Abstract
Secondary acute myeloid leukemia (sAML) poorly responds to conventional treatments and allogeneic stem cell transplantation (HSCT). We evaluated toxicity and efficacy of CPX-351 in 71 elderly patients (median age 66 years) with sAML enrolled in the Italian Named (Compassionate) Use Program. Sixty days treatment-related mortality was 7% (5/71). The response rate at the end of treatment was: CR/CRi in 50/71 patients (70.4%), PR in 6/71 (8.5%), and NR in 10/71 (19.7%). After a median follow-up of 11 months relapse was observed in 10/50 patients (20%) and 12 months cumulative incidence of relapse (CIR) was 23.6%. Median duration of response was not reached. In competing risk analysis, CIR was reduced when HSCT was performed in first CR (12 months CIR of 5% and 37.4%, respectively, for patients receiving (=20) or not (=30) HSCT, p = 0.012). Twelve-months OS was 68.6% (median not reached). In landmark analysis, HSCT in CR1 was the only significant predictor of longer survival (12 months OS of 100 and 70.5%, for patients undergoing or not HSCT in CR1, respectively, p = 0.011). In conclusion, we extend to a real-life setting, the notion that CPX is an effective regimen for high risk AML patients and may improve the results of HSCT.
Subject terms: Combination drug therapy, Drug development
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous disease including approximately one fourth of cases secondary to previous hematological disorders (sAML) or developing after chemotherapy or radiotherapy (tAML)1–5. sAMLs and tAMLs are more frequent in older patients and their prognosis is often worsened by the presence of adverse/complex cytogenetics, high risk molecular aberrations, and impaired performance status6–8. These unfavorable biologic and clinical features deeply affect the efficacy of conventional treatment that is, generally, able to induce less than 40% short-term complete remissions with poor tolerability9,10. Allogeneic stem cell transplantation (HSCT) is the only curative therapeutic option in this unfavorable setting11. However, HSCT feasibility and overall results are impaired by the low efficacy of available induction therapies, the high median age and comorbidity burden of the majority of the patients8,11. Recently, targeted drugs such as gemtuzumab ozogamicin (GO) and midostaurin showed promising efficacy in de novo AML but data on elderly AML patients are lacking12,13. CPX-351 (VYXEOS®, Jazz Pharmaceuticals) is a liposomal encapsulation of cytarabine and daunorubicin, with a molar ratio of 5:114,15. A phase II study and a phase III randomized trial have shown an increased progression free survival (PFS) and overall survival (OS) in patients with sAMLs and tAMLs receiving CPX-351, compared to individuals treated with standard 3 + 7 chemotherapy. Indeed, the best outcome was observed in patients who were consolidated with HSCT16,17. The drug has, therefore, received Food and Drug Administration (FDA) and European Medicine Agency (EMA) approval for the treatment of adult patients with tAMLs, sAMLs, or AML with morphologic myelodysplasia-related changes (MRC–AML). Efficacy and toxicity data were subsequently confirmed in a phase 4, multicenter, single-arm, open-label early access program conducted in the United States18.
However, the feasibility and activity of CPX have never been evaluated outside formal clinical trials, where inclusion criteria may have produced a positive patient selection bias. Thus, the aim of the present study was to evaluate, in a cohort of patients treated according to the Italian Compassionate Use Program (CUP), the therapeutic role of CPX-351 in a real-life setting with particular focus on the outcome of patients who received HSCT in first CR.
Material and methods
Italian compassionate use program
The Italian compassionate use program (CUP) for CPX-351 (Vyxeos®) started on June 2018 and ended in July 2019. A total of 38 Italian Centers were included in the CUP and 33 of them actually enrolled patients. Main enrollment criteria were diagnosis of sAML or tAML according to WHO 2016 definitions and cardiac ejection fraction >50%1. The complete checklist for inclusion and exclusion criteria adopted in the CUP is provided in the supplementary materials section.
Following FDA and EMA approval, CUP treatment was designed and performed according to the phase III trial by Lancet et al.17 and consisted in:
- Induction course with a CPX-351 dose of 44 mg/m2 (daunorubicin 44 mg/m2 plus cytarabine 100 mg/m2) repeated on day 1, 3, and 5. A second induction with the same dose of the drug given on day 1 and 3 was allowed for patients failing to achieve at least CRi after the first induction cycle.
- Up to two consolidation courses with a CPX-351 dose of 29 mg/m2 (daunorubicin 29 mg/m2 plus cytarabine 65 mg/m2) repeated on day 1 and 3 were scheduled for each patient.
Allogeneic stem cell transplantation consolidation was allowed in every phase of treatment, as per internal guidelines and clinical evaluation.
