantly increased the burden of acute exacerbation risk for COPD (Li et al., 2016). Another group reported that ambient air pollution levels were negatively associated with lung function (post- or pre-bronchodilator %predicted forced expiratory volume in 1 s (FEV1), and forced vital capacity (FVC)) and were associated with methacholine responsiveness (PC20) in children with asthma (Ierodiakonou et al., 2016). Moreover, in an animal study, PM2.5 exposure was found to aggravate cigarette smoke-induced pulmonary emphysema changes (airspace enlargement) and inflammatory responses in mice (Zhao et al., 2019). The findings from these reports indicate that it is important to examine the effect of HSA-Trx on the exacerbation of various respiratory diseases by urban aerosols.
5. Conclusions
We revealed here that the intravenous administration of HSA-Trx prevents urban aerosol-dependent lung injury and inflammatory responses. Moreover, HSA-Trx treatment significantly suppressed urban aerosol-dependent ROS production and neutrophilic inflammation, specifically NET induction. Therefore, we believe that HSA-Trx may be a potential candidate drug to prevent the onset or exacerbation of lung injury caused by air pollutants.
Funding
This research was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (KAKENHI KIBAN (C) 18K06691).
Author contributions
Participated in research design: Ken-ichiro Tanaka, Yu Ishima, Masahiro Kawahara. Conducted experiments: Ken-ichiro Tanaka, Maho Kubota, Tomoko Hayase, Mamika Miyaguchi, Nahoko Kobayashi, Mayumi Ikeda. Contributed new reagents or analytic tools: Mayumi Ikeda, Yu Ishima. Performed data analysis: Ken-ichiro Tanaka, Maho Kubota, Yu Ishima, Masahiro Kawahara. Wrote or contributed to the writing of the manuscript: All authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Katie Oakley, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
This paper has been recommended for acceptance by Wen Chen.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2020.115787.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Cohen J.I., Roychowdhury S., DiBello P.M., Jacobsen D.W., Nagy L.E. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology. 2009;49:1709–1717. doi: 10.1002/hep.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M., Tanaka R., Chuang V.T., Ishima Y., Taguchi K., Watanabe H., Maruyama T., Otagiri M. Human serum albumin-thioredoxin fusion protein with long blood retention property is effective in suppressing lung injury. J. Contr. Release. 2011;154:189–195. doi: 10.1016/j.jconrel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Grabcanovic-Musija F., Obermayer A., Stoiber W., Krautgartner W.D., Steinbacher P., Winterberg N., Bathke A.C., Klappacher M., Studnicka M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Ichinose T., Yoshida S., Ito T., He C., Yoshida Y., Arashidani K., Takano H., Sun G., Shibamoto T. PM2.5-induced lung inflammation in mice: differences of inflammatory response in macrophages and type II alveolar cells. J. Appl. Toxicol. 2017;37:1203–1218. doi: 10.1002/jat.3482. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Ierodiakonou D., Zanobetti A., Coull B.A., Melly S., Postma D.S., Boezen H.M., Vonk J.M., Williams P.V., Shapiro G.G., McKone E.F., Hallstrand T.S., Koenig J.Q., Schildcrout J.S., Lumley T., Fuhlbrigge A.N., Koutrakis P., Schwartz J., Weiss S.T., Gold D.R., Childhood Asthma Management Program Research, G., Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J. Allergy Clin. Immunol. 2016;137:390–399. doi: 10.1016/j.jaci.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Chuang V.T., Ishima Y., Nakajou K., Furukawa M., Watanabe H., Maruyama T., Otagiri M. Albumin fusion of thioredoxin--the production and evaluation of its biological activity for potential therapeutic applications. J. Contr. Release. 2010;147:17–23. doi: 10.1016/j.jconrel.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Kluchova Z., Petrasova D., Joppa P., Dorkova Z., Tkacova R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol. Res. 2007;56:51–56. doi: 10.33549/physiolres.930884. [DOI] [PubMed] [Google Scholar]
