Abstract
Coronavirus disease (COVID-19) has been identified as a pandemic by the World Health Organization (WHO). It was initially detected in Wuhan, China and spread to other cities of China and all countries. It has caused many deaths and the number of infections became greater than 18 million as of 5 August 2020. This study aimed to analyze the situation of COVID-19 in Thailand and the challenging disease control by employing a dynamic model to determine prevention approaches. We employed a statistical technique to analyze the ambient temperature influencing the cases. We found that temperature was significantly associated with daily infected cases (p-value <0.01). The SEIR (Susceptible Exposed Infectious and Recovered) dynamic approach and moving average estimation were used to forecast the daily infected and cumulative cases until 16 June as a base run analysis using STELLA dynamic software and statistical techniques. The movement of people, both in relation to local (Thai people) and foreign travel (both Thai and tourists), played a significant role in the spread of COVID-19 in Thailand. Enforcing a state of emergency and regulating social distancing were the key factors in reducing the growth rate of the disease. The SEIR model reliably predicted the actual infected cases, with a root mean square error (RMSE) of 12.8. In case of moving average approach, RMSE values were 0.21, 0.21, and 0.35 for two, three and five days, respectively. The previous records were used as input for prediction that caused lower values of RMSE. Two-days and three-days moving averages gave the better results than SEIR model. The SEIR model is suitable for longer period prediction, whereas the moving average approach is suitable for short term prediction. The implementation of interventions, such as governmental regulation and restrictions, through collaboration among various sectors was the key factor for controlling the spreading of COVID-19 in Thailand.
Keywords: COVID-19, Disease control, Thailand, Spread of disease, SEIR model, Moving average model
Graphical abstract
Highlights
-
•
COVID-19 disease has been determined as a pandemic, and has spread to all countries.
-
•
Temperature is associated with the daily infected COVID-19 cases in Thailand.
-
•
The spread of the disease in Thailand was associated with social activities, the enforcement by regulation and partnership among the organization are the key roles for spreading control.
-
•
Moving average model is suitable for short term prediction, SEIR model can be used for longer period prediction. Shorter moving day averages provided better prediction results than longer days.
1. Introduction
Currently, the novel coronavirus, SARS-CoV-2 might be identified as the biggest world health threat of this century. The virus has now been detected in most countries in a variety of situations. COVID-19 is a contagious infection from this coronavirus. Various countries have observed cases from December 2019 to the present [[1]1] and the world faces a severe and acute public health emergency from the emerging and ongoing spread of COVID-19 as a global pandemic level [[2], [3],15]. The first cases were determined as unexplained pneumonia cases, in China. COVID-19 cases have spread to most areas rapidly due to many factors, such as the movement of people, international travel, culture, and socio-economic factors and status. During the first period of this year (January to March), people contracting COVID-19 in Asia, Europe and the USA slightly increased. The daily activity of people infected with COVID-19, not having symptoms might have generated the rapid spread of the disease. The first case of COVID-19 in Thailand was detected 22 January 2020. The number of infected cases slightly increased at a slow rate in the first period until mid-March. After mid-March, the number of new cases sharply increased until 26 March at an average rate of 25.1% per day. The governmental policy and related public health interventions changed the trend of the confirmed positive cases. This period can be classified in two sections, namely, (I) sharp increases, and (II) gradually increasing.
In addition, enhancing people's awareness and implementing regulations also constituted key factors in achieving these goals of controlling disease spread. The distribution of COVID-19 disease was not only within Bangkok, the capital city, where it was first detected, but it spread to all regions of the country due to the social, economic and environmental factors such as travelling and close interaction of people. Other influencing factors involved were the international travel of Thais and foreigners around the world.
In addition, the number of reported cases varied depending on each country because the testing and contact tracing required knowledge and technology. One important factor is the high cost of testing, meaning some countries could not allocate sufficient budget for widespread testing in the community. Therefore, the number of infected cases reported by each country might significantly differ from reality. Many scientific research institutes have endeavored to forecast the number of infected cases. Hospitalization numbers, reported deaths or recovered cases in various conditions have been estimated at global or country levels based on available current data and using different modeling approaches. This paper aimed to present the distribution and pattern of COVID-19 disease and prediction of number cases in Thailand. In addition, we have attempted to forecast the COVID-19 cases in terms of the number of infected and new cases in Thailand using the dynamic SEIR (Susceptible Exposed Infectious and Recovered) model [[4], [5],], and moving average prediction approaches.