Written informed consent for treatment and data collection as well as EC approval was obtained for each patient enrolled in the CUP. A total of 73 patients have been treated in 33 different Centers during the CUP. CRF forms were sent to all Centers for data collection. The study was conducted according to the Declaration of Helsinki.
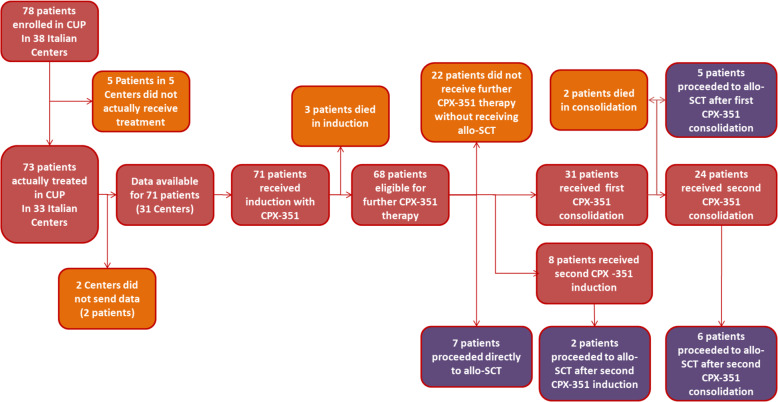
Thirty-one/33 Centers (93.9%) provided complete data, accounting for 71/73 treated patients (97%).
Diagnostic work-up
Conventional cytogenetic analysis with q-banding was performed locally in all patients and cytogenetic abnormalities were graded according to Medical Research Council Criteria7. Molecular work-up was performed as per local standard in all Centers and included NPM1 and FLT3-ITD mutational status in almost all patients, whereas TP53 mutations were evaluated in 37/71 patients (42.3%). Other mutations such as IDH1, IDH2, and RUNX1 were evaluated only in a small minority of patients (data not shown). European Leukemia Net 2017 (ELN 2017) risk score was adopted for risk definition in all patients3.
Response assessment, and adverse events definition
Complete response (CR) was defined by bone marrow blast count <5% with complete platelets and neutrophil count recovery, complete response with incomplete count recovery (CRi) was defined by <5% bone marrow blast count without platelet or neutrophil count recovery, whereas partial response (PR) was defined by a count recovery with a marrow blast count between 5 and 25% and reduced more than 50% from baseline, as per conventional IWG definitions3. Response assessment timing was chosen in each Center as per clinical standards. Minimal residual disease (MRD) assessment was not a mandatory endpoint of the study, and was performed according to internal clinical practice, following ELN suggestions3. WT1–MRD assessment was performed as recommended by ELN19. Flow cytometry-based MRD assessment data were available from 17/31 Centers (54.8%) for 40/71 patients (56.3%), whereas WT1-based MRD was available for 15/31 (48.4%) Centers for 38/71 patients (53.5%).
Adverse events during treatment were defined and graded according to common terminology criteria for adverse events (CTCAE).
Statistical analysis
The cumulative incidence of relapse (CI) at various time points was calculated in competing risk analysis by counting nonrelapse mortality (NRM) as a competing event. Dichotomous variables were compared using the Chi-Square test or by Fisher’s exact test when necessary. Continuous variables were compared using Student’s T-test, or if normal distribution could not be confirmed, by Wilcoxon’s rank test. A multivariate logistic regression model was built for risk of death and risk of relapse assessment, and included only variables that reached a p value of at least <0.100 in early univariate analysis. Overall survival (OS) was calculated from the first day of treatment until death by any cause, or last follow-up20.
A separate landmark analysis was performed in order to evaluate the impact of transplantation in comparison with other variables on OS, including only patients alive and in CR after day 90. Survival curves were built using the Kaplan–Meier method, and univariate survival analysis was performed using the Log-rank test. A Cox proportional Hazard model was built for each multivariate survival analysis, including only the variables that respected proportional risk assumption. All statistical analyses, except competing risk analysis, were performed using IBMSPSS v22© running on a Debian (Linux) operating system. Competing risk analyses were performed using the Fine and Gray sub distribution relative hazard method, and compared with Gray’s test using R statistical software (www.r-project.com), running on a Debian (Linux) operating system20.
All two-tailed p values <0.05 were considered statistically significant.
Results
Patients
Patient’s features are summarized in Table 1.
Table 1.