- Kodama A., Watanabe H., Tanaka R., Tanaka H., Chuang V.T., Miyamoto Y., Wu Q., Endo M., Hamasaki K., Ishima Y., Fukagawa M., Otagiri M., Maruyama T. A human serum albumin-thioredoxin fusion protein prevents experimental contrast-induced nephropathy. Kidney Int. 2013;83:446–454. doi: 10.1038/ki.2012.429. [DOI] [PubMed] [Google Scholar]
- Lamb N.J., Gutteridge J.M., Baker C., Evans T.W., Quinlan G.J. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 1999;27:1738–1744. doi: 10.1097/00003246-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Klingmuller K., Pozzer A., Poschl U., Fnais M., Daiber A., Munzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019;40:1590–1596. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhou X., Tan H., Hu Y., Zhang L., Liu S., Dai M., Li Y., Li Q., Mao Z., Pan P., Su X., Hu C. Neutrophil extracellular traps contribute to the pathogenesis of acid-aspiration-induced ALI/ARDS. Oncotarget. 2018;9:1772–1784. doi: 10.18632/oncotarget.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li H., Li H., Guo W., An Z., Zeng X., Li W., Li H., Song J., Wu W. Amelioration of PM2.5-induced lung toxicity in rats by nutritional supplementation with fish oil and Vitamin E. Respir. Res. 2019;20:76. doi: 10.1186/s12931-019-1045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sun S., Tang R., Qiu H., Huang Q., Mason T.G., Tian L. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou Q., Liang Y., Pan W., Bei Y., Zhang Y., Wang J., Jiao Z. miR-486 inhibits PM2.5-induced apoptosis and oxidative stress in human lung alveolar epithelial A549 cells. Ann. Transl. Med. 2018;6:209. doi: 10.21037/atm.2018.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Kou X., Xie L., Cheng F., Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ. Sci. Pollut. Res. Int. 2015;22:20167–20176. doi: 10.1007/s11356-015-5222-z. [DOI] [PubMed] [Google Scholar]
- Liu C.W., Lee T.L., Chen Y.C., Liang C.J., Wang S.H., Lue J.H., Tsai J.S., Lee S.W., Chen S.H., Yang Y.F., Chuang T.Y., Chen Y.L. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-kappaB-dependent pathway. Part. Fibre Toxicol. 2018;15:4. doi: 10.1186/s12989-018-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Nakamura H., Shioji K., Tanito M., Oka S., Ahsan M.K., Son A., Ishii Y., Kishimoto C., Yodoi J. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110:1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- Losacco C., Perillo A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018;25:33901–33910. doi: 10.1007/s11356-018-3344-9. [DOI] [PubMed] [Google Scholar]
- Mori I., Sun Z., Ukachi M., Nagano K., McLeod C.W., Cox A.G., Nishikawa M. Development and certification of the new NIES CRM 28: urban aerosols for the determination of multielements. Anal. Bioanal. Chem. 2008;391:1997–2003. doi: 10.1007/s00216-008-2076-y. [DOI] [PubMed] [Google Scholar]
- Nadeem A., Raj H.G., Chhabra S.K. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation. 2005;29:23–32. doi: 10.1007/s10753-006-8965-3. [DOI] [PubMed] [Google Scholar]
- Nishida K., Watanabe H., Ogaki S., Kodama A., Tanaka R., Imafuku T., Ishima Y., Chuang V.T., Toyoda M., Kondoh M., Wu Q., Fukagawa M., Otagiri M., Maruyama T. Renoprotective effect of long acting thioredoxin by modulating oxidative stress and macrophage migration inhibitory factor against rhabdomyolysis-associated acute kidney injury. Sci. Rep. 2015;5:14471. doi: 10.1038/srep14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinamonti S., Leis M., Barbieri A., Leoni D., Muzzoli M., Sostero S., Chicca M.C., Carrieri A., Ravenna F., Fabbri L.M., Ciaccia A. Detection of xanthine oxidase activity products by EPR and HPLC in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary disease. Free Radic. Biol. Med. 1998;25:771–779. doi: 10.1016/s0891-5849(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Porto B.N., Stein R.T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan G.J., Lamb N.J., Tilley R., Evans T.W., Gutteridge J.M. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am. J. Respir. Crit. Care Med. 1997;155:479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- Riva D.R., Magalhaes C.B., Lopes A.A., Lancas T., Mauad T., Malm O., Valenca S.S., Saldiva P.H., Faffe D.S., Zin W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011;23:257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Sarma J.V., Ward P.A. Oxidants and redox signaling in acute lung injury. Comp. Physiol. 2011;1:1365–1381. doi: 10.1002/cphy.c100068. [DOI] [PubMed] [Google Scholar]