2. Method
This research involves two parts, namely, (i) the study of associations between weather factors and the number of COVID-19 confirmed cases, and (ii) the development of a predictive model for COVID-19 transmission.
2.1. The study of COVID-19 infected case and associated weather data
The weather data, including ambient temperature, precipitation and wind speed were obtained from the official website of the Department of Meteorology, Thailand. The weather monitoring stations covered all provinces in Thailand, provided the daily mean values of each parameter used in the analysis. Descriptive statistics were analyzed, namely, minimum, maximum, mean, median, standard deviation in quartiles 1 and 3. We collected published health data from the Ministry of Public Health, which is available from the official website (http://covid19.ddc.moph.go.th/th) [6]. The Spearman's correlation of weather factors with the daily reported COVID-19 cases was analyzed using SPSS (version 18). The analysis employed a spreadsheet for the susceptible and infection rate, recovered rate and death rate. The daily temperature and cumulative infected cases were analyzed for their association at the outbreak period.
2.2. Predictive modeling development
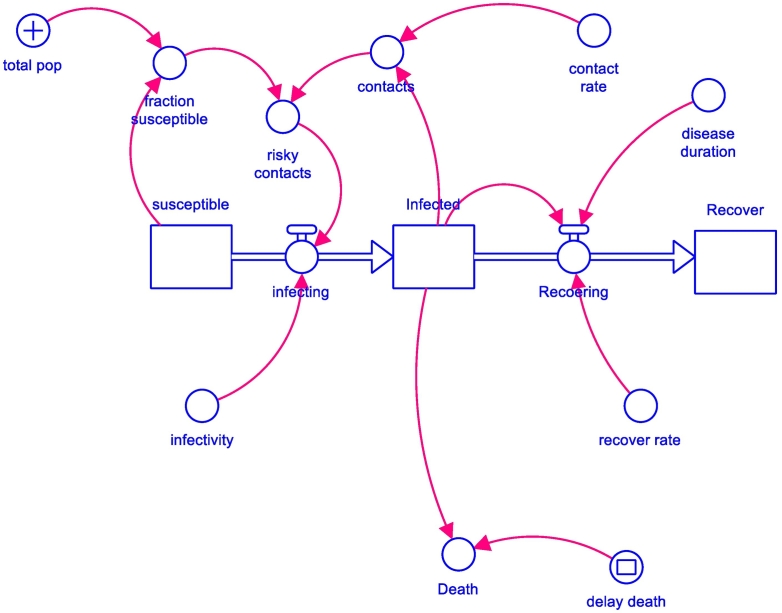
The prediction was performed using the dynamic SIR (Susceptible Infection and Recover) concept model [7], a well-validated model, particularly useful for analyzing the spread of infectious diseases. However, for this paper the SIR was modified to SEIR (Susceptible Exposure Infection and Recover) model [[8], [9], [10], [11]] because the key role of exposure or contact was the most influential factor of disease transmission. All SEIR parameters were dynamic over time, so this research was designed using the dynamic equations to estimate the baseline situation and determine the relevant scenarios [12,13]. The SEIR model concept is illustrated in Fig. 1.
Fig. 1.
SEIR modeling concept (+ the enforcement, − balancing relationship) (29).
New cases could be detected by examination under potential risk conditions, such as contact with an individual with COVID-9. The starting point of the model is when the first infected case has contracted and transmitted the disease to others. It might be only one or many individuals depending on the COVID-19 positive person's social activity. The individual with COVID-19 might spread the disease to others either one-by-one or in many cases simultaneously. The contacted individuals might not become infected due to various factors, such as health status, personal hygiene or environmental health precautions. We estimated the number of infected cases from the number of detected cases. The rate of transmission could be calculated from the available data of the Ministry of Public Health. The characteristics of COVID-19 disease were also analyzed, such as incubation and infection periods. The recovery and death rates were also estimated from the related information such as health data.
The causal loop was converted to a computer diagram using a license STELLA Software to generate the equation as a baseline study using the current parameters for Thailand, such as the rates contact, infection and recovery. Up to this stage, the model focused on the number of infected cases with intervention measures.
In addition, we employed the moving average approach from the previous detected cases for prediction the infected cases by three categories, namely: two, three- and five-days moving average. The pattern or trends of detected cases were used for future prediction. The RMSE (Root Mean Square Error) was used to determine the goodness of fit of each model mentioned above.