Patients characteristics.
| Variable | N (%) |
|---|---|
| Age | |
| <70 years | 51 (71.8) |
| >70 years | 20 (28.2) |
| Sex | |
| Male | 39 (54.9) |
| Female | 32 (45.1) |
| WBC | |
| <30 × 109/L | 60 (84.5) |
| >30 × 109/L | 11 (15.5) |
| Marrow blasts | |
| <30% | 22 (31.0) |
| >30% | 49 (69.0) |
| Previous HMA | |
| No | 54 (76.1) |
| Yes | 17 (23.9) |
| NPM1 (evaluated in 68/71, 96%) | |
| Wild type | 63 (92.7) |
| Mutated | 5 (7.3) |
| FLT3-ITD (evaluated in 69/71, 97%) | |
| Negative | 64 (92.8) |
| Positive | 5 (7.2) |
| TP53 (evaluated in 37/71, 52%) | |
| Wild type | 24 (64.9) |
| Mutated | 13 (35.1) |
| Karyotype | |
| Favorable | 3 (4.7) |
| Intermediate | 36 (50.2) |
| Poor | 32 (45.1) |
| CPX-351 indication | |
| s-AML | 36 (50.2) |
| t-AML | 22 (31.0) |
| MDS-related changes | 13 (18.8) |
| ELN 2017 | |
| Low/Int. | 8 (11.3) |
| Intermediate | 24 (33.8) |
| High | 39 (54.9) |
Patients were included in the CUP study from June 2018 to July 2019. One Center enrolled more than ten patients, three Centers enrolled four patients, five Centers enrolled three patients, whereas two or one patients were enrolled in 6 and 16 Centers, respectively. Median follow-up was 11 months (95% CI 10.47–11.53 months). Median age was 66 years (range 52–79).
CPX-351 was used in 22 patients (31%) for tAML, in 36 patients (51%) for sAML, evolving from MDS (31) or CMMoL (five patient), whereas in 13 patients CPX-351 was given for morphological MDS-related changes only (18%). Among 36 patients with a previous formal diagnosis of MDS or CMMoL, 17 (47%) had already received hypomethylating agents, for a median of four cycles (range 1–78) (Table 1).
62 (88%) patients had at least one comorbidity, the most frequent being hypertension (20 patients), type II diabetes (14 patients), COPD (13 patients), cardio-vascular disease (nine patients), hypothyroidism (seven patients), NASH (three patients). 25 (35%) patients had been previously diagnosed with another neoplasia, 23 of them had already received chemo or radiotherapy. Notably, four patients had prior autologous hematopoietic stem cell transplantation for low grade lymphoma (n. two patients) and multiple myeloma (n. two patients). Six patients (9%) presented with ECOG 3–4 upon enrollment.
Median WBC count at diagnosis was 3.3 × 109/L (range 0.4–213). Karyotype was abnormal in 40 patients (56.3%). The most frequent abnormalities were complex karyotype (18 patients), deletion of chromosome 7, (eight patients), del(5q) (seven patients). NPM1 mutations were found 5/68 patients (7.4%), whereas FLT3-ITD mutations occurred in 5/69 patients (7.2%). None of the patient had concomitant FLT3-ITD and NPM1 mutation. TP53 mutations were found in 13/37 assessed patients (35.1%). In almost all cases (12/13), the mutation was found in the context of a complex karyotype.
Treatment overview
All 71 patients received CPX-351 first induction. Three patients (4.2%) died before response assessment, so that 68 patients were eligible for further CPX-351 therapy as per protocol.
Seven (11%) patients proceeded directly to HSCT after first CPX-351 induction, whereas 22 patients did not receive further treatment, mostly for lack of response or disease progression (n. 12), impossibility to get CPX-351 due to termination CUP and lack of commercial drug (n. 6), patient refusal (n. 4). Therefore, 39 proceeded with CUP treatment program; 8/68 (12%) patients received second CPX-Induction, whereas 31/68 (46%) patients proceeded with first CPX-351 consolidation course with 24 (77%) of them receiving also the second course. Two patients died during consolidation therapy and 60 days treatment-related mortality was, therefore, 5/71 (7%). 5 out of 31 patients (16%) proceeded to HSCT after the first CPX-351 consolidation cycle, whereas six patients received HSCT after the second consolidation course and two after second induction. Two more patients received HSCT: one with progressive disease after loss of CR previously achieved with CPX-351 and one as consolidation of CR achieved with second line therapy after failure of CPX-351 induction. Main reason for not performing HSCT were poor performance status, comorbidities, and patient refusal. A detailed overview of patient flow in the CUP program is provided in Fig. 1.
Fig. 1. Treatment overwiev.
Patients enrollment and treatment in Compassionate Use Program.
Treatment toxicities
Mortality rate following first CPX-351 course was 4.2% (3/71). 57 (80.3%) patients experienced grade >1 adverse events (AEs) during induction.