- Simpson J.L., Grissell T.V., Douwes J., Scott R.J., Boyle M.J., Gibson P.G. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M., Schins R.P., Hertel O., Loft S. Transition metals in personal samples of PM2.5 and oxidative stress in human volunteers. Cancer Epidemiol. Biomark. Prev. 2005;14:1340–1343. doi: 10.1158/1055-9965.EPI-04-0899. [DOI] [PubMed] [Google Scholar]
- Stoiber W., Obermayer A., Steinbacher P., Krautgartner W.D. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules. 2015;5:702–723. doi: 10.3390/biom5020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki H., Nakamura H., Nishio A., Nakase H., Ueno S., Uza N., Kido M., Inoue S., Mikami S., Asada M., Kiriya K., Kitamura H., Ohashi S., Fukui T., Kawasaki K., Matsuura M., Ishii Y., Okazaki K., Yodoi J., Chiba T. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Tanaka K.I., Tamura F., Sugizaki T., Kawahara M., Kuba K., Imai Y., Mizushima T. Evaluation of lecithinized superoxide dismutase for the prevention of acute respiratory distress syndrome in animal models. Am. J. Respir. Cell Mol. Biol. 2017;56:179–190. doi: 10.1165/rcmb.2016-0158OC. [DOI] [PubMed] [Google Scholar]
- Tanaka R., Ishima Y., Enoki Y., Kimachi K., Shirai T., Watanabe H., Chuang V.T., Maruyama T., Otagiri M. Therapeutic impact of human serum albumin-thioredoxin fusion protein on influenza virus-induced lung injury mice. Front. Immunol. 2014;5:561. doi: 10.3389/fimmu.2014.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Watanabe H., Kodama A., Chuang V.T., Ishima Y., Hamasaki K., Tanaka K., Mizushima T., Otagiri M., Maruyama T. Long-acting human serum albumin-thioredoxin fusion protein suppresses bleomycin-induced pulmonary fibrosis progression. J. Pharmacol. Exp. Therapeut. 2013;345:271–283. doi: 10.1124/jpet.112.201814. [DOI] [PubMed] [Google Scholar]
- Tao L., Gao E., Bryan N.S., Qu Y., Liu H.R., Hu A., Christopher T.A., Lopez B.L., Yodoi J., Koch W.J., Feelisch M., Ma X.L. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected] Proc. Natl. Acad. Sci. U. S. A. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka S., Amaya F., Hashimoto S., Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxidants Redox Signal. 2008;10:739–753. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- Ueda S., Nakamura T., Yamada A., Teratani A., Matsui N., Furukawa S., Hoshino Y., Narita M., Yodoi J., Nakamura H. Recombinant human thioredoxin suppresses lipopolysaccharide-induced bronchoalveolar neutrophil infiltration in rat. Life Sci. 2006;79:1170–1177. doi: 10.1016/j.lfs.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li M., Stadler S., Correll S., Li P., Wang D., Hayama R., Leonelli L., Han H., Grigoryev S.A., Allis C.D., Coonrod S.A. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Biermann M.H., Brauner J.M., Liu Y., Zhao Y., Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front. Immunol. 2016;7:302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xue L., Li B., Tian H., Zhang Z., Tao S. Therapeutic potential of bixin in PM2.5 particles-induced lung injury in an Nrf2-dependent manner. Free Radic. Biol. Med. 2018;126:166–176. doi: 10.1016/j.freeradbiomed.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li M., Wang Z., Chen J., Zhao J., Xu Y., Wei X., Wang J., Xie J. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir. Res. 2019;20:120. doi: 10.1186/s12931-019-1081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.