3. Result
3.1. Situation of COVID-19 in Thailand
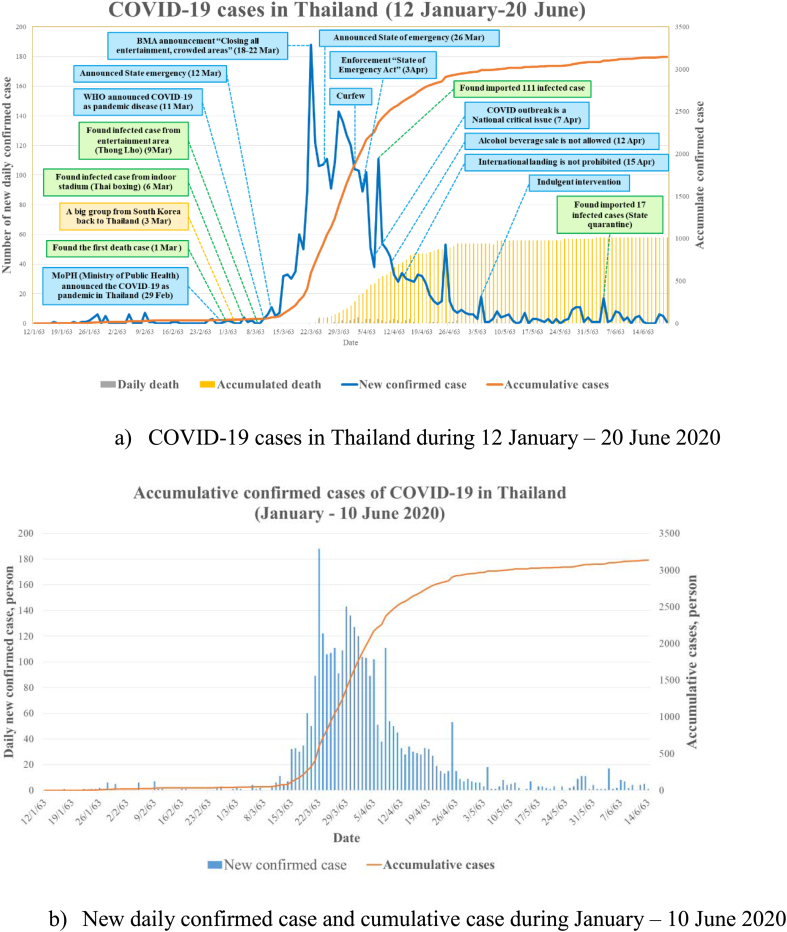
This analysis shows the evolution of COVID-19 cases in Thailand from the first case until 6 July 2020. The majority of positive cases were male, at 56.7%. The average age on positive individuals was 42.5 and 35.7 for males and females, respectively. The maximum age for males was 84, whereas for females was 80 years. The minimum age was found at only five months of age [14]. The timeline of COVID-19 cases in Thailand and critically related events is illustrated in Fig. 2. The first phase was initiated from the first infected case in Thailand 22 January until 15 March 2020, characterized by the low slope of cumulative cases. The second phase could be identified from 16 March when the cumulative cases reached 100 and over. On 16 March the cumulative cases totaled 111, and the slope from this day on sharply increased with the number of infected people reaching 1000 cases on 26 March, as Fig. 2. The timeline of confirmed cases and interventions from the first case to 20 June 2020 is shown in Fig. 2.
Fig. 2.
Timeline of COVID-19 situation and its related events in Thailand from 12 January to 20 June 2020.
Regarding cases resulting in death, the average was 1.14% of patients testing positive for COVID-19 by national registered laboratory, which was much lower than the global average of 5.4% (as of April 4, 2020) [16]. The increasing rate of the positive cases in Thailand was not as sharp as reported in EU countries or the USA. Many reasons include the high temperature (35–39.9 degree Celsius) in hot season and the culture of Thai people using “Wai”, pressing both hands together to show respect or apologize, instead of handshaking. This Thai greeting is a lack of body contact might have played a role in lowering the transmission rate.