Most of the AEs were infections, with fever of unknown origin (FUO) in 20/71 (28%), sepsis in 20/71 (28%), pneumonia in eight patients (11.3%), including two Pneumocistis jirovecii-related pneumonia (PCP), invasive fungal infections in three patients (4.2%). Mucositis was reported in five patients (7%), whereas a self-resolving diffuse skin rash was observed in 18/71 patients (25.4%). Four patients experienced alopecia (5.6%). Most of the AEs were easily manageable and resolved completely. Median time to neutrophil >0.5 × 109/L and platelet >25 × 109/L recovery was 38 (range 12–60) and 28 (range 12–60) days, respectively.
Median time from first day of induction cycle and first day of second cycle was 55 days (range 33–101). Second cycle therapy-related mortality was 5.1% (2/39). Grade >1 AEs were reported in 25/39 patients (64.1%) during second cycle, including FUO in nine patients (23.7%), sepsis in 3 (7.7%), pneumonia in 3 (7.7%), including 1 PCP pneumonia, mucositis in 2 (2.8%), and diffuse skin rash in 8 (20.5%).
Overall, 60 days treatment-related mortality was 5/71 (7%), due to uncontrolled infections (n. three patients) or bleeding (n. two patients).
Response evaluation
Response to first induction was evaluated in 68/71 surviving patients after a median of 36 days from first day of CPX-351 administration. CR was observed in 38/71 patients (53.5%) whereas CRi and PR were reported in 8/71 (11.3%) and 6/71 patients (8.5%), respectively. Sixteen patients did not respond (NR) (22.5%) and three patients died before response evaluation (4.2%), two because of uncontrolled CNS bleeding and one because of severe pneumonia. Among the 40 patients undergoing MFC MRD analysis, MRD negative CR was reached in 15 patients (37.5%). 21 (53.8%) of the 38 patients evaluated with WT1-based MRD assessment achieved WT1–MRD negativity.
At the end of CPX treatment (EOT) 50/71 (70.4%) patients were in CR, 6/71 (8.5%) in PR and 10/71 (14.1%) were refractory, whereas treatment-related mortality was 7% (five patients).
Specifically, one CR patient died during first consolidation because of uncontrolled infection and one CRi patient lost response while the other 44 maintained CR/CRi at EOT. Among PR patients, one died after second cycle because of uncontrolled infection, two achieved CR, four maintained PR, and one lost response at EOT. Six of the 14 NR patients received second induction: 4 and 2 of them achieved CR and PR, respectively, at EOT.
Response probability was not influenced by any of the analyzed variables (Table 2). In particular, the response rate was not lower in patients who had failed previous HMA for MDS (CR rate of 10/17 and 40/54, for patients with or without previous HMA treatment, respectively, p = 0.361), in patients harboring TP53 mutations (CR rate of 10/13 and 18/24 for patients with or without TP53 mutations, respectively, p = 1.000), in patients with high-risk disease according to ELN 2017 (CR rate of 25/32 and 25/39, for patients with low/intermediate vs. high-risk disease, respectively, p = 0.291). CR rates reported in patients with high risk cytogenetic features (CR rate 19/32, 59.4%), such as complex karyotype (CR rate of 11/18, 61.5%) or deletion of chromosome 5 or 7 (CR rate 8/15, 53.5%) were not significantly different from those observed in patients with either normal or nonhigh risk cytogenetic (CR rate of 31/37, p = 0.074). Age at diagnosis did not impact on CR probability both if evaluated continuously or with any chosen cut-off. Multivariate logistic regression analysis showed that only high risk cytogenetics and marrow blast count above 30% had a borderline, nonsignificant, impact on response probability (p = 0.057 and 0.062, respectively).
Table 2.
Complete remission analysis.