3.2. Distribution of COVID-19 in Thailand
The first COVID-19 case was detected in Bangkok in January. It spread to other areas after this first detection. The sharp increase in COVID-19 case from March was an alarming event for the Thai government to act. Consequently, the regulation of closing restaurants and entertainment venues was strictly employed. The trend of increasing cases in other provinces was also unexpected; increasing numbers of cases were detected from 29 March. Therefore, the Royal Thai government announced a state of emergency in Thailand. However, many local organizations at provincial level had already declared their own emergency state. At that time, there were no infected cases in some provinces during the early declaration. However, it remained crucial to enforce the regulations throughout the country; all organizations were required to follow the government policy in their sector. For example, a curfew was imposed from 10:00 pm to 05:00 am. All department stores were closed, except for food and beverage outlets. During the first period, COVID-19 spread mainly in Bangkok, and spread to other areas, as indicated in Fig. 3. The updated records as of 18 July 2020, showed 53% of total infected cases were in Bangkok as illustrated in Fig. 4.
Fig. 3.
Occurrence of COVID-19 cases in Bangkok and other provinces (during 15 March – 4 July 2020).
3.3. Weather parameters and correlation with daily COVID-19 cases
During the study period, descriptive statistics were used to analyze data (Table 1). Ambient temperature across Thailand during the study period differ much for the whole country; the standard deviations of maximum, minimum and mean temperature in degree Celsius were approximately 1.69, 1.67 and 1.57, respectively. The coefficients of variation (CV) were 4.82, 7.43 and 5.47 for maximum, minimum and mean temperatures, respectively. The average maximum and minimum temperatures were 38.1 and 31.1o Celsius, respectively, whereas the minimum and maximum average temperatures were 25.7 and 31.5o Celsius, respectively. During the studied period, almost regions were summer season, however, there was some precipitation in the Southern region of Thailand. Due to the variation of precipitation across the country, the related coefficient of variation was high (61.8%), so this parameter was not analyzed to find any correlation with COVID-19 cases.
Table 1.
Weather data of temperature (in degree Celsius), wind speed and precipitation in Thailand during the studied period (January-4 April 2020.
| Statistic | Max T | Min T | Mean T | WS (m/s) | Precipitation(mm) |
|---|---|---|---|---|---|
| Minimum | 31.11 | 19.10 | 25.68 | 5.16 | 5.94 |
| Q1 (Quartile) | 33.53 | 20.73 | 27.05 | 5.74 | 10.92 |
| Mean | 35.00 | 22.43 | 28.71 | 6.20 | 25.10 |
| Standard deviation | 1.69 | 1.67 | 1.57 | 0.60 | 15.51 |
| Median | 34.88 | 22.79 | 28.85 | 6.12 | 18.06 |
| Q3 (Quartile) | 36.12 | 23.85 | 29.98 | 6.57 | 38.84 |
| Maximum | 38.06 | 25.03 | 31.51 | 8.09 | 52.89 |
| CV (Coefficient of Variation, %) | 4.82 | 7.43 | 5.47 | 9.71 | 61.81 |
Because our available data of COVID-19 cases and weather variables, namely, maximum, minimum and average temperatures from January until 4 April 2020 showed non-normal distribution, Spearman's correlation was used for this study. Details of correlation are presented in Table 2. We used regression analysis to create the equation model using accumulated confirmed cases as the dependent variable and temperature categories as independent variables. We found a significant association (p-value <0.001) as presented in Table 3.
Table 2.
Spearman's correlations among weather parameters and daily accumulated COVID-19 cases.
| Minimum daily temperature | Average daily temperature | Maximum daily temperature | Daily cumulative COVID-19 cases | |
|---|---|---|---|---|
| Minimum daily temperature (Min T) | 1 | 0.912** | 0.938** | 0.732* |
| Average daily temperature (Mean T) | 1 | 0.942** | 0.751* | |
| Maximum daily temperature (Max T) | 1 | 0.694* | ||
| Daily cumulative COVID-19 cases | 1 |
Note: * p-value is 0.05, ** p-value is 0.01.
Table 3.
Association of cumulative COVID-19 confirmed cases and temperature with different models.
| Parameter | Correlation with Accumulate COVID case | R2 | Model | Regression model | Significance |
|---|---|---|---|---|---|
| Minimum temperature | 0.599 | 0.40 | Linear regression | −3776.45 + 181.69*Min T | <0.001 |
| 0.587 | 0.35 | Logarithmic | −11,959.65 + 3944.5*ln(Min T) | <0.001 | |
| 0.575 | 0.33 | Inverse | 4119.60–85,243.23(1/Min T) | <0.001 | |
| 0.730 | 0.53 | Quadratic | 44,466.87–4182.39*MixT+49.54*(Min T)2 | <0.001 | |
| Average temperature | 0.654 | 0.43 | Linear regression | −5745.72 + 210.52*Mean T | <0.001 |
| 0.643 | 0.41 | Logarithmic | −19,583.02 + 5924.48*ln(Mean T) | <0.001 | |
| 0.633 | 0.40 | Inverse | 6105.65–166,250.10(1/Mean T) | <0.001 | |
| 0.792 | 0.63 | Quadratic | 80,241.76–5806.13*MeaT+104.94*(Mean T)2 | <0.001 | |
| Maximum temperature | 0.624 | 0.39 | Linear regression | −6234.54 + 186.64*Max T | <0.001 |
| 0.617 | 0.38 | Logarithmic | −22,640.74 + 6453.90*ln(Max T) | <0.001 | |
| 0.609 | 0.37 | Inverse | 6668.12–222,461.09(1/Max T) | <0.001 | |
| 0.688 | 0.47 | Quadratic | 54,534.76–3287.53*Max T + 49.54*(Max T)2 | <0.001 |
3.4. Prediction of daily infected cases
Many news sources stated that the number of COVID-19 cases in Thailand would present an exponential growth rate, and some researchers reported that the growth rate of 33% infection would remain [17,18]. The number of positive cases in Thailand would reach 300,000 within 15 April 2020, if intervention was not strictly performed [19]. This was also the reason the authors investigate the prediction with or without intervention enforcement.
The causal loop diagram shown in Fig. 1 was transferred to the diagram in Fig. 4 and then converted to eqs. (1 to 4) using the SEIR approach. For example, the number of positive cases depended on the number of individuals with COVID-19 and their contacts in each period. The SEIR model has been applied in the study of many infectious diseases [20,21]. The equations of the SEIR model are shown below.
Fig. 4.
Proportion of local infection and state quarantine of COVID-19 in Thailand.
N is the number of the population.
S is the number of the suspected population.
μ is the increasing or decreasing rate of the population.
| (2) |
I is the number of the infected population.
α is the probability of changing from S to E.
| (3) |
E is the number of the exposed population.
β is the probability of changing from E to I.
| (4) |
δ is the probability of changing from I to R.
3.4.1. Base run
We simulated the base run using the current situation and using the implementation approach of intervention, namely, reduced contacting rate. At the base run study, 85% of no close contact or reduction of exposure (social distancing) was performed according to the enforcement of interventions such as the State Emergency Act, curfew, closing many high transmission risk places (indoor stadiums, department stores, restaurants and others). The result of predicted cumulative positive cases compared with the actual cumulative cases is presented in Fig. 5. We also calculated the root mean square errors (RMSE) of the predictive and actual values, using the RMSE equation below.
Fig. 5.
Predicted infected COVID-19 cases and actual cases as of 10 June 2020.
Where yi is the actual numbers, and y'i is the predictive values. The calculated RMSE was 12.8%. In addition, the correlation of predictive and actual values was 0.84 (R2 = 0.71) as illustrated in Fig. 6.
Fig. 6.
Daily predicted positive COVID-19 cases and actual cases as of 10 June 2020.
In addition, the authors used the moving average model for prediction; namely, two, three- and five-days moving averages. Johns Hopkins University used five days moving average for prediction [22]. In addition, other researchers also employed this technique to forecast COVID-19 cases [23]. The results of two, three- and five-days moving averages are presented in Fig. 7. We found that two- and three-days moving average provided better results than five days moving average. The correlation between actual values and two, three- and five-days moving averages were 0.98, 0.98 and 0.94, respectively. The RMSE were 0.21, 0.21 and 0.35, respectively, as illustrated in Table 4 and Fig. 7.
Fig. 7.
Two, three- and five-days moving averages to predict COVID-19.
Table 4.