| Variable | N (%) | CR–CRi (%) | p-value (univariate) | Hazard ratio (95% CI)* | p-value (multiv.) |
|---|---|---|---|---|---|
| Overall | 71 (100%) | 50 (70.4%) | – | – | – |
| Age | |||||
| <70 years | 51 (71.8) | 37 (72.5) | 0.531 | 0.901 (0.639–1.271) | – |
| >70 years | 20 (28.2) | 13 (65.0) | |||
| Sex | |||||
| Male | 39 (54.9) | 29 (74.4) | 0.446 | 0.821 (0.495–1.363) | – |
| Female | 32 (45.1) | 21 (65.6) | |||
| WBC | |||||
| <30 × 109/L | 60 (84.5) | 42 (70.0) | 1.000 | 1.020 (0.825–1.262) | – |
| >30 × 109/L | 11 (15.5) | 8 (72.7) | |||
| Marrow blasts | |||||
| <30% | 22 (31.0) | 19 (86.4) | 0.055 | 0.376 (0.124–1.136) | 0.062 |
| >30% | 49 (69.0) | 31 (63.3) | |||
| Previous HMA | |||||
| No | 54 (76.1) | 40 (74.1) | 0.361 | 0.833 (0.598–1.162) | – |
| Yes | 17 (23.9) | 10 (58.8) | |||
| NPM1 | |||||
| Wild type | 63 (92.6) | 42 (66.7) | 0.176 | 1.194 (1.012–1.235) | 0.150 |
| Mutated | 5 (7.4) | 5 (100) | |||
| FLT3-ITD | |||||
| Negative | 64 (92.8) | 45 (70.3) | 1.000 | 0.965 (0.825–1.119) | – |
| Positive | 5 (7.2) | 3 (60.0) | |||
| TP53 | |||||
| Wild type | 24 (64.9) | 18 (75.0) | 1.000 | 1.037 (0.605–1.776) | – |
| Mutated | 13 (35.1) | 10 (76.9) | |||
| Karyotype | |||||
| Fav./Int. | 39 (54.9) | 31 (79.5) | 0.074 | 0.614 (0.342–1.105) | 0.057 |
| Poor | 32 (45.1) | 19 (59.4) | |||
| Therapy related | |||||
| No | 49 (69.0) | 37 (75.5) | 0.260 | 0.752 (0.515–1.158) | – |
| Yes | 22 (31.0) | 13 (59.1) | |||
| ELN 2017 | |||||
| Low/Int. | 32 (45.1) | 25 (78.1) | 0.296 | 0.667 (0.343–1.297) | – |
| High | 39 (54.9) | 25 (64.1) | |||
*Hazard ratio calculation refers to the first row of each variable.
Detailed analysis of CR probability is shown in Table 2.
Cumulative incidence of relapse
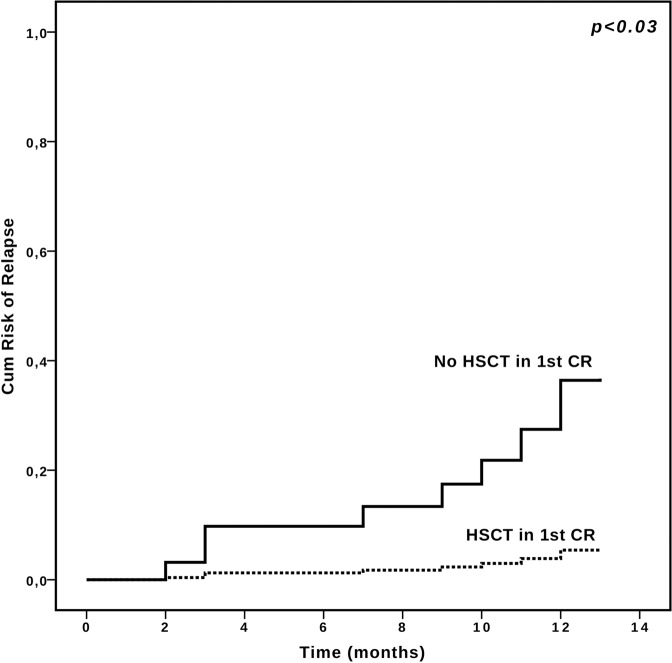
After a median follow-up of 11 months (95% CI 10.47–11.53 months), relapse was observed in 10/50 responding patients (20%) and 12 months CIR was 23.6%, whereas median duration of response was not reached.
In competing risk analysis, a lower CIR was observed only when consolidation therapy with HSCT was performed in first CR after CPX-351 therapy, (12 months CIR of 5% and 37.4%, respectively, for patients receiving or not HSCT, respectively, p = 0.012, Fig. 2). A trend towards reduced CIR was observed among patients with MFC MRD negative CR, however without reaching statistical significance (12 months CIR of 11.1% and 36.7%, respectively, for patients with MRD negative or positive CR, p = 0.151). WT1-based MRD analysis led to superimposable results (data not shown).
Fig. 2. Relapse Risk in responding patients according to transplantation.
Cumulative risk of relapse in patients achieving complete remission (CR), receiving or not hematopoietic stem cell transplantation (HSCT).
Multivariate analysis confirmed that HSCT consolidation was the only independent predictor of lower CIR (p < 0.05, data not shown).
Overall survival
Overall, 21 patients died (29.6%), mostly because of refractory disease (n = 16).
Twelve-months OS was 68.6% (median not reached, Fig. 3). In univariate analysis, survival probability was affected only by cytogenetic risk (12 months OS of 78.6 and 56.6%, for patients with favorable/intermediate and poor risk karyotype, respectively, p < 0.05). Failure of previous HMA therapy, ELN 2017 risk score, as well as presence of TP53 mutation did not impact on survival (Table 3). Notably, MRD status after cycle 1 did not affect survival (12 months OS of 71.1 % vs. 84.0% for MRD-negative and MRD-positive patients, respectively, p = 0.414). WT1-based MRD analysis led to similar results (data not shown).
Fig. 3. Overall Survival.
Overall Survival in the whole cohort from the time of enrollment in Compassionate Use Program.
Table 3.
Overall survival analysis.
| Variable | Alive (%) | 12-month OS (%) | Median OS | p-value (univariate) | p-value (multiv.) |
|---|---|---|---|---|---|
| Overall | 50/71(70.4) | 68.6 | NR | – | – |
| Age | |||||
| <70 years | 38/51 (78.5) | 73.0 | NR | 0.241 | – |
| >70 years | 12/20 (60.0) | 58.3 | NR | ||
| Sex | |||||
| Male | 27/39 (69.2) | 68.0 | NR | 0.877 | – |
| Female | 23/32 (71.9) | 69.3 | NR | ||
| WBC | |||||
| <30 × 109/L | 43/60 (71.7) | 70.0 | NR | 0.490 | – |
| >30 × 109/L | 7/11 (63.6) | 62.3 | NR | ||
| Marrow blasts | |||||
| <30% | 17/22 (77.3) | 76.7 | NR | 0.371 | – |
| >30% | 33/49 (67.3) | 64.9 | NR | ||
| Previous HMA | |||||
| No | 16/54 (70.4) | 68.9 | NR | 0.945 | – |
| Yes | 15/17 (70.6) | 69.1 | NR | ||
| NPM1 | |||||
| Wild type | 43/63 (68.3) | 66.6 | NR | 0.162 | – |
| Mutated | 5/5 (100) | 100 | NR | ||
| FLT3-ITD | |||||
| Negative | 45/64 (70.3) | 68.3 | NR | 0.570 | – |
| Positive | 3/5 (60.0) | 60.0 | NR | ||
| TP53 | |||||
| Wild type | 20/24 (83.3) | 83.1 | NR | 0.081 | 0.570 |
| Mutated | 7/13 (53.8) | 51.9 | NR | ||
| Karyotype | |||||
| Fav./Int. | 31/39 (79.5) | 78.6 | NR | 0.049 | 0.051 |
| Poor | 19/32 (59.4) | 56.6 | NR | ||
| Therapy related | |||||
| No | 35/49 (71.4) | 68.7 | NR | 0.717 | – |
| Yes | 15/22 (68.2) | 68.2 | NR | ||
| ELN 2017 | |||||
| Low/Int. | 26/32 (88.9) | 79.9 | NR | 0.073 | 0.071 |
| High | 24/39 (64.7) | 59.5 | NR | ||
| MRD TP1 | |||||
| Negative | 11/15 (73.3) | 71.1 | NR | 0.414 | – |
| Positive | 21/25 (84.0) | 84.0 | NR | ||
Multivariate OS analysis confirmed that karyotype was the only independent predictor of survival, however with only borderline significance (p = 0.051). Detailed OS analysis is provided in Table 3.
In order to assess the impact of HSCT in first CR and the correlation with the other variables, a landmark model was applied, including only patients alive and in CR at day 90. A total of 20/50 (40%) patients achieving CR with CPX-351 underwent HSCT consolidation (Fig. 1). Median age of patients submitted to HSCT was 65.5 years (range 54–73). Four of them (20%) had failed previous HMA for MDS, four had t-AML (20%), 13 had high risk disease according to ELN 2017 (65%). Cytogenetic was unfavorable in nine patients (45%), with deletion of chromosome 5 or 7 in 6 and complex karyotype in 3. Among the 13 patients tested, 3 (23.1%) had a TP53 mutation.
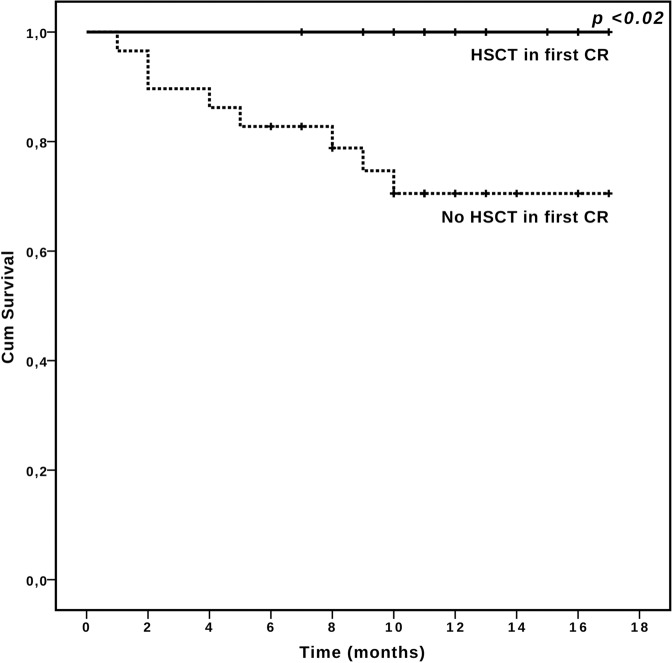
In landmark analysis, HSCT performed in first CR after CPX-351 was the only significant predictor of longer survival (12 months OS of 100 and 70.5%, for patients receiving or not HSCT in CR1, respectively, p = 0.011, Fig. 4). None of the other variables affected survival according to landmark analysis.