The goodness of fits of models, advantage and disadvantage of used models in this study.
| Model | Correlation of actual values and prediction values | RMSE value (%) | Advantages | Disadvantage | |
|---|---|---|---|---|---|
| SEIR (Susceptible, Exposure, Infection and Recover) | 0.84 | 12.8 | The advantages of this model are; long term prediction. It can be used for various scenarios study that link to the actual situation. |
The complicated model needs various inputs and understanding. The influencing factors such as intervention and its enforcement, individual implementation are required as inputs for model simulation. | |
| Moving average | Two days | 0.98 | 0.21 | It can be used for short term prediction. Only previous records are used for prediction. It can be used to understand the behaviour of the disease |
The limitation for long term prediction. The influencing factors are not used for forecasting. |
| Three days | 0.98 | 0.21 | |||
| Five days | 0.94 | 0.23 | |||
3.4.2. The burden of disease control
Regarding Thai confirmed cases of COVID-19, we found that imported cases were the key influencing factors, and the interaction of people in many areas such as entertainment places like Thai boxing stadiums and bars in a closed hall or a room were the main spreading factors to other areas. Other important factors included attending crowded cultural and religious ceremonies, and incorrectly performed self-quarantine. The practice of isolating to prevent contacting others was important to reduce the growth rate and disease spread in Thailand, as reported by other researchers [5]. Community health volunteers play a strong role in the Thai health system and they can cover the whole country. The partnership among the involved and responsible organizations in each area and individual cooperation were the key role for disease spreading control.
4. Discussion
From the records, COVID-19 in Thailand was mainly found in Bangkok, which was the original source of spreading. Bangkok is the center of various aspects in this country and serves as the gateway for foreigner visitors to Thailand. Moreover, foreigners typically visit Bangkok because of the available tourist attractions. In addition, many international and local flights are offered to many places that are convenient and comfortable [17]. In addition, health volunteering in the capital city cannot be easily performed due to the complexity of the health system, whereas health volunteering in rural or other provinces was simplified regarding the duties of volunteers.
A strong correlation between temperature and the number of COVID-19 confirmed cases, we found a similar result of average temperature and confirmed cases in Jakarta, the capital city of Indonesia located in the same tropical climate zone [[24], [25], [26], [27]]. Another study conducted in a subtropical zone like China also reported that temperature was positively related to the number of infected cases [28].
The predicted values deviated from actual values due to many influencing factors such as the quarantine, social distancing and individual health status. The individual health and quarantine were not used in this mode. The moving average prediction method provided better results than the SEIR model for this study. Fewer moving average days (two or three days) produced better results than more days (five days). However, the limitation of moving average approaches is that they cannot predict cases in the long term, compared with the SEIR model. In order to improve the performance of SEIR model, the details of influencing factors should be investigated as model input for further studies.
5. Conclusion
COVID-19 disease was determined as a pandemic by WHO [3,29]. The increasing confirmed cases of COVID-19 in Thailand can be classified in two phases; namely, phase I (slow increase from the first case until accumulating 100 cases) and phase II (sharp increase from reaching 100 cases until 8 April 2020). Regarding our model prediction, we found that moving average approaches gave better results than the SEIR model. The shorter moving average (i.e. period of days) also provided better results than a longer moving average. However, the moving average approach is only suitable for short term prediction, whereas SEIR modeling can be used for longer prediction. Controlling the disease spread was strictly enforced through the collaboration of central and local governments, partnerships among stakeholders and people in their community. This highlighted that social distancing and personal hygiene and should be performed rigorously and consistently across sectors [4,30]. Isolating confirmed cases also played a key role in reducing daily spread of the disease [5]. Moreover, the Thai government employed health volunteer at the community level to act by isolating at risk members in the community. They screened all entering people in the community by following the Ministry of Public Health procedure and examined the fever by using a thermometer. The government should support stockpiling of medical supplies, such as suitable personal protective equipment, and make them available to medical and health care providers, the special place for COVID-19 screening and others. Further, the private sector should join forces with government agencies to provide masks, and alcohol gel hand sanitizer to the general public. Currently the infected cases of COVID-19 in Thailand situation is controllable due to the strength point of the Thai public health system and the results of the model would benefit the government in controlling disease transmission. However, it is very interesting to investigate more about the transmission of disease from animal to human and the interaction of different aspects of government, influential socio-economic aspects of disease transmission. Moreover, the medical aspects and public health facilities and management system would be the important factors of disease control.
Acknowledgments
Acknowledgment
The authors would like to thank the Ministry of Public Health for their published data of COVID-19 cases and the Department of Meteorology, Thailand for providing weather data.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Author statement.