Fig. 4. Overall Survival in responding patients according to transplantation.
Landmark Overall Survival analysis in patients alive and in complete remission (CR) at day 90, receiving or not hematopoietic stem cell transplantation (HSCT).
Multivariate analysis confirmed that HSCT in first CR was the only independent predictor for OS (p < 0.05). Detailed overview of the landmark model is provided in supplementary material (Supplemental Table 1).
Discussion
As the outcome of sAML and tAML is unsatisfactory with conventional chemotherapy, and the probability of performing HSCT is low, the results produced by CPX-351 represent a remarkable improvement in the treatment of these diseases8–10,16,17,21–23. Here, in a real life multicenter setting, we confirm the observation of the low toxicity and the high efficacy of CPX-351, and report an increased number of patients undergoing transplant16–18.
Indeed, despite the prolonged hematological recovery after CPX-351 induction, related to the extended drug exposure15,24,25, the incidence of mucositis, severe infectious complications and the mortality rate at 30 and 60 days were lower than those observed after conventional intensive chemotherapy2,9, and comparable to what reported in previous CPX trials16–18. It should be noted that our patients had multiple comorbidities, including concomitant active neoplasms, that in many cases would have precluded their enrollment into phase II–III clinical trials with CPX-35116–18.
Response to treatment was comparable to previous reports16–18. Notably CR probability was not affected by any of the most relevant prognostic factors, such as ELN 2017 risk3 and unfavorable cytogenetics7. Interestingly even the presence of TP53 mutation in the context of a complex karyotype did not impact on the CR rate. This is in contrast with a previous paper showing the detrimental influence of TP53 mutations on CR/CRi rate in patients treated with CPX-351 induction26, but consistent with more recent data from a French Group27, thus suggesting the need of further studies in this setting. In addition, CR rate was not lower among patients progressed under hypomethylating therapy for MDS. This observation, if confirmed in larger series of patients, may have a great clinical value as many trials have reported the lack of activity of conventional treatment after failure of azacitidine or decitabine28,29.
As expected, the duration of response was shorter in patients who cannot proceed to HSCT consolidation, but still satisfactory given the overall poor prognosis of our cohort. The best outcome was observed in patients who promptly underwent transplant after achieving CR. In this view, Lancet et al. showed that the outcome of patients who were transplanted following CPX therapy was significantly better compared to that observed in patients undergoing transplant after conventional 3 + 7 therapy, due to a lower transplant related mortality (TRM) and a reduced post-transplant relapse rate17. The transplant rate in our series (40% among patients achieving CR after CPX-351, 28.2% overall) was slightly higher than that reported in phase II and III trials16,17 and double than that reported in the extended access program by Roboz et al.18. The growing awareness of the lower TRM of HSCT after CPX-351 induction probably led Italian hematologists to a more aggressive transplant policy including the use of alternative donors. Despite the high median age of transplanted patients, no transplant related deaths have been so far reported. It might be speculated that the reduction of TRM was related to the lower extra hematological toxicity during CPX-351 induction and consolidation courses. It is unclear whether the reduced post-transplant relapse rate with CPX-351 might be due to a deeper leukemic cells clearance prior to transplant, as MRD was not evaluated in the phase II and III trials16,17. Considering that pretransplant MRD has a strong impact on postHSCT relapse risk30,31, further trials with CPX-351 should include evaluation of MRD before HSCT. In our study, we evaluated MRD with either MFC or WT1 levels showing that near 50% of complete remissions were MRD negative. Preliminary data from a French compassionate program reported a similar rate of MRD negativity evaluated with next generation sequencing26. In our study, however, albeit showing a trend toward reduced relapse risk, MRD-negativity did not result in better clinical outcome possibly due to the relatively low number of patients.
An alternative explanation is that MRD assessment after the first CPX course may not represent the most informative time point. Indeed, Buccisano et al. showed that MRD analysis after the second chemotherapy course had the highest prognostic value32.
In conclusion, Italian CUP experience confirms that CPX-351 is an effective regimen for high risk AML patients treated with a curative aim. CPX-351 can induce good quality remissions with acceptable toxicity in the majority of patients, and increases results of HSCT, through a reduction of TRM and post-transplant relapse rate. Furthermore, the lower incidence of severe complications expected with CPX-351, compared to conventional treatment, may reasonably increase the number of elderly patients receiving intensive induction and HSCT consolidation. For frail subjects, CPX-351 cannot be recommended as it induces the long-lasting aplasia requiring prolonged hospitalizations. Further studies are needed to investigate the potential role of CPX-351 in combination with other innovative therapeutic agents33 and to identify the factors predicting response34.
Supplementary information
Author contributions
F.G., P.M., M.C., L.F., L.P., and R.M.L. wrote the manuscript. F.G., P.M., L.F., M.C., M.G., S.G., G.R., M.R., G.B., M.D.A., A.B., B.S., P.Z., A.M.S., F.G., G.P., P.M., M.C., S.D.A., E.A., A.C., C.P., F.P., A.C., C.G., M.M., C.A., N.F., G.R., M.C., F.C.S., A.T., G.R., and F.F. provided and revised the data and revised the manuscript. F.G., G.R., F.F., L.P., and R.M.L. designed the research. P.M. and L.F. collected the data. F.G. performed the analysis. F.G., P.M., M.C., G.R., F.F., L.P., and R.M.L. revised the final version of the manuscript. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised: Livio Pagano, Roberto Massimo Lemoli
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41408-020-00361-8).
References
- 1.Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman MA. A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells Mol. Dis. 2013;50:119–130. doi: 10.1016/j.bcmd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Dohner H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miesner M, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified”(AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC) Blood. 2010;116:2742–2751. doi: 10.1182/blood-2010-04-279794. [DOI] [PubMed] [Google Scholar]
- 5.Granfeldt Ostgard LS, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J. Clin. Oncol. 2015;33:3641–3649. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 6.Leith CP, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. doi: 10.1182/blood.V89.9.3323. [DOI] [PubMed] [Google Scholar]
- 7.Grimwade D, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 8.Fianchi L, et al. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am. J. Hematol. 2015;90:E80–E85. doi: 10.1002/ajh.23966. [DOI] [PubMed] [Google Scholar]
- 9.Rai KR, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58:1203–1212. doi: 10.1182/blood.V58.6.1203.1203. [DOI] [PubMed] [Google Scholar]
- 10.Yates JW, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother. Rep. 1973;57:485–488. [PubMed] [Google Scholar]
- 11.Fianchi L, et al. Therapy-related myeloid neoplasms: clinical perspectives. Onco Targets Ther. 2018;11:5909–5915. doi: 10.2147/OTT.S101333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone RM, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomized controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim WS, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine: daunorubicin formulation, in bone marrow xenografts. Leuk. Res. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Kim HP, et al. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp. Hematol. 2011;39:741–750. doi: 10.1016/j.exphem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Lancet JE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancet JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 2018;36:2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roboz GJ, et al. Final safety and efficacy results from the CPX-351 early access program for older patients with high-risk or secondary acute myeloid leukemia. Leuk. Lymph. 2020;61:1188–1194. doi: 10.1080/10428194.2020.1725503. [DOI] [PubMed] [Google Scholar]
- 19.Cilloni D, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J. Clin. Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 20.Delgado J, et al. Survival analysis in hematologic malignancies: recommendations for clinicians. Haematologica. 2014;99:1410–1420. doi: 10.3324/haematol.2013.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appelbaum FR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayser S, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 23.Bejar R, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J. Clin. Oncol. 2014;32:2691–2698. doi: 10.1200/JCO.2013.52.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman EJ, et al. First in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman EJ, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemia. Leuk. Res. 2012;36:1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg AD, et al. TP53 mutations predict poorer responses to CPX-351 in acute myeloid leukemia. Blood. 2018;132:1433. doi: 10.1182/blood-2018-99-117772. [DOI] [Google Scholar]
- 27.Chiche E, et al. CPX-351 induces deep response and suppress the impact of poor prognosis mutations (TP53, ASXL1, RUNX1 and EVI-1) defined by ELN 2017 in t-AML and MRC-AML: a report from a multicentric French cohort. Blood. 2019;134:1355. doi: 10.1182/blood-2019-125623. [DOI] [Google Scholar]
- 28.Jabbour E, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prébet T, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J. Clin. Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley SA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102:865–873. doi: 10.3324/haematol.2016.159343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guolo F, et al. Combining flow cytometry and WT1 assessment improves the prognostic value of pre-transplant minimal residual disease in acute myeloid leukemia. Haematologica. 2017;102:e348–e351. doi: 10.3324/haematol.2017.167254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buccisano F, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119:332–341. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- 33.Di Nardo CD, et al. Venetoclax combined with decitabine or azacitidine in treatment naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talati C, et al. Genomic landscape impacts induction outcome with CPX-351 in patients with acute myeloid leukemia. Blood. 2018;132:2741. doi: 10.1182/blood-2018-99-117412. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.