References
- 1.Manav R. 2020. Bhatnagar, COVID-19: Mathematical Modeling and Predictions. @IIT Delhi. [Google Scholar]
- 2.Patrick G.T., Walker C.W., Watson Oliver. The global impact of COVID-19 and strategies for mitigation and suppression. In: Walker C.W. Patrick G.T., Watson Oliver., editors. WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis. Abdul Latif Jameel Institute for Disease and Emergency Analytics, Imperial College London; 2020. [Google Scholar]
- 3.WHO Coronavirus Disease (COVID-19) Pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from.
- 4.Prem K. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5(5):e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellewell J. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health, M.O.P Covid-19 Infected Situation Reports. 2020. http://covid19.ddc.moph.go.th/en Available from.
- 7.Lin Q. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int. J. Infect. Dis. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H. Improved epidemic dynamics model and its prediction for COVID-19 in Italy. Appl. Sci. 2020:10(14). doi: 10.1016/j.ijid.2020.02.058. [DOI] [Google Scholar]
- 9.He S., Peng Y., Sun K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 2020;101:1667–1680. doi: 10.1007/s11071-020-05743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J. Thorac. Dis. 2020;12(3):165–174. doi: 10.21037/jtd.2020.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carcione J.M. A simulation of a COVID-19 epidemic based on a deterministic SEIR model. Front. Public Health. 2020;8(230) doi: 10.3389/fpubh.2020.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucharski A.J.E.A. Early dynamics of transmission and control of 2019-ncov: a mathematical modelling study. The Lancet Infectious Diseases. 2020;20 doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read J.M., Bridgen J.R., Cummings D.A., Ho A., Jewell C. Novel coronavirus 2019-ncov: early estimation of epidemiological parameters and epidemic predictions. P.medRxiv. 2020 doi: 10.1101/2020.01.23.20018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Disease Control M.O.P.H. Covid-19 Infected Situation Reports. 2020. http://covid19.ddc.moph.go.th/en Available from.
- 15.Worldometer Coronavirus Cases. 2020. https://www.worldometers.info/coronavirus/ Available from.
- 16.the Center for Systems Science and Engineering (CSSE) J.H.U. Coronavirus COVID-19 Global Cases the Center for Systems Science and Engineering (CSSE) 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 4 April 2020; Available from.
- 17.Tantrakarnapa K., Bhopdhornangkul B., Nakhaapakorn K. Influencing factors of COVID-19 spreading: A case study of Thailand. J. Public Health. 2020:1–7. doi: 10.1007/s10389-020-01329-5. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.hfocus Trends of COVID-19 in Thailand 2020 [cited 2020 June 20] https://www.hfocus.org/content/2020/03/18753 Available from:
- 19.Prachachat, prachachat.net (on line newspaper) It is an on line newspaper, prachachat.net (on line newspaper) https://www.prachachat.net/general/news-436343. Prachachat.news; 2020. The Prediction of COVID-19 Cases by Siriraj Hospital (in Thai)https://www.prachachat.net/general/news-436343 Bangkok. [Google Scholar]
- 20.Annas S. 2020. Stability Analysis and Numerical Simulation of SEIR Model for Pandemic COVID-19 Spread in Indonesia; p. 139. Chaos, Solitons & Fractals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martelloni G., Martelloni G. 2020. Modelling the Downhill of the Sars-Cov-2 in Italy and a Universal Forecast of the Epidemic in the World; p. 139. Chaos, Solitons & Fractals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.University, J.H New Cases of COVID-19 In World Countries. https://coronavirus.jhu.edu/data/new-cases 2020 [cited 2020 18 July]; Available from.
- 23.He Y.-T. Moving-average based index to timely evaluate the current epidemic situation after COVID-19 outbreak. medRxiv bioRxiv. 2020 doi: 10.1101/2020.03.24.20027730. [DOI] [Google Scholar]
- 24.Wang J.T.K., Feng K., Lin X., Weifeng L., Chen K., Wang F. High temperature and high humidity reduce the transmission of COVID-19. SSRN Electron. J. 2020 https://www.cebm.net/study/covid-19-high-temperature-and-high-humidity-reduce-the-transmission-of-covid-19/ [Google Scholar]
- 25.Xie J. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosepu R. Correlation between weather and Covid-19 pandemic in Jakarta. Indonesia. Sci Total Environ. 2020;725:138436. doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y.A.J.X. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dashraath P. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardoulakis S. COVID-19 environmental transmission and preventive public health measures. Aust. N. Z. J. Public Health. 2020 doi: 10.1111/1753-6405.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]